Abstract
Objectives
In older adults, traditional metrics derived from polysomnography (PSG) are not well correlated with subjective sleep quality. Little is known about whether the association between PSG and subjective sleep quality changes with age, or whether quantitative assessment of EEG (qEEG) is associated with sleep quality. We therefore examined the relationship between subjective sleep quality and objective sleep characteristics (standard polysomnography and qEEG) across middle to older adulthood.
Methods
Using a cross-sectional analyses of 3,173 community-dwelling men and women ages 39–90 participating in the Sleep Heart Health Study, we examined the relationship between a morning rating of the prior night’s sleep quality (Sleep Depth and Restfulness) and polysomnographic and qEEG descriptors of that single night of sleep, along with clinical and demographic measures. Multivariable models were constructed using two machine learning methods, lasso penalized regressions and random forests.
Results
Little variance was explained across models. Greater objective sleep efficiency, reduced wake after sleep onset and fewer sleep to wake stage transitions were each associated with higher sleep quality; qEEG variables contributed little explanatory power. The oldest adults reported the highest sleep quality even as objective sleep deteriorated, such that they would rate their sleep better given the same level of sleep efficiency. Despite this, there were no major differences in the predictors of subjective sleep across the age span.
Conclusion
Standard metrics derived from polysomnography, including qEEG, contribute little to explaining subjective sleep quality in middle-aged to older adults. The objective correlates of subjective sleep quality do not appear to systematically change with age, despite a change in the relationship between subjective sleep quality and objective sleep efficiency.
Keywords: Sleep quality, machine learning, polysomnography, aging, sex differences
1. Introduction
Subjective sleep quality is increasingly recognized as an important aspect of sleep health with implications for functional impairment, morbidity and mortality [1]. Despite this, surprisingly little is known about the subjective and objective sleep correlates that inform sleep quality, nor how they vary across age. It is clear that objective sleep quality worsens with age; studies utilizing polysomnography (PSG) confirm that age-related changes in sleep predispose older individuals to increased fragmentation and wakefulness at night [2]. Moreover, the prevalence of sleep disorders, including insomnia, periodic limb movements, and sleep disordered breathing, increase with age in both sexes [3–5]. Despite the deterioration in objective sleep quality, subjective sleep quality reports appear to plateau or even improve with advancing age [6, 7]. The objective, demographic, and clinical variables that influence subjective sleep quality, and their relationship to advancing age, remains incompletely understood.
Using novel machine learning algorithms, we have recently shown in older adults that traditional PSG metrics, demographics, and clinical characteristics do not predict subjective reports of sleep quality well [8]. Quantitative EEG (qEEG) characteristics have been postulated as promising [9] in providing more informative indices that may explain subjective sleep quality reports, but to date have not been systematically examined. Here, we sought to replicate and broaden our findings by examining qEEG characteristics in predicting subjective sleep quality alongside demographic and clinical factors in midlife and beyond.
2. Methods
2.1 Study Population
Participants in these analyses were enrolled in the Sleep Heart Health Study (SHHS), a multi-center cohort study on the consequences of sleep-disordered breathing. A total of 6,441 participants over the age of 39 who had no history of positive airway pressure treatment, tracheostomy, or home oxygen therapy (all of which would limit polysomnographic interpretations of sleep apnea) completed a baseline examination between 1995 and 1998. Of these, 5,804 had overnight data available for analysis. Participants were excluded from present analyses if both subjective sleep quality reports were missing (n=101), if all EEG data were missing (n=222), and if the PSG record was of poor quality or incomplete to prevent accurate estimation of sleep staging percentage (n=101). Additionally, given the limitations of home testing devices, recordings were excluded where signal failures resulted in an incomplete capture of time in bed period (n=2,146). A final sample of 3,173 was included in subsequent analyses. Excluded and included subjects differed slightly but significantly on age (included vs. excluded subjects mean 62.73 vs 63.62; p<0.01, t-test; Cohen’s d = 0.08), BMI (28.4 vs 27.9; p<0.001, t-test; d = 0.10), and race (greater number of African American participants excluded; p<0.0001, χ2 test; Cramer’s V = 0.07). Excluded and included subjects did not differ by sex.
