Abstract
In this study, batch and column experiments were conducted to evaluate the feasibility of using persulfate oxidation to treat groundwater contaminated by landfill leachate (CGW). In batch experiments, persulfate was compared with H2O2, and permanganate for oxidation of organic compounds in CGW. It was also compared with the potential of biodegradation for contaminant removal from CGW. Persulfate was observed to be superior to H2O2 and permanganate for degradation of total organic carbon (TOC) in the CGW. Conversely, biodegradation caused only partial removal of TOC in CGW. In contrast, persulfate caused complete degradation of the TOC in the CGW or aged CGW, showing no selectivity limitation to the contaminants. Magnetite (Fe3O4) enhanced degradation of leachate compounds in both CGW and aged CGW with limited increase in persulfate consumption and sulfate production. Under dynamic flow condition in 1-D column experiments, both biodegradation and persulfate oxidation of TOC were enhanced by Fe3O4. The enhancement, however, was significantly greater for persulfate oxidation. In both batch and column experiments, Fe3O4 by itself caused minimal consumption of persulfate and production of sulfate, indicating that magnetite is a good persulfate activator for treating CGW in heterogeneous systems The results of the study show that the persulfate-based in-situ chemical oxidation (ISCO) method has great potential to treat the groundwater contaminated by landfill leachate.
Keywords: Persulfate oxidation, Magnetite, Biodegradation, Landfill leachate, Contaminated groundwater
1. Introduction
Rapid worldwide economic development in recent years has caused massive generation of industrial and municipal solid waste (MSW). Landfill disposal is currently the most used method for municipal and industrial solid waste management [1, 2]. A main disadvantage of this method is leachate generation, which occurs through rainfall percolation and a series of physicochemical and biological processes in the solid waste [3]. Landfill leachate typically contains a wide variety of organic and inorganic constituents [4]. Landfill leachate can be categorized into young leachate (typically ≤2 years since generation) and mature leachate (typically ≥5 years since generation), depending on the fraction of the organic compounds that are refractory to biodegradation [5]. Due to these refractory compounds, landfill leachate can be a source of long term contamination for soil and groundwater [3, 6, 7]. Different methods, including biological, physical and chemical oxidation processes, have been reported for leachate treatment [8-12].
Advanced oxidation processes (AOPs) such as Fenton oxidation [13, 14], Fenton-like oxidation [15], ozone oxidation [16], photocatalytic oxidation [17], and electrochemical oxidation [5] have been extensively tested for the treatment of landfill leachate. Even though these processes are effective for landfill leachate treatment, their potential application for soil and groundwater remediation poses challenges. Photocatalytic oxidation and electrochemical treatment are not applicable to in situ remediation. While Fenton reagent (Fe-activated hydrogen peroxide) has been used for in situ chemical oxidation (ISCO), hydrogen peroxide suffers from rapid decomposition in the subsurface, resulting in the generation of heat and gas, causing a series of issues, including safety, relatively short radius of influence, and low remediation efficiency [18]. In addition, due to the limited aqueous solubility and inefficient dispersion of ozone gas in heterogeneous subsurface environments, as well as the requirement of on-site generation systems, the application of ozone remediation has also been hindered [19]. Persulfate (PS, S2O82−) has been used successfully for in-situ chemical oxidation (ISCO) treatment of groundwater contaminated by organic compounds [20-23]. Persulfate is relatively safe to handle, as it is stable under normal conditions. Furthermore, persulfate is highly soluble in water, it can be conveniently used to mix with soil or injected at high concentrations [21]. In addition, it is more persistent than hydrogen peroxide and thus has a larger radius of influence in the subsurface [22]. Persulfate is a strong oxidant with a high redox potential of 2.01 V. When activated, persulfate produces SO4•− and HO• radicals which are even more powerful for contaminant degradation (standard redox potentials are 2.6 V and 2.8 V, respectively [23]). Importantly, persulfate is effective for a wide range of compounds including many that are refectory to other treatment methods.
Persulfate oxidation has been applied to degrade a variety of contaminants. For example, effective degradation was also reported for petroleum-hydrocarbons [20, 22] chlorinated ethylenes [24-26], chlorinated alkanes [27, 28], dioxane [29, 30], methyl tert-butyl ether (MTBE) [20, 31], polycyclic aromatic hydrocarbons (PAHs) [32-34]. Several studies have reported that persulfate was applied to remove COD and color, and improve biodegradability for landfill leachate in above-ground treatment systems. Deng et al used heat to improve persulfate oxidation for simultaneous removal of refractory organic contaminants and ammonia in landfill leachate [4]. Shabiimam and Dikshit used low pH to enhance persulfate oxidation for removing total organic carbon and color from stabilized leachate [35]. Chou et al enhanced persulfate oxidation to treat mature landfill leachate by using microwaves [36].
As noted above, several methods exist for persulfate activation, including heat [37-39], base activation [40-42], ultrasonication [43], UV irradiation [44], electrochemical [45], microwave [36], and transition metals [26, 46, 47]. The majority of these methods are not practical for in-situ applications, with transition metals and base the primary activators employed. Ferrous iron (Fe2+) is commonly used to catalyze persulfate oxidation [48-50]. However, overall oxidation potential is often limited in subsurface systems due to issues related to Fe2+ availability. In addition, Fe2+ can scavenge SO4•− at a rate higher than its generation [51]. Zero-valent iron (Fe°) and iron bearing minerals such as geothite, hematite, pyrite, ilmenite, ferrihydrite, lepidocrocite, and magnetite have been studied for heterogeneous persulfate activation [52-58]. Recently, magnetite (Fe3O4) was reported to be an effective catalyst as compared to Fe (III) oxides for heterogeneous catalytic oxidation of organic pollutants [55, 59, 60]. Magnetite is a focus due to its ubiquitous presence in the subsurface environment [61, 62]. In addition, application of Fe3O4 to persulfate-based chemical oxidation processes for contaminant removal from wastewater has been proven to be successful [63-66]. These results lead to the contention of applying magnetite to persulfate-based ISCO technology for treatment of groundwater contaminated by landfill leachate.
The objective of this study is to investigate the persulfate-based oxidation method for removal of contaminants in landfill leachate contaminated groundwater (CGW), and the potential of persulfate-based ISCO technology for remediation of CGW. The performance of persulfate was compared with two other typical ISCO oxidants (permanganate and H2O2). Persulfate-based oxidation was also compared with biodegradation (associated with natural attenuation) for overall evaluation of effectiveness. The use of magnetite (Fe3O4) as an activator for enhancing persulfate-based oxidation was also examined. In addition to batch experiments, column experiments were conducted to investigate the performance of the method under dynamic flow conditions.
2. Materials and methods
2.1. Materials
Landfill leachate was collected from the leachate storage tank of Heimifeng landfill (Changsha, Hunan Province, China). The leachate collected was stored in a tinted glass bottle with zero headspace in a refrigerator at 4 °C. Before use, the raw leachate was centrifuged at a rotating speed of 7000 r/min for 20 min, and the supernatant was filtered through a 0.45 μm membrane to remove suspended solids that may interfere with the reactions for dissolved organic matter (DOM) or sample analysis in the following experiments. This is the stock landfill leachate, and the water quality parameters were analyzed and presented in Table 1. Due to a low BOD5/COD ratio (<0.12) and high NH4+-N concentration, the leachate was classified as mature leachate [67].
Table 1.
