Abstract
Terpyridine and quaterpyridine-based complexes allow wide light harvesting of the solar spectrum. Terpyridines, with respect to bipyridines, allow for achieving metal-complexes with lower band gaps in the metal-to-ligand transition (MLCT), thus providing a better absorption at lower energy wavelengths resulting in an enhancement of the solar light-harvesting ability. Despite the wider absorption of the first tricarboxylate terpyridyl ligand-based complex, Black Dye (BD), dye-sensitized solar cell (DSC) performances are lower if compared with N719 or other optimized bipyridine-based complexes. To further improve BD performances several modifications have been carried out in recent years affecting each component of the complexes: terpyridines have been replaced by quaterpyridines; other metals were used instead of ruthenium, and thiocyanates have been replaced by different pinchers in order to achieve cyclometalated or heteroleptic complexes. The review provides a summary on design strategies, main synthetic routes, optical and photovoltaic properties of terpyridine and quaterpyridine ligands applied to photovoltaic, and focuses on n-type DSCs.
Keywords: dye-sensitized solar cells, polypyridines, Ru(II) complexes, terpyridines, quaterpyridines
1. Introduction
Dye-sensitized solar cells (DSCs) are photoelectrochemical devices able to convert sunlight into electricity [1]. The architecture and operating principles of these devices have already been extensively reviewed in the literature [2,3,4,5,6], and the photosensitizer represents one of the key components of this device. Different kinds of sensitizers [3,4] have been used so far, including Ru complexes [7], porphyrines [5], phtalocyanines, metal-free dyes [6] (including squaraines [8,9,10], cyanines [11,12], and push-pull dyes [13]).
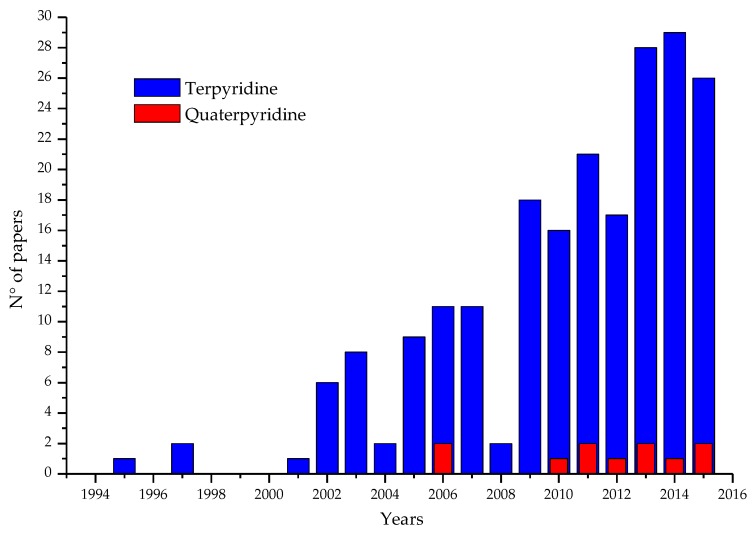
Since 1997 [14] the interest in 2,2’:6’,2’’-terpyridine (tpy) as ligands in organometallic sensitizers for DSC applications has constantly grown and, in the last three years, more than 80 papers and patents concerning this subject were published. Interest on 2,2’:6’,2’’:6’’,2’’’-quaterpyridines (qtpy) is more recent and has resulted in more than 10 papers (Figure 1).
Figure 1.
Publications concerning the use of terpyridines (blue) and quaterpyridines (red) in DSCs. Source: SciFinder (January 2016) [15].
While the general use of polypyridines in Ru complexes sensitizers has already been deeply reviewed in the past by Islam [16], Vougioukalakis [17], and Adeloye [18], or for the electrolytes by Bignozzi et al. [19], no insight about the specific structure–properties relationships of tpy and qtpy complexes in the same field have been provided. Thus, we drew our attention on these panchromatic sensitizers with a particular focus on cells performances and device investigation. For this reason works dealing only with computational investigation [20] will not be taken into consideration.
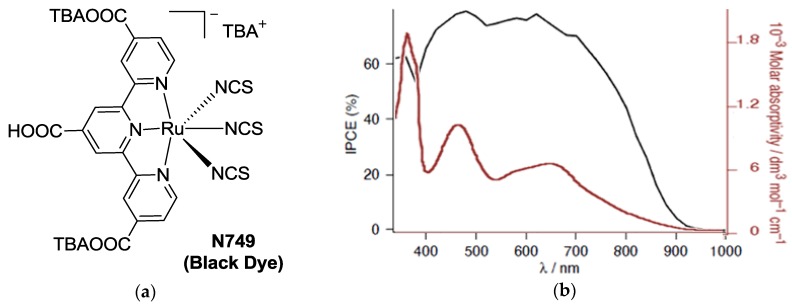
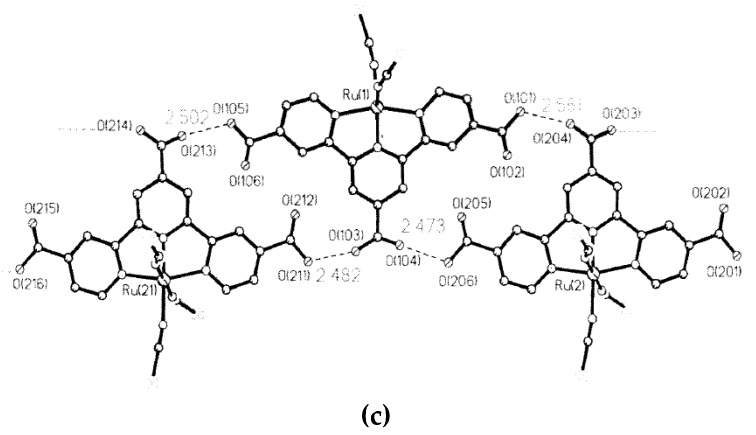
The first use of tpy ligands in DSCs technology was pioneered by Nazeeruddin et al. [14], providing good performances owing to their broader absorption with respect to the standard bipyridine-based Ru complexes. The structure proposed in 1997 by the EPFL researchers was named N749 or Black Dye (BD), thanks to its panchromatic absorption (Figure 2, top) and represents a benchmark standard as tpy complex sensitizer. In this dye, ruthenium(II) is complexed by a tpy, the 4,4’,4’’-tricarboxy-2,2’:6’,2’-terpyridine (tctpy) and three isothiocyanate ancillary ligands. X-ray diffraction showed a slightly distorted octahedral coordination around the Ru atoms by the three nitrogen donors of tctpy and three nitrogen of isothiocyanate ligands. Very strong intermolecular bonds account for bidimensional arrays, in which the distance between the planes prevents π-stacking between the tpy rings (Figure 2, bottom) [21]. The final BD was prepared by titration with tetrabutylammonium hydroxide in order to deprotonate two of the three carboxylic functions, which proved to be a crucial feature for performances’ optimization.
Figure 2.
(a) Black Dye (BD) or N749 structure; (b) light absorption spectrum (red) and IPCE (black) [12] (Adapted from Ref 12 with permission of The Royal Society of Chemistry); and (c) crystal structure showing intermolecular hydrogen bonding [21] (Reprinted with permission from Nazeeruddin, M. K.; Péchy, P.; Renouard, T.; Zakeeruddin, S. M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; Spiccia, L.; Deacon, G. B.; Bignozzi, C. A.; Grätzel, M. Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. Copyright 2001 American Chemical Society).
Comparing to bipyridine structures, terpyridines allow to achieve lower band gap for the metal to ligand transition (MLCT), thus providing a better absorption at lower energies and, therefore, broader solar harvesting. The conversion efficiency of BD was first reported as 10.4% (TiO2: 18 μm, dye: 0.2 mM ethanol + 20 mM sodium taurodeoxycholate, electrolyte: 0.6 M DMPII (1,2-dimethyl-3-propylimidazolium iodide), 0.1 M I2, 0.5 M t-bupy (t-butylpyridine), 0.1 M LiI in methoxyacetonitrile) [21], and after further structural tuning (see Section 3.2.5), it was improved up to 11.2% (TiO2: 15 + 7 μm; dye 0.3 mM ethanol / t-butanol 1:1 with 0.6 mM of tetra-butylammonium deoxycholate and 1 mM deoxycholic acid (DCA) as co-adsorbate; electrolyte: 0.6 M DMPII, 0.05 M I2, 0.5 M t-bupy, 0.1 M LiI, 0.1 M GuNCS (guanidinium thiocyanate) in CH3CN) [22]. Despite the wider absorption, performances of BD are not superior to N719 [23] (Figure 3) or other optimized bipyridines complexes [24]. This behavior has been attributed to a lower molar extinction coefficient (7640 M−1·cm−1 in DMF) [21] and worse surface coverage of titania [25].
Figure 3.
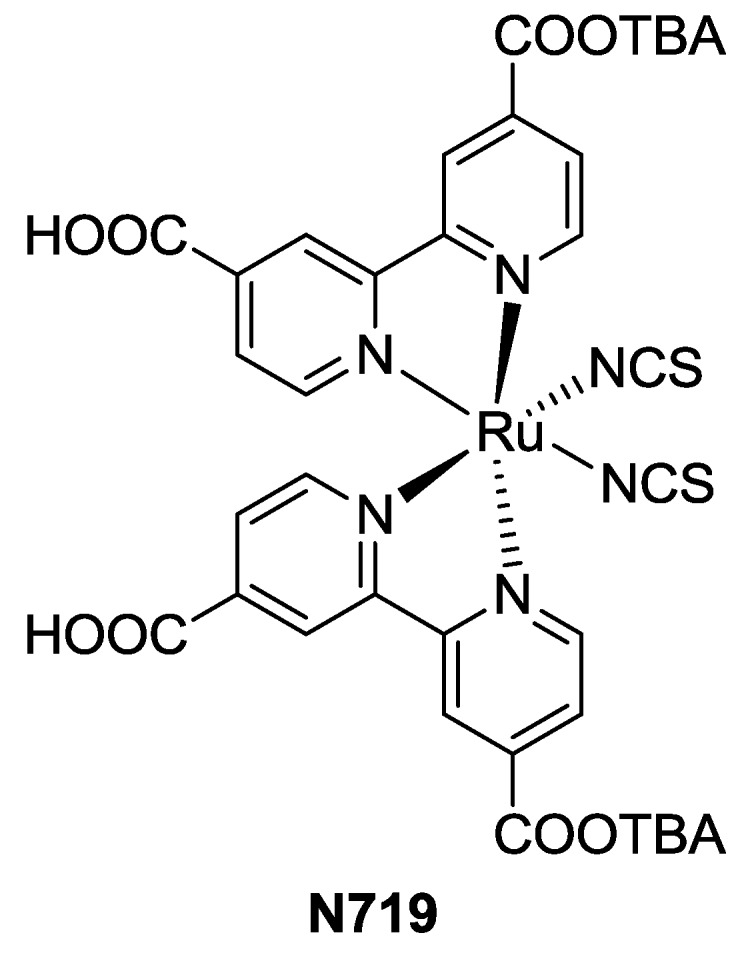
N719 structure.
With the aim to further improve BD performance, several modifications have been carried out concerning each component of the complex. In order to increase the molar extinction coefficient and other features ruthenium was substituted with other metals; thiocyanates were replaced with different pinchers in order to obtain cyclometalated or heteroleptic complexes; and the terpyridine ligand was substituted with a quatertpyridine in order to extend the π-conjugation.
The state of the art of polypyridine structures designed to further improve BD performances is summarized in the next sections. After a survey on the synthetic pathways to obtain tpy and qtpy structures, the three main types of changes underlined before (metal centre, ancillary, and tpy ligands) and their effect on DSCs performances will be taken into account in order to outline a structure-property relationship. Moreover, we remind that DSCs are a complex multivariate system [26], with different components and variables, and that a direct correlation between the photosensitizers’ molecular structures and related efficiencies can sometimes lead to inaccurate conclusions. For this reason, we selected literature examples where an internal standard reference (BD, 719 or N3) is reported in order to compare the characteristics of the novel structures. Moreover, specific conditions have been added to selected references.
2. Synthesis
The terpyridine structure was first synthesized in 1932 by Morgan and Burstall [27] as a byproduct of bipyridine synthesis, obtained by dehydrogenation of pyridine in the presence of anhydrous ferric chloride. Nowadays, several synthetic pathways have been developed [28,29,30], allowing this ligand to reach large applications such as uses in the preparation of Co(II) [31], Os(II) [32], Ru(II) [33] Ir(II) [34,35], Pd(II), Pt(II), and Au(III) complexes [36], supramolecular complexes [37,38,39,40], molecular wires [41], polymers [42], in the surface functionalization of nanostructures [43], in the conjugation with amino acids [44], biomacromolecules [45], in the coupling with inorganic nanoparticles [46], and have shown their remarkable activity in other fields such as sensing [47] and catalysis [48,49]. We will report briefly the main strategies used to obtain tpy ligands focusing on the structure–properties relationship in DSCs.
2.1. Terpyridine Core
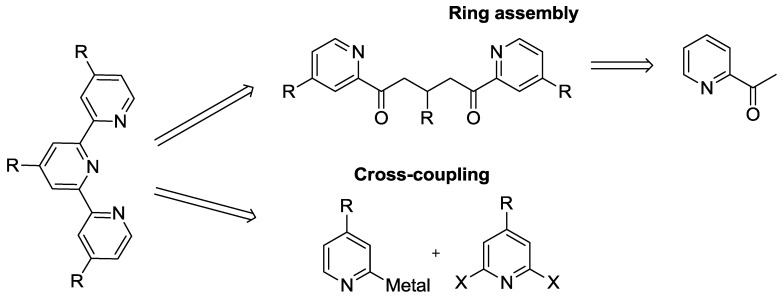
Tpy structures are mainly prepared through two basic synthetic approaches, which involve either ring assembly or coupling methodologies, as summarized in Scheme 1.