All participants provided informed consent and all procedures were approved by institutional review boards at respective sites. Further information about the study methods and design are available elsewhere [10]. All data for this project was downloaded from the National Sleep Research Resource (NSRR) website [11].
2.2 Polysomnography
Sleep studies were completed in-home and unattended using polysomnography (PS-2 system; Compumedics, Abbotsford, Australia; see ref [10] for more information). A 12-channel recording montage included C3/A2 and C4/A1 electroencephalograms (EEG), bilateral electrooculograms (EOG), and a chin electromyogram (EMG) to gauge sleep; thoracic and abdominal respiratory inductance plethysmography (respiratory effort); nasal-oral thermocouple (airflow); finger pulse oximetry (blood oxygenation); and electrocardiogram (heart rate). All PSG data were scored in 30-second epochs according to standard criteria [12, 13]. Methods for determining interscorer reliability are reported elsewhere [12, 14]. Variables of interest included polysomnography-determined total sleep time, wake after sleep onset (WASO), percentage of time spent in each sleep stage, latency to REM sleep, the number of sleep to wake shifts per hour, the number of deep (N3) to lighter (N1/N2) stage shifts per hour, and sleep efficiency. Apneahypopnea index, defined as the number of respiratory events with oxygen desaturation ≥ 4% per hour, was also used.
In additional to traditional metrics derived from EEG, quantitative measures of EEG spectral activity in various power bands were also examined. Spectral analysis was completed using methods for automated artifact detection and removal [15] and wavelet decomposition [16] (see NSRR [11] for complete information). All spectral output was reviewed by a technician, and problematic studies were discussed by an international team of experts. Variables of interest included log-transformed REM and NREM alpha (8–12 Hz), beta (15–20 Hz), delta (1–4 Hz), sigma (12–15 Hz), high-frequency sigma (14–15 Hz), slow oscillations (1 Hz) and theta (4–8 Hz) across the night.
2.3 Sleep Quality Ratings
Participants completed a morning sleep diary following their polysomnographic monitoring on which they rated the quality of their prior night’s sleep using 5-point Likert-type scales, with higher scores indicating higher quality, along two dimensions: “Sleep Depth” (Light to Deep) and “Sleep Restfulness” (Restless to Restful). These ratings of sleep quality have been used in previous research [17].
2.4 Self-reported Sleep Variables
Participants reported in hours their habitual sleep duration on weekdays and weekends, which were collapsed to a weighted average reflecting habitual sleep duration across the week. Daytime sleepiness was assessed using the Epworth Sleepiness Scale [18].
2.5 Demographic and Clinical Variables
All participants reported race, ethnicity, sex and level of education attained (grouped as <12 years, 12–16 years, >16 years). The following variables were also reported and used in present models: use of benzodiazepines or antidepressants or any reported sleep medication use (overlapping categories); history of medical illness (including diabetes, stroke, or heart disease [myocardial infarction, angina, bypass or angioplasty]); caffeine use (in drinks per day); alcohol use (in drinks per week); smoking status (current, former, never); body mass index and waist circumference (in centimeters); health status (Physical Component Scores and Mental Component Scores of the Medical Outcomes Study 36-item short-form health survey (SF-36) [19], along with self-reported ratings of health on a “very good” to “very poor” 1–5 Likert-type scale; and marital status (married, divorced/separated, widowed, unmarried, refused/unknown). To capture the impact of both trait and state levels of stress on subjective sleep quality, responses to a single question on the SF-36, “During the past 4 weeks, how much of the time have you felt calm and peaceful?” (all, most, some, a little, none of the time) and an answer to the question, “How stressful was your day today?” (typical, less, more) were included.
