Abstract
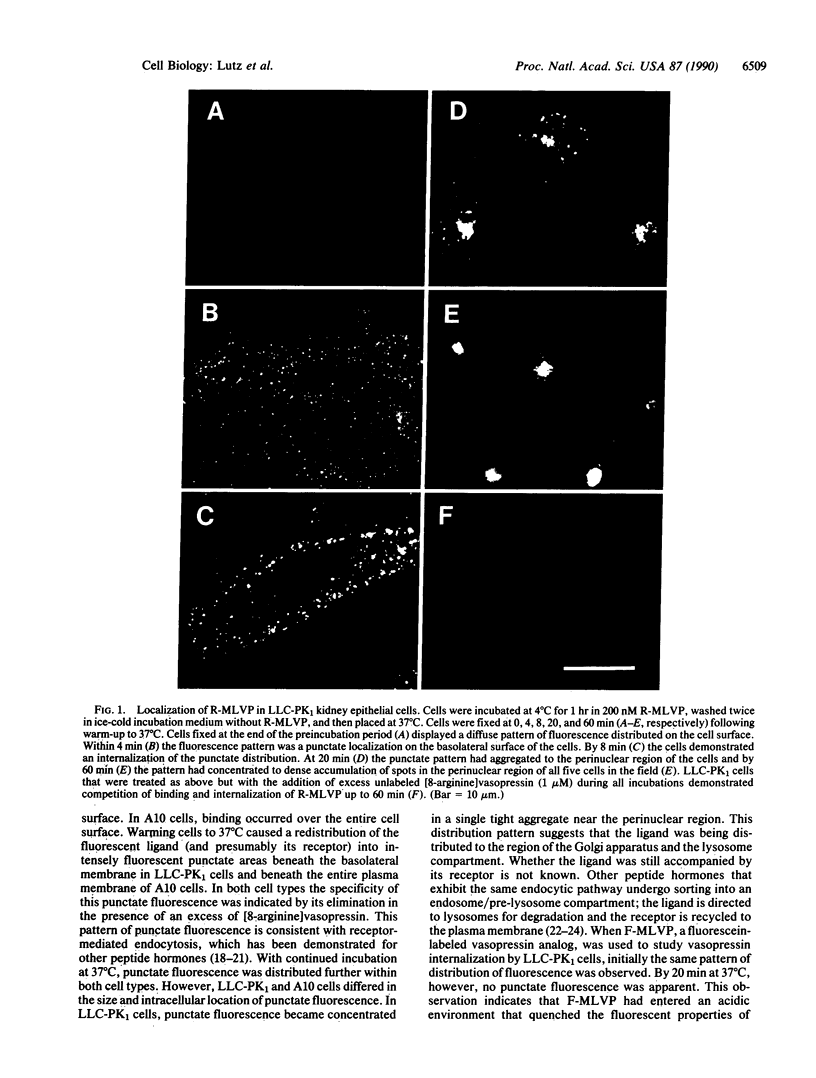
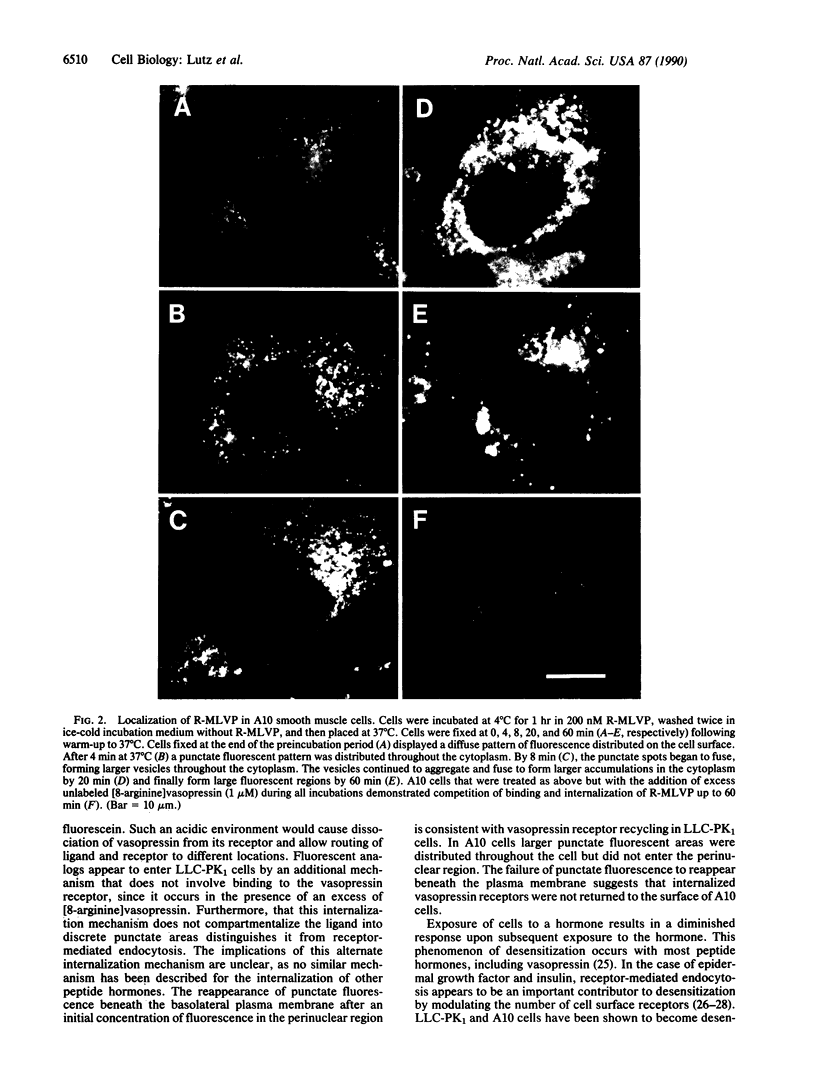
To determine whether receptor-mediated endocytosis occurs in vasopressin-responsive cells, we developed a model system using synthetic fluorescent-labeled vasopressin analogs and A10 (smooth muscle) and LLC-PK1 (kidney epithelial) cells in culture; these cell lines express V1 and V2 vasopressin cell surface receptor types, respectively. We used epifluorescence microscopy to examine the binding, internalization, and intracellular destination of [1-(2-mercapto)propionic acid,8-lysine-N6-carboxytetramethylrhodamine] vasopressin (R-MLVP) and [1-(2-mercapto)propionic acid,8-lysine-N6-carboxyfluorescein]vasopressin (F-MLVP) in these cells. The rhodamine-labeled fluorescent vasopressin analog, R-MLVP, initially bound in a diffuse manner at the cell surface of both A10 and LLC-PK1 cells and could be displaced by excess unlabeled [8-arginine]vasopressin. After incubation at 37 degrees C, bound ligand rapidly aggregated into small clusters or patches, which were internalized in a manner consistent with receptor-mediated endocytosis. Subsequent processing of internalized ligand-receptor complexes appeared to differ between A10 and LLC-PK1 cells. In the case of LLC-PK1 cells, ligand was delivered to a tightly focused lysosome compartment in the perinuclear region of the cell, and receptor molecules were replenished at the cell surface. The lysosomal location of ligand was supported by the quenching of fluorescence in the internalized vesicles when F-MLVP was used as a fluorescent tracer. In the case of A10 cells, ligand became localized to a vesicular compartment and reappearance of receptor at the cell surface was limited. Our data are consistent with the occurrence of receptor-mediated endocytosis of vasopressin in cells with V1 and V2 receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar N., Nambi P., Stassen F. L., Crooke S. T. Vascular vasopressin receptors mediate phosphatidylinositol turnover and calcium efflux in an established smooth muscle cell line. Life Sci. 1986 Jul 7;39(1):37–45. doi: 10.1016/0024-3205(86)90435-2. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986 Mar;68(3):375–381. doi: 10.1016/s0300-9084(86)80004-9. [DOI] [PubMed] [Google Scholar]
