Abstract
Inorganic-organic hydride perovskites bring the hope for fabricating low-cost and large-scale solar cells. At the beginning of the research, two open questions were raised: the hysteresis effect and the role of chloride. The presence of chloride significantly improves the crystallization and charge transfer property of the perovskite. However, though the long held debate over of the existence of chloride in the perovskite seems to have now come to a conclusion, no prior work has been carried out focusing on the role of chloride on the electronic performance and the crystallization of the perovskite. Furthermore, current reports on the crystal structure of the perovskite are rather confusing. This article analyzes the role of chloride in CH3NH3PbI3-xClx on the crystal orientation and provides a new explanation about the (110)-oriented growth of CH3NH3PbI3 and CH3NH3PbI3-xClx.
Keywords: solar cells, perovskites, X-ray diffraction, phase transitions
1. Introduction
Since the first organic-inorganic halide perovskite solar cell was reported [1], perovskites have attracted growing interest and the power conversion efficiency (PCE) has reached 20.1% [2]. It is not very common that a photovoltaic device can experience such a rapid development. While the structure of the cells evolved from sensitized meso-structure to planar structure [3], both inorganic and organic materials can be applied as electron and hole transfer materials [4]. Furthermore, by tuning the composition of the perovskite, the band gap can be easily modified [5]. Given the numerous advantages of perovskite, a clear understanding of the crystal structure is crucial and the role of chloride in the formation of CH3NH3PbI3-xClx (hereafter, we use MA short for CH3NH3) is one of the most pressing topics.
It has been reported that the presence of chloride in the perovskite improves the uniformity of its layer [6] and results in an increase of the carriers’ diffusion length from ca. 100 nm to over 1 μm [7]. However, the long held debate over of the existence of chloride in the perovskite seems to have now come to a conclusion. First, when synthesizing the perovskite by the one step method with precursor solution of MACl and PbI2 (1:1 molar ratio) in anhydrous N,N-dimethylformamide (DMF), the resulting crystal is not MAPbI2Cl but a mixture of MAPbI3 and MAPbCl3 [8]. This provides direct evidence that chloride (Cl−) cannot substitute iodine (I−) in the perovskite to form a stable crystal. Then, two contradictory results were then reported. X-ray photoelectron spectroscopy (XPS) showed that the molar ratio C:N:Pb:I:Cl of the perovskite is ca. 1:1:1:2:1, when prepared from a precursor of MAI:PbCl2 (molar ratio 3:1) [9]. On the other hand, energy dispersive X-ray (EDX) analysis showed that no Cl− was present in the perovskite prepared from PbI2 + MAI + MACl [10]. Noting that the XPS was unable to determine the existence of MAPbI2Cl crystal and that EDX has its detecting limitation, more precise characterizations were needed. Later on, the simultaneous Fourier transform infrared spectroscopy analysis of the expelled gas during the decomposition of MAPbI3-xClx showed the presence of Cl−, angle-resolved XPS [11] and X-ray fluorescence spectroscopy (XFS) [12] not only confirmed the existence of Cl−, but also showed that Cl− was located at the interface between the perovskite and the electron transport TiO2 layer, and not in the perovskite structure [11,12]. Moreover, scanning transmission microscopy-energy dispersive spectroscopy (STEM-EDS) detected no trace of Cl− in the perovskite. Even though there is a strong Cl− signal, no N was observed indicting the presence of only PbCl2 [13]. Thus, Cl− only appears at the interface between MAPbI3 and the anode. Two more reports have further confirmed this conclusion. XPS analysis showed only weak Cl− signal after etching the surface of MAPbI3-xClx by a 50 nm thickness [14]. Hard X-ray photoelectron spectroscopy and fluorescence yield X-ray absorption spectroscopy showed no Cl− at the surface of MAPbI3-xClx with higher average concentration of Cl throughout the perovskite layer at the deep beneath [15]. Here, we refer to MAPbI3-xClx as MAPbI3 that is prepared using chloride-containing precursors. However, as the condition for depositing MAPbI3-xClx differs, Cl− may still remain in the resulting perovskite layer. For instance, X-ray absorption near edge structure (XANES) results showed that x = 0.05 ± 0.03 Cl atoms per formula unit remain in the films after annealing at 95 °C for 120 min [16]. The results from photothermal induced resonance (PTIR) showed that the MAPbI3-xClx film consists of a mixture of Cl-rich (xlocal < 0.3) and Cl-poor phases after a mild annealing (60 °C, 60 min) and homogeneous Cl-poorer (xlocal < 0.06) phase upon further annealing (110 °C) [17].
In addition, first-principles calculation results provide some good explanation. For the crystal structure, Cl− concentration was found below 3%–4% [8] and if the Cl− ions enter the crystal structure, they preferentially occupy the apical positions in the PbI4X2 octahedra [18]. For the electronic property, while the molecular orientations of CH3NH3+ result in three times larger photocurrent response than the ferroelectric photovoltaic BiFeO3, Cl− substitution at the equatorial site induces a larger response than does substitution at the apical site [19]. Results also showed that, using Cl− precursor can avoid forming the PbI defects [20]. Introducing Cl− would reduce the lattice constant which can inhibit the formation of interstitial defects [21]. As excitons may be screened by collective orientational motion of the organic cations, Cl− might hinder this motion and results in better transport properties [22]. Little difference of electronic properties was represent among orthorhombic, tetragonal and cubic phases of MAPbI3 [23], however, the valance-band-maximum and conduction-band-minimum states can be mainly derived from iodine ions at some unique positions, Cl− substitution can strengthen the unique position of the ions and result in more localized charge density [24]. Thus, lower carrier recombination rate and enhanced carrier transport ensued. For the interface, the (001) and (110) surfaces tend to favor hole injection to 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)-9,9′-spirobifluorene (Spiro-MeOTAD), while the (100) surface facilitates electron transfer to [6,6]-phenyl C61-butyric acid methyl ester (PCBM) [25]. A better structural matching between adjacent rows of perovskite surface halides and TiO2 under coordinated titanium may be the reason for the (110)-oriented growth of MAPbI3-xClx and MAPbI3 [26]. Interfacial Cl− may thus further stabilize the (110) surface and modify the interface electronic structure between MAPbI3 and TiO2 [26].
Despite the absence of Cl− in the perovskite, it still played an important role in the crystallization process. For instance, the morphology of MAPbI3-xClx was compared with MAPbI3 [27] and a model in which the Cl− rich phase modifies the morphologies of perovskite was proposed and fit well with the results from scanning electron microscopy (SEM) [27]. In addition, the transmission electron microscopy (TEM) of freeze-dried perovskite MAPbI3−xClx precursor solution showed the presence of PbCl2 nanoparticles [28] and this is in agreement with the dynamic light scattering (DLS) investigations of MAPbI3−xClx precursor solution [29]. Thus, references [28,29] further proved the model of the heterogeneous nucleation by PbCl2 nanoparticles proposed in reference [27]. However, the formation mechanism of the crystal structure remains undermined and this will be discussed in the following parts of this article.