2.6 Data Analytic Strategy
Missing data were imputed using the Expectation Maximization algorithm in version 1.7.3 of the “Amelia II” package in R [20]. Where p values were considered, the Benjamini & Hochberg [21] method was used to control the false discovery rate. All p values reported are two-tailed.
Traditional methods for determining variable selection and importance (e.g., stepwise regression) are known to overfit training data and perform poorly in predicting new data [22, 23]. Instead, we used two machine learning methods built for multivariable analyses and designed to minimize bias and error: lasso penalized regressions [24] and random forests [25]. A fuller description of both methods as applied to sleep data can be found in Ref [8]. R packages “glmnet” and “rfsrc” were used to run lasso and random forest analyses, respectively. Key outcome measures of interest included the relative importance of various predictor variables.
The two measures of sleep quality (Sleep Depth and Restfulness) were treated as continuous in regression modeling for two key reasons. First, nonparametric random forest models are robust to violations of non-normality, making them a suitable fit for modeling ordinal data. Second, all sleep quality responses appeared normally distributed (methods for testing normal distribution assumptions were not employed given large sample size).
To evaluate whether variables selected by the lasso had statistically significant effect sizes, along with the ordering of these selected variables, we randomly split half of the data into training and test sets. We initially selected variables using lasso on our training set, then used our test set to run an ordinary least squares regression on those variables.
3. Results
The sample was predominantly white, college-educated and in good self-reported health, with similar numbers of men and women. Table 1 lists descriptive information for all demographic and clinical variables considered in machine learning models.
Table 1.
Demographic, clinical and polysomnographic information for study sample.
| Variable | N=3,173 |
|---|---|
| Age, M ± SD | 62.73 ± 11.33 |
| Sex, No. Female (%) | 1,639 (51.65) |
| Race, No. (%) | |
| White | 2,702 (85.16) |
| African American | 234 (7.37) |
| Hispanic | 176 (5.55) |
| Other | 61 (1.92) |
| Education, No. (%) | |
| ≤ 10 years | 259 (8.16) |
| 11–15 years | 1588 (50.05) |
| 16–20 years | 1157 (36.46) |
| > 20 years | 169 (5.33) |
| Health, No. (%) | |
| Excellent | 501 (15.79) |
| Very Good | 1200 (37.82) |
| Good | 1103 (34.76) |
| Fair | 326 (10.27) |
| Poor | 43 (1.36) |
| SF-36 Physical Health, M ± SD | 47.88 ± 9.56 |
| SF-36 Mental Health, M ± SD | 53.33 ± 8.18 |
| SF-36 Calm, M ± SD | 2.78 ± 1.19 |
| Self-Reported Stress | 1.48 ± 0.74 |
| Habitual Sleep Duration, hours | 7.13 ± 1.15 |
| Epworth Sleepiness Scale > 10, No. (%) | 781 (24.61) |
| Waist Circumference, M ± SD | 96.16 ± 13.64 |
| Body Mass Index (BMI), M ± | SD 27.95 ± 4.98 |
| Usual alcohol intake per week, M ± SD | 3.01 ± 5.36 |
| Daily cups of caffeinated drinks, M ± SD | 2.58 ± 2.58 |
| Taking Benzodiazepines1, No. (%) | 182 (5.74) |
| Taking Antidepressants1, No. (%) | 218 (6.87) |
| Taking sleeping pills2, No. (%) | 201 (6.33) |
| Smoking Status, No. (%) | |
| Never | 1507 (47.49) |
| Former | 1369 (43.15) |
| Current | 297 (9.36) |
| History of Diabetes, No. (%) | 217 (6.84) |
| History of Coronary Heart Disease, No. (%) | 477 (15.03) |