- Enns C. A., Larrick J. W., Suomalainen H., Schroder J., Sussman H. H. Co-migration and internalization of transferrin and its receptor on K562 cells. J Cell Biol. 1983 Aug;97(2):579–585. doi: 10.1083/jcb.97.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Grier C. E., 3rd, Nambi P., Aiyar N., Crooke S. T. Molecular mechanisms of homologous and heterologous desensitization mediated by vasopressin in smooth muscle cells. J Biol Chem. 1989 Apr 5;264(10):5384–5391. [PubMed] [Google Scholar]
- Hazum E., Cuatrecasas P., Marian J., Conn P. M. Receptor-mediated internalization of fluorescent gonadotropin-releasing hormone by pituitary gonadotropes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6692–6695. doi: 10.1073/pnas.77.11.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Brandt B. L. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res. 1976 Mar 15;98(2):349–366. doi: 10.1016/0014-4827(76)90446-8. [DOI] [PubMed] [Google Scholar]
- Lai W. H., Cameron P. H., Wada I., Doherty J. J., 2nd, Kay D. G., Posner B. I., Bergeron J. J. Ligand-mediated internalization, recycling, and downregulation of the epidermal growth factor receptor in vivo. J Cell Biol. 1989 Dec;109(6 Pt 1):2741–2749. doi: 10.1083/jcb.109.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester B. R., Sheppard J. R., Burman M., Somkuti S. B., Stassen F. L. Desensitization of LLC-PK1 cells by vasopressin results in receptor down-regulation. Mol Cell Endocrinol. 1985 May;40(2-3):193–204. doi: 10.1016/0303-7207(85)90175-3. [DOI] [PubMed] [Google Scholar]
- Linderman J. J., Lauffenburger D. A. Analysis of intracellular receptor/ligand sorting in endosomes. J Theor Biol. 1988 May 21;132(2):203–245. doi: 10.1016/s0022-5193(88)80157-7. [DOI] [PubMed] [Google Scholar]
- Lutz W. H., Londowski J. M., Kumar R. The synthesis and biological activity of four novel fluorescent vasopressin analogs. J Biol Chem. 1990 Mar 15;265(8):4657–4663. [PubMed] [Google Scholar]
- Mills J. W., Macknight A. D., Dayer J. M., Ausiello D. A. Localization of [3H]ouabain-sensitive Na+ pump sites in cultured pig kidney cells. Am J Physiol. 1979 Mar;236(3):C157–C162. doi: 10.1152/ajpcell.1979.236.3.C157. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kahane I., Cuatrecasas P. Receptor-mediated internalization of fluorescent chemotactic peptide by human neutrophils. Science. 1979 Sep 28;205(4413):1412–1414. doi: 10.1126/science.472759. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ J., DEINHARDT F. Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology. 1960 Oct;12:316–317. doi: 10.1016/0042-6822(60)90205-1. [DOI] [PubMed] [Google Scholar]