2. Methods for Fabricating MAPbI3-xClx
In Section 3, we discuss the crystal structure of MAPbI3-xClx according to the deposition method. As the fabrication methods were discussed in detail in reference [30], here we add a brief introduction about the preparation methods of MAPbI3-xClx. For the one-step deposition method, MAI:PbI2/PbCl2 (molar ratio 1:1 or 3:1) [31,32] were dissolved in γ-butyrolactone (GBL) or DMF, spin-coated on the substrates and annealed to form perovskite. Different annealing conditions result in different morphology of the MAPbI3-xClx layer. While a rapid thermal annealing at 130 °C resulted in micron-sized perovskite grains [33], two-step annealing, such as 90 °C for 30 min then at 100 °C for 2 min [34] or 60 °C then ramping to 90 °C [35], resulted in optimal PCE on poly(3,4-ethylenedioxythiophene) poly(styrene-sulfonate) (PEDOT:PSS) substrates. A full coverage of perovskite can be achieved by rapid cooling after annealing [36]. To increase the solubility of Cl−, 1,8-diiodooctane [37] or other alkyl halide additives [38] or dimethyl sulfoxide [9] can be employed. Adding poly-(vinylpyrrolidone) (PVP) can also improve the surface coverage of perovskite [39]. It is interesting to note that, for MAPbI3−xClx, a simple annealing step is enough to form a good coverage [6,40], but for MAPbI3, a special step, such as multi-deposition [41], adding N-cyclohexyl-2-pyrrolidone (CHP) [42], fast deposition [43,44,45], or air flow during spin coating [46,47], is needed.
The sequential deposition method was mainly applied for MAPbI3 perovskite. In a typical synthesis, the solution of PbI2 in DMF was spun on a substrate as the first step then the substrate was dipped in a solution of MAI in 2-propanol (IPA) to form MAPbI3 crystals as the second step [48]. For the inclusion of chloride, in the first step the PbCl2 can be mixed with PbI2 in DMF or dimethyl sulfoxide (DMSO) [49,50,51,52], and/or the second step MACl can be added [53,54,55]. For vapor based deposition methods, the MAPbI3-xClx can be formed by co-evaporating MAI and PbCl2 onto the substrates [56,57] or by reacting PbCl2 on substrates with MAI vapor [58,59].
3. The Crystal Structure Form and Formation
3.1. Crystal Structure of MAPbI3 Layer
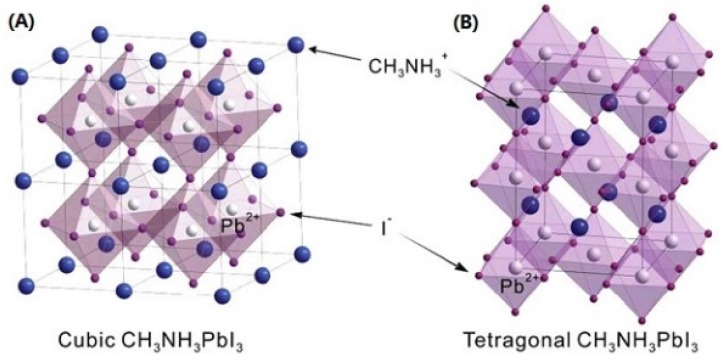
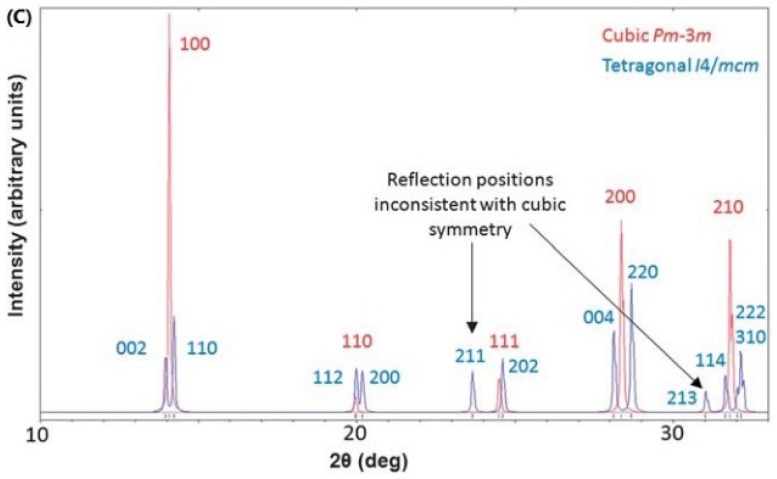
The parameters and transitions of phases of bulk MAPbI3 were included in references [60,61]. Here, we focus on the tetragonal and cubic phases [62]. In fact, there are no critical differences between the two phases, except a slight rotation of PbI6 octahedra along the c-axis. The atomic structures of MAPbI3 of the two phases are shown in Figure 1A,B. Thus, the tetragonal phase can be treated as a pseudocubic phase with a* = a/, c* = c/2 [63]. Below 54 °C, the cubic phase of MAPbI3 can be transformed into the tetragonal phase [60], and the opposite transition occurs by annealing at 100 °C for 15 min [41]. In Figure 1C, the X-ray diffraction (XRD) patterns of the two phases are shown. After transformation to the tetragonal phase, the (100) and (200) peaks of cubic MAPbI3 split, also new (211) and (213) peaks show up. Here, we use the peak splitting as indictor for phase transformation. Analysis of the MAPbI3-xClx usually shows the cubic phase of MAPbI3, however, with a much more preference along (100) and (200). This will be discussed in the Section 3.2 and Section 3.3.
Figure 1.
(A) Atomic models of MAPbI3 with cubic phase; and (B) tetragonal phase; (C) the calculated XRD patterns for MAPbI3 in both phases. (A) and (B) are reprinted from reference [64], Copyright © IOP Publishing. Reproduced with permission. All rights reserved; (C) is reprinted from reference [65], Copyright © 2013, Royal Society of Chemistry.
Another phase which should be noted is the amorphous phase. Pair distribution function analysis of X-ray scattering showed that after annealing at 100 °C for 30 min, the MAPbI3 in meso-porous TiO2 has about 30 atom% in medium range crystalline order and the other 70 atom% in a disordered state with a coherence length of 1.4 nm [66]. The poor crystallization of the MAPbI3 in meso-porous TiO2 was studied by high-resolution TEM [67]. Quartz crystal microbalance measurements suggest that during the sequential method only half of PbI2 is converted to MAPbI3 instantly, while the other half is involved in reversible transformation with MAPbI3. Additionally, the amorphous character with a very small average crystallite size may be present after the transformation as previously discussed [68]. The amorphous phase may also present during the initially deposited MAPbI3-xClx, as indicated by the envelope in some XRD spectra. In reference [69], the amorphous phase MA5PbCl4I3 was also mentioned. Moreover, both XRD and photoluminescence studies of MAPbI2Cl (2MAPbI3+MAPbCl3) indicate the existence of the amorphous phase [70].
3.2. Converting Lead Halides to Perovskite
In the sequential deposition method, PbI2 or/and PbCl2 were first dissolved in a solvent. As PbI2 crystal has a layered structure, DMF can intercalate into the PbI2 interlayer space and screen PbI2 via Pb-O bonding [71,72,73]. When DMF is intercalated, the XRD peak of the PbI2 (001) plane red shifts from 14.8° to 7.94° [72,73]. The red-shift of this XRD peak to 9.17° also indicates the intercalation of DMSO [43]. While PbCl2 doesn’t possess a similar layered structure as PbI2, its solubility is poor where PbCl2 nanoparticles may only suspend in the solvent [28]. However, depositing a mixture of PbI2 and PbCl2 on the substrates result in a new PbICl phase [74], whose crystal structure is similar to PbCl2 [75].