| Marital Status, No. (%) | |
| Married | 2494 (78.60) |
| Widowed | 256 (8.07) |
| Divorced or Separated | 311 (9.80) |
| Never Married | 99 (3.12) |
| Total Sleep Time, M ± SD | 357.04 ± 61.95 |
| Wake After Sleep Onset, M ± SD | 57.69 ± 40.91 |
| Sleep Efficiency, M ± SD | 81.80 ± 10.16 |
| Stage 1 Percent, M ± SD | 5.41 ± 3.94 |
| Stage 2 Percent, M ± SD | 56.67 ± 11.76 |
| Stage 3/4 Percent, M ± SD | 18.16 ± 11.96 |
| REM Percent, M ± SD | 19.75 ± 6.16 |
| REM Latency, M ± SD | 88.33 ± 55.42 |
| Sleep to Wake Shifts per hour, M ± SD | 3.81 ± 1.83 |
| Deep to Light Stage Shifts per hour, M ± SD | 2.99 ± 1.90 |
| Stage 2 to Stage 1 Shifts per hour, M ± SD | 0.03 ± 0.08 |
| Respiratory Disturbance Index, M ± SD | 8.30 ± 12.05 |
| Alpha NREM, M ± SD | 3.89 ± 1.70 |
| Alpha REM, M ± SD | 2.24 ± 1.74 |
| Beta NREM, M ± SD | 0.69 ± 1.55 |
| Beta REM, M ± SD | 0.69 ± 1.66 |
| Delta NREM, M ± SD | 30.90 ± 1.58 |
| Delta REM, M ± SD | 10.23 ± 1.55 |
| High-frequency Sigma NREM, M ± | SD 1.78 ± 1.70 |
| High-frequency Sigma REM, M ± | SD 1.02 ± 1.66 |
| Sigma NREM, M ± SD | 2.29 ± 1.70 |
| Sigma REM, M ± SD | 1.26 ± 1.70 |
| Slow Oscillations NREM, M ± SD | 102.33 ± 1.78 |
| Slow Oscillations REM, M ± SD | 29.51 ± 1.70 |
| Theta NREM, M ± SD | 7.24 ± 1.62 |
| Theta REM, M ± SD | 3.98 ± 1.66 |
Note:
Within two weeks of the visit;
At least one day per week
Lasso regression indicated that the model variables had relatively low predictive value for Sleep Depth (r2=7%) and Restfulness (r2=13%) (Table 2). While of low predictive value, sleep efficiency, age, WASO, and habitual sleep duration were the top predictors for both aspects of sleep quality. We used the results of the lasso regression to form the basis of an ordinary least squares (OLS) regression. After separating data into training and test sets, we entered all significant predictors (i.e., factors with non-zero coefficients in a lasso training model) into an OLS regression using the test set, in order to reduce bias and generate accurate p values [22]. After correcting for multiple comparisons, this OLS model predicting Sleep Depth retained three variables with significant p values: sleep efficiency (p<0.01), age (p<0.05) and habitual sleep duration (p<0.05). There were four variables predicting Sleep Restfulness: WASO (p<0.01), age (p<0.05), race (p<0.05) and habitual sleep duration (p<0.05). Sleep efficiency was likely not returned in the latter model because it was highly collinear with WASO, and lasso models often drop one of two collinear predictors. Indeed, when WASO was excluded from analyses, sleep efficiency emerged as the strongest predictor of Sleep Restfulness, and when Sleep Efficiency was excluded from analysis, WASO emerged as a top predictor of Sleep Depth (results not shown). The top five variables returned (regardless of significance value) are displayed in Table 2. Adjusted R2 estimates indicated that the variables in the OLS models accounted for only 9–11% of the variance. To both independently confirm the results of the lasso and OLS models and determine the relative importance of the predictors, we also performed random forest regression. The top predictors were WASO, age, total sleep time, and sleep efficiency, but R2 estimates indicate that the random forest models predicting Sleep Depth and Restfulness accounted for only 8 and 9% of the variance, respectively (Table 2).
Table 2.