Scheme 1.
Retrosynthetic pathways to tpy core.
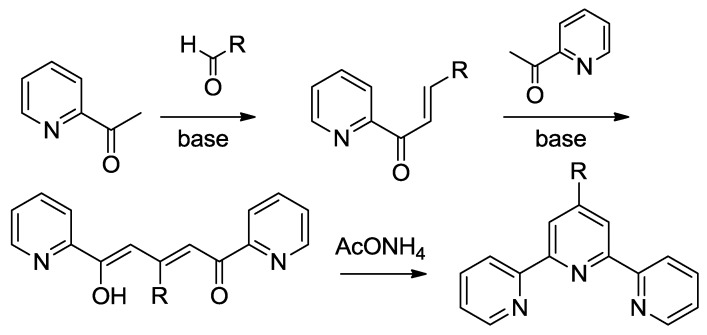
The first route has been formerly reviewed in 1976 by Kröhnke [50], who reported the synthesis of α,β-unsaturated ketones from 2-acetyl derivatives of pyridine and aldehydes. Then, the intermediate reacts with another 2-acetylpyridine to form a 1,5-diketone that can undergo cyclization to pyridine thanks to ammonia sources such as AcONH4 (Scheme 2). A series of modifications to this procedure has been proposed in order to increase yields or improve the synthetic pathway sustainability [28,51].
Scheme 2.
Example of the Kröhnke pathway.
The second strategy exploits recent advances in organometallic reactions (cross-coupling in Scheme 1). The electron poor pyridines are less effective in the Suzuki reaction [52] due to the weaker electrophilicity of pyridyl-boronates with respect to other organometallic reagents, such as the organo-tin involved in Stille reaction [53].
Noteworthy, the synthetic pathway used to achieve 4,4’,4’’-tricarboxy-2’,6’-terpyridine (tctpy) for Black Dye [54] involves the formation of the terpyridine core starting from 4-ethyl pyridine refluxed with Pd/C over nine days. This procedure was further improved by Dehaudt et al. [55]. Among the other possible strategies to obtain a tpy core, it is worth noting an inverse Diels-Alder reaction on 1,2,4-triazine that uses 2,5-norbornadiene as dienophile [56].
2.2. Functionalization of Terpyridines
In order to design complexes suitable for DSCs applications a series of modifications has to be taken into consideration, with the aim of introducing anchoring moieties, donor groups, bulky alkyl chains, or extending the π-conjugation. Cross-coupling reactions represent the most frequently used synthetic tool, while more specific pathways include the formation of carboxylic acid by furan degradation [57,58,59,60]. Other common syntheses are dealing with pyridine functionalizations; for example, the pyridine N-oxide is used as an intermediate to obtain halogen and pyrrolidinyl functionalizations [61,62], while 4-pyridones analogues are used to have access to halogens or triflates derivatives [63]. Husson et al. reviewed the derivatizations with thienyl [56] and furanyl [64] moieties while recently Woodward et al. [65] reported a synthetic strategy to further extend the scope and number of the anchoring moieties on oligopyridines.
2.3. Quaterpyridine Synthesis and Complex Formation
The synthesis and functionalization of qtpy usually exploit the same synthetic strategies used for tpy, namely Kröhnke and coupling reactions. In the latter case N-methyliminodiacetic acid (MIDA [66]) boronates have been successfully applied as key reagents to obtain quaterpyridine ligands in good yields [67] through Suzuki-Miyaura reaction.
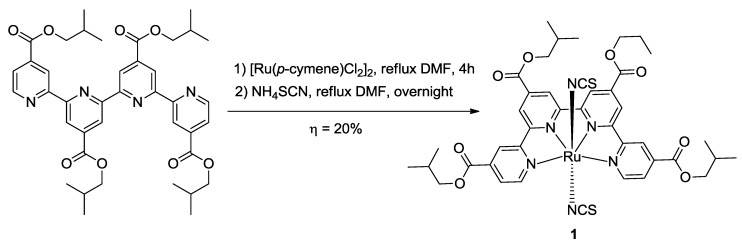
In order to obtain Ru(II) complexes of polypyridines, Adeloye et al. [18] used Ru p-cymene or Ru(III)Cl3 as starting materials and they substituted the chlorines with thiocyanates or other ancillary ligands. Exploiting microwave-assisted synthesis, a facile procedure to obtain a functionalized qtpy ligand and its trans-dithiocyanato ruthenium complex has been reported [68] (Scheme 3).
Scheme 3.
Microwave-assisted synthesis of the trans-Ru (II) complex [68].
3. Modifications of Black Dye and Structure-Properties Relationships on Devices
3.1. Terpyridine modification
In this section, tpy based ruthenium complexes bearing three thiocyanates as ancillary ligands will be reviewed, outlining structural modifications on tpy ligand and their effects on DSCs performances.
Molecular engineering on tpy ligands has commonly the aim to extend π-conjugation in order to increase the molar extinction coefficient and further stabilize the LUMO level. In this way more photons can be harnessed and converted thanks to a simultaneous hyperchromic effect and bathochromic shift in the absorption spectra, respectively. Other common structural modifications are the substitution of one of the three pyridines with either a donor group (such as triphenyl amine), in order to enhance the push-pull system character, or a hydrophobic group, in order to reduce recombination with the electrolyte. Particularly interesting are the structural variations related to the anchoring moieties. The tctpy used in BD offers three possible anchoring points, allowing a proper sensitizer-semiconductor coupling and improving the stability of the device. Moreover, alternative anchoring groups, with respect to the carboxylic acid functionality, have been tested. Zakeeruddin [25] proposed a terpyridine functionalized with a phosphonic acid group on 4’-position with the purpose of overcoming the slow desorption of the carboxyl anchoring group from the semiconductor surface in presence of water. Waser [69] proposed a tpy bearing a phosphonic acid functionality, coupled with TiO2 for DSCs and water splitting applications, while Anthonysamy et al. [70] proposed a 4’-methacryloyloxymethylphenyl moiety as an anchoring group.
As far as the carboxyl anchoring group is concerned, in 2002 Wang et al. [71] tested a 4’-carboxyphenyl substitution (Figure 4), obtaining an appreciable bathochromic shift with respect to N3 (cis-diisothiocyanato-bis(2,2’-bipyridyl-4,4’-dicarboxylic acid) ruthenium(II)), but a sensible loss in short circuit current in comparison with BD occurred, which can be explained by the fewer grafting points on the structure.
Figure 4.
Structure proposed by Wang et al. and N3 dye [71].
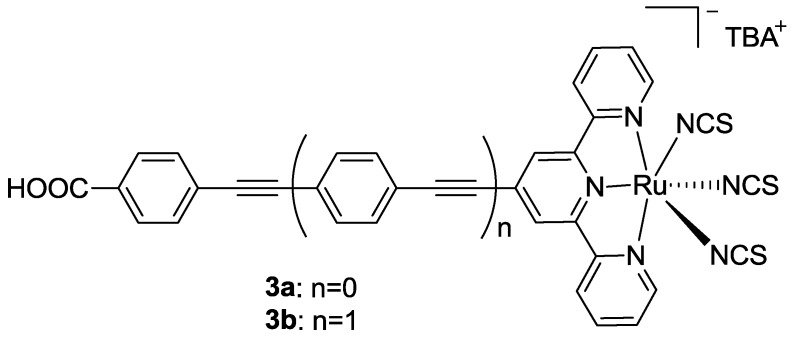
Funaki et al. [72] proposed a similar substitution, in which phenylene ethylene moieties (3a in Figure 5) were introduced between the COOH functionality and the tpy core, obtaining a better charge injection (12.8 mA/cm2) with respect to dye 2 (6.1 mA/cm2), even if a thicker TiO2 (36 μm vs. 10 μm) and higher light intensity (100 mW/cm-2 vs. 78 mW/cm-2) were used. The injection efficiency proved to be lower with respect to BD (16.7 mA/cm2), tested in the same conditions. Moreover, when the spacer was represented by two phenylene ethynylene units (3b in Figure 4) a higher molar extinction coefficient and slight bathochromic shift were obtained, but a significantly lower Jsc value was observed (5.7 mA/cm2) which was ascribed to an increased dye aggregation.
Figure 5.
Complexes reported by Funaki et al. [72].
McNamara et al. [73] reported a ligand similar to 2 bearing a hydroxamic acid instead of the carboxyl moiety. The dye showed promising properties but was not tested on any device.
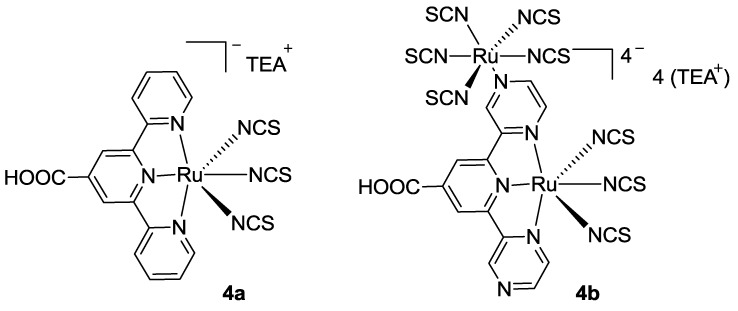
In 2010, Vougioukalakis et al. [74] synthesized a 4’-carboxyterpyridine acid Ru(II) complex (4a in Figure 5). With the purpose of increasing the chelating sites, the two outer pyridine rings were also substituted with pyrazine, which resulted in the coordination of a second Ru(II) atom (4b in Figure 6).
Figure 6.
Complexes with one (4a) or two (4b) metal centers [74].
The overall performances were worse with respect to BD, even if a better absorption on TiO2 was recorded, due to the greater flexibility of the dyes bearing only one anchoring group, which accounts for a higher number of molecules adsorbed on the surface. Complex 4a, whose structure is similar to dye 2, showed similar Jsc (6.19 mA/cm2), but its absorption was hypsochromically shifted with respect to BD. The 2,6-dipyrazinylpyridine ligand (complex 4b) led to overall lowest performances with 0.27 mA/cm2 charge injection and 0.02% efficiency (TiO2: 22 μm, dye 0.3 mM ethanol, electrolyte PMII Ionic Salt, Dyesol). Further improvements in the number of chelated Ru(II) atoms have been reported by Manriquez et al. [75] in the preparation of supramolecular structures.
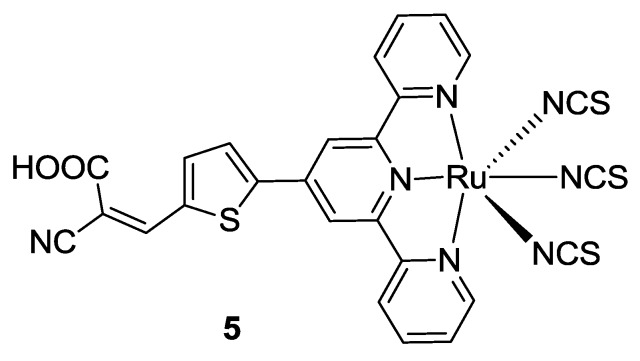
Very recently, Kaniyambatti [76] reported a tpy substituted in 4’- with a cyanoacrylic acid moiety via a thiophene bridge (5 in Figure 7). The modification leads again to a hypsochromic shift in the absorption spectrum coupled with a higher molar extinction coefficient owing to the extended π-conjugation and strong auxochrome resulting from the thiophene moiety.
Figure 7.
Terpyridine with a cyanoacrylic acid moiety [76].
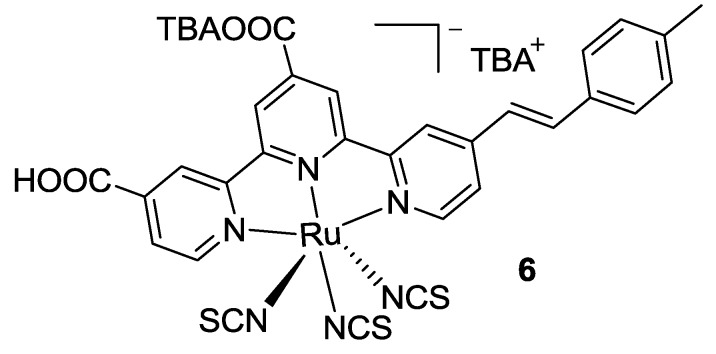
In 2013, Numata et al. [77] proposed a double anchored tpy bearing a 4-methylstyryl substituted in 4’’-position (6 in Figure 8) in order to extend the π-conjugation and to obtain better charge injection with respect to N749. This complex achieved a higher molar extinction coefficient especially on the π-π* transition, and a better IPCE in the same region, which led to an improved efficiency with respect to BD (η = 11.1% ; TiO2: 25 μm; dye: 0.3 mM acetonitrile / t-butanol 1:1, 24 h + 20 mM CDCA, electrolyte: 0.05 mM I2, 0.1 M LiI, DMPII, 0.2 M t-bupy in CH3CN).
Figure 8.
4-Methylstyryl substituted and double-anchored tpy (HIS-2) [77].
