Abstract
Centrosome- and chromatin-based microtubule nucleation pathways have been implicated in spindle assembly. Using total internal reflection fluorescent microscopy and Xenopus egg extracts, Petry et al. demonstrate that new microtubules can also nucleate and branch out from existing ones in animal cells.
The mitotic spindle apparatus consists of a dense network of interacting microtubules (MTs) exhibiting highly dynamic structures and behaviors. How MTs, along with associated motors and other proteins, organize into a spindle has been one of biology’s most fascinating questions. Although decades of study might leave the impression that most of the important mechanisms have already been discovered, in this issue of Cell, Petry et al. (2013) uncover an important aspect of MT nucleation in animal cells that calls for a revision of spindle assembly theories.
Two MT nucleation pathways, namely, centrosome-based and chromatin-based MT nucleation, have been strongly implicated in spindle assembly in animal cells. Whereas cells lacking centrosomes use the chromatin-based MT nucleation pathway, both nucleation pathways are involved in spindle assembly in cells containing centrosomes (Figure 1B). As MTs are nucleated, many MT-associated proteins further regulate their organization into a spindle containing two antiparallel MT arrays that position chromosomes at the middle of the spindle to form the meta-phase plate. It has been suggested that spindle MTs could arise by nucleation from existing MTs in the spindle (Clausen and Ribbeck, 2007; Goshima et al., 2008). However, until now, no direct observation of this process had been possible, likely because of the difficulty of detailed observations in the dense spindle using conventional fluorescent microscopy. Using total internal reflection microscopy, the authors observe MT-based nucleation of MTs in Xenopus egg extract, an in vitro assay system that recapitulates spindle assembly in animals. They show that new MTs emanate from existing MTs with the same polarity and with low branch angles (Figure 1A).
Figure 1. Consequences of Microtubule Nucleation from Existing Microtubules.
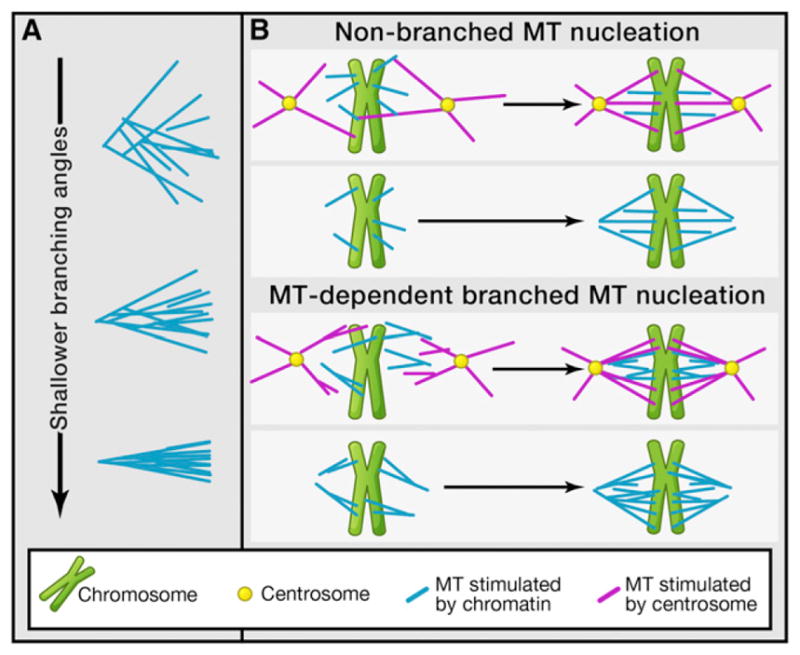
(A) Effect of the branching angle on the density of microtubules (MTs). As the branching angle decreases, the MTs form a tighter, denser cluster, thus enhancing the organization of MTs during spindle assembly.
(B) Spindle MT nucleation can occur at centrosomes or independent of centrosomes. With MT-branched MT nucleation, the number of MTs increases, leading to denser spindles and possibly a more optimal morphology.
This exciting discovery has a number of significant implications regarding the mechanism of spindle assembly. One salient feature of the spindle in animal cells is its sharp boundaries, where the high MT density drops off quickly at the edge of the spindle. Considering that motor proteins are known to slide MTs apart within the spindle, it is unclear why the sliding MTs do not protrude the edge of the spindle. Previously, computer simulations have suggested an autocatalytic, positive-feedback loop in MT production. This loop coupled with the dynamic instability of MTs and the chromatin-produced gradient of RanGTP, a small G protein that regulates multiple aspects of mitosis, including spindle assembly (Kalab et al., 2002; Li and Zheng, 2004), could lead to the high MT density observed around the chromatin (Clausen and Ribbeck, 2007; Loughlin et al., 2010). In these models, dynamic instability helps eliminating MTs that are not part of the spindle, thereby enabling the focusing of the MTs into a single structure. As pointed out by Petry et al. (2013), MT-dependent MT nucleation acts to close this positive-feedback loop, offering an efficient means to quickly amplify the number of MTs during spindle assembly and leading to high MT density. In their observations, the authors find that catastrophes (the shift from a growing to a shrinking MT) were rare. Instead, they find that nucleation occurs at shallow angles relative to the existing MTs (Figure 1A), and so the array of MTs forms a tight bundle of MTs that grows with one polarity in each half of the spindle, explaining why the spindle does not expand laterally, but rather emerges as a tightly focused bundle of MTs (Figure 1B).