Top five predictors of sleep quality according to machine learning method, listed in descending order of importance.
| Depth (Light—Deep) | Restfulness (Restless—Restful) | |
|---|---|---|
| L | Sleep Efficiency | Sleep Efficiency |
| A | Age | Wake After Sleep Onset |
| S | Wake After Sleep Onset | Age |
| S | Habitual Sleep Duration | Habitual Sleep Duration |
| O | Total Sleep Time | NREM Fast Sigma |
| r2=0.07 | r2=0.13 | |
|
| ||
| Depth (Light—Deep) | Restfulness (Restless—Restful) | |
|
| ||
| O | Sleep Efficiency | Wake After Sleep Onset |
| L | Age | Age |
| S | Habitual Sleep Duration | Race |
| Wake After Sleep Onset | Habitual Sleep Duration | |
| Epworth Sleepiness Scale | NREM Fast Sigma | |
| r2=0.09 | r2=0.11 | |
|
| ||
| Depth (Light—Deep) | Restfulness (Restless—Restful) | |
|
| ||
| R F | Wake After Sleep Onset | Age |
| A O | Sleep Efficiency | Wake After Sleep Onset |
| N R | Age | Sleep Efficiency |
| D E | Total Sleep Time | Total Sleep Time |
| O S | NREM Fast Sigma | NREM Delta |
| M T | ||
| r2=0.08 | r2=0.09 | |
Note: The models above include all 48 variables listed in Table 1.
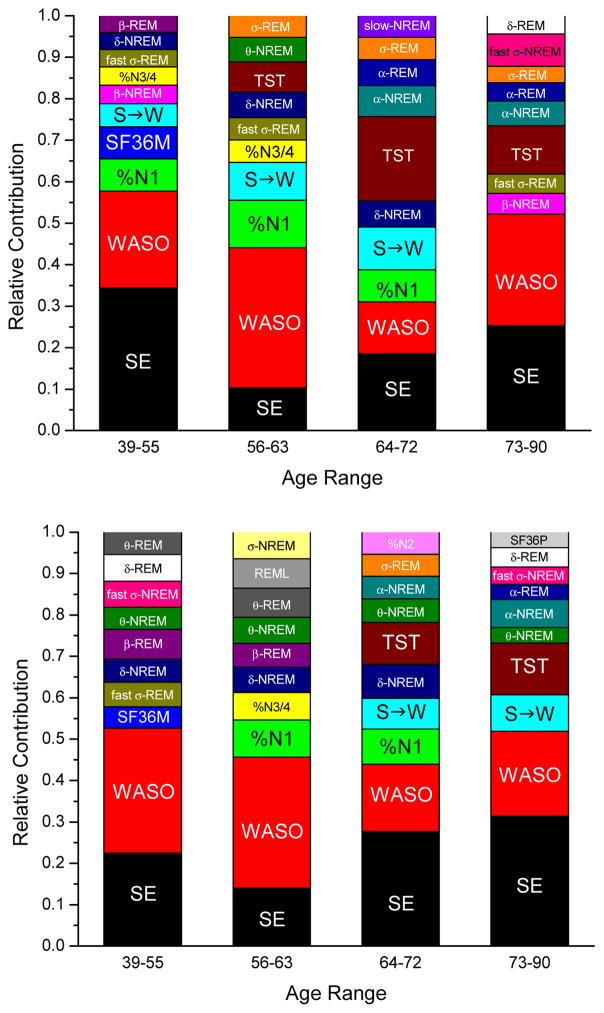
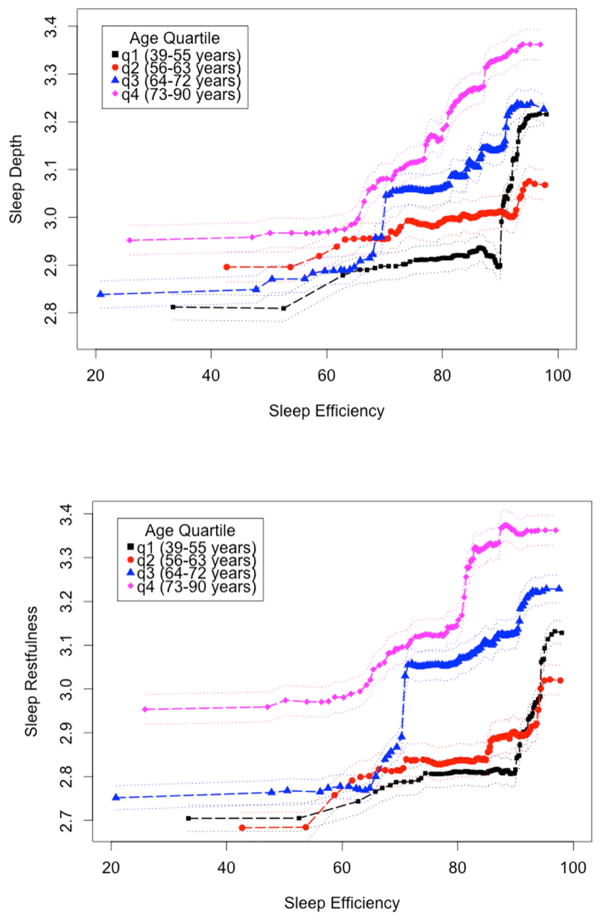
Given our a priori assumption that there is a relationship between sleep and age, and given that age was a significant predictor in all models described above, we stratified the sample into quartiles (39–55, 56–63, 64–72, and 73–90 years of age) and conducted new random forest models within each age group to ascertain how predictors might vary as a function of age. Traditional PSG metrics (sleep efficiency, WASO, percentage of Stage 1 sleep, sleep to wake stage shifts) were most important in explaining subjective sleep quality across models (Figure 1). The relative contributions of various predictors of subjective sleep quality did not, however, appear to change as a function of age. Objective sleep efficiency was among the most important of the predictor variables (i.e., its removal from machine learning models resulted in sizeable increases in predictive error). We thus examined the relationship between objective sleep efficiency and subjective sleep quality in the random forest models, allowing all other variables to simultaneously enter the model (partial dependence plot, Figure 2). The relationship suggests that, at any given level of objective sleep efficiency, individuals in the older two cohorts will rate their subjective sleep quality as progressively better relative to the younger two cohorts.
Figure 1.