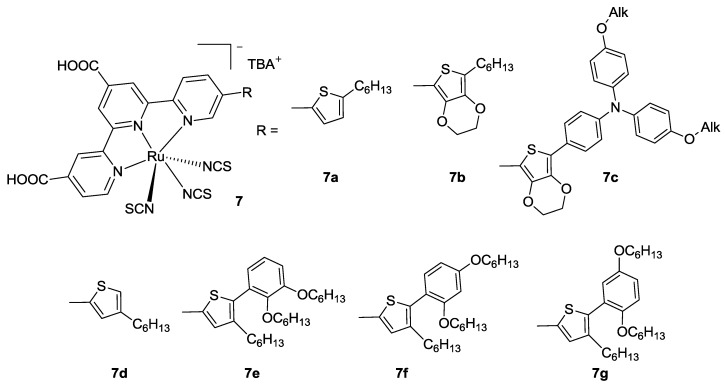
In 2011 Yang et al. [78] tested a series of 4,4'-dicarboxy terpyridine bearing a thiophene or a 3,4-ethylenedioxythiophene in 5’’ position (7a,b in Figure 9). The substitution of the latter with a triphenylamino moiety (7c) resulted in better performances with respect to BD tested in the same conditions (η = 8.29% vs. 6.89%; TiO2: 10 μm + 5 μm, dye: 0.3 mM ethanol + 10 mM chenodeoxycholic acid (CDCA), electrolyte: 0.6 M MDPII, 0.5 M t-bupy, 0.05 M I2, 0.1 M LiI in CH3CN), owing to the higher molar extinction coefficients in the high energy region of the spectrum. Substitution with hexyl-EDOT (7b, EDOT: 3,4-ethylenedioxythiophene) afforded even higher efficiency (η = 10.3% with TiO2: 15 + 5 μm). Similar modifications have been taken into consideration by Kimura et al. [79] (7d–g in Figure 9). In the series, structures with hindered hexyloxy-substituted rings resulted in better performances, probably because of the hindrance of alkyl chains towards the electrolyte, thus avoiding the redox couple to interact with titania and considerably reducing the dark current. Among these, the best results were obtained when the electron donor hexyloxy groups on the phenyl ring are in ortho or para positions (7f in Figure 9).
Figure 9.
Series of 5’’-substituted tpy proposed by Yang (7a-c) [78]; and Kimura (7d-g) [79].
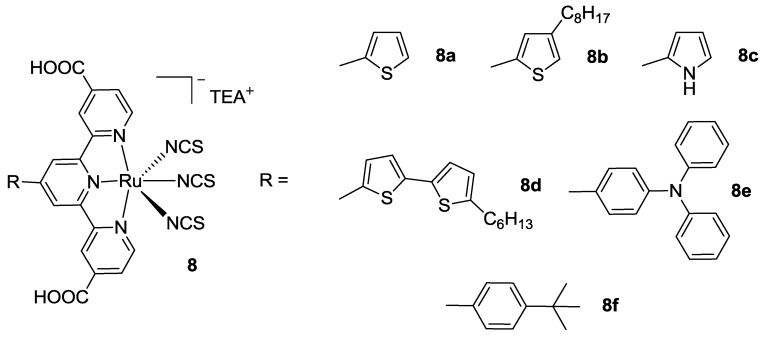
Very recently, Dehaudt [80] and Koyyada [81] proposed a simple synthetic pathway to achieve 4’-substituted Black Dye analogs (Figure 10) using octylthiophene (8b) and hexyl bithiophene (8d), pyrrole (8c), triphenylamine (8e), t-butyl phenyl (8f), phenoxazine, and phenothiazine groups. While these modifications did not allow to achieve better results respect to the BD in terms of efficiency, they gave an insight into the structure-property relationships, as well as fundamental issues about charge transfer, polarization, or binding. Thienyl-substituted analogues showed better performances with respect to triphenylamino donors, giving an efficiency of 5.57% (TiO2: 14 + 3 μm, dye: 0.5 mM ethanol / t-butanol + 10 mM CDCA, electrolyte: 0.5 M DMPII, 0.5 M t-bupy, 0.1 M LiI, 0.05 M I2 in CH3CN).
Figure 10.
4’ substituted Black Dye analogs [80].
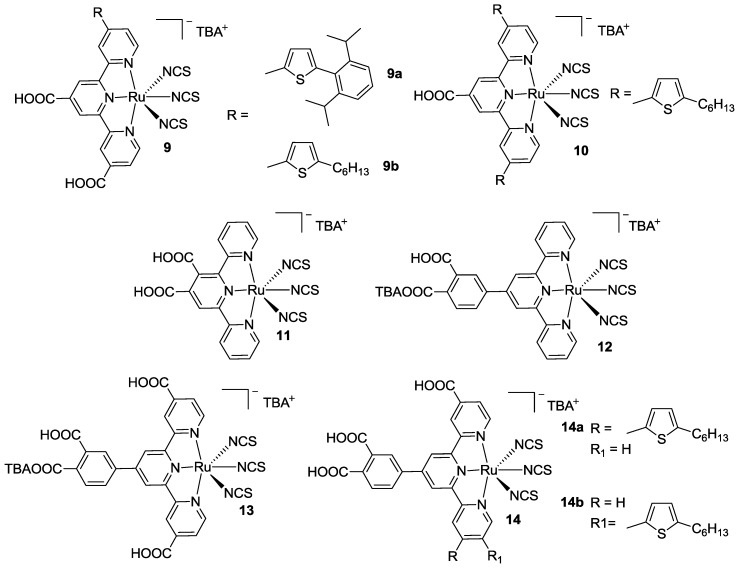
Ozawa et al. proposed a series of tpy having anchoring groups either in the classical 4-, 4’- and 4’’-positions or 3’-, 4’-positions, obtaining mono, bis, tri, and tetra-anchored complexes (Figure 11) [82,83]. Substitution with hexylthiophene in 3- or 4-positions was also investigated by impedance spectroscopy (EIS) and open circuit voltage decay (OCVD), revealing that charge recombination with electrolyte solution is largely promoted when compared to the carboxylic-modified one (Figure 10) [84,85]. Efficiencies close to the BD reference were recorded for the tetra-anchored complex 13, and for the 4’’-thienyl dicarboxy substituted complexes 9. The symmetric substitution with two hexyltiophene groups was also taken into consideration [86,87].
Figure 11.
Quaterpyridine Ligand
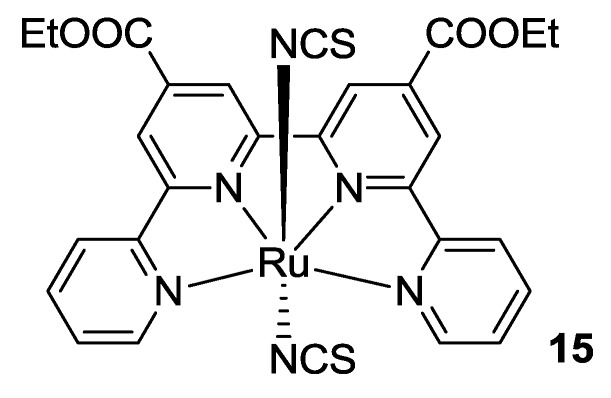
Tpy modification included the design of tetrapyridines as tetradentate ligands, that were proposed in order to avoid the geometrical isomerism of bipyridine complexes that leads to cis and trans conformers, showing different optical properties [88]. In fact, trans isomers of bipyridines complexes show better photophysical properties, but they are converted by thermal and photoinduced isomerization to the more stable cis isomers that, unfortunately, show worse panchromatic absorption. Tetradentate ligands, owing to their planar structure, coordinate the ruthenium in the plane and only leave apical position available for ancillary ligands, thus avoiding the isomerization and ensuring better solar harvesting features. The first example of a tetradentate ligand for DSCs applications was proposed in 2001 by Renouard et al. [89] who synthesized a 6,6’-bis-benzimidazol-2-yl-2,2’-bipyridine and a 2,2’:6’,2’’:6’’,2’’’-quaterpyridine bearing ethyl ester functionalities. The qtpy ligand was then characterized for DSCs applications as a complex with Ruthenium (15, Figure 12) [90]. The ester moieties showed poor adsorption on TiO2; thus, a further hydrolysis step proved mandatory in order to anchor the dye to the semiconductor surface. Thiocyanate ancillary ligands resulted in blue shifted absorption with respect to chlorine ones due to the stronger σ-acceptor properties of SCN. Remarkable conversion efficiency was recorded, up to 940 nm with 75% IPCE in the plateau region and 18 mA/cm2 Jsc (TiO2: 12 μm, dye: 0.3 mM ethanol / DMSO 95:5, electrolyte: 0.6 M DMPII, 0.1 M I2, 0.5 M t-bupy, 0.1 M LiI in methoxyacetonitrile).
Figure 12.
The first qtpy complex applied in DSCs by Renouard et al. [90].
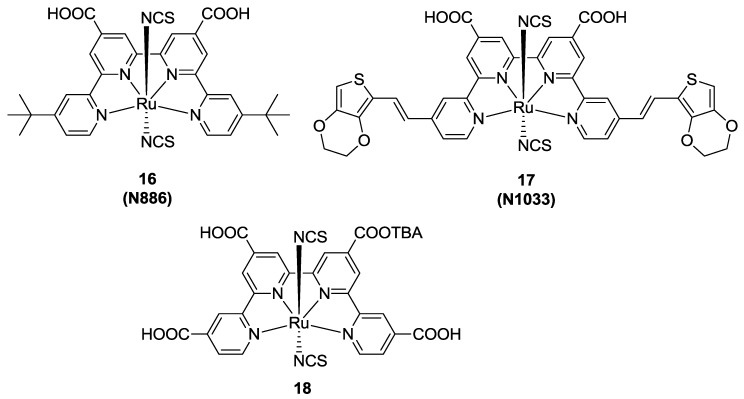
A further investigation was reported by Barolo et al. [91], in 2006, with the lateral functionalization of the quaterpyridines with t-butyl moieties as electron-releasing, bulky groups (16, Figure 13). The proposed dye, named N886, showed remarkable differences between protonated and non-protonated forms. Wider absorption with respect to N719 was reported, together with a lower molar extinction coefficient and unfavourable alignment of its excited state (as demonstrated by DFT calculations). With the purpose of overcoming these drawbacks, in 2011 the same research group proposed to substitute t-butyls with EDOT-vinylene groups, to further extend the π-conjugation (N1033, Figure 13) [92]. This complex showed a lower energy gap and a broad IPCE curve having still 33% conversion at 800 nm. The poorer efficiency with respect to N886 was ascribed to a lower driving force for electron injection, that limits the open circuit potential. The same drawback was also reported for a qtpy substituted with four COOH anchoring moieties (18, Figure 13) [68] but its high charge injection and an optimization of the electrolyte composition led to a record efficiency for qtpy Ru-complexes of 6.53% (TiO2: 12 + 5 μm, dye: 0.18 mM t-butanol / CH3CN 1:1 with 10% DMF, electrolyte: 1.0 M dimethylimidazolium iodide, 0.03 M I2, 0.1M CDCA, 0.1M GuSCN, 0.23 M LiI in valeronitrile / CH3CN 15:85). Co-sensitization with D35, in order to enhance conversion at higher frequencies, was also reported.
Figure 13.
3.2. Substitution of Ancillary Ligands: Heteroleptic and Cyclometalated Complexes
A further modification on terpyridine complexes involved the substitution of commonly used thiocyanate ligands with other ancillary ligands. The monodentate thiocyanate ligand has the role to tune the spectral and redox properties of the sensitizers acting on the destabilization of the metal t2g orbital [93]. By exchanging these ligands with σ-donor groups, it was possible to tune the photochemical properties of the complex, and to minimize the drawbacks associated with these monoanchored ligands. In fact, the possible formation of isomers, owing the bidentate character of the thiocyanate ligand causes a decrease in the synthetic yield [21,78,94]. Moreover the weak Ru-NCS bond itself leads to a decreased stability of the complex and, more importantly, thiocyanate lacks of an effective chromophore that could improve IPCE, particularly at shorter wavelengths. All these features encouraged the engineering of new heteroleptic cyclometalated complexes starting from Black Dye, by exchanging one or more thiocyanate ligands. A drawback affecting this kind of modification is the destabilisation of HOMO orbitals that can lead to a lower driving force in the dye regeneration by the electrolyte.
Strategies for the design of Ru tridentate heterocyclic ligands tailored to tune the properties of the excited state were recently reviewed by Pal et al. [95]. Medlycott [96] in 2005 surveyed the strategies for improving the photophysical properties of tridentate ligands commonly considered weaker than bipyridine ones, and Hammarstrom et al., in 2010 [97], investigated the possibility to expand their bite angle. In the following paragraphs we will report an overview of ancillary ligands properly synthesized to tune the photoelectrochemical properties of tpy for applications in DSCs.
3.2.1. Bipyridines
Ancillary ligand exchange was pioneered in 1997 by Zakeeruddin et al. [25] who substituted two of the three thiocyanates with a 4,4’-dimethyl-2,2’-bipyridine. In this case, the tpy ligand was not represented by tctpy, but by a simpler tpy with a phosphonic acid anchoring group (Figure 14).
Figure 14.
First example of tpy Ru-complex showing a bipyridine instead of two thiocyanates [25].