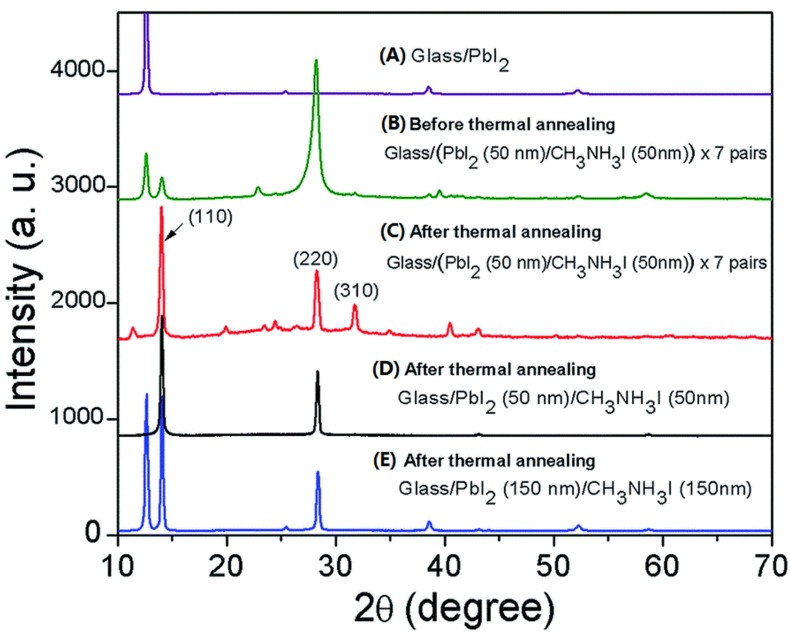
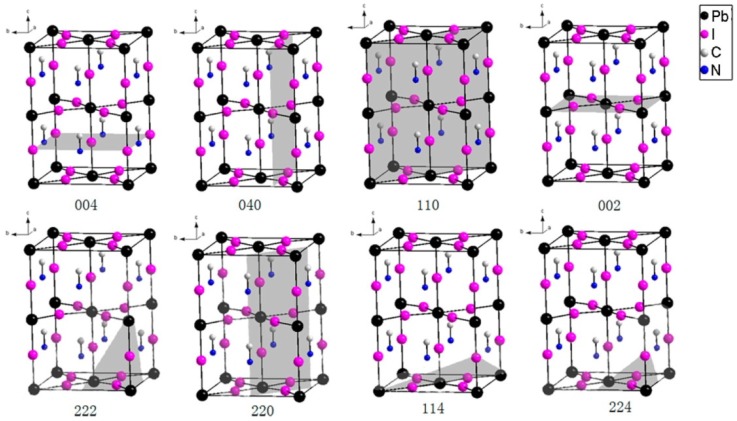
At the beginning of the reaction of PbI2 and MAI, a predominant peak at (220) appeared (as shown in Figure 2B). In other words, the MAPbI3 preferentially grows along (220) plane at first. The annealing process increases the long range crystalline order and results in the predominant (110) peak instead. Noting the (220) is only a short range of (110), thus, another possible reason for the (110)-oriented growth of MAPbI3-xClx and MAPbI3 may be because layered crystal structure of PbI2 (growth along (001) planes of PbI2 like the liquid catalyst cluster model mentioned in reference [76]). The lattice planes of tetragonal MAPbI3 are showed in Figure 3.
Figure 2.
XRD patterns of glass substrates with vapor deposition of (A) a layer of PbI2; and (B) a layer of PbI2 followed by a layer of MAI, repeating this step 7 times; XRD pattern of the (C) annealed 7-time deposited PbI2/MAI layer; (D) 1-time deposited PbI2 (50 nm thickness)/MAI (50 nm thickness) layer; and (E) 1-time deposited PbI2 (150 nm thickness)/MAI (150 nm thickness) layer. Reprinted from reference [77], Copyright © 2015, Royal Society of Chemistry.
Figure 3.
Crystallographic (lattice) planes (in gray) of tetragonal MAPbI3. Reprinted from reference [78], Copyright © 2015, American Chemical Society.
For PbCl2, Cl− was detached from PbCl2 when the PbCl2 was evaporated on the MAI substrate [79] and all the atoms of lead halide were dissociated during the crystal formation of the perovskite [80]. Thus, except the speed and the way of breaking the lead halide, the following steps should be similar with the one step method (Section 3.3) for converting PbI2 or PbCl2 with MAI to the perovskite. However, the situation in the presence of MACl may be different. As less energy is needed for MACl than MAI to undergo phase transition from solid to gas [69], it may be easier for MACl than MAI to diffuse into the PbI2 and cause the crystallization of perovskite [81]. However, as Cl− cannot be incorporated into MAPbI3 crystal structure, the MAI and MACl may compete with each other to determine the result crystal, because only MAPbI3 or MAPbCl3 was formed when PbI2 was soaked in 80 mM MAI + 40 mM MACl or in 40 mM MAI + 80 mM MACl, respectively [80]. Thus, the incorporation of some amount of MACl managed to modify the morphology of the perovskite and resulted in better performance of the solar cells.
3.3. One Step Deposition of MAPbI3-xClx
The better crystallization of MAPbI3-xClx along (110) and (220) plane of the tetragonal phase or (100) and (200) planes of the cubic phase may be due to the lowered cubic-tetragonal phase transition temperature of MAPbI3−xClx after the incorporation of Cl− [82]. A clear cubic-tetragonal phase transition temperature of MAPbI3 was detected by differential scanning calorimeter (DSC) analysis [65], however no such phase transition was observed for MAPbI3−xClx [83]. To explain the absence of the phase transition for MAPbI3−xClx, we first study the crystallization process of MAPbI3-xClx by one step deposition method.
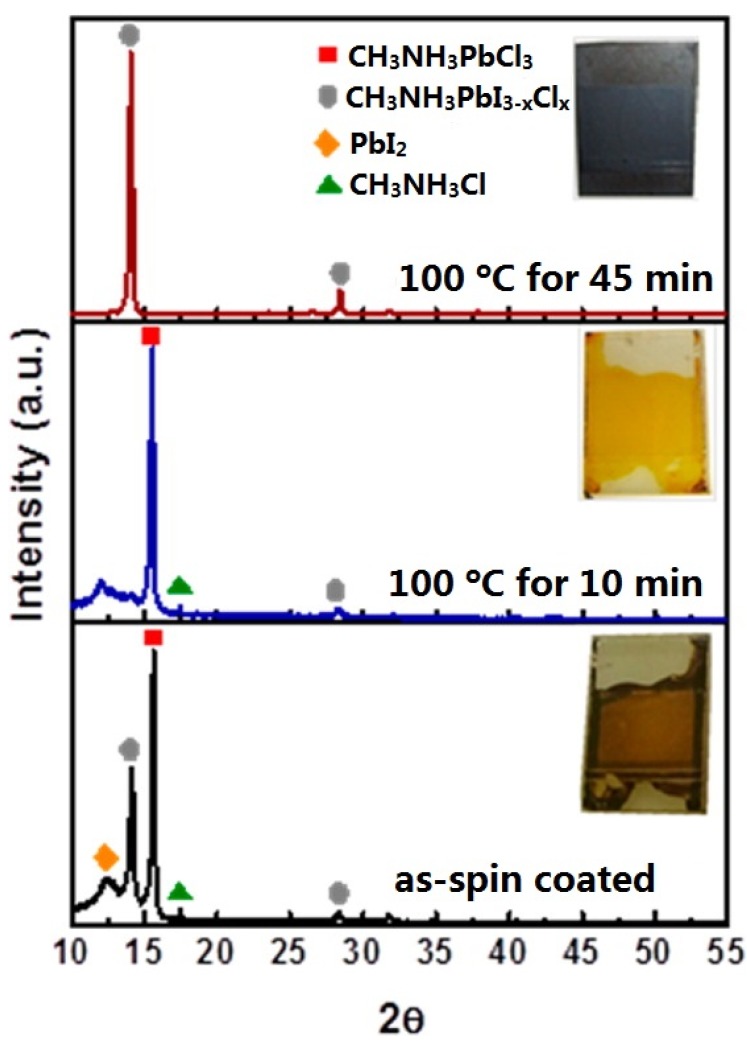
Detail information about crystal formation process of MAPbI3 is summarized in reference [85]. For MAPbI3-xClx, the transformation from the intermediate phase to the perovskite is determined as 80 °C by in situ grazing incidence wide-angle X-ray scattering (GIWAXS) [86]. Figure 4 presents a clearer picture of the crystal formation of MAPbI3-xClx. The 15.7° and 31.5° peaks are associated with the (100) and (200) diffraction peaks of MAPbCl3 [82]. These peaks were also observed in references [27,87,88]. In Figure 4, it is interesting to note that MAPbI3 was formed first for the as-spin coated film but converted to MAPbCl3 after annealing at 100 °C for 10 min, and then MAPbCl3 was converted back to MAPbI3 after 45 min of annealing [84]. Further annealing would result in the decomposition of MAPbI3 to PbI2, but this occurred after conversion to the intermediate phase to MAPbI3 [89]. Because MAPbCl3 is in a cubic phase, we suppose that MAPbCl3 may cause a template effect for the cubic MAPbI3 phase.