- Roy C., Hall D., Karish M., Ausiello D. A. Relationship of (8-lysine) vasopressin receptor transition to receptor functional properties in a pig kidney cell line (LLC-PK1). J Biol Chem. 1981 Apr 10;256(7):3423–3427. [PubMed] [Google Scholar]
- Ryan K. L., Thornton R. M., Proppe D. W. Vasopressin contributes to maintenance of arterial blood pressure in dehydrated baboons. Am J Physiol. 1989 Feb;256(2 Pt 2):H486–H492. doi: 10.1152/ajpheart.1989.256.2.H486. [DOI] [PubMed] [Google Scholar]
- Salzman N. H., Maxfield F. R. Fusion accessibility of endocytic compartments along the recycling and lysosomal endocytic pathways in intact cells. J Cell Biol. 1989 Nov;109(5):2097–2104. doi: 10.1083/jcb.109.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbraccia P., Wong K. Y., Brunetti A., Rafaeloff R., Trischitta V., Hawley D. M., Goldfine I. D. Insulin down-regulates insulin receptor number and up-regulates insulin receptor affinity in cells expressing a tyrosine kinase-defective insulin receptor. J Biol Chem. 1990 Mar 25;265(9):4902–4907. [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sonne O. Receptor-mediated endocytosis and degradation of insulin. Physiol Rev. 1988 Oct;68(4):1129–1196. doi: 10.1152/physrev.1988.68.4.1129. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Heckman G., Schmidt D., Aiyar N., Nambi P., Crooke S. T. Identification and characterization of vascular (V1) vasopressin receptors of an established smooth muscle cell line. Mol Pharmacol. 1987 Mar;31(3):259–266. [PubMed] [Google Scholar]
- Wells A., Welsh J. B., Lazar C. S., Wiley H. S., Gill G. N., Rosenfeld M. G. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990 Feb 23;247(4945):962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Dixon B. S., Dillingham M. A., Garcia-Sainz J. A., Anderson R. J. Pertussis toxin prevents homologous desensitization of adenylate cyclase in cultured renal epithelial cells. J Biol Chem. 1986 Feb 5;261(4):1503–1506. [PubMed] [Google Scholar]