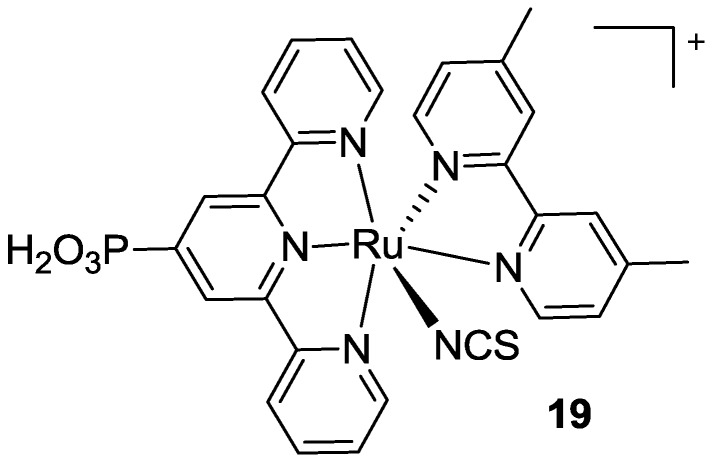
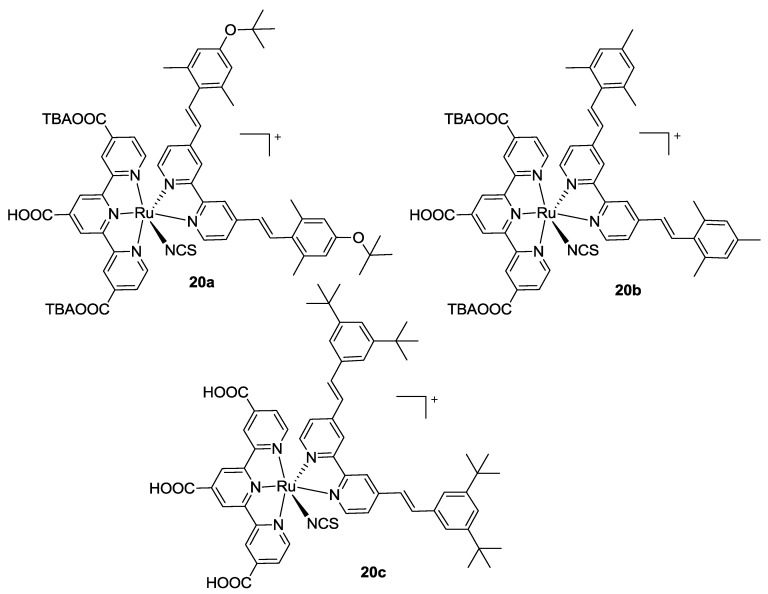
This research topic became of interest again when, in 2011, Chandrasekharam et al. [98] proposed to substitute two thiocyanate ancillary ligands with a bipyridine having electron donor styryl moieties in 4,4’- position (20a,b, Figure 15). Worse panchromatic behavior was observed with respect to BD, but also better performances in device, owing to an increased molar extinction coefficient in the visible region. A low value of fill factor led to a 3.36% best efficiency, higher with respect to that of BD evaluated in the same conditions (TiO2: 9 + 4.8 μm, ethanol solution, Z580 electrolyte: 0.2 M I2, 0.5 M GuSCN, 0.5 M N-methylbenzimidazole in [bmim] [I] / 1-ethyl-3-methylimidazolium tetracyanoborate 65:35). Similar bipyridines, slightly modified in the styryl substitution, were also tested by Giribabu et al. [99] (20c, Figure 15). A more positive oxidation potential with respect to BD under the same conditions has been reported (0.78V vs. 0.60V) which was associated with a more negative reduction (−1.30V vs. −1.10V) explaining the loss in panchromatic absorption.
Figure 15.
Monothiocyanate complexes proposed by Chandrasekharam [98] (top) and Giribabu [99] (bottom).
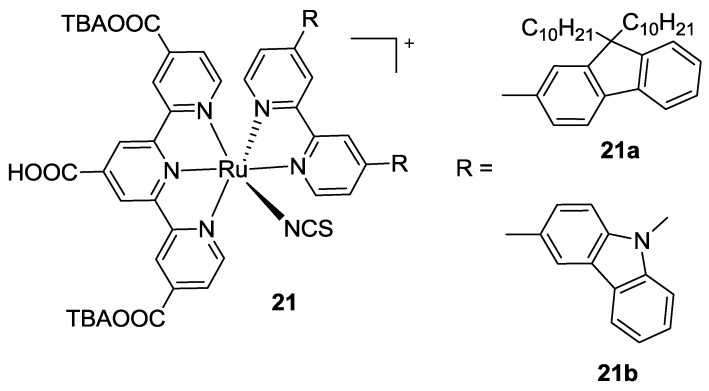
Very recently, Koyyada et al. [100] reported other bipyridines 4,4’- substituted with fluoren-2-yl (21a in Figure 16) or carbazol-3-yl (21b) groups, as ancillary ligands. Even if the proposed structures reported good molar extinction coefficients and favourable oxidation and reduction potentials, the overall performances were quite low, mainly due to the poor generated photocurrent that was possibly related to an unfavorable localization of LUMO, far from the anchoring sites on titania.
Figure 16.
Bipyridine ancillary ligands with fluoren-2-yl or carbazol-3-yl substitutions [100].
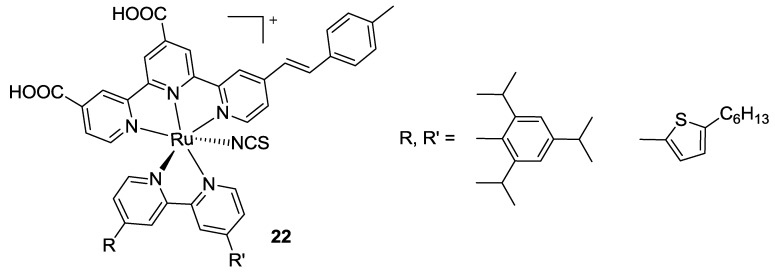
In 2015 Pavan Kumar et al. [101] modified complex 6 [77] by substituting two thiocyanates with an asymmetrical bipyridine ligand bearing hexylthiophene and mesityl subtituents on each pyridine ring (22, Figure 17).
Figure 17.
Ancillary ligands modifications of complex 6 [101].
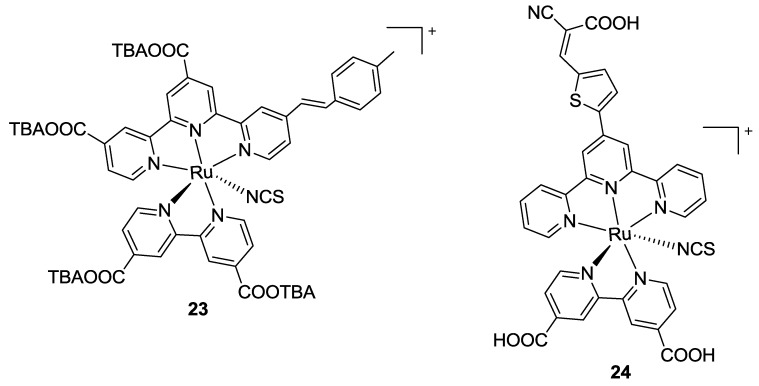
In the same paper, a Ru complex was reported, in which the bipyridine bears two carboxyl substituents. While having four anchoring groups, this complex led to lower efficiencies (23, Figure 18). With similar purposes, Kanniyambatti [76] modified complex 5, achieving a three-anchored sensitizer (24, Figure 18) with higher molar extinction coefficient and higher efficiency with respect to both complex 5 and BD tested in the same conditions (η = 7.5 vs 6.1%; TiO2: 10 + 4 μm, dye: 0.5 mM t-butanol / acetonitrile 1:1 with with CDCA 0.5 mM, electrolyte: 0.6 M [bmim][I], 0.03 M I2, 0.1 M GuSCN and 0.5 M t-bupy in CH3CN / valeronitrile 85:15).
Figure 18.
Four (23) and three (24) anchored complexes by Pavan Kumar [101] and Kanniyambatti [76].
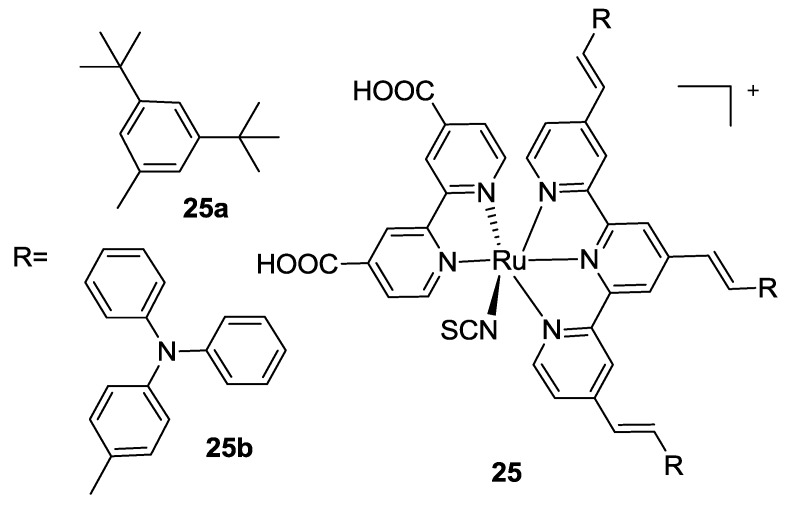
All these modifications were in line with the results from Giribabu, who proposed a Ru complex with 4,4’-dicarboxybipyridine and a tpy ligand bearing the same electron donor in 4,4’,4’’- positions (t-butyl or biphenyl amino substituted styryl moieties) (25a-b, Figure 19) [102]. In this case, a further enhancement in π-conjugation led to increased molar extinction coefficients and improved performances. Similar complexes that bear donating groups on the terpyridine and electron withdrawing/grafting moieties on a bidentate ligand have been proposed by Mosurkal [103], Erten-Ela [104] and, more recently, by Mongal [105]. In the first case, the anchoring moiety was provided by 4,4’-dicarboxy-2,2’-bipyridine. Mono and dinuclear ruthenium complexes were compared on the device, where the latter one gave better performances. In the second case, the bidentate ligand was represented by a phenantroline substituted with phenyl sulfonic acid moieties in order to graft and sensitize TiO2 and ZnO.
Figure 19.
Tpy extended with substituted stiryl moieties by Giribabu [102].
3.2.2. Bis-Terpyridine
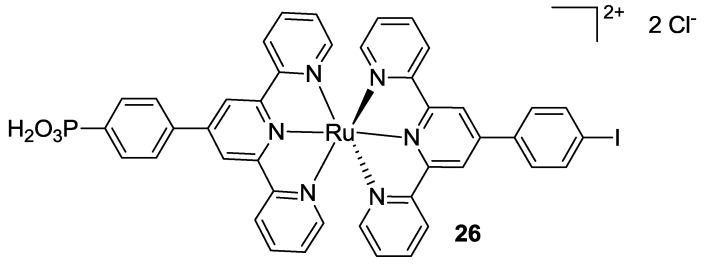
Stergiopoulos et al., in 2005 [106], replaced all the thiocyanates with another terpyridine. In the resulting heteroleptic complex, one tpy was substituted in 4’- with a p-iodophenyl moiety and the other one with a p-phenylphosphonic acid, in order to allow the grafting to TiO2 semiconductor in a solid state device (26, Figure 20).
Figure 20.
Bis-tpy complex proposed by Stergiopoulos et al. [106].
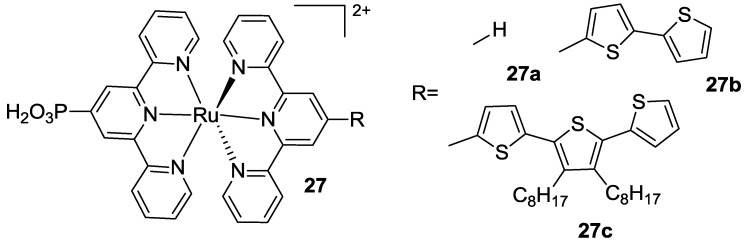
In the same year Houarner et al. [107] proposed another bis-tpy complex with a phosphonic acid as the anchoring group on one terpyridine and oligothiophene moieties on the other one, in order to increase the interaction between dye and hole transporting material (27, Figure 21). Low performances of this class were attributed to an undesired localisation of the LUMO orbital on thiophenes and, as a consequence, to a difficult charge injection into the TiO2. In order to improve the performances, the same group in 2007 introduced an unconjugated bridge between the tpy and the polythiophene moiety [108].
Figure 21.
A first series of bis-tpy complexes proposed by Houarner et al. [107].
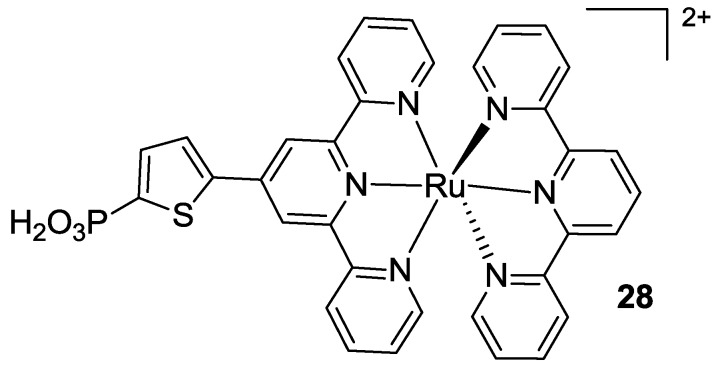
Further improvements to the Houarner series were reported in 2007 [109] by introducing a thiophene π-conjugated bridge between the terpyridine and the phosphonate anchoring group, improving the photoconversion efficiency (28, Figure 22). The thiophene spacer proved to be an interesting and efficient relay in the molecular design; however, overall low efficiencies were obtained, owing to a lower driving force for charge injection.
Figure 22.
A structural variation of bis-py Ru complex proposed by Houarner et al. [109].
Krebs and his research group [110] further investigated bis-tpy Ru complexes using bromophenyl, carboxyphenyl, carboxyl acid [111], and ester moieties in order to compare their anchoring properties. Ester moieties showed weaker absorption to TiO2 with respect to carboxylic acid and non-symmetric complexes reported efficiencies three times higher with respect to symmetric ones. The same group [112,113] and Chan [114] studied bis-tpy Ru-complexes in conjugated polymers, and their application to polymeric solar cells [112,113]. Tpy-bearing polyphenylene-vinylene and thienyl-fluorene units were exploited in order to incorporate the resulting Ru complexes in the polymer chains; carboxyl acid functionalization of the bipyridine moieties resulted in improved efficiency. Caramori et al. [115], using an heteroleptic thienylterpyridine Ru complex, improved the electron collection efficiency owing to an electrolyte based on the combination of cobalt and iron polypyridine complexes.