Figure 4.
XRD patterns and optical images (insets) of MAPbI3-xClx film during annealing. Reprinted from reference [84], Copyright © 2015, American Chemical Society.
In addition, the MAI:PbI2 (molar ratio 3:1) precursor solution on compact TiO2 can also form MAPbI3 with a predominant (110) plane, but the annealing temperature need to be above 150 °C [84,88,90]. The different sublimation temperature of MAI and MACl and the evidence of residue MAI or MACl in the resulting perovskite may explain the higher annealing temperature needed for MAPbI3 [84,91].
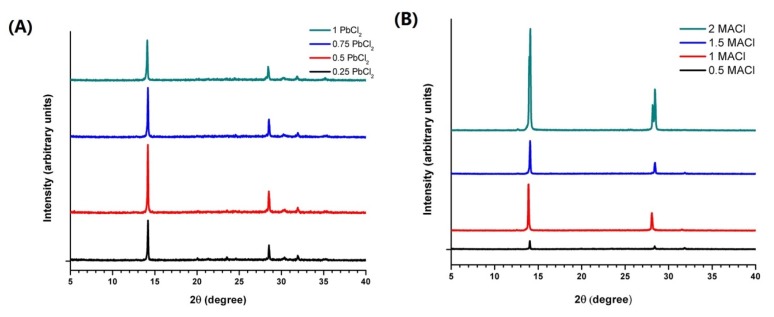
The XRD patterns of the resulting MAPbI3-xClx prepared from different chloride-containing precursors are summarized in Figure 5. All the patterns showed predominant crystallization along the (110) and (220) planes. Interestingly, the (220) peak split at a high MACl (x = 2) concentration in Figure 5B [27]. This split was also observed in reference [29]. In the sequential deposition method, the (110) and (220) crystallization preference may be due to an in situ transformation process [92] of PbI2 to MAPbI3, as discussed in Section 3.2. However, the PbI6 octahedra are more likely to be fully dissociated in the one step precursor solution. [29,93,94,95] As MACl does not fit in the MAPbI3 structure, it could be possible that MACl may be expelled along the (110) planes of the MAPbI3 and that is why the MAPbI3-xClx always showed the (110) and (220) orientation preference. This assumption can be proved by (220) peak split in Figure 5B, as excess of MACl breaks down the crystal range along (110) planes resulting in peak split. However, MAI can fit in the MAPbI3 structure, (110)-oriented growth is just the result of cubic phase in high temperature (150 °C in refereneces [84,88,90]). Surprisingly, a main XRD peak of (310) was observed for the one step deposition prepared MAPbI3 [96]. The main peak of (310), which is distinct from the (110) peak, may have resulted from the fact that the MAI was added into the precursor solution after the PbI2 was completely dissolved instead of both MAI and PbI2 being present at the same time [96], or the fact that the (310) plane of MAPbI3 may match the crystal structure of the substrate better. Then the magnitude of the (110) peak of MAPbI3-xClx and the (310) peak of MAPbI3 further increases after 5 weeks [96]. Thus, we believe that the annealing process may only reinforce the crystallization preference as it is initially formed and the effects of substrate also contribute to the crystal structure formation of the perovskite in some cases. Returning to Figure 5, if excess of MACl breaks down the growth along the (110) plane, we believe MACl can also break down the crystalline order range. Since a large amount of MAPbI3 existed in the amorphous phase form, the cubic phase of MAPbI3 may be more favorable in short crystalline order range than the tetragonal phase.
Figure 5.
XRD patterns of MAPbI3-xClx prepared from (A) precursor solution xPbCl2+yPbI2+zMAI (x = 0.25, 0.5, 0.75 and 1; y = 1 − x; z = 3 × x + y) in DMF; and (B) precursor solution 1PbI2+1MAI+xMACl (x = 0.5, 1, 1.5 and 2). Reprinted from reference [27], Copyright © 2014, American Chemical Society.
There are other influences associated with Cl−. Increasing the temperature during the soaking of the PbI2 substrate in MAI + MACl IPA solution can improve the (110) orientation of MAPbI3-xClx where the high temperature facilitates the expelling of MACl [97]. Annealing the MACl:PbI2 (3:1) precursor on compact TiO2 at 60 °C for 10 min followed by 100 °C for 20 min instead of gradually heating from 25 to 100 °C for 45 min resulted in the (200) crystal plane of MAPbI3-xClx being vertically aligned on the substrate [98]. The tetragonal phase MAPbI3−xClx was occasionally found on compact TiO2 substrate [53], while the cubic phase always occurred in meso-porous substrate, where the trapped MACl in meso-porous structure [8] helps the formation of cubic phase. While the size of MAPbI3 crystal grains are smaller but the degree of crystallinity improves in the presence of MACl [27,54], the sequential deposited MAPbI3-xClx results in (001) elongated crystals [13].