Water quality of the stock landfill leachate
| Parameter | Concentration (mg/L) |
|---|---|
| TOC | 3415 ± 102 |
| COD | 6912 ± 297 |
| BOD5 | 565 ± 19 |
| BOD5/COD | <0.1 |
| NH4+–N | 1845 ± 18 |
| NO3−–N | 18.1 ± 6.6% |
| NO2−–N | 0.71 ± 0.56% |
| Lithium (Li) | 8.4 |
| Sodium (Na) | 2139 |
| Potassium (K) | 1399 |
| Magnesium (Mg) | 236 |
| Calcium (Ca) | 184 |
| Iron (Fe) | 1.7 |
| Manganese (Mn) | 0.3 |
| Nickel (Ni) | 0.2 |
| Cobalt (Co) | 0.07 |
| Strontium (Sr) | 0.75 |
| Copper (Cu) | < 0.005 (D.L) |
| Selenium (Se) | < 0.075 (D.L) |
| Plumbum (Pb) | < 0.042 (D.L) |
| pH | 7.82 |
D.L: Detection Limit
Ultrapure water was used to represent groundwater for dilution of landfill leachate to produce CGW due to the very high salt concentrations in the leachate (the salt concentrations in CGW were significantly higher than those in typical groundwater) (dilution factor was 50). The organic compounds in the CGW were analyzed using gas chromatography-mass spectrometry (Shimadzu GCMS-QP2010, Japan) (Fig. S1, SI). The total organic carbon (TOC) was determined by TOC analyzer (Shimadzu TOC-VCPH, Japan). Details for GC-MS and TOC analysis are presented in Analytical Methods (Section 2.5). The results show that the major species detected by the GC-MS were low molecular weight hydrocarbons and oxygenated hydrocarbon derivatives (Table 2). Some of the compounds, such as trans-2-Dodecen-1-ol, trifluoroacetate and diethyl phthalate, are refractory compounds that have long life times in the environment. The TOC in CGW was measured to be 68 mg/L.
Table 2.
Organic compounds in the landfill leachate contaminated groundwater identified by GC-MS
| Formula | Organic compounds |
|---|---|
| C6H14 | Pentane, 3-methyl- |
| C6H14 | n-Hexane |
| C6H12 | Cyclopentane, methyl- |
| C9H18O2 | Nonanoic acid |
| C10H20O2 | n-Decanoic acid |
| C7H12O4 | Propanedioic acid, diethyl- |
| C13H22O | 5,9-Undecadien-2-one, 6,10-dimethyl- |
| C12H26O | 1-Dodecanol |
| C16H34 | Hexadecane |
| C12H26O | 1-Dodecanol |
| C8H16O | Cyclohexanol, 2,4-dimethyl- |
| C12H24O2 | Dodecanoic acid |
| C12H26O | 1-Dodecanol |
| C16H34 | Hexadecane |
| C12H14O4 | Diethyl Phthalate |
| C14H26O3 | Undecanoic acid, 4,8-dimethyl-10-oxo- |
| C19H40 | Pentadecane, 2,6,10,14-tetramethyl- |
| C13H28O | n-Tridecan-1-ol |
| C17H36 | Heptadecane |
| C19H40 | Pentadecane, 2,6,10,14-tetramethyl- |
| C11H24 | Nonane, 3,7-dimethyl- |
| C14H30O2 | Ethanol, 2-(dodecyloxy)- |
| C14H28O2 | Tetradecanoic acid |
| C14H30O | 1-Tetradecanol |
| C14H23F3O2 | trans-2-Dodecen- 1-ol, trifluoroacetate |
| C6H10N6O3 | Furazan-3-carboxamide |
| C14H28O2 | Tetradecanoic acid |
| C15H30 | 1-Pentadecene |
| C17H36O | n-Heptadecanol-1 |
| C17H36 | Heptadecane |
| C14H30O2 | Ethanol, 2-(dodecyloxy)- |
| C16H34 | Hexadecane |
| C15H30O3 | Methoxyacetic acid, dodecyl ester |
| C14H28O2 | Tetradecanoic acid |
| C7H12N2O4 | 5-Cyano-desoxinojirimycin |
| C12H19NO7 | 1,3-Dioxolane-4-acetic acid |
| C15H28O2 | Cyclopentadecanone, 2-hydroxy- |
| C15H30O2 | Pentadecanoic acid |
| C16H22O4 | 1,2-Benzenedicarboxylic acid |
| C15H32O | n-Pentadecanol |
| C25H36O7 | 4,13,20-Tri-O-methylphorbol 12-acetate |
| C17H34O2 | Hexadecanoic acid, methyl ester |
| C18H34O2 | Oleic Acid |
| C17H16OS | 3,3-Di-p-tolyl-thietan-2-one |
| C16H32O2 | n-Hexadecanoic acid |
| C19H40O | n-Nonadecanol-1 |
| C16H33I | Hexadecane, 1-iodo- |
The aged CGW was produced by aging CGW to remove the degradable compounds. Briefly, CGW in a 4-L flask was placed in an incubation chamber at 25 °C until TOC in the CGW stabilized (~15 days). The incubation continued for another 20 days to obtain the aged CGW. The DOM in the aged CGW typically consists of compounds refractory to biodegradation, such as fulvic acid [68].
Sodium persulfate (Na2S2O8, ≥98.0%) and hydrogen peroxide (H2O2, 30%) were purchased from Sinopharm Chemical Reagent, Shanghai, China. Potassium Permanganate (KMnO4, ≥99.5%) was from Kaixin Chemical Reagent (Hengyang, China). The magnetite (Fe3O4, 99%) was obtained from Aladdin Industrial Corporation (Shanghai, China) and was characterized by electron scanning microscopy-energy dispersive X-ray spectroscopy (SEM-EDS) (Image shown in Fig. S10, SI). The particle size of the Fe3O4 solids is ~1 μm. The other chemicals were of reagent grade and obtained from Hengxing Chemical Preparation Corporation Co., Ltd (Tianjin, China). All solutions were prepared using ultra-pure water produced by UPT-II-40 (Ulupure, Chengdu, China) with an electrical resistivity of 18.3 MΩ ·cm.
The quartz sand used for the column experiments was obtained from Zhilei Building Co. Ltd (Guangzhou, China). The sand was ultrasonically cleaned, air-dried and then screened. The segment with the size of 100-120 mesh (0.125-0.147 mm) was used in the experiments.
2.2. Batch degradation experiments
Persulfate was first compared with permanganate and H2O2 (not Fenton reagent) for oxidative degradation of the organic compounds in CGW. 40-mL borosilicate vials were used as the reactors for this experiment. 20 mL of oxidant solution at concentration of 86 mM and 20 ml of CGW were mixed in a vial, and the vial was closed using a cap with polytetrafluoroethylene lined septa. Multiple vials were placed in a thermostat water bath (25 ± 1 °C) with a lid, which maintained the reaction in the dark. At predetermined time intervals, three vials as replicates were sacrificed for sample analysis. These vials were first transferred to an ice-water bath to quench oxidation reactions, and then the solution was immediately analyzed for TOC, oxidant concentration, and pH. In the experiment in which persulfate was used as the oxidant, sulfate concentration was also measured.
A complementary experiment was conducted to examine the impact of biodegradation (natural attenuation) on TOC. The experiment was conducted by following the same procedures as used in the oxidative degradation experiment except that the 20 mL of oxidant solution was replaced by ultrapure water. TOC and pH in the samples were measured at predetermined time intervals.
A control experiment was conducted to test for possible loss of organic compounds due to adsorption to vials, photochemical decomposition, or other unidentified physicochemical processes during the course of the experiment. For this test, 1 g/L of sodium azide (a bactericide) solution was used in place of the oxidation solution, which was used to inhibit microbial activity.
2.3. Effect of Fe3O4 on degradation of leachate compounds by persulfate
15 ml of persulfate solution at concentration of 86 mM and 15 mL CGW were mixed in a 40-ml borosilicate vial. 0.6 g of Fe3O4 was added into the mixed solution to yield a solid-liquid ratio of 1:50 (w/w). Then three glass beads with a diameter of 4-5 mm were added into each vial for better agitation during the experiment. These vials were closed using caps with polytetrafluoroethylene lined septa and then placed in a reciprocal water-bath shaker running at 300 rpm (25 ± 1 °C). At predetermined time intervals, three vials were sacrificed for sample analysis. These vials were first transferred to an ice-water bath to quench reaction. Then the samples were filtered through a 0.22-μm glass fiber membrane to remove the Fe3O4 solids. The filtrate was immediately analyzed for the concentration of TOC, persulfate, sulfate, ferrous ion and total iron and pH. Control experiments were conducted to examine whether Fe3O4 caused biodegradation or adsorption. In the control experiment to see whether Fe3O4 caused biodegradation, 1 g/L of sodium azide solution was used in place of the oxidant solution. Using sodium azide was to inhibit activity of microorganisms and thus prevent biodegradation (removing the impact of microbial activity from the reaction). In the other control experiment to see whether Fe3O4 caused adsorption, the oxidant solution was replaced with ultrapure water (sodium azide was avoided) and the test lasted for only 2 h.
The degradation of organic compounds in aged CGW by Fe3O4-activated persulfate was also investigated following the methods described above.