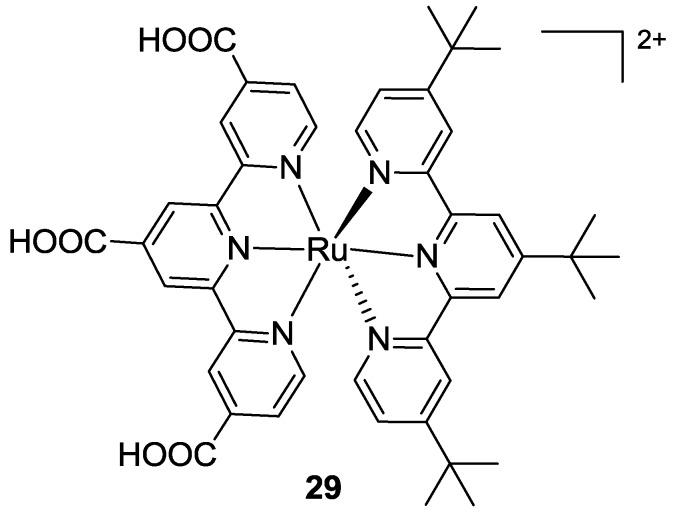
Very recently, Koyyada [100] replaced all thiocyanates in the BD structure with a tris (t-butyl) tpy, thus maintaining tctpy as the anchoring moiety (29 in Figure 23). The complex showed good optical properties, with a hypsochromic shift in the visible range of the spectrum and a higher molar extinction coefficient respect to BD, but the overall performances were quite low.
Figure 23.
Modification of the BD structure with tris (t-butyl) tpy [100].
3.2.3. Phenylpyridine and Pyrimidine
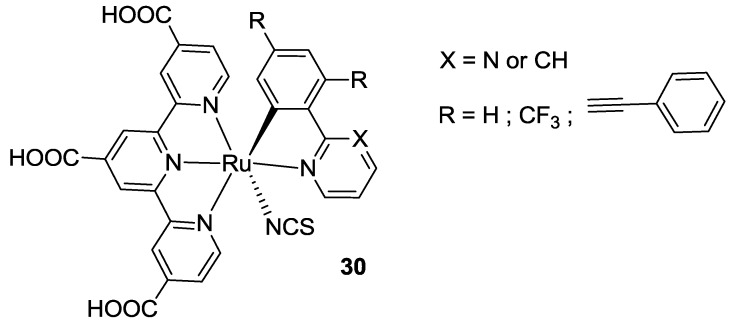
Funaki investigated the possibility to maintain the same terpyridine ligand of Black Dye, tctpy, substituting two thiocyanates with a series of C^N bidentate ligands (30 in Figure 24) [116,117,118]. These complexes were designed in order to utilize ancillary ligands with stronger donor properties with respect to thiocyanates in order to destabilize the t2g HOMO orbital, to reduce the band gap and to harness lower energy regions of the solar spectrum. 2-Phenylpyridines as such, and those substituted in 4’ position with a phenyl ethynyl group [118], were used to obtain cyclometalated ruthenium(II) complexes. The wider π-extension allowed to obtain higher molar extinction coefficients and a higher charge injection with an IPCE value of 10% at 900 nm. The main drawback of these complexes was a low oxidation potential that reduced the driving force for dye regeneration. In order to raise the HOMO level and ease the dye regeneration by iodine, the same group [116] extended the C^N ligands series to 2-phenylpyrimidines, substituted on the phenyl ring with trifluoromethyl groups. The CF3 group further reduces the electron donor behavior of the ligand and stabilizes the HOMO level. In this way a 10.7% efficiency was obtained, with respect to 10.1% of BD tested in the same conditions (TiO2: 25 + 6 μm, dye: 0.4 mM ethanol with 40 mM DCA, electrolyte: 0.6 M DMPII, 0.05 M I2, 0.1 M LiI, 0.5 M t-bupy in CH3CN). These ligands were further investigated in 2013 [117] by computational studies.
Figure 24.
C^N bidentate ligands proposed by Funaki et al. for Ru(II)-complexes [116,117,118].
3.2.4. β Diketonate Ligands
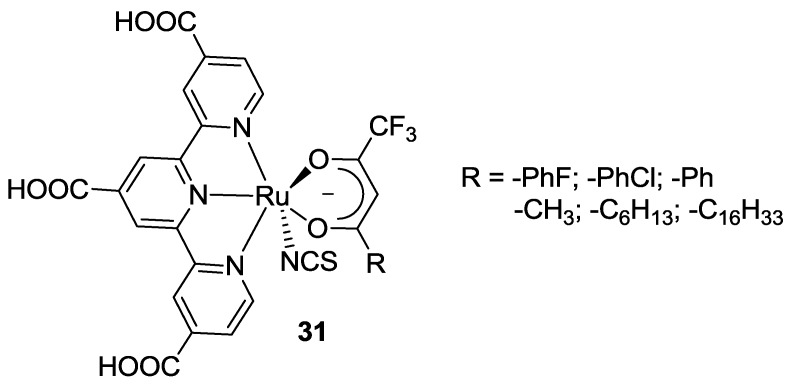
A series of β-diketonate ligands (31 in Figure 25) was investigated by Islam et al. [119,120,121,122,123,124,125] as ancillary ligands alternative to thiocyanates in the BD structure. The strong σ-donating nature of the negatively-charged oxygen donor atom destabilizes the ground-state energy level of the dye compared to BD, leading to a shift of the MLCT transitions to lower energies. In 2002 [125] a Ru(II) complex with 1,1,1-trifluoropentane-2,4-dionato ligand showed efficient panchromatic sensitization of nanocrystalline TiO2 solar cells. Additionally, a longer alkyl chain (using 1,1,1-trifluoroeicosane-2,4-dionato ligand) [122] prevented surface aggregation of the sensitizer and allowed to avoid or reduce the use of chenodeoxycholic acid. The use of longer alkyl chains may protect the TiO2 surface, through steric hindrance and hydrophobic effect, preventing the access of electrons to the redox electrolyte, favouring a higher Voc. On the other hand, the bulky alkyl group may not only facilitate the ordered molecular arrangement on the TiO2 surface, but also keep dye molecules far away each other, thus suppressing intermolecular dye interaction and increasing Jsc [126].
Figure 25.
β-diketonates ligands by Islam et al. [119,120,121,122,123,124,125].
In 2006 [123] the same group further modified the β-diketonate ligand with a halogen p-chlorophenyl group. Aryl substituents with different electron-donating strength were allowed to control the shift of the low-energy MLCT band and Ru oxidation potential. A very efficient sensitization (η = 9.1%; TiO2: 20 μm, dye: 0.2 mM CH3CN / t-butanol 1:1 with 20 mM DCA, electrolyte: 0.6 M DMPII, 0.05 M I2, 0.1 M LiI, 0.07 M t-bupy in CH3CN), with an IPCE greater than 80% in the whole visible range extending up to 950 nm was obtained. Further substituted β-diketonate ligands were tested in 2011 [119] showing a great potential to tune the photochemical properties.
3.2.5. Pyrazolyl Ligands
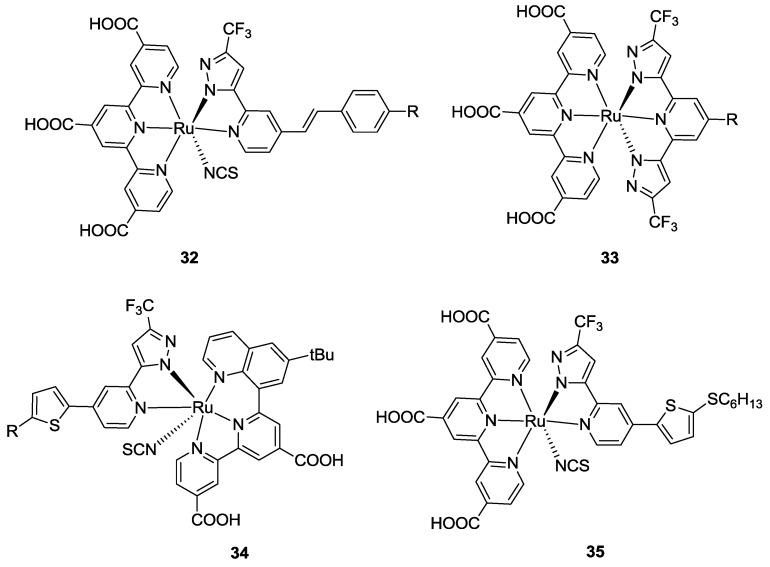
Novel N^N bidentate ligands, different from the bipyridines, were proposed by Chen et al. [127]. A series of 2-(pyrazol-3-yl)pyridine ligands were used as an alternative to thiocyanate in BD and tested in cells (32, Figure 26). These dyes overcome the efficiency of BD tested in the same conditions (η = 10.05 vs 9.07% ; TiO2: 18 + 4 μm, dye: 0.3 mM DMF / t-butanol 1:1 with 10 mM DCA, electrolyte: 0.6 M DMPII, 0.1 M I2, 0.1 M LiI, 0.5 M t-bupy in CH3CN) due to their higher molar extinction coefficients between 400 and 550 nm and their extended absorption up to 850 nm, as a consequence of the HOMO destabilization by the pyrazole. The same group reported, in 2011 [128], a series of tridentate 2,6-bis(3-pyrazolyl)pyridine ligands bearing various substitutions in 4- position (33, Figure 26). The reported IPCE spectra showed a worse sensitization in the NIR region with respect to N749 but a better conversion in the visible range which accounts for efficiencies up to 10.7% (TiO2: 15 + 5 μm, dye: 0.3 mM ethanol / DMSO 4:1 with 1M CDCA, electrolyte: 0.6 M DMPII, 0.1 M I2, 0.5 M t-bupy, 0.1 M LiI in CH3CN). The results were explained by the bulky ligand effect, which may allow better packing of the dye molecules on the TiO2 surface and prevent interfacial charge recombination. On the other hand, the contribution of the pyridine in the ligand, which is neutral with respect to the negatively charged thiocyanates, might allow the negative dipole moment to be localized closer to the surface, thus affording a higher Voc. Further investigations on these complexes were carried out by replacing the tctpy with a dicarboxytpy ligand substituted in the 5- or 6- position of a terminal pyridyl unit with π-conjugated thiophene pendant chains, obtaining good stability and performances with respect to BD [129]. More recently, the terminal pyridyl unit of the tctpy was replaced with variously substituted quinolines (34, Figure 26) reaching good performances (η = 10.19%; TiO2: 15 + 7 μm, dye: 0.3 mM ethanol / t-butanol 1:1 with 0.6 mM of tetra-butylammonium deoxycholate, electrolyte: 0.6 M DMPII, 0.05 M LiI, 0.05 M I2, 0.5 M t-bupy in CH3CN) [130]. In this new family of complexes, electron-donating-bulky t-butyl substituents on quinoline gave better performances with respect to the electron-withdrawing COOH group. With the t-butyl group, in fact, a blueshift for transitions at lower energies was reported together with a hyperchromic effect that improved IPCE and Jsc. Further modifications to the bidentate ancillary ligands led to a best result efficiency (η = 11.16%; with the addition of 1mM DCA as co-adsorbate to the dye solution and electrolyte: 0.1 M LiI and 0.1 M GuNCS, 0.5 M t-bupy in CH3CN) [22] when tctpy and hexylthiothienyl-substituted pyrazolyl-pyridine were used to complex ruthenium (II) (35, Figure 25). This complex showed worse conversion in the NIR spectral region but improved IPCE in the visible one with respect to BD, thus determining a better efficiency in the same conditions.
Figure 26.
Pyridyl-pyrazolate ligand and quinolyl-bipyridine ligand for Ru(II)-complexes [22,127,128,129].
Recently, Chang et al. [131] reported pyrazolyl-pyridine ancillary ligands bearing a series of donor groups in which the simplest substituents (such as t-butyl group) leads to better efficiency with respect to the triphenylamino and benzothiadiazolylgroups.
3.2.6. Phenyl Bipyridines
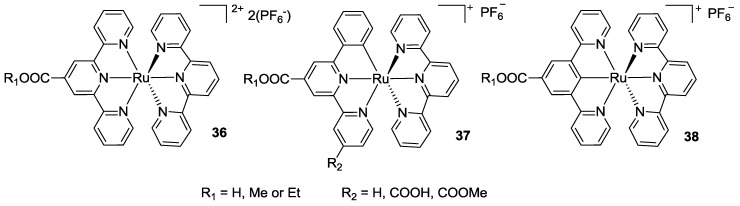
In 2007 Wadman et al. [132] compared a bis-tpy Ru complex bearing one carboxyl group in the 4- position, with two structurally homolog complexes in which the tpy was replaced by 6-phenylbipyridines, with one or two carboxyl groups (36 and 37, Figure 27). The N^N’^C Ru(II)-complex with two anchoring groups showed performances similar to N719. Thus, in 2010 [133], the same group further extendend the series, including N^C^N’ ligands based on 3,5-bis(2-pyridyl)benzoic acid (38, Figure 27).
Figure 27.
Tpy and phenyl-bipyridines complexes investigated by Wadman et al. [132].
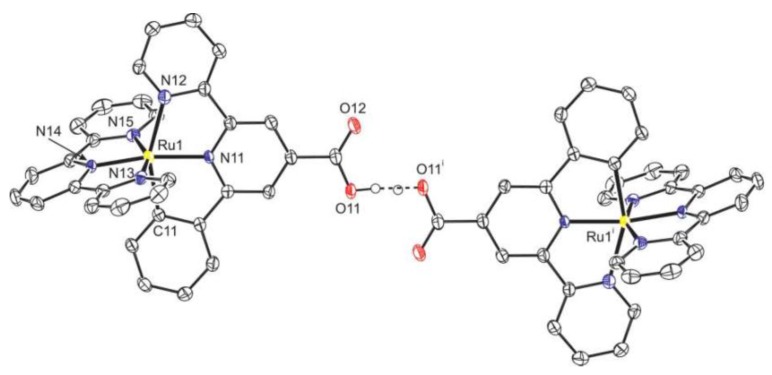
X-ray structural determination on the mono carboxyl complex (37, R1 = H, R2 = COOH) showed a distorted octahedral coordination, with the cyclometalated ligand perpendicular to the terpyridine and elongation of the nitrogen to Ru bond opposite to the C-Ru bond. In the solid state, the complex forms dimers via hydrogen bonds between the carboxyl functions (Figure 28).