This narrow profile afforded by MT-dependent MT nucleation may also help to understand how MTs capture chromosomes efficiently. Previously, it has been suggested that search and capture, whereby MTs search space and capture chromosomes as they are found, requires a means for biasing MT dynamics toward chromosomes (Wollman et al., 2005). RanGTP may provide this bias, as it is formed around the chromosomes. The MT-dependent MT nucleation may help to amplify the effect of the RanGTP gradient as newly nucleated MTs will emanate in directions close to the existing ones, furthering the preferred direction.
In addition to uncovering an important process during spindle assembly, Petry et al. (2013) also provide clues that may lead to deciphering the molecular mechanism that underlies this phenomenon. By manipulating different proteins previously shown to regulate MT nucleation, the authors demonstrate that the well-known MT nucleator γ-tubulin ring complex (γTuRC) (Zheng et al., 1995) provides the primary nucleating activity, whereas augmin, known to increase MT density within the spindle (Goshima et al., 2008), is required for the MT-dependent branching nucleation. Interestingly, TPX2, a MT-binding protein implicated in chromatin-based MT nucleation, and RanGTP strongly stimulate branching MT nucleation synergistically.
Using immunoprecipitation, the authors show that TPX2, γTuRC, and augmin bind to one another in Xenopus egg extracts. Because TPX2 directly bind to MTs, one idea is that TPX2 stimulates branching MT nucleation by bringing γTuRC and augmin to MTs. TPX2 is an effector of RanGTP (Gruss et al., 2001). The latter promotes the binding of TPX2 to Aurora A kinase by displacing importin α and β bound to the nuclear localization signal (NLS) localized within the N-terminal half of TPX2 (NT-TPX2). This, in turn, leads to kinase activation and proper spindle assembly (Tsai et al., 2003). RanGTP might displace importin α and β from TPX2, which enhances the formation of the complex containing TPX2, γTuRC, and augmin. This displacement, however, seems unlikely because the authors show that the C-terminal half of TPX2 (CT-TPX2), which does not contain NLS, is sufficient to stimulate branching MT nucleation. It would be interesting to test whether RanGTP further enhances branching MT nucleation stimulated by CT-TPX2. If it does, RanGTP may function through factors other than TPX2 to promote branching MT nucleation. Ultimately, to understand the MT branching nucleation, it will be important to reconstitute the process in vitro using purified components. By uncovering a number of players, this paper has put the field in a strong position to achieve the goal.
Clearly, a number of open questions remain. For example, positive-feedback schemes, such as the one reported here, require a means for turning them off. In the present context, is this achieved by tubulin depletion? The authors also observe that cytoplasmic dynein rapidly transported MTs but does this influence spindle morphology, and if so how? Nevertheless, through their discoveries, Petry et al. (2013) have shown that animal cells have their own way to efficiently nucleate MTs in the same orientation, and this will keep those interested in the spindle apparatus busy for a long time to come.
Acknowledgments
Y.Z. and P.A.I. are supported by the National Institutes of Health GM56312 and GM086704, respectively.
References
- Clausen T, Ribbeck K. PLoS ONE. 2007;2:e244. doi: 10.1371/journal.pone.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD. J Cell Biol. 2008;181:421–429. doi: 10.1083/jcb.200711053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW. Cell. 2001;104:83–93. doi: 10.1016/s0092-8674(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Kalab P, Weis K, Heald R. Science. 2002;295:2452–2456. doi: 10.1126/science.1068798. [DOI] [PubMed] [Google Scholar]
- Li HY, Zheng Y. Genes Dev. 2004;18:512–527. doi: 10.1101/gad.1177304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin R, Heald R, Nédélec F. J Cell Biol. 2010;191:1239–1249. doi: 10.1083/jcb.201006076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. Cell. 2013;152:768–777. doi: 10.1016/j.cell.2012.12.044. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y. Nat Cell Biol. 2003;5:242–248. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- Wollman R, Cytrynbaum EN, Jones JT, Meyer T, Scholey JM, Mogilner A. Curr Biol. 2005;15:828–832. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison T. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]