2.4. Column experiments
The column used in the experiments was 2 cm in inner diameter and 20 cm in length. It was constructed of glass with all connectors constructed of stainless steel. Two column experiments were conducted to test degradation of TOC in CGW. In one experiment the column was packed with the silica sand, and in the other the column was packed sequentially with silica sand (2.5 cm), a mixture of Fe3O4 and sand at a mass ratio of 1:9 (15 cm), and silica sand (2.5 cm). The packed columns were flushed with CO2 to displace air, then vertically oriented and saturated with degassed water using a valveless piston pump (QG-6, Fluid Metering INC, USA), and then oriented horizontally for the experiments.
Before the column experiment, volatility of the leachate compounds in CGW in reservoir (incompletely sealed) was examined. Ultrapure water and CGW at a ratio of 1:1 (v:v) were mixed in the reservoir. The reservoir was sampled at predetermined time intervals and TOC were measured. Results demonstrated that volatilization caused minimal loss of TOC from the reservoir (Fig. S5, SI).
In each column experiment, two solutions were used for injection. The first solution was a mixture of ultrapure water and CGW at a ratio of 1:1 (v:v). This was used to test biodegradation in the column. The second solution was a mixture of persulfate solution (86 mM) and CGW at a ratio of 1:1 (v:v), which was used to test oxidative degradation. The solution was injected into the column at a flow rate of 0.05 ml/min, which is equivalent to a mean pore-water velocity of 0.045 cm/min. After 7-8 pore volumes of injection, the influent was switched to water. Effluent samples were collected in glass tubes placed in an ice-water bath so as to quench the reaction during sample collection. The samples were then weighed and analyzed for pH and the concentrations of TOC, persulfate, sulfate, ferrous ion, and total iron. The concentrations of TOC and persulfate in the reservoir were measured before, during, and after each experiment. These measured values were used as the reservoir concentrations (C0) correspondingly for the effluent samples collected at different times. Between injection of the two solutions, the column was flushed with degassed water for at least 10 pore volumes.
An additional set of experiments was conducted to test degradation of aged CGW by persulfate in the Fe3O4 column. The injection solution was prepared by mixing persulfate solution (86 mM) and aged CGW at a ratio of 1:1 (v:v). The procedure of the experiment was identical as that used for the column experiment for CGW treatment.
2.5. Analytical methods
The organic compounds in the CGW were analyzed using gas chromatography-mass spectrometry (Shimadzu GCMS-QP2010, Japan) equipped with a DB-5ms capillary column (50 m × 0.25 mm ID × 0.25 μm). The temperature for the GC-MS interface and ion source were 250 °C and 220 °C, respectively. The mass spectrometer was operated in the electron ionization (EI) mode at 70 eV and helium (flow rate of 1 ml min−1) was used as carrier gas. The injection port was maintained at 300 °C. The column temperature was held at 80 °C for 1 min, and then programmed at 12 °C/min to 200 °C (hold 1 min), 8 °C/min to 280 °C (hold 15 min), then at 2 °C/min to 300 °C. The total organic carbon (TOC), which is used to represent the total concentration of contaminants in CGW, was determined using a TOC analyzer (Shimadzu TOC-VCPH, Japan) after dilution of the samples. The concentration of H2O2 and persulfate were measured using the iodide-spectrophotometric method described by Zhong et al [30]. The concentration of permanganate ion (MnO4−) was measured with a UV-spectrophotometer (UV-2250, Shimadzu) at a wavelength of 525 nm [69, 70]. Sulfate, ferrous ion and total dissolve iron were measured using a Hach DR2800 spectrophotometer (Loveland, CO) based on the modulated Hach methods (No. 8051, 8146, 8008). The pH of samples was determined using PHS-3C pH meter (Leici Instrument Co., Shanghai).
3. Results and discussion
3.1. Batch experiments
3.1.1. Comparison between oxidants for degradation of leachate TOC
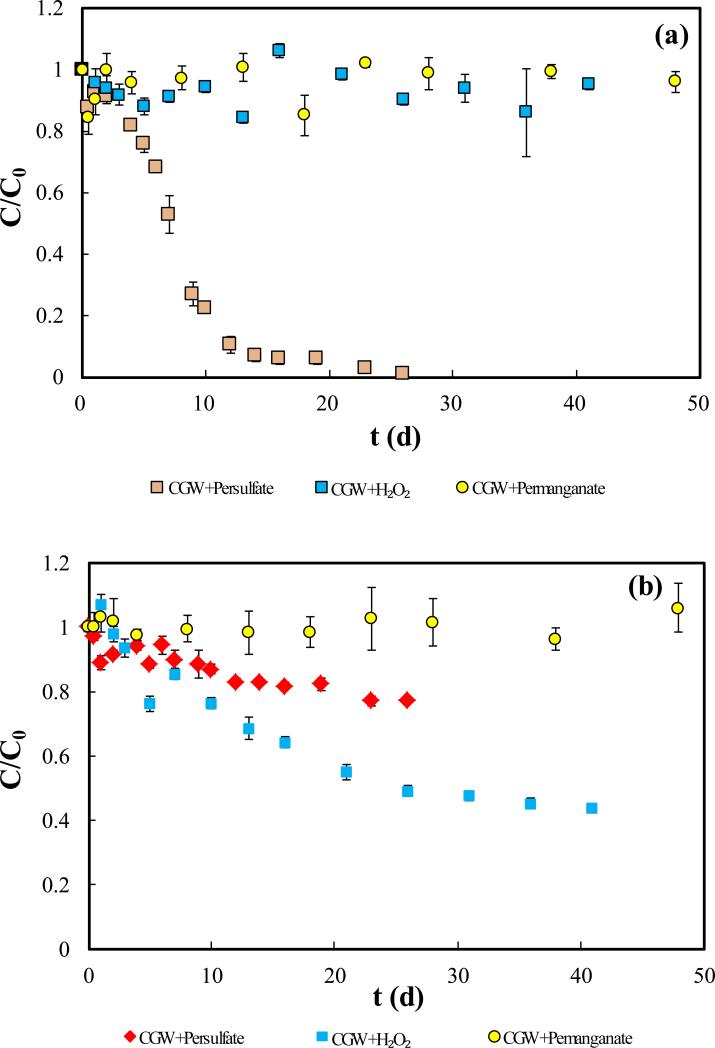
The results of degradation of TOC in CGW by the three oxidants (persulfate, H2O2, and permanganate) are shown in Fig. 1. TOC decreases significantly in the first 10 d (total removal percentage ~80%) for persulfate, and complete removal is observed in 26 d (Fig. 1(a)). Biodegradation is unlikely to have contributed significantly to the TOC removal, due to suppression of microbial activity by the high oxidation-reduction potential (2.01 V) and low pH (2~3) in the solution. In contrast to persulfate, TOC degradation by H2O2 and permanganate was not significant over the entire period of the experiment (50 d). These results show that persulfate is the strongest among the three oxidants for leachate TOC degradation.
Fig.1.
Comparison between oxidants for degradation of TOC in CGW. (a) Relative concentration of TOC versus time; (b) Relative concentrations of oxidants versus time. CGW in legend represents leachate contaminated groundwater.
The results of oxidant consumption during the experiment are presented in Fig. 1(b). Permanganate concentration is stable for the entire testing period, indicating no consumption. This result, together with the observed absence of TOC removal, shows that permanganate is ineffective for degradation of leachate TOC. The concentration of H2O2 is observed to decrease significantly. However, there was minimal removal of TOC by H2O2. This indicates that H2O2 experienced significant non-oxidative decomposition, consistent with its lower stability. Persulfate exhibited a 20% reduction by the end of the experiment. The relative oxidative effectiveness of H2O2 and persulfate observed herein are similar to the results in our prior study for 1,4-dioxane oxidation [30]. A possible reason could be a lower concentration of radicals in H2O2 solution than in persulfate solution, which was indicated by the much lower intensity of EPR signal of HO• in H2O2 solution [30]. Results of this comparison show that persulfate is superior to the other two commonly used ISCO oxidants, H2O2 and permanganate, for degradation of TOC in CGW.