Figure 28.
Crystal structure of complex 37 in form of its dimer [132] (Adapted from Ref 131 with permission of The Royal Society of Chemistry).
N^N’^C cyclometalated compounds showed better sensitization properties respect to the bis-tpy complexes; while the lower efficiencies of the N^C^N' complexes were ascribed to a LUMO localization which prevented an efficient electron injection into the TiO2 conduction band. The replacement of a coordinative Ru-N bond with a covalent carbon-ruthenium bond led to a redshift and to a broadening in the optical absorption of the corresponding ruthenium complex. Functionalization on the N^C^N’ ligand with another tpy resulted in the synthesis of dinuclear Ru(II)-complexes [134].
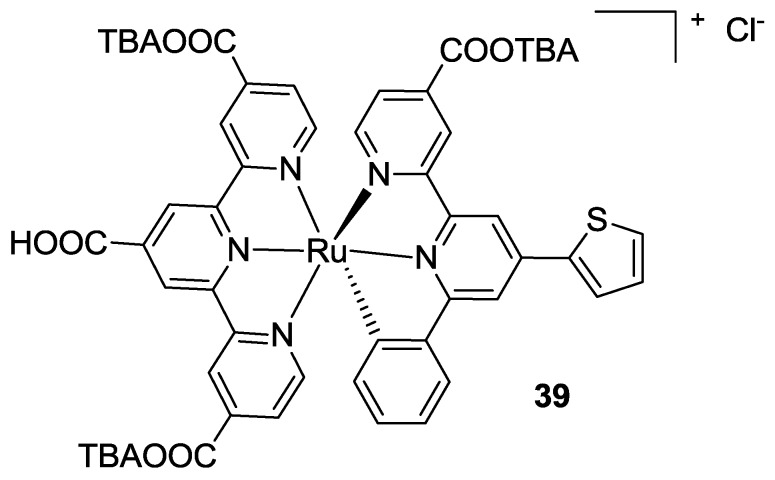
Kisserwan et al. [135] further engineered the 6-phenyl-2,2’-bipyridyl (C^N^N’) ligand with a thiophene and carboxylic acid moieties in the 4- and 4’- positions of the bipyridine moiety (39, Figure 29). The thienyl group was chosen with the purpose of increasing the molar extinction coefficient, while COOH had the aim to further strengthen the coupling with TiO2. With respect to Wadman’s works, tctpy was used instead of tpy. The work focused more on electrolyte composition than on sensitizer design, providing better performances when CuI was used as an additive. The same group in 2012 [57] extendend the investigation on the 6-phenyl-2,2’-bipyridyl (C^N^N’) ligand, studying the influence of either donor or acceptor substituents on the phenyl and the presence of COOH on the bipyridine. When the thienyl group was replaced by COOH, lower efficiencies were observed, attributed to a less efficient electron injection. The best sensitizer was also studied for its long-term stability, showing better results when compared to N719.
Figure 29.
Bis-tpy-based Ru(II) complex proposed by Kisserwan et al. [135].
In 2011, Robson et al. [136] published an extensive study in which a series of asymmetric bis-tridentated ruthenium complexes was synthesized, whose ligands ranged from terpyridine (N^N’^N’’) to phenyl-bipyridine (C^N^N’) and di-(2-pyridyl)-benzene (N^C^N’), bearing anchoring electron-withdrawing groups on one ligand and, on the other, a thienyl-triphenylamino group as donor counterpart (40, Figure 30). A thorough investigation of the photophysical and electrochemical properties was pursued in order to understand the role of the organometallic bond and terminal substituents and to tune the energetic levels. Broad absorption spectra were generated in Ru(II) complexes containing an organometallic bond because of the electronic dissymmetry about the octahedral Ru(II) center. The intensity of the spectra in the visible region was enhanced when the organometallic bond was orthogonal to the principal axis (i.e., C^N^N’ ligand). When the anchoring ligand is represented by a N^C^N’ tridentate combination, the LUMO is placed remotely from TiO2, and this prevents an efficient charge injection. On the other hand, if the organometallic bond is placed on the donor ligand, HOMO level can be localized either on the triphenyl amino moiety or on Ru(II), maximizing light harvesting in the visible region; while, at the same time, the LUMO on the anchoring ligand ensures an efficient electron transfer towards the semiconductor surface. The highest recorded efficiency reached 8.02% (TiO2: 15 + 4.5 μm, dye: 0.3 mM ethanol, Z1137 electrolyte: 1.0 M 1,3-dimethylimidazolium iodide, 60 mM I2, 0.5 M t-bupy, 0.05 M NaI, 0.1 M GuNCS in CH3CN / valeronitrile 85:15).
Figure 30.
Robson et al. [136] series of bis-tridentated ruthenium complexes bearing triphenyl amino groups.
3.2.7. Dipyrazinyl-Pyridine
Another series of bis-tridentate complexes was reported in 2007 by Al-mutlaq et al. [137] using dipyrazinyl-pyridine ligands with different substituents on 4’- position, and cathecol moieties as grafting groups (41, Figure 31). In comparison to homolog complexes with terpyridine, dipyrazinyl-pyridine led to higher oxidation potential. Exchanging SCN improved HOMO and LUMO while substituting tpy with dipyrazinyl-pyridine lowered these values.
Figure 31.
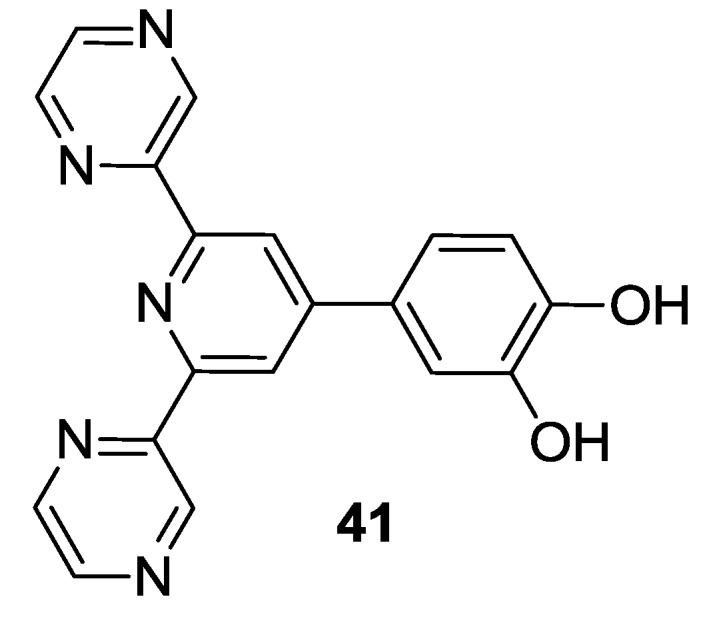
Example of dipyrazinyl-pyridine ligand [135].
Sepehrifard et al. [138,139] investigated a series of homoleptic bis-tridentate ruthenium complexes, employing both tpy and dipyrazinyl-pyridine ligands. The poorer performances of the latter ones were attributed to lower LUMO levels and weaker bonding to TiO2. The best results were obtained with terpyridine ligands bearing COOH grafting groups (1.53% efficiency) while the use of dipyrazinyl-pyridine ligands, ester groups or the introduction of a phenylene spacer between the pyridine and the anchoring group all resulted in lower efficiencies.
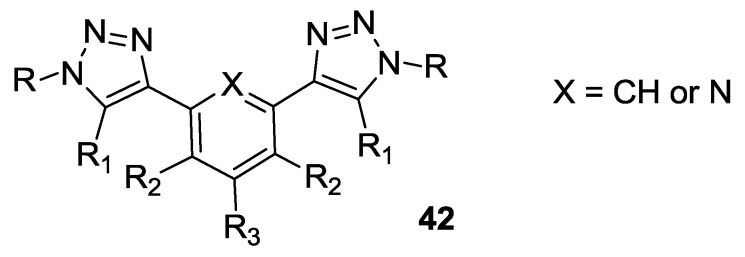
3.2.8. Triazolate
Schulze et al. investigated triazolate as chelating moiety in a series of N^C^N’ cyclometalated ligands [140] and N^N’^N’’ ligands [141]. 1,3-Di(4-triazolyl)benzene and 2,5-di(4-triazolyl)pyridine were used in association with tctpy as the grafting moiety (42, Figure 32). In the case of the N^C^N’ ligand, the substitution with electron-withdrawing groups such as F or NO2 stabilizes the HOMO energy level providing blueshift and loss in charge injection, while hydrophobic alkyl chains are expected to be beneficial for the long-term stability. The relatively low efficiency obtained as the best result (η = 4.9%; TiO2: 12 + 3 μm, dye: 0.25 mM methanol, electrolyte: 0.6 M 1,3-dimethylimidazolium iodide, 0.06 M I2, 0.1 M LiI, 0.5 M t-bupy, 0.1 M GuSCN in CH3CN) in the case of the N^N’^N’’ ligand with respect to N749 (6.1% in the same conditions, dipping solution in ethanol) was explained by loss in panchromatic absorption.
Figure 32.
3.2.9. Other Ligands
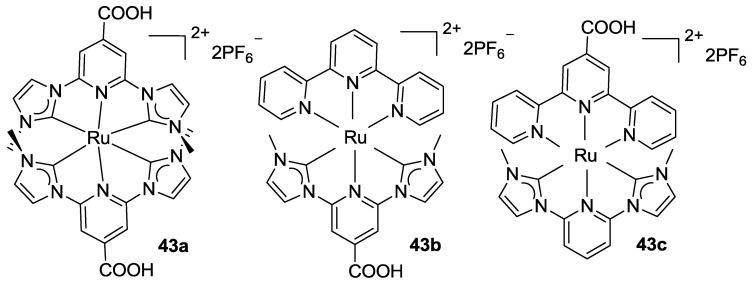
C^N^C’ ligands have been tested by Park et al. [142] in a series of bis-tridentate ruthenium complexes, exploiting N-heterocyclic carbenes such as 2,6-bis-(3-methylimidazolium-1-yl)pyridine (43a-c, Figure 33).
Figure 33.
Ru(II) complexes proposed by Park et al. [142].
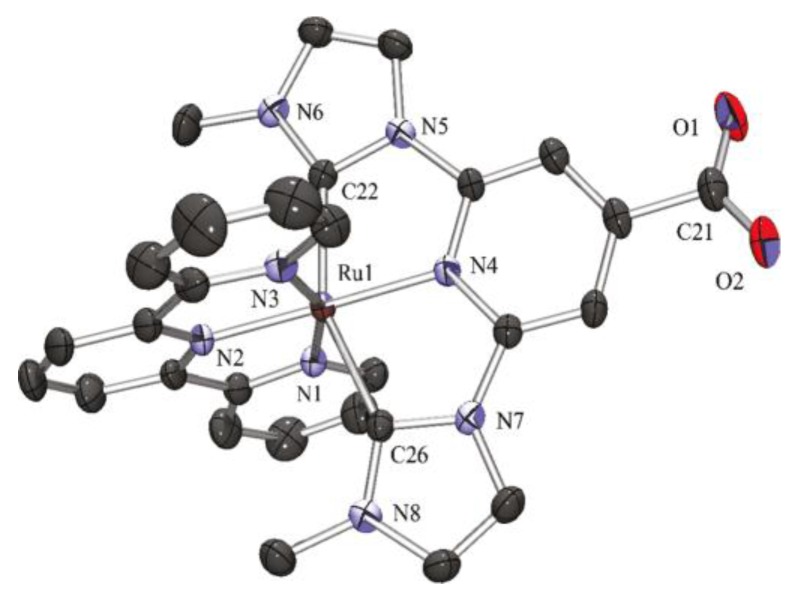
X-ray crystal structure of 43b shows a typical geometry with both ligands coordinated in a meridional fashion; bond distances between Ru and the coordinated N or C are similar and the carboxyl function is deprotonated (Figure 34). Overall efficiencies were far from N719 tested in the same conditions, a result that was mainly attributed to low charge injection.
Figure 34.
ORTEP drawing of complex 43b [142] (Reprinted with permission from Park, H.-J.; Kim, K. H.; Choi, S. Y.; Kim, H.-M.; Lee, W. I.; Kang, Y. K.; Chung, Y. K. Unsymmetric Ru(II) Complexes with N-Heterocyclic Carbene and/or Terpyridine Ligands: Synthesis, Characterization, Ground- and Excited-State Electronic Structures and Their Application for DSSC Sensitizers. Inorg. Chem. 2010, 49, 7340–7352. Copyright 2010 American Chemical Society).
Bonacin et al. [143] proposed a complex of Ru(II) with carboxyphenyl tpy, thiocyanate, and 8-hydroxy quinoline in order to host a carboxymethyl cyclodextrin anchored to TiO2. Even if poor results were reported (ascribed to high HOMO potential and low regeneration), the host-guest interaction of the dye with the cyclodextrin increased the performances by preventing dye aggregation and limiting the dark current.