4. Conclusions
In this article, the location of Cl− and its influence on the crystal morphology of MAPbI3-xClx is summarized, where the deposition methods (one step deposition, sequential deposition and vapor based deposition) for MAPbI3-xClx are reviewed. Furthermore, the cubic and tetragonal phases of MAPbI3 are elucidated and the crystallization process of MAPbI3-xClx is also summarized. Detailed information about the crystal structure with variable deposition parameters is also discussed. Though a recent report showed that Cl− mainly improves the carrier transport at the perovskite/Spiro-MeOTAD and perovskite/TiO2 interfaces, rather than within the perovskite crystals, the authors of reference [99] more recently spatially resolved photoluminescence decay results showed less recombination in the high chlorine concentration region [100]. Thus, the effect of high concentration of Cl− on the morphologies and electronic properties of the perovskite can still not be ignored. Additionally, whether Cl− is predominantly present as a substituent for I−, as an interstitial, or at the surface of the crystal, remains unclear [101] and this is worth further investigation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kojima A., Teshima K., Shirai Y., Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem Soc. 2009;131:6050–6051. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- 2.Yang W.S., Noh J.H., Jeon N.J., Kim Y.C., Ryu S., Seo J., Seok S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science. 2015;348:1234–1237. doi: 10.1126/science.aaa9272. [DOI] [PubMed] [Google Scholar]
- 3.Kim H.S., Im S.H., Park N.G. Organolead halide perovskite: New horizons in solar cell research. J. Phys. Chem. C. 2014;118:5615–5625. doi: 10.1021/jp409025w. [DOI] [Google Scholar]
- 4.Luo S., Daoud W.A. Recent progress in organic-inorganic halide perovskite solar cells: Mechanisms and materials design. J. Mater. Chem. A. 2015;3:8992–9010. doi: 10.1039/C4TA04953E. [DOI] [Google Scholar]
- 5.Mohammad K.N., Gao P., Gratzel M. Organohalide lead perovskites for photovoltaic applications. Energ. Environ. Sci. 2014;7:2448–2463. [Google Scholar]
- 6.Dualeh A., Tétreault N., Moehl T., Gao P., Nazeeruddin M.K., Grätzel M. Effect of annealing temperature on film morphology of organic-inorganic hybrid pervoskite solid-state solar cells. Adv. Funct. Mater. 2014;24:3250–3258. doi: 10.1002/adfm.201304022. [DOI] [Google Scholar]
- 7.Stranks S.D., Eperon G.E., Grancini G., Menelaou C., Alcocer M.J.P., Leijtens T., Herz L.M., Petrozza A., Snaith H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science. 2013;342:341–344. doi: 10.1126/science.1243982. [DOI] [PubMed] [Google Scholar]
- 8.Colella S., Mosconi E., Fedeli P., Listorti A., Gazza F., Orlandi F., Ferro P., Besagni T., Rizzo A., Calestani G., et al. MAPbI3−xClx mixed halide perovskite for hybrid solar cells: The role of chloride as dopant on the transport and structural properties. Chem Mater. 2013;25:4613–4618. doi: 10.1021/cm402919x. [DOI] [Google Scholar]
- 9.Conings B., Baeten L., de Dobbelaere C., D’Haen J., Manca J., Boyen H.-G. Perovskite-based hybrid solar cells exceeding 10% efficiency with high reproducibility using a thin film sandwich approach. Adv. Mater. 2014;26:2041–2046. doi: 10.1002/adma.201304803. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Zhu K. CH3NH3Cl-assisted one-step solution growth of CH3NH3PbI3: Structure, charge-carrier dynamics, and photovoltaic properties of perovskite solar cells. J. Phys. Chem. C. 2014;118:9412–9418. doi: 10.1021/jp502696w. [DOI] [Google Scholar]
- 11.Colella S., Mosconi E., Pellegrino G., Alberti A., Guerra V.L.P., Masi S., Listorti A., Rizzo A., Condorelli G.G., De Angelis F., et al. Elusive presence of chloride in mixed halide perovskite solar cells. J. Phys. Chem. Lett. 2014;5:3532–3538. doi: 10.1021/jz501869f. [DOI] [PubMed] [Google Scholar]
- 12.Unger E.L., Bowring A.R., Tassone C.J., Pool V.L., Gold-Parker A., Cheacharoen R., Stone K.H., Hoke E.T., Toney M.F., McGehee M.D. Chloride in lead chloride-derived organo-metal halides for perovskite-absorber solar cells. Chem. Mater. 2014;26:7158–7165. doi: 10.1021/cm503828b. [DOI] [Google Scholar]
- 13.Dar M.I., Arora N., Gao P., Ahmad S., Grätzel M., Nazeeruddin M.K. Investigation regarding the role of chloride in organic-inorganic halide perovskites obtained from chloride containing precursors. Nano Lett. 2014;14:6991–6996. doi: 10.1021/nl503279x. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi N., Yanagida M., Shirai Y., Masuda T., Han L., Miyano K. Hysteresis-free and highly stable perovskite solar cells produced via a chlorine-mediated interdiffusion method. J. Mater. Chem. A. 2015;3:12081–12088. doi: 10.1039/C5TA01668A. [DOI] [Google Scholar]
- 15.Starr D.E., Sadoughi G., Handick E., Wilks R.G., Alsmeier J.H., Kohler L., Gorgoi M., Snaith H.J., Bar M. Direct observation of an inhomogeneous chlorine distribution in CH3NH3PbI3-xClx layers: Surface depletion and interface enrichment. Energ. Environ. Sci. 2015;8:1609–1615. doi: 10.1039/C5EE00403A. [DOI] [Google Scholar]
- 16.Pool V.L., Gold-Parker A., McGehee M.D., Toney M.F. Chlorine in PbCl2-derived hybrid-perovskite solar absorbers. Chem. Mater. 2015;27:7240–7243. doi: 10.1021/acs.chemmater.5b03581. [DOI] [Google Scholar]
- 17.Chae J., Dong Q., Huang J., Centrone A. Chloride incorporation process in CH3NH3PbI3−xClx perovskites via nanoscale bandgap maps. Nano Lett. 2015;15:8114–8121. doi: 10.1021/acs.nanolett.5b03556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosconi E., Amat A., Nazeeruddin M.K., Grätzel M., De Angelis F. First-principles modeling of mixed halide organometal perovskites for photovoltaic applications. J. Phys. Chem. C. 2013;117:13902–13913. doi: 10.1021/jp4048659. [DOI] [Google Scholar]
- 19.Zheng F., Takenaka H., Wang F., Koocher N.Z., Rappe A.M. First-principles calculation of the bulk photovoltaic effect in CH3NH3PbI3 and CH3NH3PbI3−xClx. J. Phys. Chem. Lett. 2014;6:31–37. doi: 10.1021/jz502109e. [DOI] [PubMed] [Google Scholar]
- 20.Buin A., Pietsch P., Xu J., Voznyy O., Ip A.H., Comin R., Sargent E.H. Materials processing routes to trap-free halide perovskites. Nano Lett. 2014;14:6281–6286. doi: 10.1021/nl502612m. [DOI] [PubMed] [Google Scholar]
- 21.Du M.H. Efficient carrier transport in halide perovskites: Theoretical perspectives. J. Mater. Chem. A. 2014;2:9091–9098. doi: 10.1039/c4ta01198h. [DOI] [Google Scholar]
- 22.Even J., Pedesseau L., Katan C. Analysis of multivalley and multibandgap absorption and enhancement of free carriers related to exciton screening in hybrid perovskites. J. Phys. Chem. C. 2014;118:11566–11572. doi: 10.1021/jp503337a. [DOI] [Google Scholar]
- 23.Yin W.-J., Shi T., Yan Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv. Mater. 2014;26:4653–4658. doi: 10.1002/adma.201306281. [DOI] [PubMed] [Google Scholar]