3.1.2. Oxidation of TOC in CGW by persulfate
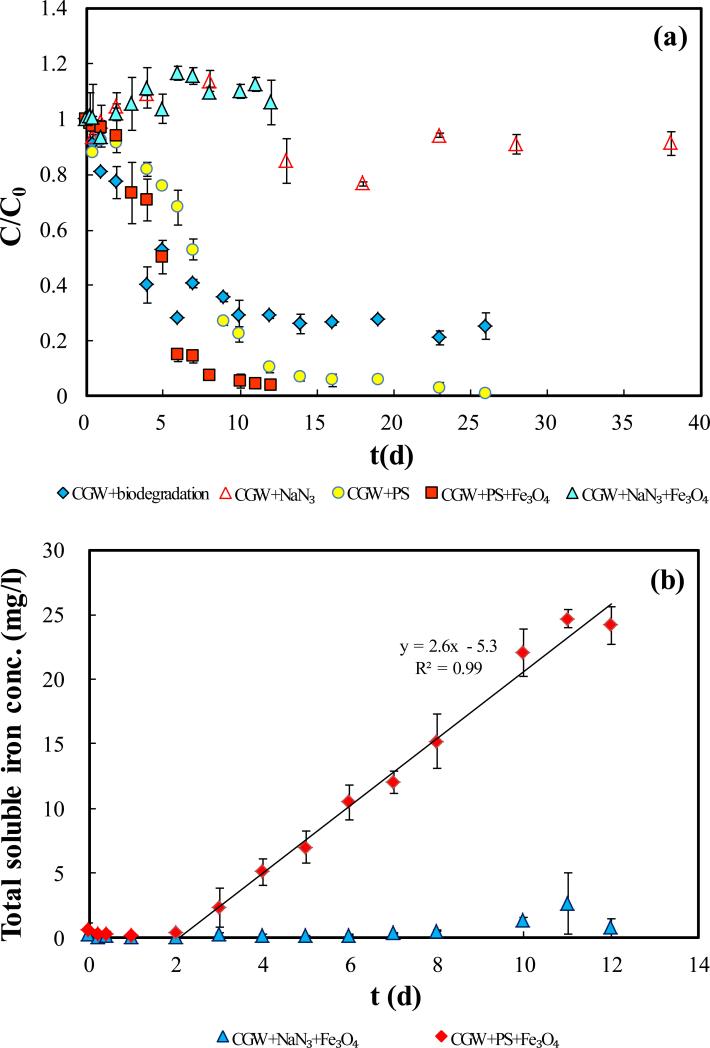
Biodegradation, persulfate-based degradation, and Fe3O4-activated persulfate degradation of TOC in CGW were compared and the results are presented in Fig. 2. In the biodegradation experiment in which persulfate is absent (CGW+biodegradation in Fig. 2(a)), TOC in CGW decreases rapidly in the first 5 d, showing a degradation rate even higher than for persulfate-based oxidative degradation (CGW+PS in Fig. 2(a)). The relative TOC concentration (C/C0), however, stabilizes after 10 days at a level of ~30%. In the control experiment with NaN3 as the bacterial metabolism inhibitor (CGW+NaN3 in Fig. 2(a)), no significant decrease of TOC is observed during the entire phase of the experiment. The result confirms that TOC loss in the experiment is not due to adsorption to vials, photochemical decomposition, volatilization, or other physicochemical processes. Incompleteness of the degradation indicates that biodegradation is not effective for removal of the refractory species in the CGW. This is in contrast to persulfate-based chemical oxidation, which causes complete degradation.
Fig.2.
Degradation of TOC in CGW in batch reactor system. Controls of CGW + NaN3, CGW + NaN3 + Fe3O4 are also included to examine physicochemical degradation of leachate compounds in the absence of persulfate. (a) Relative concentration of TOC; (b) Concentrations of total soluble iron; (c) Relative concentrations of sulfur species. PS ,TS and CGW in legend represent persulfate, total sulfur and leachate contaminated groundwater, respectively.
The presence of Fe3O4 does not cause decrease of TOC in CGW if the biological activity is suppressed by NaN3 (CGW+NaN3+Fe3O4 in Fig. 2(a)), and no production of soluble Fe was observed (CGW+NaN3+Fe3O4 in Fig. 2(b)), showing that Fe3O4 by itself does not cause degradation. In addition, the relative concentration of TOC (C/C0) remained stable at 1 for 2 h in the presence of Fe3O4 in the CGW (no persulfate and no sodium azide) (Fig. S8, SI), indicating no adsorption of TOC to Fe3O4. The Fe3O4, however, significantly enhances degradation of TOC by persulfate, causing a reduction in the time for complete degradation compared to persulfate alone (Fig. 2(a)). Persulfate-based reaction generally causes pH in the system to decrease [40]. As shown in Fig. S3, SI, the presence of Fe3O4 caused earlier stabilization of pH than in the absence of Fe3O4, which also indicates faster reaction. This result shows that the reaction between persulfate and the contaminants can be enhanced in the presence Fe3O4, likely due to heterogeneous activation of persulfate by Fe3O4. Hou et al also have reported that persulfate can be activated by Fe3O4, which increased the degradation efficiency of tetracycline by 50.5% [66].
Production of soluble Fe started at 2 d and the Fe concentration increased linearly with time of contact (CGW+PS+Fe3O4 in Fig. 2(b)). This is in contrast to the minimal soluble Fe production in the entire phase of experiment in the absence of persulfate (CGW+NaN3+Fe3O4 in Fig. 2(b)). The result shows that persulfate reacts with the Fe3O4 particles during the degradation process, which follows a zero-order kinetics shown by the linearity observed (the zero-order rate constant is 2.6 mg/(L·d)). Such zero-order kinetics implies a surface reaction mechanism for persulfate activation.
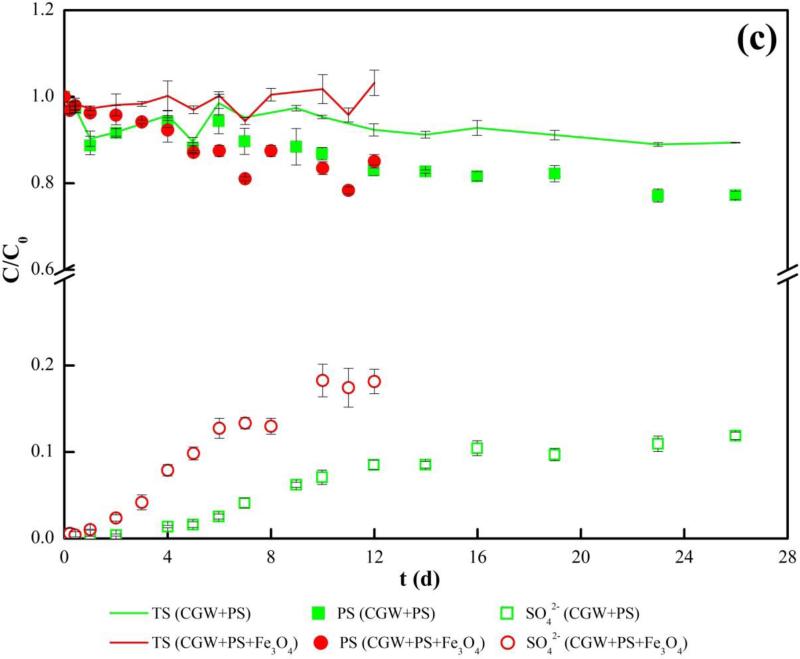
The results of persulfate consumption and sulfate production are presented in Fig. 3(c). For the entire phase of experiment, the total sulfur concentration, which is the sum of concentration of persulfate and sulfate, remains stable at the initial concentration (C/C0=1). The result indicates that sulfate is the only product for persulfate decomposition and no precipitation of persulfate or sulfate occurs. Two stages of sulfate production are observed and they are separated at 6 d and 12 d in the presence and absence of Fe3O4, respectively, corresponding to the phases of ongoing and post contaminant degradation. By the end of 12 days when ~95% of the TOC was removed by persulfate in the presence or absence of Fe3O4, the relative concentrations of persulfate remaining in the system were similar at 81% and 85%. This result shows that consumption of persulfate by Fe3O4 for leachate compound degradation is limited. The results of persulfate consumption and sulfate production are in contrast to the results of our previous study on activation of persulfate by iron filings for 1,4-dioxane degradation, for which very significant decrease and increase of persulfate and sulfate concentrations, respectively, were observed in the first 15 minutes [30].
Fig.3.