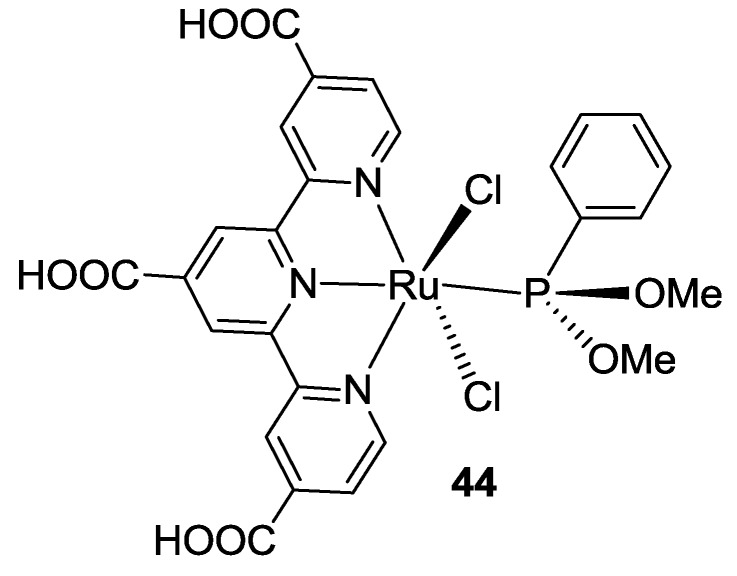
Kinoshita et al. [144,145] spent efforts in order to further extend the absorption of BD. In conventional Ru(II) complexes, short-lived 1MLCT states immediately relax to long-lived 3MLCT states through intersystem crossing. The spin-forbidden singlet-to-triplet transition from HOMO to 3MLCT has been observed for a phosphine-coordinated Ru(II) sensitizer (44 in Figure 35), providing light conversion up to 1000 nm and unprecedented charge injection (26.8 mA/cm-2). Unfortunately no evidence about long-term stability of this complex was reported.
Figure 35.
Phosphine-coordinated Ru(II) sensitizer by Kinoshita et al. [144].
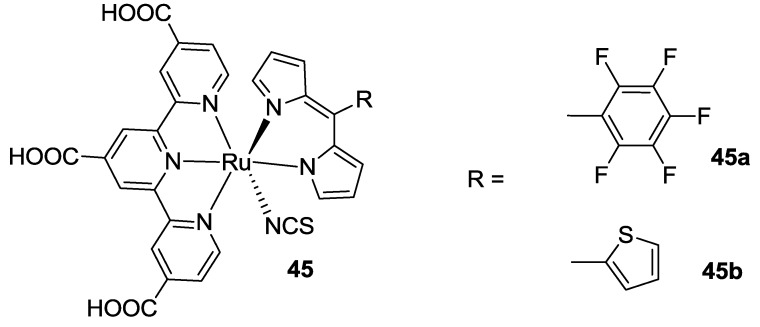
Recently, Li used 2,2’-dipyrromethanes as N^N’ bidentate ligand in order to substitute thiocyanates in the BD structure. The dipyrromethanes having 5-pentafluorophenyl and 2-thienyl substituents gave IPCE curves showing a sensitization up to 950 nm (45, Figure 36) [146].
Figure 36.
2,2’-Dipyrromethane by Li et al. [146].
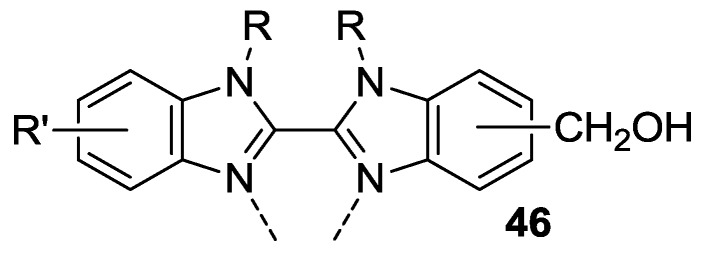
A bidentate benzimidazole was tested by Swetha et al. [147] as ancillary ligand in a Ru complex with tctpy, showing blueshifted absorption and a higher molecular extinction coefficient in the high energy region of the solar spectrum with respect to N749, which accounted for a better IPCE in the 400–640 nm range and a 6.07% efficiency (46, Figure 37; dye: 0.3 mM CH3CN / n-butanol 1:1 with 20 mM DCA, electrolyte: 0.5 M DMPII, 0.05 M I2, 0.1 M LiI CH3CN / butanol 1:1).
Figure 37.
Benzimidazole ligand tested by Swetha et al. [147].
3.3. Exchange of Metal Center
Terpyridine complexes with other metals were reported by Bignozzi’s group, who complexed osmium with tctpy, various bipyridines and pyridylquinoline [148,149,150]. The idea was to further broaden absorption spectra thanks to Os(II) complexes characterized by high spin-orbit coupling constant that allows the direct population of low energy, spin-forbidden, 3MLCT states. No significant differences in IPCE values were found in the case of the various Os complexes showing values up to 50% at 900 nm and 70% in the visible region. A better stability was ascribed to Os complexes with respect to the Ru case, even though these complexes showed lower light conversion.
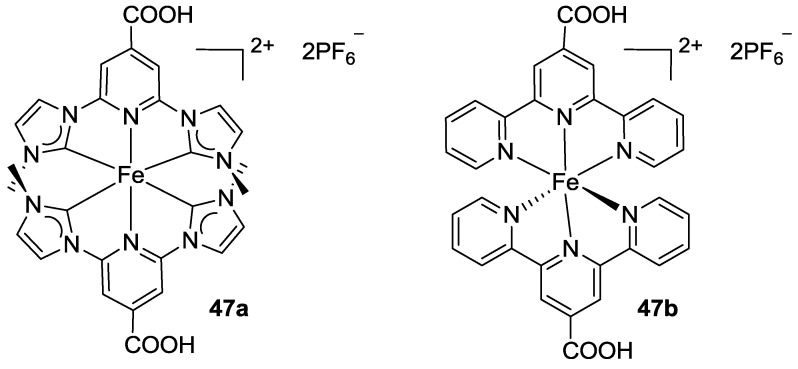
Lapides and co-workers, in 2013 [151], tested another element of the eighth group, iron, using terpyridines as ligands in a supramolecular structure with Ru, as a multicomponent film deposed on TiO2. An improved stability of the ruthenium dye was reported, even if these structures have not been tested on DSCs devices. More recently, Duchanois [152] reported a homoleptic iron complex bearing tridentate bis-carbene (C^N^C’) ligands for sensitization of TiO2 photoanodes (homologous to the Ru complex 43a), and compared it with a bis-tpy iron complex (47a,b, Figure 38). A considerable stabilization of 3MLCT state was obtained for the cyclometalated complex, but still low performances were recorded with respect to the reference sensitizers.
Figure 38.
Iron complexes reported by Duchanois [152].
Since platinum(II) complexes usually display an intense charge-transfer absorption band in the visible region, Kwok et al. [153], in 2010, proposed a complex of platinum with tctpy and various alkynyl ancillary ligands, reaching up to 3.6% efficiency.
Shinpuku et al. [154] synthesized a series of new complexes of iridium with tpy and biphenylpyridine. Cyclometalated iridium complexes were commonly exploited in light source devices as OLEDs and showed narrower absorption spectra respect to Ruthenium ones due to more energetic MLCT transition. A shorter portion of the solar spectrum was harnessed and a lower Jsc was detected, nevertheless a 2.16% efficiency and long lived excited-state lifetime were reported.
The interest for d10 metal ions complexes such as Zn-porphyrines has grown in photonic applications, ranging from OLEDs and LECs to DSCs technologies. Bozic-Weber et al. [155,156,157] synthesized bis-tpy Zn heteroleptic complexes for TiO2 sensitization. Terpyridines substituted with various anchoring and triphenylamino moieties extended with benzothiadiazole-diphenylamino units gave efficiencies between 0.5% and 1%. Housecroft et al. reviewed sensitizers made of Earth-abundant metals, concerning copper [158] and other d-block metals [159].
4. p-Type
Tpy complexes have been investigated also for the sensitization of p-type semiconductors. In p-type DSCs, the rules for sensitizers design are inverted with respect to classical n-type DSC cells. In fact, in these devices, the excited dye has to inject holes from HOMO to the conduction band of a p-semiconductor [160].
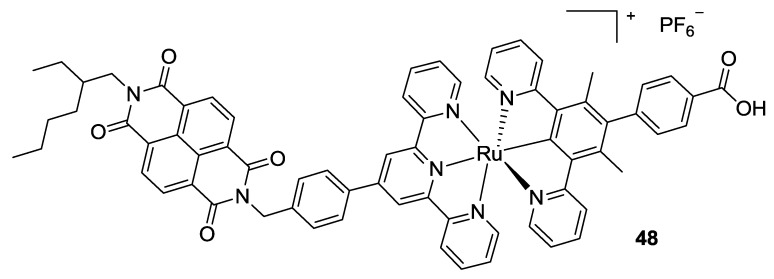
Ji et al., in 2013 [161], proposed a cyclometalated (N^C^N’)-(N^N’^N’’) Ru[II] chromophore to sensitize NiO (48, Figure 39). The N^C^N’ ligand was employed as anchoring moiety, while the tpy ligand was functionalized in the 4’ position with a substituted naphthalenediimide (NDI) in order to withdraw electrons from the NiO surface. This dye was studied by femtosecond transient absorption spectroscopy, and results showed a slower charge recombination in the NDI-substituted complex. Perylene imides have been recently used by Sariola-Leikas et al. [162] as bridge groups to obtain supramolecular structures for TiO2 sensitization in solid state devices.
Figure 39.
NDI-Tpy proposed by Ji et al. [161].
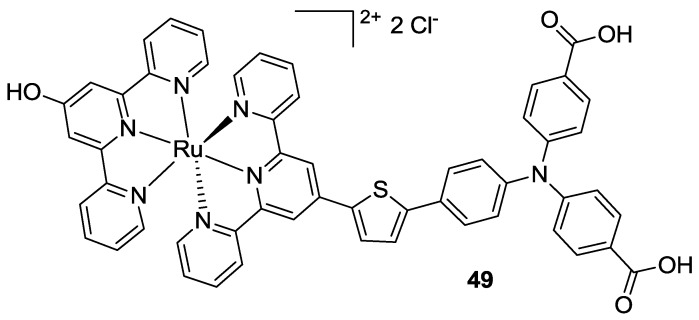
In 2014 both Constable [163] and Wood [164] proposed heteroleptic tpy complexes for sensitization of p-type semiconductors. The latter used a triphenyl amino moiety as anchoring donor group to increase the hole injection achieving efficiencies in pDSCs between 0.07 and 0.09 (49, Figure 40). Both bis-tpy and phenylbipy-tpy complexes were investigated showing better performances with iodine electrolyte with respect to the Co-based one, which was ascribed to high charge recombination with NiO.
Figure 40.
“K1” structure proposed by Wood et al. [164].
5. Co-Sensitization
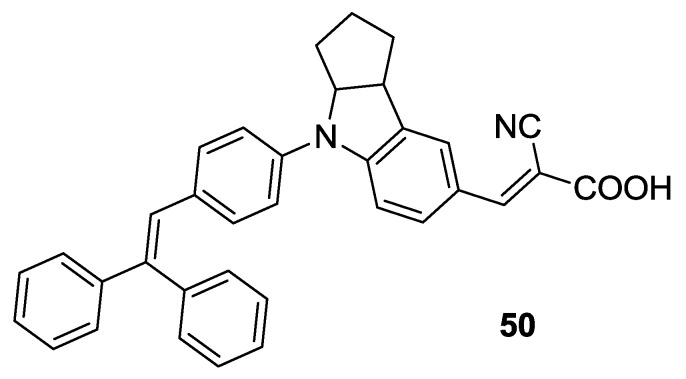
The Black Dye has also been used in cocktail with other sensitizers characterized by higher molar extinction coefficient in the high energy regions of the spectrum, in order to increase the IPCE at lower wavelengths. Ogura et al. [165] used BD in combination with the push-pull indoline dye D131 (50, Figure 41), reaching a conversion efficiency of 11.0% (working electrode was made with different layers of TiO2 mixtures with increasing amounts of polystyrene; 0.19 mM D131 and 0.56 mM BD in CH3CN / t-butanol 1:1, electrolyte: 0.15 M NaI, 0.075 M I2, 1.4 M DMPII, CH3CN / methoxyacetonitrile 9:1). Ozawa et al. [166] optimized this system using 20 mM chenodeoxycholic acid achieving a 11.6% efficiency with a TiO2 film with 45 μm thickness (0.14 mM D131 and 0.2 mM BD in 1-propanol, electrolyte: 0.05 M I2, 0.1 M LiI, 0.6 M DMPII, 0.3 M t-bupy in CH3CN).
Figure 41.
Sharma [167] proposed the cosensitization of a modified BD complex with a Zn porphyrin, with a recorded efficiency of 8.15%. Bahreman [168] synthesized a Ru complex in which a tpy was covalently bound to rhodamine B through an ethanolamine spacer, thus pursuing an energy transfer by “reverse“ FRET.
6. Summary and Outlook
The literature offers multiple choices in order to tune the photoelectrochemical properties of terpyridine-based complexes such as the Black Dye, ranging from the modification of the donor and acceptor ligands to the exchange of the metal center with other cations. The increase of the molar extinction coefficient has been commonly pursued by extending the π-conjugation on the ligands. Different anchoring moieties were compared, among which COOH turned out as one of the most effective groups. Isothiocyanate was often substituted by different ancillary ligands in order to improve long-term stability and the synthetic yield of complexation; bidentate and tridentate ligands that exploit coordination through N or C atoms have been tested in order to achieve a better sensitization. Tetradentate ligands have been used in order to further enlarge the spectral absorption properties.
Few outlines can be depicted in this scenario for the design of future complexes: (1) better stability can be achieved avoiding the use of monodentate SCN ancillary ligands; (2) better performances are offered in the case of heteroleptic complexes (the homoleptic ones have an unfavourable symmetric charge distribution); (3) hydrophobic substitutions on the ligands are able to reduce the electron recombination; (4) a better coupling between the complex and semiconductor can be achieved when COOH moieties are used as attaching groups. Overall, a wise approach is requested in order to tune the energy levels far enough to reach panchromatic absorption, but not too much in order not to exceed the limit for a good regeneration rate by the electrolyte and a good electron injection driving force. Furthermore, the use of tpy complexes nowadays goes beyond the traditional role as sensitizers. Cobalt complexes have been reported as redox mediators, by exploiting the interaction of the EDOT-substituted complex with a PEDOT-covered counter-electrode (PEDOT: poly(3,4-ethylenedioxythiophene) [169]. By finely tuning the single DSC components and their interaction, a further increase of DSC performances will be possible.