- 24.Li D., Liang C., Zhang H., Zhang C., You F., He Z. Spatially separated charge densities of electrons and holes in organic-inorganic halide perovskites. J. Appl. Phys. 2015;117:074901. doi: 10.1063/1.4909102. [DOI] [Google Scholar]
- 25.Yin J., Cortecchia D., Krishna A., Chen S., Mathews N., Grimsdale A.C., Soci C. Interfacial charge transfer anisotropy in polycrystalline lead iodide perovskite films. J. Phys. Chem. Lett. 2015:1396–1402. doi: 10.1021/acs.jpclett.5b00431. [DOI] [PubMed] [Google Scholar]
- 26.Mosconi E., Ronca E., De Angelis F. First-principles investigation of the Tio2/organohalide perovskites interface: The role of interfacial chlorine. J. Phys. Chem. Lett. 2014;5:2619–2625. doi: 10.1021/jz501127k. [DOI] [PubMed] [Google Scholar]
- 27.Williams S.T., Zuo F., Chueh C.-C., Liao C.-Y., Liang P.-W., Jen A.K.Y. Role of chloride in the morphological evolution of organo-lead halide perovskite thin films. Acs Nano. 2014;8:10640–10654. doi: 10.1021/nn5041922. [DOI] [PubMed] [Google Scholar]
- 28.Tidhar Y., Edri E., Weissman H., Zohar D., Hodes G., Cahen D., Rybtchinski B., Kirmayer S. Crystallization of methyl ammonium lead halide perovskites: Implications for photovoltaic applications. J. Am. Chem. Soc. 2014;136:13249–13256. doi: 10.1021/ja505556s. [DOI] [PubMed] [Google Scholar]
- 29.Yan K., Long M., Zhang T., Wei Z., Chen H., Yang S., Xu J. Hybrid halide perovskite solar cell precursors: Colloidal chemistry and coordination engineering behind device processing for high efficiency. J. Am. Chem. Soc. 2015;137:4460–4468. doi: 10.1021/jacs.5b00321. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L., Zhang D., Ma Y., Lu Z., Chen Z., Wang S., Xiao L., Gong Q. Morphology control of the perovskite films for efficient solar cells. Dalton Trans. 2015;44:10582–10593. doi: 10.1039/C4DT03869J. [DOI] [PubMed] [Google Scholar]
- 31.Kim H.S., Lee C.R., Im J.H., Lee K.B., Moehl T., Marchioro A., Moon S.J., Humphry-Baker R., Yum J.H., Moser J.E., et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9% Sci. Rep. 2012;2 doi: 10.1038/srep00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee M.M., Teuscher J., Miyasaka T., Murakami T.N., Snaith H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science. 2012;338:643–647. doi: 10.1126/science.1228604. [DOI] [PubMed] [Google Scholar]
- 33.Saliba M., Tan K.W., Sai H., Moore D.T., Scott T., Zhang W., Estroff L.A., Wiesner U., Snaith H.J. Influence of thermal processing protocol upon the crystallization and photovoltaic performance of organic-inorganic lead trihalide perovskites. J. Phys. Chem. C. 2014;118:17171–17177. doi: 10.1021/jp500717w. [DOI] [Google Scholar]
- 34.Hsu H.-L., Chen C., Chang J.-Y., Yu Y.-Y., Shen Y.-K. Two-step thermal annealing improves the morphology of spin-coated films for highly efficient perovskite hybrid photovoltaics. Nanoscale. 2014;6:10281–10288. doi: 10.1039/C4NR02751E. [DOI] [PubMed] [Google Scholar]
- 35.Kang R., Kim J.-E., Yeo J.-S., Lee S., Jeon Y.-J., Kim D.-Y. Optimized organometal halide perovskite planar hybrid solar cells via control of solvent evaporation rate. J. Phys. Chem. C. 2014;118:26513–26520. doi: 10.1021/jp508015c. [DOI] [Google Scholar]
- 36.Guo Y., Liu C., Inoue K., Harano K., Tanaka H., Nakamura E. Enhancement in the efficiency of an organic-inorganic hybrid solar cell with a doped P3HT hole-transporting layer on a void-free perovskite active layer. J. Mater. Chem. A. 2014;2:13827–13830. doi: 10.1039/C4TA02976C. [DOI] [Google Scholar]
- 37.Liang P.-W., Liao C.-Y., Chueh C.-C., Zuo F., Williams S.T., Xin X.-K., Lin J., Jen A.K.Y. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 2014;26:3748–3754. doi: 10.1002/adma.201400231. [DOI] [PubMed] [Google Scholar]
- 38.Chueh C.-C., Liao C.-Y., Zuo F., Williams S.T., Liang P.-W., Jen A.K.Y. The roles of alkyl halide additives in enhancing perovskite solar cell performance. J. Mater. Chem. A. 2014;3:9058–9062. doi: 10.1039/C4TA05012F. [DOI] [Google Scholar]
- 39.Ding Y., Yao X., Zhang X., Wei C., Zhao Y. Surfactant enhanced surface coverage of CH3NH3PbI3-xClx perovskite for highly efficient mesoscopic solar cells. J. Power Sources. 2014;272:351–355. doi: 10.1016/j.jpowsour.2014.08.095. [DOI] [Google Scholar]
- 40.Eperon G.E., Burlakov V.M., Docampo P., Goriely A., Snaith H.J. Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 2014;24:151–157. doi: 10.1002/adfm.201302090. [DOI] [Google Scholar]
- 41.Takeo O., Masahito Z., Yuma I., Atsushi S., Kohei S. Microstructures and photovoltaic properties of perovskite-type CH3NH3PbI3 compounds. Appl. Phys. Express. 2014;7 doi: 10.7567/APEX.7.121601. [DOI] [Google Scholar]
- 42.Jeon Y.-J., Lee S., Kang R., Kim J.-E., Yeo J.-S., Lee S.-H., Kim S.-S., Yun J.-M., Kim D.-Y. Planar heterojunction perovskite solar cells with superior reproducibility. Sci. Rep. 2014;4 doi: 10.1038/srep06953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeon N.J., Noh J.H., Kim Y.C., Yang W.S., Ryu S., Seok S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014;13:897–903. doi: 10.1038/nmat4014. [DOI] [PubMed] [Google Scholar]
- 44.Xiao M., Huang F., Huang W., Dkhissi Y., Zhu Y., Etheridge J., Gray-Weale A., Bach U., Cheng Y.-B., Spiccia L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. 2014;126:10056–10061. doi: 10.1002/ange.201405334. [DOI] [PubMed] [Google Scholar]
- 45.Jung J.W., Williams S.T., Jen A.K.Y. Low-temperature processed high-performance flexible perovskite solar cells via rationally optimized solvent washing treatments. Rsc Adv. 2014;4:62971–62977. doi: 10.1039/C4RA13212B. [DOI] [Google Scholar]
- 46.Ito S., Tanaka S., Vahlman H., Nishino H., Manabe K., Lund P. Carbon-double-bond-free printed solar cells from TiO2/CH3NH3PbI3/CuSCN/Au: Structural control and photoaging effects. Chem. Phys. Chem. 2014;15:1194–1200. doi: 10.1002/cphc.201301047. [DOI] [PubMed] [Google Scholar]
- 47.Huang F., Dkhissi Y., Huang W., Xiao M., Benesperi I., Rubanov S., Zhu Y., Lin X., Jiang L., Zhou Y., et al. Gas-assisted preparation of lead iodide perovskite films consisting of a monolayer of single crystalline grains for high efficiency planar solar cells. Nano Energy. 2014;10:10–18. doi: 10.1016/j.nanoen.2014.08.015. [DOI] [Google Scholar]
- 48.Burschka J., Pellet N., Moon S.J., Humphry-Baker R., Gao P., Nazeeruddin M.K., Gratzel M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature. 2013;499:316–320. doi: 10.1038/nature12340. [DOI] [PubMed] [Google Scholar]
- 49.Ma Y., Zheng L., Chung Y.-H., Chu S., Xiao L., Chen Z., Wang S., Qu B., Gong Q., Wu Z., et al. A highly efficient mesoscopic solar cell based on CH3NH3PbI3-xClx fabricated via sequential solution deposition. Chem. Commun. 2014;50:12458–12461. doi: 10.1039/C4CC01962H. [DOI] [PubMed] [Google Scholar]