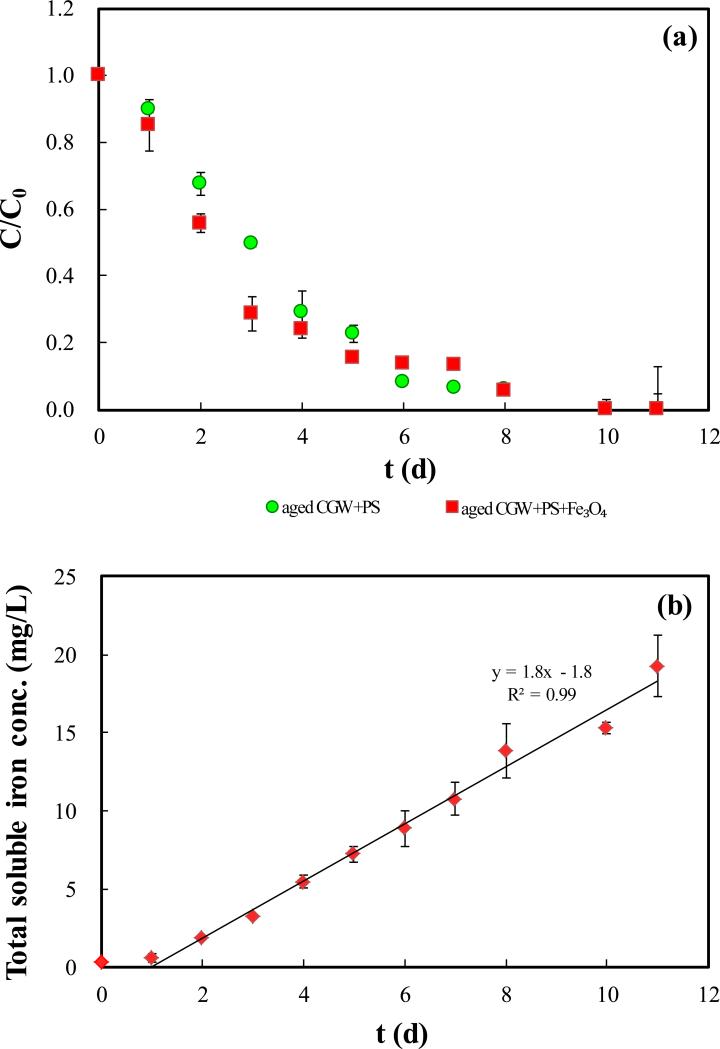
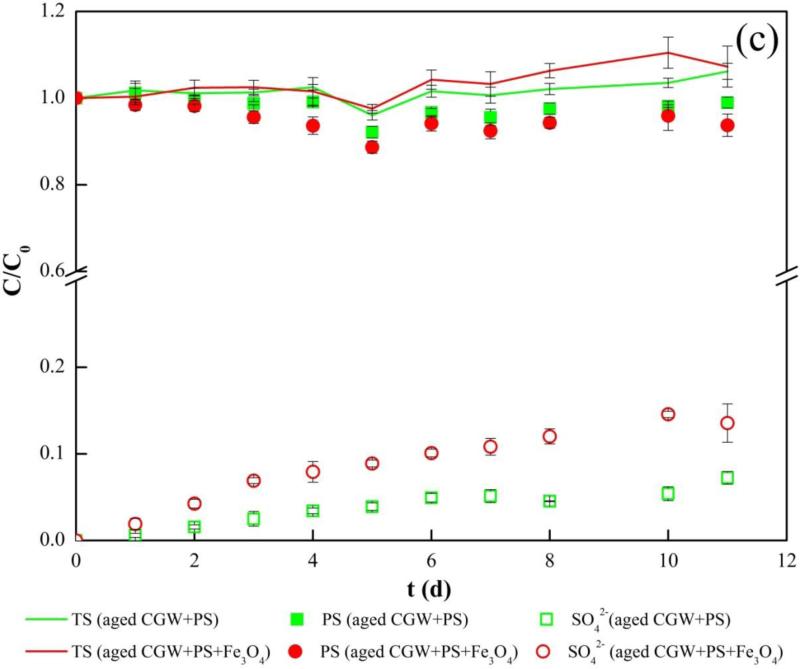
Degradation of TOC in aged CGW by persulfate in batch reactor system (a) Relative concentration of TOC; (b) Concentration of total iron; (c) Relative concentrations of sulfur species. PS, TS and aged CGW in legend represent persulfate, total sulfur and aged leachate contaminated groundwater, respectively.
Based on the pattern of TOC degradation, soluble Fe production, and sulfate production, a surface activation mechanism is speculated for Fe3O4 to activate persulfate to degrade the leachate compounds in CGW. Persulfate was activated at the surface of Fe3O4 solids to generate sulfate radicals via surface reaction. The radicals attack the leachate compounds in the neighboring solution and cause TOC degradation, or they may react with surface structures of Fe3O4 to produce soluble Fe. Degradation of leachate compounds seems to be preferential to reaction with surface structures of Fe3O4, as indicated by the delayed production of soluble Fe (Fig.2(b)) and a significantly lower sulfate production rate in the second stage when the leachate compounds were depleted (Fig.2(c)).
3.1.3. Degradation of TOC in aged CGW
Results of TOC removal by persulfate in aged CGW are presented in Fig. 3(a). TOC removal is complete in either presence or absence of Fe3O4. However, the degradation is faster in the presence of Fe3O4 in the first 3 days, yielding 21% more removal of TOC than in the absence of Fe3O4. Production of soluble Fe starts at 1 d, and the Fe concentration again increases linearly with time under acidic condition (Fig. S9, SI). The zero-order rate constant for Fe production is 1.8 mg/(L•d) (Fig. 3(b)), which is smaller than for TOC removal for CGW (2.6 mg/(L·d)). Production of sulfate over time shows a two-stage pattern, compromising a fast stage and a slow stage, which are separated at 3 d and 6 d in the presence and absence of Fe3O4, respectively (Fig. 3(c)).
3.1.4. The pseudo-first-order rate constants of TOC degradation
Due to the stoichiometrically great excess of oxidant to contaminants as indicated by the large initial molar ratio between oxidant and TOC (10:1), The reactions are assumed to follow pseudo-first order kinetics and the reaction rate constants are presented in Fig. S2, SI and Table 3. The pseudo-first-order rate constant for TOC biodegradation in CGW (leachate only) is 0.14 d−1. The rate constant for TOC degradation by persulfate alone is 0.16 d−1, which is similar to that for biodegradation. The rate constant in the presence of both persulfate and Fe3O4 is 0.27 d−1, which is significantly higher than that for persulfate alone. This result demonstrates that the reaction between persulfate and the contaminants can be enhanced by Fe3O4. The rate constant for TOC removal by persulfate in aged CGW is 0.34 d−1, which is larger than for CGW, indicating that persulfate can degrade compounds refractory to biodegradation even faster than the biodegradable compounds. The comparison of the reaction rate not only shows that persulfate-based chemical oxidation is superior to biodegradation, but also indicates that Fe3O4 can effectively activate persulfate to provide stronger oxidation condition for TOC degradation in CGW.
Table 3.
Pseudo-first-order reaction rate constant k and half-life t1/2 of TOC in batch experiments
| Reactants | Rate constant k (day−1) | Half-life t1/2 (day) |
|---|---|---|
| CGW + biodegradation | 0.14 | 5.0 |
| CGW + NaN3 | 0.009 | 77 |
| CGW + persulfate | 0.16 | 4.3 |
| CGW + hydrogen peroxide | 0.002 | 347 |
| CGW + permanganate | 0.001 | 693 |
| CGW + NaN3 + Fe3O4 | −0.011 | n.a |
| CGW + persulfate + Fe3O4 | 0.27 | 2.6 |
| Aged CGW + persulfate | 0.35 | 2.0 |
| Aged CGW + persulfate + Fe3O4 | 0.34 | 2.0 |
n.a: not available
3.2 Degradation of leachate TOC under dynamic flow conditions
The results of degradation of TOC in porous media under dynamic flow condition are presented in Fig. 4. For the sand-packed column, breakthrough curves (BTCs) of pentafluorobenzoic acid (PFBA), a nonreactive tracer, are symmetrical for arrival and elution waves, and C/C0 is approximately 0.5 at PV of 1, indicating ideal transport and uniform packing of the column (Fig. S4, SI). In the absence of persulfate (leachate only), C/C0 of TOC increases and forms plateau with C/C0 of 1, and then decreases and forms a secondary plateau with C/C0 of 0.6 at PV of 5 (Fig. 4(a)). Such a decrease of C/C0 and formation of the secondary plateau can be a result of accumulative growth of the microbes in the column (i.e., formation of biofilm), which can enhance efficiency of biodegradation. The plateau C/C0 of the BTC in the presence of persulfate, however, is close to 1, indicating insignificant degradation. The hydraulic residence time (tr) in the column was ~9 h. Such a short residence time is the likely reason for the minimal degradation (no significant degradation is observed within 9 h in batch experiment).