Top ten predictors of sleep quality by random forest models, stratified by age quartile, for Sleep Depth (top) and Sleep Restfulness (bottom).
Note: The models above include all 48 variables listed in Table 1. See text for details on each measure. Education, level of education; Fast, High-Frequency Sigma; HST, habitual sleep duration; %N1, %N2, %N3/4, percent of sleep spent in NREM stage 1, NREM stage 2, and NREM stage 3 or 4, respectively; S->W, number of transitions between sleep and wake; RDI, Respiratory Disturbance Index; REML, latency to REM sleep; SE, sleep efficiency; SF36M, Mental Component Score of the Medical Outcomes Study 36-item short-form survey; SF36P, Physical Component Score of the Medical Outcomes Study 36-item short-form survey; TST, total sleep time; Slow, slow oscillations; WASO, wake after sleep onset
Figure 2.
Partial dependence plots of objective sleep efficiency predicting subjective sleep depth and sleep restfulness, stratified by age quartile.
4. Discussion
Our data confirm our recent finding that the standard clinical metrics derived from overnight polysomnography are not well correlated with the subjective phenomenon of sleep quality, at least as captured by the scales used in this study (Sleep “Restfulness” and “Depth”). Furthermore, including qEEG correlates does not help capture the subjective phenomenology we are recording. These findings are consistent with our previous paper [8], though the previous data were limited to older adults (ages 73 and above); our current data span the range of 39–90 years of age. While it is possible that stronger associations exist between qEEG, PSG-derived variables and subjective sleep quality for adults younger than 39 years of age, our data indicate that these variables do not adequately capture several features of subjective sleep quality in midlife and beyond. Our analyses also addressed subjective sleep quality immediately after a PSG study. One might expect that the immediately proximal PSG night may be most predictive of sleep assessments made the next morning. That we found only weak relationships to exist lends further support to the limitations of PSG in predicting subjective sleep quality. We did not examine the relationship between these predictor variables and other possible functions of sleep (e.g., increase of objective alertness, improvement in memory function, removal of cerebral waste). It is quite possible that the variables that we find are uninformative vis-a-vis subjective sleep quality may be useful in the assessment of the quality of sleep relevant to alternate functions.