- 50.Zheng L., Ma Y., Chu S., Wang S., Qu B., Xiao L., Chen Z., Gong Q., Wu Z., Hou X. Improved light absorption and charge transport for perovskite solar cells with rough interfaces by sequential deposition. Nanoscale. 2014;6:8171–8176. doi: 10.1039/c4nr01141d. [DOI] [PubMed] [Google Scholar]
- 51.Dar M.I., Abdi-Jalebi M., Arora N., Grätzel M., Nazeeruddin M.K. Growth engineering of CH3NH3PbI3 structures for high-efficiency solar cells. Adv. Energy Mater. 2016;6 doi: 10.1002/aenm.201501358. [DOI] [Google Scholar]
- 52.Li Y., Sun W., Yan W., Ye S., Peng H., Liu Z., Bian Z., Huang C. High-performance planar solar cells based on CH3NH3PbI3-xClx perovskites with determined chlorine mole fraction. Adv. Funct. Mater. 2015;25:4867–4873. doi: 10.1002/adfm.201501289. [DOI] [Google Scholar]
- 53.Docampo P., Hanusch F., Stranks S.D., Döblinger M., Feckl J.M., Ehrensperger M., Minar N.K., Johnston M.B., Snaith H.J., Bein T. Solution deposition-conversion for planar heterojunction mixed halide perovskite solar cells. Adv. Energy Mater. 2014;4 doi: 10.1002/aenm.201400355. [DOI] [Google Scholar]
- 54.Jiang M., Wu J., Lan F., Tao Q., Gao D., Li G. Enhancing the performance of planar organo-lead halide perovskite solar cells by using mixed halide source. J. Mater. Chem. A. 2014;3:963–967. doi: 10.1039/C4TA05373G. [DOI] [Google Scholar]
- 55.Dong Q., Yuan Y., Shao Y., Fang Y., Wang Q., Huang J. Abnormal crystal growth in CH3NH3PbI3-xClx using a multi-cycle solution coating process. Energ. Environ. Sci. 2015;8:2464–2470. doi: 10.1039/C5EE01179E. [DOI] [Google Scholar]
- 56.Liu M., Johnston M.B., Snaith H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature. 2013;501:395–398. doi: 10.1038/nature12509. [DOI] [PubMed] [Google Scholar]
- 57.Ono L.K., Wang S., Kato Y., Raga S.R., Qi Y. Fabrication of semi-transparent perovskite films with centimeter-scale superior uniformity by the hybrid deposition method. Energ. Environ. Sci. 2014;7:3989–3993. doi: 10.1039/C4EE02539C. [DOI] [Google Scholar]
- 58.Leyden M.R., Ono L.K., Raga S.R., Kato Y., Wang S., Qi Y. High performance perovskite solar cells by hybrid chemical vapor deposition. J. Mater. Chem. A. 2014;2:18742–18745. doi: 10.1039/C4TA04385E. [DOI] [Google Scholar]
- 59.Chen C.-W., Kang H.-W., Hsiao S.-Y., Yang P.-F., Chiang K.-M., Lin H.-W. Efficient and uniform planar-type perovskite solar cells by simple sequential vacuum deposition. Adv. Mater. 2014;26:6647–6652. doi: 10.1002/adma.201402461. [DOI] [PubMed] [Google Scholar]
- 60.Poglitsch A., Weber D. Dynamic disorder in methylammoniumtrihalogenoplumbates (ii) observed by millimeter-wave spectroscopy. J. Chem. Phys. 1987;87:6373–6378. doi: 10.1063/1.453467. [DOI] [Google Scholar]
- 61.Stoumpos C.C., Malliakas C.D., Kanatzidis M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013;52:9019–9038. doi: 10.1021/ic401215x. [DOI] [PubMed] [Google Scholar]
- 62.Kawamura Y., Mashiyama H., Hasebe K. Structural study on cubic–tetragonal transition of CH3NH3PbI3. J. Phy. Soc. Jpn. 2002;71:1694–1697. doi: 10.1143/JPSJ.71.1694. [DOI] [Google Scholar]
- 63.Eperon G.E., Stranks S.D., Menelaou C., Johnston M.B., Herz L.M., Snaith H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energ. Environ. Sci. 2014;7:982–988. doi: 10.1039/c3ee43822h. [DOI] [Google Scholar]
- 64.Chen Z., Li H., Tang Y., Huang X., Ho D., Lee C.-S. Shape-controlled synthesis of organolead halide perovskite nanocrystals and their tunable optical absorption. Mater. Res. Express. 2014;1 doi: 10.1088/2053-1591/1/1/015034. [DOI] [Google Scholar]
- 65.Baikie T., Fang Y.N., Kadro J.M., Schreyer M., Wei F.X., Mhaisalkar S.G., Graetzel M., White T.J. Synthesis and crystal chemistry of the hybrid perovskite CH3NH3PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A. 2013;1:5628–5641. doi: 10.1039/c3ta10518k. [DOI] [Google Scholar]
- 66.Choi J.J., Yang X.H., Norman Z.M., Billinge S.J.L., Owen J.S. Structure of methylammonium lead iodide within mesoporous titanium dioxide: Active material in high-performance perovskite solar cells. Nano Lett. 2014;14:127–133. doi: 10.1021/nl403514x. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Y., Vasiliev A.L., Wu W., Yang M., Pang S., Zhu K., Padture N.P. Crystal morphologies of organolead trihalide in mesoscopic/planar perovskite solar cells. J. Phys. Chem. Lett. 2015;6:2292–2297. doi: 10.1021/acs.jpclett.5b00981. [DOI] [PubMed] [Google Scholar]
- 68.Harms H.A., Tetreault N., Pellet N., Bensimon M., Gratzel M. Mesoscopic photosystems for solar light harvesting and conversion: Facile and reversible transformation of metal-halide perovskites. Faraday Discuss. 2014;176:251–269. doi: 10.1039/C4FD00160E. [DOI] [PubMed] [Google Scholar]
- 69.Dualeh A., Gao P., Seok S.I., Nazeeruddin M.K., Grätzel M. Thermal behavior of methylammonium lead-trihalide perovskite photovoltaic light harvesters. Chem Mater. 2014;26:6160–6164. doi: 10.1021/cm502468k. [DOI] [Google Scholar]
- 70.Park B.-W., Jain S.M., Zhang X., Hagfeldt A., Boschloo G., Edvinsson T. Resonance raman and excitation energy dependent charge transfer mechanism in halide-substituted hybrid perovskite solar cells. Acs Nano. 2015;9:2088–2101. doi: 10.1021/nn507345e. [DOI] [PubMed] [Google Scholar]
- 71.Wakamiya A., Endo M., Sasamori T., Tokitoh N., Ogomi Y., Hayase S., Murata Y. Reproducible fabrication of efficient perovskite-based solar cells: X-ray crystallographic studies on the formation of CH3NH3PbI3 layers. Chem. Lett. 2014;43:711–713. doi: 10.1246/cl.140074. [DOI] [Google Scholar]
- 72.Hao F., Stoumpos C.C., Liu Z., Chang R.P.H., Kanatzidis M.G. Controllable perovskite crystallization at a gas-solid interface for hole conductor-free solar cells with steady power conversion efficiency over 10% J. Am. Chem. Soc. 2014;136:16411–16419. doi: 10.1021/ja509245x. [DOI] [PubMed] [Google Scholar]
- 73.Shen D., Yu X., Cai X., Peng M., Ma Y., Su X., Xiao L., Zou D. Understanding the solvent-assisted crystallization mechanism inherent in efficient organic-inorganic halide perovskite solar cells. J. Mater. Chem. A. 2014;2:20454–20461. doi: 10.1039/C4TA05635C. [DOI] [Google Scholar]
- 74.Li Y., Cooper J.K., Buonsanti R., Giannini C., Liu Y., Toma F.M., Sharp I.D. Fabrication of planar heterojunction perovskite solar cells by controlled low-pressure vapor annealing. J. Phys. Chem. Lett. 2015;6:493–499. doi: 10.1021/jz502720a. [DOI] [PubMed] [Google Scholar]
- 75.Brixner L.H., Chen H.Y., Foris C.M. X-ray study of the PbCl2−xIx and PbBr2−xIx systems. J. Solid State Chem. 1981;40:336–343. doi: 10.1016/0022-4596(81)90400-X. [DOI] [Google Scholar]
- 76.Im J.-H., Luo J., Franckevičius M., Pellet N., Gao P., Moehl T., Zakeeruddin S.M., Nazeeruddin M.K., Grätzel M., Park N.-G. Nanowire perovskite solar cell. Nano Lett. 2015;15:2120–2126. doi: 10.1021/acs.nanolett.5b00046. [DOI] [PubMed] [Google Scholar]