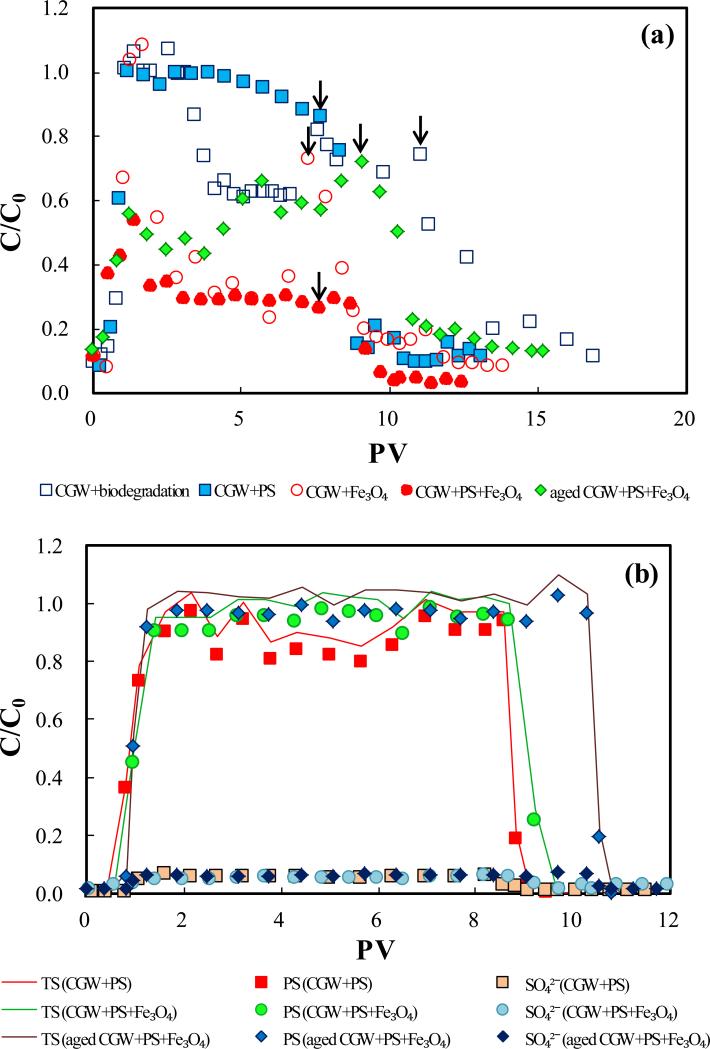
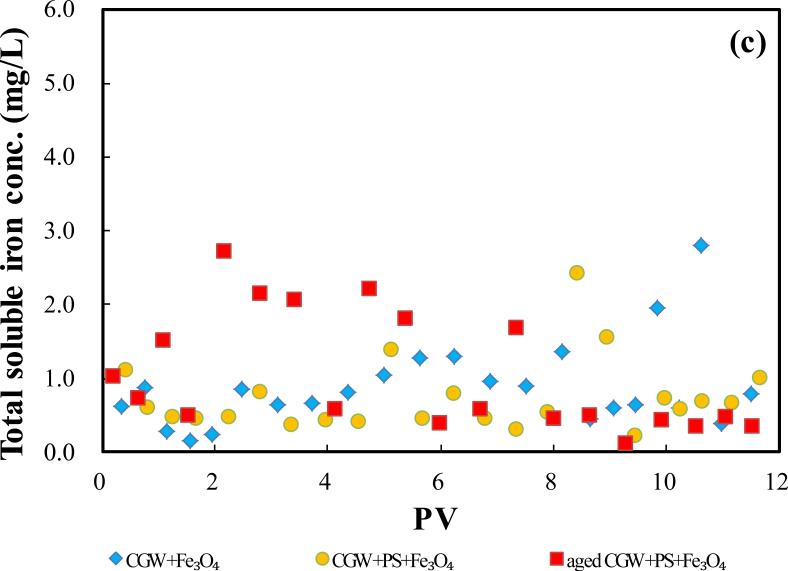
Fig.4.
Degradation of TOC in CGW and aged CGW in sand column under dynamic flow conditions. (a) Breakthrough curves of TOC; (b) Breakthrough curves of persulfate, total sulfur and sulfate; (c) Elution curves of total soluble iron. PS, TS, and CGW and aged CGW in legend represent persulfate, total sulfur, and leachate contaminated groundwater and aged leachate contaminated groundwater, respectively. Arrow indicates the start of water elution.
The presence of Fe3O4 in the sand enhanced both biodegradation and persulfate degradation of the leachate compounds. In the absence of persulfate, C/C0 increases to 1 at ~1.5 PV with injection of CGW, and then decreases rapidly to the secondary plateau of ~0.3. This C/C0 is significantly lower than that formed in the sand containing no Fe3O4, and is identical to the stabilized C/C0 for biodegradation in the batch experiment. The persulfate-based oxidation caused a significantly lower plateau of C/C0 (~0.3) for CGW, despite the limited residence time (9 h). This is in contrast to the minimal degradation of TOC in the column containing no Fe3O4. Significant removal of TOC was also observed for aged CGW in the presence of Fe3O4, shown by a plateau C/C0 significantly less than 1 (~0.6). These results indicate that Fe3O4 can significantly enhance degradation of leachate TOC by persulfate.
The BTCs of persulfate and sulfate with persulfate injection are presented in Fig. 4(b). No decrease of persulfate concentration is observed either with the presence or absence of Fe3O4, for CGW or aged CGW. Limited production of sulfate is observed and the C/C0 values of sulfate at the plateaus are similar. The results show that Fe3O4 alone does not cause significant decomposition of persulfate. Persulfate-based reaction caused pH in the system to decrease, however, no significant production of soluble Fe was observed (Fig. 4(c)). This observation shows that the reaction between persulfate and Fe3O4 is not significant in the presence of leachate compounds with maintained concentrations due to continuous injection. The results indicate the Fe3O4 can activate persulfate to preferentially react with leachate compounds, causing high efficiency of leachate compound degradation.
4. Conclusions
Persulfate is superior to hydrogen peroxide and permanganate for oxidative removal of landfill leachate compounds from contaminated groundwater. Persulfate-enhanced oxidation also has advantages over biodegradation for the leachate removal in that it shows non-selectivity to the compounds and causes complete degradation of the species that are refractory to biodegradation. Magnetite can enhance performance of persulfate for leachate compound degradation without significant consumption of the persulfate. Results of the column experiments demonstrate that significant degradation of the leachate compounds by persulfate can be achieved in porous medium containing magnetite under dynamic flow conditions.
Results of this study show that persulfate-based in-situ chemical oxidation (ISCO) method has good potential for treating groundwater contaminated by mature landfill leachate. A conceptual application of this method is the construction of persulfate-enhanced oxidation reactive zone, e.g., Fe3O4-based permeable reactive barrier or curtain wall, downgradient of the source of landfill leachate contamination. The distance between the location of contamination occurrence and the reactive zone will allow a hydraulic residence time sufficiently long for natural attenuation to remove the portion of biodegradable compounds. Persulfate is injected upgradient of the reactive zone. Perfusion and activation of persulfate in the zone is anticipated to cause complete degradation of the refractory leachate compounds.
Supplementary Material
Acknowledgements
This study was funded by the National Natural Science Foundation of China (51378192, 51378190 and 51308200). Additional support was provided by the NIEHS Superfund Research Program (P42 ES04940). The data supporting analysis and the conclusion are all included in the figures, tables, and Supporting Information file.