By examining a 50-year age span, we were able to examine how the relationship between subjective sleep quality and polysomnography indices varies with advancing age. Aging-associated changes in a variety of PSG-determined variables have been well described [2] but, at the same time, subjective reports indicate that sleep quality is better in older adults [7, 26]. Reasons for the divergence between subjective and objective measures have rarely been studied, nor how closely these two constructs map on with age. We demonstrate that one contributing factor may be that as individuals age, the relationship between sleep efficiency and both sleep depth and sleep restfulness changes such that for the same level of objective sleep efficiency, there is a greater sleep quality subjectively reported. These age-related differences are supported by non-overlapping error bars (Figure 2). The relationship between sleep efficiency and sleep quality can, in our data set, be described by a sigmoidal curve, where the linear rise of the curve occurs mainly between 65 and 95% sleep efficiency, a very common range of values that might be observed in older adults. As can be readily visualized (Figure 2), while an individual in the first age quartile (aged 39–55 years) with 85% sleep efficiency would rate themselves about 2.9 on the sleep depth scale, an individual in the fourth quartile (aged 73–90 years) with the same 85% sleep efficiency would rate themselves about 3.2 on this scale, about a 10% improvement. A note of caution must be had, of course, as these data are cross-sectional and not longitudinal. Especially as there is a change in objective sleep measures during aging, it would be of great interest to examine the same individuals over many years to determine if within a given individual the relationship between sleep efficiency and subjective sleep quality systematically changed with age. Even so, our data suggest that sleep quality reports may be less reflective of objective continuity as individuals age, and that clinicians may be cautious not to rely on these to understand actual sleep fragmentation.
Our finding that sleep efficiency has the most consistent association with subjective sleep quality is consistent with previous work. In a small sample using a longitudinal design, Akerstedt and colleagues found sleep continuity to be most important to ratings of subjective sleep quality [27]. In our recent investigation of sleep quality in a very large cohort of older adults, we similarly showed sleep efficiency to be most important to ratings of sleep quality [8]. However we should note that, while we found a strong sex effect in our older adult sample, sex was not determined to be an important predictor of subjective sleep quality in this midlife sample. To evaluate this more carefully, we stratified by sex and reran our predictive models within age quartiles. Partial dependence plots between sleep efficiency, the strongest correlate to emerge, and sleep quality ratings appeared consistent between the sexes and across ages (results not shown).
The main limitation of our current study is that we restricted the number of variables that we considered in the models to a subset of the standard metrics derivable from both PSG and qEEG. While there may be other measures we didn’t include that had greater predictive values than the ones we included, a balance must be reached between including a large number of correlates and building a model that is interpretable and meaningful. It is quite possible that there are other signals derivable from PSG or qEEG data that better reflect subjective sleep quality. We note that random forest algorithms employed here are ideal for handling any nonlinear associations that might exist in PSG or qEEG data.
Previous researchers have noted that understanding subjective sleep quality would require larger multivariable analyses and inclusion of qEEG [9]. Here we have performed such an analysis; in addition to PSG and qEEG variables, our models included a wide variety of demographic and clinical correlates, including age, sex, health status, medication use, and lifestyle factors (caffeine, alcohol, tobacco use). A further strength of this research was its focus of prior-night sleep quality, as opposed to chronic or recurrent sleep quality overall, allowing for greater specificity and interpretability. Most of the correlates examined did not contribute to explaining the variation in subjective sleep quality, indicating that these do not substantially explain variation in subjective sleep quality ratings in middle-aged to older adults. This could, alternatively, be interpreted as sleep quality being a highly individualized experience that has few universal components within the adult population. Whether this hypothesis is true, or whether we have yet to discover the biological correlates of subjective sleep quality remains an open question for future research.