- 77.Ng A., Ren Z., Shen Q., Cheung S.H., Gokkaya H.C., Bai G., Wang J., Yang L., So S.K., Djurisic A.B., et al. Efficiency enhancement by defect engineering in perovskite photovoltaic cells prepared using evaporated PbI2/CH3NH3I multilayers. J. Mater. Chem. A. 2015;3:9223–9231. doi: 10.1039/C4TA05070C. [DOI] [Google Scholar]
- 78.Zhu F., Men L., Guo Y., Zhu Q., Bhattacharjee U., Goodwin P.M., Petrich J.W., Smith E.A., Vela J. Shape evolution and single particle luminescence of organometal halide perovskite nanocrystals. Acs Nano. 2015;9:2948–2959. doi: 10.1021/nn507020s. [DOI] [PubMed] [Google Scholar]
- 79.Ng T.-W., Chan C.-Y., Lo M.-F., Guan Z.Q., Lee C.-S. Formation chemistry of perovskites with mixed iodide/chloride content and the implications on charge transport properties. J. Mater. Chem. A. 2015;3:9081–9085. doi: 10.1039/C4TA05819D. [DOI] [Google Scholar]
- 80.Moore D.T., Sai H., Tan W.K., Estroff L.A., Wiesner U. Impact of the organic halide salt on final perovskite composition for photovoltaic applications. APL Mater. 2014;2 doi: 10.1063/1.4886275. [DOI] [Google Scholar]
- 81.Xu Y., Zhu L., Shi J., Lv S., Xu X., Xiao J., Dong J., Wu H., Luo Y., Li D., et al. Efficient hybrid mesoscopic solar cells with morphology-controlled CH3NH3PbI3-xClx derived from two-step spin coating method. Acs Appl. Mater. Inter. 2015;7:2242–2248. doi: 10.1021/am5057807. [DOI] [PubMed] [Google Scholar]
- 82.Pistor P., Borchert J., Fränzel W., Csuk R., Scheer R. Monitoring the phase formation of co-evaporated lead halide perovskite thin films by in situ XRD. J. Phys. Chem. Lett. 2014;5:3308–3312. doi: 10.1021/jz5017312. [DOI] [PubMed] [Google Scholar]
- 83.Williams A.E., Holliman P.J., Carnie M.J., Davies M.L., Worsley D.A., Watson T.M. Perovskite processing for photovoltaics: A spectro-thermal evaluation. J. Mater. Chem. A. 2014;2:19338–19346. doi: 10.1039/C4TA04725G. [DOI] [Google Scholar]
- 84.Yantara N., Yanan F., Shi C., Dewi H.A., Boix P.P., Mhaisalkar S.G., Mathews N. Unravelling the effects of Cl addition in single step CH3NH3PbI3 perovskite solar cells. Chem. Mater. 2015;27:2309–2314. doi: 10.1021/cm502710r. [DOI] [Google Scholar]
- 85.Song Z., Watthage S.C., Phillips A.B., Tompkins B.L., Ellingson R.J., Heben M.J. Impact of processing temperature and composition on the formation of methylammonium lead iodide perovskites. Chem. Mater. 2015;27:4612–4619. doi: 10.1021/acs.chemmater.5b01017. [DOI] [Google Scholar]
- 86.Tan K.W., Moore D.T., Saliba M., Sai H., Estroff L.A., Hanrath T., Snaith H.J., Wiesner U. Thermally induced structural evolution and performance of mesoporous block copolymer-directed alumina perovskite solar cells. Acs Nano. 2014;8:4730–4739. doi: 10.1021/nn500526t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou H., Chen Q., Li G., Luo S., Song T.-b., Duan H.-S., Hong Z., You J., Liu Y., Yang Y. Interface engineering of highly efficient perovskite solar cells. Science. 2014;345:542–546. doi: 10.1126/science.1254050. [DOI] [PubMed] [Google Scholar]
- 88.Yu H., Wang F., Xie F., Li W., Chen J., Zhao N. The role of chlorine in the formation process of “CH3NH3PbI3-xClx” perovskite. Adv. Funct. Mater. 2014;24:7102–7108. doi: 10.1002/adfm.201401872. [DOI] [Google Scholar]
- 89.Song T.-B., Chen Q., Zhou H., Luo S., Yang Y., You J. Unraveling film transformations and device performance of planar perovskite solar cells. Nano Energy. 2015;12:494–500. doi: 10.1016/j.nanoen.2015.01.025. [DOI] [Google Scholar]
- 90.Grancini G., Marras S., Prato M., Giannini C., Quarti C., De Angelis F., De Bastiani M., Eperon G.E., Snaith H.J., Manna L., et al. The impact of the crystallization processes on the structural and optical properties of hybrid perovskite films for photovoltaics. J. Phys. Chem. Lett. 2014;5:3836–3842. doi: 10.1021/jz501877h. [DOI] [PubMed] [Google Scholar]
- 91.Pathak S., Sepe A., Sadhanala A., Deschler F., Haghighirad A., Sakai N., Goedel K.C., Stranks S.D., Noel N., Price M., et al. Atmospheric influence upon crystallization and electronic disorder and its impact on the photophysical properties of organic-inorganic perovskite solar cells. Acs Nano. 2015;9:2311–2320. doi: 10.1021/nn506465n. [DOI] [PubMed] [Google Scholar]
- 92.Yang S., Zheng Y.C., Hou Y., Chen X., Chen Y., Wang Y., Zhao H., Yang H.G. Formation mechanism of freestanding CH3NH3PbI3 functional crystals: In situ transformation vs dissolution-crystallization. Chem. Mater. 2014;26:6705–6710. doi: 10.1021/cm5028817. [DOI] [Google Scholar]
- 93.Shkrob I.A., Marin T.W. Charge trapping in photovoltaically active perovskites and related halogenoplumbate compounds. J. Phys. Chem. Lett. 2014;5:1066–1071. doi: 10.1021/jz5004022. [DOI] [PubMed] [Google Scholar]
- 94.Wang Q., Yun J.-H., Zhang M., Chen H., Chen Z.-G., Wang L. Insight into the liquid state of organo-lead halide perovskites and their new roles in dye-sensitized solar cells. J. Mater. Chem A. 2014;2:10355–10358. doi: 10.1039/c4ta01105h. [DOI] [Google Scholar]
- 95.Stamplecoskie K.G., Manser J.S., Kamat P.V. Dual nature of the excited state in organic-inorganic lead halide perovskites. Energ. Environ. Sci. 2015;8:208–215. doi: 10.1039/C4EE02988G. [DOI] [Google Scholar]
- 96.Park B.-W., Philippe B., Gustafsson T., Sveinbjörnsson K., Hagfeldt A., Johansson E.M.J., Boschloo G. Enhanced crystallinity in organic-inorganic lead halide perovskites on mesoporous Tio2 via disorder-order phase transition. Chem. Mater. 2014;26:4466–4471. doi: 10.1021/cm501541p. [DOI] [Google Scholar]
- 97.Docampo P., Hanusch F.C., Giesbrecht N., Angloher P., Ivanova A., Bein T. Influence of the orientation of methylammonium lead iodide perovskite crystals on solar cell performance. APL Mater. 2014;2 doi: 10.1063/1.4890244. [DOI] [Google Scholar]
- 98.Ishii A., Jena A.K., Miyasaka T. Fully crystalline perovskite-perylene hybrid photovoltaic cell capable of 1.2 V output with a minimized voltage lossa. APL Mater. 2014;2 doi: 10.1063/1.4895039. [DOI] [Google Scholar]
- 99.Chen Q., Zhou H., Fang Y., Stieg A.Z., Song T.-B., Wang H.-H., Xu X., Liu Y., Lu S., You J., et al. The optoelectronic role of chlorine in CH3NH3PbI3(Cl)-based perovskite solar cells. Nat. Commun. 2015;6 doi: 10.1038/ncomms8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Quilettes D.W., Vorpahl S.M., Stranks S.D., Nagaoka H., Eperon G.E., Ziffer M.E., Snaith H.J., Ginger D.S. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science. 2015;348:683–686. doi: 10.1126/science.aaa5333. [DOI] [PubMed] [Google Scholar]
- 101.Stranks S.D., Nayak P.K., Zhang W., Stergiopoulos T., Snaith H.J. Formation of thin films of organic-inorganic perovskites for high-efficiency solar cells. Angew. Chem. Int. Ed. 2015;54:3240–3248. doi: 10.1002/anie.201410214. [DOI] [PubMed] [Google Scholar]