Reference
- 1.Tengrui L, Al-Harbawi A. Bo, Characteristics of nitrogen removal from old landfill leachate by sequencing batch biofilm reactor. AJAS. 2007;4:211–214. [Google Scholar]
- 2.Kurniawan TA, Lo W.-h. Removal of refractory compounds from stabilized landfill leachate using an integrated H2O2 oxidation and granular activated carbon (GAC) adsorption treatment. Water. Res. 2009;43:4079–4091. doi: 10.1016/j.watres.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 3.Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH. Present and long-term composition of MSW landfill leachate: a review. Crit. Rev. Env. Sci. Tec. 2002;32:297–336. [Google Scholar]
- 4.Deng Y, Ezyske CM. Sulfate radical-advanced oxidation process (SR-AOP) for simultaneous removal of refractory organic contaminants and ammonia in landfill leachate. Water. Res. 2011;45:6189–6194. doi: 10.1016/j.watres.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Englehardt JD. Electrochemical oxidation for landfill leachate treatment. Waste. Manage. 2007;27:380–388. doi: 10.1016/j.wasman.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Bashir MJ, Isa MH, Kutty SRM, Awang ZB, Aziz HA, Mohajeri S, Farooqi IH. Landfill leachate treatment by electrochemical oxidation. Waste. Manage. 2009;29:2534–2541. doi: 10.1016/j.wasman.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Han D, Tong X, Currell MJ, Cao G, Jin M, Tong C. Evaluation of the impact of an uncontrolled landfill on surrounding groundwater quality, Zhoukou, China. J. Geochem. Explor. 2014;136:24–39. [Google Scholar]
- 8.Xu Z-Y, Zeng G-M, Yang Z-H, Xiao Y, Cao M, Sun H-S, Ji L-L, Chen Y. Biological treatment of landfill leachate with the integration of partial nitrification, anaerobic ammonium oxidation and heterotrophic denitrification. Bioresource. Technol. 2010;101:79–86. doi: 10.1016/j.biortech.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 9.Kurniawan TA, Lo W.-h., Chan GY. Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J. Hazard. Mater. 2006;129:80–100. doi: 10.1016/j.jhazmat.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Lin SH, Chang CC. Treatment of landfill leachate by combined electro-Fenton oxidation and sequencing batch reactor method. Water. Res. 2000;34:4243–4249. [Google Scholar]
- 11.Hilles AH, Abu Amr SS, Hussein RA, Arafa AI, El-Sebaie OD. Effect of persulfate and persulfate/H2O2 on biodegradability of an anaerobic stabilized landfill leachate. Waste. Manage. 2015;44:172–177. doi: 10.1016/j.wasman.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 12.Abu Amr SS, Aziz HA, Adlan MN, Bashir MJ. Pretreatment of stabilized leachate using ozone/persulfate oxidation process. Chem. Eng. J. 2013;221:492–499. [Google Scholar]
- 13.Mohajeri S, Aziz HA, Isa MH, Bashir MJ, Mohajeri L, Adlan MN. Influence of Fenton reagent oxidation on mineralization and decolorization of municipal landfill leachate. J. Environ. Sci. Heal. A. 2010;45:692–698. doi: 10.1080/10934521003648883. [DOI] [PubMed] [Google Scholar]
- 14.Deng Y, Englehardt JD. Treatment of landfill leachate by the Fenton process. Water. Res. 2006;40:3683–3694. doi: 10.1016/j.watres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Shu H-Y, Fan H-J, Chang M-C, Hsieh W-P. Treatment of MSW landfill leachate by a thin gap annular UV/H2O2 photoreactor with multi-UV lamps. J. Hazard. Mater. 2006;129:73–79. doi: 10.1016/j.jhazmat.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Wu JJ, Wu C-C, Ma H-W, Chang C-C. Treatment of landfill leachate by ozone-based advanced oxidation processes. Chemosphere. 2004;54:997–1003. doi: 10.1016/j.chemosphere.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Primo O, Rivero MJ, Ortiz I. Photo-Fenton process as an efficient alternative to the treatment of landfill leachates. J. Hazard. Mater. 2008;153:834–842. doi: 10.1016/j.jhazmat.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 18.I. ITRC Council TITaR, editor. Technical and Regulatory Guidance for In Situ Chemical Oxidation of Contaminated Soil and Groundwater. 2005 [Google Scholar]
- 19.Huling SG, Pivetz BE. In-situ chemical oxidation. DTIC Document. 2006 [Google Scholar]
- 20.Liang S, Kao C, Kuo Y, Chen K, Yang B. In situ oxidation of petroleum-hydrocarbon contaminated groundwater using passive ISCO system. Water. Res. 2011;45:2496–2506. doi: 10.1016/j.watres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Deng D, Yang L. Degradation of dimethyl phthalate in solutions and soil slurries by persulfate at ambient temperature. J. Hazard. Mater. 2014;271:202–209. doi: 10.1016/j.jhazmat.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Yen C-H, Chen K-F, Kao C-M, Liang S-H, Chen T-Y. Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: Feasibility and comparison with common oxidants. J. Hazard. Mater. 2011;186:2097–2102. doi: 10.1016/j.jhazmat.2010.12.129. [DOI] [PubMed] [Google Scholar]
- 23.Budaev S, Batoeva A, Tsybikova B. Degradation of thiocyanate in aqueous solution by persulfate activated ferric ion. Miner. Eng. 2015;81:88–95. [Google Scholar]
- 24.Huang K-C, Zhao Z, Hoag GE, Dahmani A, Block PA. Degradation of volatile organic compounds with thermally activated persulfate oxidation. Chemosphere. 2005;61:551–560. doi: 10.1016/j.chemosphere.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 25.Liang C, Lee I-L, Hsu I-Y, Liang C-P, Lin Y-L. Persulfate oxidation of trichloroethylene with and without iron activation in porous media. Chemosphere. 2008;70:426–435. doi: 10.1016/j.chemosphere.2007.06.077. [DOI] [PubMed] [Google Scholar]
- 26.Liang C, Liang C-P, Chen C-C. pH dependence of persulfate activation by EDTA/Fe (III) for degradation of trichloroethylene. J. Contam. Hydrol. 2009;106:173–182. doi: 10.1016/j.jconhyd.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Waldemer RH, Tratnyek PG, Johnson RL, Nurmi JT. Oxidation of chlorinated ethenes by heat-activated persulfate: kinetics and products. Environ. Sci. Technol. 2007;41:1010–1015. doi: 10.1021/es062237m. [DOI] [PubMed] [Google Scholar]
- 28.Liang C, Bruell CJ. Thermally activated persulfate oxidation of trichloroethylene: experimental investigation of reaction orders. Ind. Eng. Chem. Res. 2008;47:2912–2918. [Google Scholar]
- 29.Zhao L, Hou H, Fujii A, Hosomi M, Li F. Degradation of 1, 4-dioxane in water with heat-and Fe2+-activated persulfate oxidation. Environ. Sci. Pollut. R. 2014;21:7457–7465. doi: 10.1007/s11356-014-2668-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhong H, Brusseau ML, Wang Y, Yan N, Quig L, Johnson GR. In-situ activation of persulfate by iron filings and degradation of 1, 4-dioxane. Water. Res. 2015;83:104–111. doi: 10.1016/j.watres.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang C, Guo Y-Y, Chien Y-C, Wu Y-J. Oxidative degradation of MTBE by pyrite-activated persulfate: proposed reaction pathways. Ind. Eng. Chem. Res. 2010;49:8858–8864. [Google Scholar]
- 32.Zhao D, Liao X, Yan X, Huling SG, Chai T, Tao H. Effect and mechanism of persulfate activated by different methods for PAHs removal in soil. J. Hazard. Mater. 2013;254:228–235. doi: 10.1016/j.jhazmat.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 33.Ferrarese E, Andreottola G, Oprea IA. Remediation of PAH-contaminated sediments by chemical oxidation. J. Hazard. Mater. 2008;152:128–139. doi: 10.1016/j.jhazmat.2007.06.080. [DOI] [PubMed] [Google Scholar]
- 34.Gryzenia J, Cassidy D, Hampton D. Production and accumulation of surfactants during the chemical oxidation of PAH in soil. Chemosphere. 2009;77:540–545. doi: 10.1016/j.chemosphere.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Shabiimam M, Dikshit AK. Treatment of municipal landfill leachate by oxidants. Am. J. Environ. Eng. 2012;2:1–5. [Google Scholar]