Highlights.
We explored the relationship between subjective sleep quality and objective measures of sleep in midlife and older adults using machine learning
Polysomnography, clinical, and demographic measures did not well explain sleep quality
Quantitative EEG was not useful in predicting subjective sleep quality
Older adults reported higher sleep quality even as objective sleep deteriorated
The importance of various predictors did not change with advancing age
Acknowledgments
This study was supported by the following: HL086862 and HL075078 (SHHS) and HL114473 (NSRR); VA Sierra Pacific Mental Illness Research Education and Clinical Center (JMZ); Lucille Packard Foundation for Children’s Health, UL1TR001085, Stanford Child Health Research Institute (KAK).
Footnotes
Authorship Responsibility: Each author participated sufficiently in the work and analysis of data, as well as the writing of the manuscript.
Conflict of Interest: The authors report no conflict of interest associated with this manuscript.
Disclosure: This work was supported by NIH funding. There was no off-label or investigational use of any medication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 7.Zilli I, Ficca G, Salzarulo P. Factors involved in sleep satisfaction in the elderly. Sleep Med. 2009;10:233–9. doi: 10.1016/j.sleep.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan KA, Hirshman J, Hernandez B, Stefanick ML, Hoffman AR, Redline S, et al. When a gold standard isn’t so golden: Lack of prediction of subjective sleep quality from sleep polysomnography. Biol Psychol. 2016;123:37–46. doi: 10.1016/j.biopsycho.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9(Suppl 1):S10–7. doi: 10.1016/S1389-9457(08)70011-X. [DOI] [PubMed] [Google Scholar]
- 10.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 11.Dean DA, 2nd, Goldberger AL, Mueller R, Kim M, Rueschman M, Mobley D, et al. Scaling Up Scientific Discovery in Sleep Medicine: The National Sleep Research Resource. Sleep. 2016;39:1151–64. doi: 10.5665/sleep.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington D.C: US Department of Health, Education, and Welfare, Government Printing Office; 1968. [Google Scholar]
- 14.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–57. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 15.Buckelmuller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–6. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Aeschbach D, Lockyer BJ, Dijk DJ, Lockley SW, Nuwayser ES, Nichols LD, et al. Use of transdermal melatonin delivery to improve sleep maintenance during daytime. Clin Pharmacol Ther. 2009;86:378–82. doi: 10.1038/clpt.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laffan A, Caffo B, Swihart BJ, Punjabi NM. Utility of sleep stage transitions in assessing sleep continuity. Sleep. 2010;33:1681–6. doi: 10.1093/sleep/33.12.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 20.Honaker J, King G, Blackwell M. Amelia II: A program for missing data. J Stat Software. 2011;45:1–47. [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B (Methodological) 1995:289–300. [Google Scholar]
- 22.Hastie T, Tibshirani R, Friedman J, Franklin J. The elements of statistical learning: data mining, inference and prediction. Math Intelligencer. 2005;27:83–5. [Google Scholar]
- 23.Harrell F, Lee KL, Mark DB. Tutorial in biostatistics multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Tibshirani R. Regression shrinkage and selection via the lasso. J Royal Stat Soc B (Methodological) 1996:267–88. [Google Scholar]
- 25.Breiman L. Random forests. Machine learning. 2001;45:5–32. [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Akerstedt T, Hume K, Minors D, Waterhouse J. The meaning of good sleep: a longitudinal study of polysomnography and subjective sleep quality. J Sleep Res. 1994;3:152–8. doi: 10.1111/j.1365-2869.1994.tb00122.x. [DOI] [PubMed] [Google Scholar]