- 36.Chou Y-C, Lo S-L, Kuo J, Yeh C-J. Microwave-enhanced persulfate oxidation to treat mature landfill leachate. J. Hazard. Mater. 2015;284:83–91. doi: 10.1016/j.jhazmat.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RL, Tratnyek PG, Johnson ROB. Persulfate persistence under thermal activation conditions. Environ. Sci. Technol. 2008;42:9350–9356. doi: 10.1021/es8019462. [DOI] [PubMed] [Google Scholar]
- 38.Liang CJ, Bruell CJ, Marley MC, Sperry KL. Thermally activated persulfate oxidation of trichloroethylene (TCE) and 1, 1, 1-trichloroethane (TCA) in aqueous systems and soil slurries. Soil and sediment contamination: An international journal. 2003;12:207–228. [Google Scholar]
- 39.Gu X, Lu S, Li L, Qiu Z, Sui Q, Lin K, Luo Q. Oxidation of 1, 1, 1-trichloroethane stimulated by thermally activated persulfate. Ind. Eng. Chem. Res. 2011;50:11029–11036. [Google Scholar]
- 40.Liang C, Wang Z-S, Bruell CJ. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere. 2007;66:106–113. doi: 10.1016/j.chemosphere.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Marchesi M, Thomson NR, Aravena R, Sra KS, Otero N, Soler A. Carbon isotope fractionation of 1, 1, 1-trichloroethane during base-catalyzed persulfate treatment. J. Hazard. Mater. 2013;260:61–66. doi: 10.1016/j.jhazmat.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Liang C, Guo Y-Y. Remediation of diesel-contaminated soils using persulfate under alkaline condition. Water. Air. Soil. Poll. 2012;223:4605–4614. [Google Scholar]
- 43.Price GJ, Clifton AA, Keen F. Ultrasonically enhanced persulfate oxidation of polyethylene surfaces. Polymer. 1996;37:5825–5829. [Google Scholar]
- 44.Lau TK, Chu W, Graham NJ. The aqueous degradation of butylated hydroxyanisole by UV/S2O82−: study of reaction mechanisms via dimerization and mineralization. Environ. Sci. Technol. 2007;41:613–619. doi: 10.1021/es061395a. [DOI] [PubMed] [Google Scholar]
- 45.Yuan S, Liao P, Alshawabkeh AN. Electrolytic manipulation of persulfate reactivity by iron electrodes for trichloroethylene degradation in groundwater. Environ. Sci. Technol. 2013;48:656–663. doi: 10.1021/es404535q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anipsitakis GP, Dionysiou DD. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B- Environ. 2004;54:155–163. [Google Scholar]
- 47.Killian PF, Bruell CJ, Liang C, Marley MC. Iron (II) activated persulfate oxidation of MGP contaminated soil. Soil. Sediment. Conta. 2007;16:523–537. [Google Scholar]
- 48.Liang C, Bruell CJ, Marley MC, Sperry KL. Persulfate oxidation for in situ remediation of TCE. II. Activated by chelated ferrous ion. Chemosphere. 2004;55:1225–1233. doi: 10.1016/j.chemosphere.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 49.Xu X-R, Li X-Z. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep. Purif. Technol. 2010;72:105–111. [Google Scholar]
- 50.Rao Y, Qu L, Yang H, Chu W. Degradation of carbamazepine by Fe (II)-activated persulfate process. J. Hazard. Mater. 2014;268:23–32. doi: 10.1016/j.jhazmat.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Wan J, Ma Y, Wang Y, Huang M. Influence of particle size of zero-valent iron and dissolved silica on the reactivity of activated persulfate for degradation of acid orange 7. Chem. Eng. J. 2014;237:487–496. [Google Scholar]
- 52.Teel AL, Ahmad M, Watts RJ. Persulfate activation by naturally occurring trace minerals. J. Hazard. Mater. 2011;196:153–159. doi: 10.1016/j.jhazmat.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Ahmad M, Teel AL. Watts, Persulfate activation by subsurface minerals. J. Contam. Hydrol. 2010;115:34–45. doi: 10.1016/j.jconhyd.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Gregory KB, Larese-Casanova P, Parkin GF, Scherer MM. Abiotic transformation of hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine by FeII bound to magnetite. Environ. Sci. Technol. 2004;38:1408–1414. doi: 10.1021/es034588w. [DOI] [PubMed] [Google Scholar]
- 55.Matta R, Hanna K, Chiron S. Fenton-like oxidation of 2, 4, 6-trinitrotoluene using different iron minerals. Sci. Total. Environ. 2007;385:242–251. doi: 10.1016/j.scitotenv.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 56.Weng C-H, Tao H. Highly efficient persulfate oxidation process activated with Fe0 aggregate for decolorization of reactive azo dye Remazol Golden Yellow. Arab. J. Chem. 2015 [Google Scholar]
- 57.Weng C-H, Tsai K-L. Ultrasound and heat enhanced persulfate oxidation activated with Fe 0 aggregate for the decolorization of CI Direct Red 23. Ultrason. Sonochem. 2016;29:11–18. doi: 10.1016/j.ultsonch.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Liu N, Ding F, Weng C-H, Hwang C-C, Lin Y-T. Minimizing the interference of carbonate ions on degradation of SRF3B dye by Fe0-aggregate-activated persulfate process. Sep. Purif. Technol. 2016 [Google Scholar]
- 59.Hanna K, Kone T, Medjahdi G. Synthesis of the mixed oxides of iron and quartz and their catalytic activities for the Fenton-like oxidation. Catal. Commun. 2008;9:955–959. [Google Scholar]
- 60.Xue X, Hanna K, Abdelmoula M, Deng N. Adsorption and oxidation of PCP on the surface of magnetite: kinetic experiments and spectroscopic investigations. Appl. Catal. B-Environ. 2009;89:432–440. [Google Scholar]
- 61.Gorski CA, Scherer MM. Influence of magnetite stoichiometry on FeII uptake and nitrobenzene reduction. Environ. Sci. Technol. 2009;43:3675–3680. doi: 10.1021/es803613a. [DOI] [PubMed] [Google Scholar]
- 62.Vikesland PJ, Heathcock AM, Rebodos RL, Makus KE. Particle size and aggregation effects on magnetite reactivity toward carbon tetrachloride. Environ. Sci. Technol. 2007;41:5277–5283. doi: 10.1021/es062082i. [DOI] [PubMed] [Google Scholar]
- 63.Hung C-M, Chen C-W, Jhuang Y-Z, Dong C-D. Fe3O4 Magnetic Nanoparticles: Characterization and Performance Exemplified by the Degradation of Methylene Blue in the Presence of Persulfate. J. Adv. Oxid. Technol. 2016;19:43–51. [Google Scholar]
- 64.Fang G-D, Dionysiou DD, Al-Abed SR, Zhou D-M. Superoxide radical driving the activation of persulfate by magnetite nanoparticles: implications for the degradation of PCBs. Appl. Catal. B-Environ. 2013;129:325–332. [Google Scholar]
- 65.Usman M, Faure P, Ruby C, Hanna K. Application of magnetite-activated persulfate oxidation for the degradation of PAHs in contaminated soils. Chemosphere. 2012;87:234–240. doi: 10.1016/j.chemosphere.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Hou L, Zhang H, Xue X. Ultrasound enhanced heterogeneous activation of peroxydisulfate by magnetite catalyst for the degradation of tetracycline in water. Sep. Purif. Technol. 2012;84:147–152. [Google Scholar]
- 67.Renou S, Givaudan J, Poulain S, Dirassouyan F, Moulin P. Landfill leachate treatment: review and opportunity. J. Hazard. Mater. 2008;150:468–493. doi: 10.1016/j.jhazmat.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 68.He P.-j., Xue J.-f., Shao L.-m., Li G.-j., Lee D-J. Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill. Water. Res. 2006;40:1465–1473. doi: 10.1016/j.watres.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 69.Liao X, Zhao D, Yan X. Determination of potassium permanganate demand variation with depth for oxidation–remediation of soils from a PAHs-contaminated coking plant. J. Hazard. Mater. 2011;193:164–170. doi: 10.1016/j.jhazmat.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 70.Mahmoodlu MG, Hartog N, Hassanizadeh SM, Raoof A. Oxidation of volatile organic vapours in air by solid potassium permanganate. Chemosphere. 2013;91:1534–1538. doi: 10.1016/j.chemosphere.2012.12.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.