Acknowledgments
The authors gratefully acknowledge financial support of the DSSCX project (PRIN 2010-2011, 20104XET32) from MIUR and Università di Torino (Ricerca Locale ex-60%, Bando 2014).
Abbreviations
The following abbreviations are used in this manuscript:
- BD or N479
Black dye
- [bmim][I]
1-butyl-3-methyl imidazolium iodide
- CDCA
Chenodeoxycholic acid
- DSCs
Dye sensitized Solar Cells
- DCA
Deoxycholic acid
- DMPII
1,2-dimethyl-3-propylimidazolium iodide
- EDOT
3,4-ethylenedioxythiophene
- EIS
Impedance spectroscopy
- FRET
Förster Resonance Energy Transfer
- GuNCS
guanidinium thiocyanate
- IPCE
Incident photon to current efficiency
- Jsc
Short circuit current
- LEC
Light-emitting Electrochemical Cell
- MLCT
Metal to ligand charge transfer
- NDI
Naphthalenediimide
- OCVD
Open Circuit Voltage Decay
- OLED
Organic Light Emitting Diode
- PEDOT
Poly(3,4-ethylenedioxythiophene)
- qtpy
2,2':6',2":6",2"'-Quaterpyridine
- SCN
Thiocyanate
- TBA
Tetrabutylammonium
- t-bupy
(t-butylpyridine)
- tctpy
4,4’,4’’-Tricarboxyl-2,2’:6’,2’’-terpyridine
- TEA
Tetraethylammonium
- tpy
2,2’:6’,2’’Terpyridine
- Voc
Open circuit voltage
Author Contributions
DS, NB and PQ conceived and drafted the review. DS and CM screened the search results and extracted data from papers. CB and GV coordinated and supervised the project. All authors analyzed and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.O'Regan B., Grätzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353:737–740. doi: 10.1038/353737a0. [DOI] [Google Scholar]
- 2.Hagfeldt A., Boschloo G., Sun L., Kloo L., Pettersson H., Kalyanasundaram K. Dye-Sensitized Solar Cells. Chem. Rev. 2010;110:6595–6663. doi: 10.1021/cr900356p. [DOI] [PubMed] [Google Scholar]
- 3.Park J., Viscardi G., Barolo C., Barbero N. Near-infrared sensitization in dye-sensitized solar cells. Chimia. 2013;67:129–135. doi: 10.2533/chimia.2013.129. [DOI] [PubMed] [Google Scholar]
- 4.Barbero N., Sauvage F. Low Cost Electricity Production From Sunlight: Third-Generation Photovoltaics and the Dye-Sensitized Solar Cell. In: Moya X., Munoz-Rojas D., editors. Materials for Sustainable Energy Applications: Conversion, Storage, Transmission and Consumption. CRC Press; Boca Raton, FL, USA: 2016. pp. 87–147. [Google Scholar]
- 5.Higashino T., Imahori H. Porphyrins as excellent dyes for dye-sensitized solar cells: recent developments and insights. Dalton Trans. 2015;44:448–463. doi: 10.1039/C4DT02756F. [DOI] [PubMed] [Google Scholar]
- 6.Mishra A., Fischer M.K.R., Bäuerle P. Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. Engl. 2009;48:2474–2499. doi: 10.1002/anie.200804709. [DOI] [PubMed] [Google Scholar]
- 7.Abbotto A., Barolo C., Bellotto L., De Angelis F., Grätzel M., Manfredi N., Marinzi C., Fantacci S., Yum J.-H., Nazeeruddin M.K. Electron-rich heteroaromatic conjugated bipyridine based ruthenium sensitizer for efficient dye-sensitized solar cells. Chem. Commun. 2008;42:5318–5320. doi: 10.1039/b811378e. [DOI] [PubMed] [Google Scholar]
- 8.Saccone D., Galliano S., Barbero N., Viscardi G., Barolo C. Polymethine dyes in hybrid photovoltaics: structure-properties relationships. Eur. J. Org. Chem. 2015 doi: 10.1002/ejoc.201501598. in press. [DOI] [Google Scholar]
- 9.Park J., Barolo C., Sauvage F., Barbero N., Benzi C., Quagliotto P., Coluccia S., Di Censo D., Grätzel M., Nazeeruddin M.K., et al. Symmetric vs. asymmetric squaraines as photosensitisers in mesoscopic injection solar cells: A structure–property relationship study. Chem. Commun. 2012;48:2782–2784. doi: 10.1039/c2cc17187b. [DOI] [PubMed] [Google Scholar]
- 10.Park J., Barbero N., Yoon J., Dell’Orto E., Galliano S., Borrelli R., Yum J.-H., Di Censo D., Grätzel M., Nazeeruddin M.K., et al. Panchromatic symmetrical squaraines: a step forward in the molecular engineering of low cost blue-greenish sensitizers for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2014;16:24173–24177. doi: 10.1039/C4CP04345F. [DOI] [PubMed] [Google Scholar]
- 11.Magistris C., Martiniani S., Barbero N., Park J., Benzi C., Anderson A., Law C., Barolo C., O’Regan B. Near-infrared absorbing squaraine dye with extended π conjugation for dye-sensitized solar cells. Renew. Energy. 2013;60:672–678. doi: 10.1016/j.renene.2013.06.018. [DOI] [Google Scholar]
- 12.Ono T., Yamaguchi T., Arakawa H. Study on dye-sensitized solar cell using novel infrared dye. Sol. Energy Mater. Sol. Cells. 2009;93:831–835. doi: 10.1016/j.solmat.2008.09.038. [DOI] [Google Scholar]
- 13.Pydzińska K., Ziółek M. Solar cells sensitized with near-infrared absorbing dye: Problems with sunlight conversion efficiency revealed in ultrafast laser spectroscopy studies. Dyes Pigm. 2015;122:272–279. doi: 10.1016/j.dyepig.2015.07.003. [DOI] [Google Scholar]
- 14.Nazeeruddin M.K., Pechy P., Grätzel M. Efficient panchromatic sensitization of nanocrystalline TiO2 films by a black dye based on a trithiocyanato-ruthenium complex. Chem. Commun. 1997;18:1705–1706. doi: 10.1039/A703277C. [DOI] [Google Scholar]
- 15.Scifinder, 2016. Chemical Abstracts Service; Columbus, OH, USA: 2016. [(accessed on 15 January 2016)]. Available online: http://www.cas.org/products/scifinder. [Google Scholar]
- 16.Islam A., Sugihara H., Arakawa H. Molecular design of ruthenium(II) polypyridyl photosensitizers for efficient nanocrystalline TiO2 solar cells. J. Photochem. Photobiol. A. 2003;158:131–138. doi: 10.1016/S1010-6030(03)00027-3. [DOI] [Google Scholar]
- 17.Vougioukalakis G.C., Philippopoulos A.I., Stergiopoulos T., Falaras P. Contributions to the development of ruthenium-based sensitizers for dye-sensitized solar cells. Coord. Chem. Rev. 2011;255:2602–2621. doi: 10.1016/j.ccr.2010.11.006. [DOI] [Google Scholar]
- 18.Adeloye A.O., Ajibade P.A. Towards the development of functionalized polypyridine ligands for Ru(II) complexes as photosensitizers in dye-sensitized solar cells (DSSCs) Molecules. 2014;19:12421–12460. doi: 10.3390/molecules190812421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bignozzi C.A., Argazzi R., Boaretto R., Busatto E., Carli S., Ronconi F., Caramori S. The role of transition metal complexes in dye sensitized solar devices. Coord. Chem. Rev. 2013;257:1472–1492. doi: 10.1016/j.ccr.2012.09.008. [DOI] [Google Scholar]
- 20.Fantacci S., Lobello M.G., De Angelis F. Everything you always wanted to know about black dye (but were afraid to ask): A DFT/TDDFT investigation. Chimia. 2013;67:121–128. doi: 10.2533/chimia.2013.121. [DOI] [PubMed] [Google Scholar]
- 21.Nazeeruddin M.K., Péchy P., Renouard T., Zakeeruddin S.M., Humphry-Baker R., Comte P., Liska P., Cevey L., Costa E., Shklover V., et al. Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J. Am. Chem. Soc. 2001;123:1613–1624. doi: 10.1021/ja003299u. [DOI] [PubMed] [Google Scholar]
- 22.Wang S.-W., Chou C.-C., Hu F.-C., Wu K.-L., Chi Y., Clifford J.N., Palomares E., Liu S.-H., Chou P.-T., Wei T.-C., et al. Panchromatic Ru(II) sensitizers bearing single thiocyanate for high efficiency dye sensitized solar cells. J. Mater. Chem. A. 2014;2:17618–17627. doi: 10.1039/C4TA04483E. [DOI] [Google Scholar]
- 23.Nazeeruddin M.K., De Angelis F., Fantacci S., Selloni A., Viscardi G., Liska P., Ito S., Takeru B., Grätzel M. Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 2005;127:16835–16847. doi: 10.1021/ja052467l. [DOI] [PubMed] [Google Scholar]
- 24.Sauvage F., Decoppet J.-D., Zhang M., Zakeeruddin S.M., Comte P., Nazeeruddin M., Wang P., Grätzel M. Effect of sensitizer adsorption temperature on the performance of dye-sensitized solar cells. J. Am. Chem. Soc. 2011;133:9304–9310. doi: 10.1021/ja110541t. [DOI] [PubMed] [Google Scholar]
- 25.Zakeeruddin S.M., Nazeeruddin M.K., Pechy P., Rotzinger F.P., Humphry-Baker R., Kalyanasundaram K., Grätzel M., Shklover V., Haibach T. Molecular engineering of photosensitizers for nanocrystalline solar cells: Synthesis and characterization of Ru dyes based on phosphonated terpyridines. Inorg. Chem. 1997;36:5937–5946. doi: 10.1021/ic970008i. [DOI] [PubMed] [Google Scholar]
- 26.Gianotti V., Favaro G., Bonandini L., Palin L., Croce G., Boccaleri E., Artuso E., van Beek W., Barolo C., Milanesio M. Rationalization of dye uptake on titania slides for dye-sensitized solar cells by a combined chemometric and structural approach. Chem. Sus. Chem. 2014;7:3039–3052. doi: 10.1002/cssc.201402194. [DOI] [PubMed] [Google Scholar]
- 27.Morgan G.T., Burstall F.H. 3. Dehydrogenation of pyridine by anhydrous ferric chloride. J. Chem. Soc. 1932:20–30. doi: 10.1039/jr9320000020. [DOI] [Google Scholar]
- 28.Heller M., Schubert U.S. Syntheses of functionalized 2,2′:6′,2′′-terpyridines. Eur. J. Org. Chem. 2003;2003:947–961. doi: 10.1002/ejoc.200390150. [DOI] [Google Scholar]
- 29.Fallahpour R.A. Synthesis of 4’-substituted-2,2':6', 2 -terpyridines. Synthesis. 2003;35:155–184. doi: 10.1055/s-2003-36811. [DOI] [Google Scholar]
- 30.Cargill Thompson A.M.W. The synthesis of 2,2′:6′,2″-terpyridine ligands — versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997;160:1–52. doi: 10.1016/S0010-8545(96)01283-0. [DOI] [Google Scholar]
- 31.Hayami S., Komatsu Y., Shimizu T., Kamihata H., Lee Y.H. Spin-crossover in cobalt(II) compounds containing terpyridine and its derivatives. Coord. Chem. Rev. 2011;255:1981–1990. doi: 10.1016/j.ccr.2011.05.016. [DOI] [Google Scholar]
- 32.Arrigo A., Santoro A., Puntoriero F., Lainé P.P., Campagna S. Photoinduced electron transfer in donor–bridge–acceptor assemblies: The case of Os(II)-bis(terpyridine)-(bi)pyridinium dyads. Coord. Chem. Rev. 2015;304-305:109–116. doi: 10.1016/j.ccr.2014.09.019. [DOI] [Google Scholar]
- 33.Sauvage J.P., Collin J.P., Chambron J.C., Guillerez S., Coudret C., Balzani V., Barigelletti F., De Cola L., Flamigni L. Ruthenium(II) and Osmium(II) Bis(terpyridine) Complexes in Covalently-Linked Multicomponent Systems: Synthesis, Electrochemical Behavior, Absorption Spectra, and Photochemical and Photophysical Properties. Chem. Rev. 1994;94:993–1019. doi: 10.1021/cr00028a006. [DOI] [Google Scholar]
- 34.Flamigni L., Collin J.P., Sauvage J.P. Iridium terpyridine complexes as functional assembling units in arrays for the conversion of light energy. Acc. Chem. Res. 2008;41:857–871. doi: 10.1021/ar700282n. [DOI] [PubMed] [Google Scholar]
- 35.Baranoff E., Collin J.P., Flamigni L., Sauvage J.P. From ruthenium(II) to iridium(III): 15 years of triads based on bis-terpyridine complexes. Chem. Soc. Rev. 2004;33:147–155. doi: 10.1039/b308983e. [DOI] [PubMed] [Google Scholar]
- 36.Eryazici I., Moorefield C.N., Newkome G.R. Square-planar Pd(II), Pt(II), and Au(III) terpyridine complexes: their syntheses, physical properties, supramolecular constructs, and biomedical activities. Chem. Rev. 2008;108:1834–1895. doi: 10.1021/cr0781059. [DOI] [PubMed] [Google Scholar]
- 37.Hofmeier H., Schubert U.S. Recent developments in the supramolecular chemistry of terpyridine-metal complexes. Chem. Soc. Rev. 2004;33:373–399. doi: 10.1039/b400653b. [DOI] [PubMed] [Google Scholar]
- 38.Schubert U.S., Hofmeier H., Newkome G.R. Modern Terpyridine Chemistry. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2006. [Google Scholar]
- 39.Gao Y., Rajwar D., Grimsdale A.C. Self-Assembly of Conjugated Units Using Metal-Terpyridine Coordination. Macromol. Rapid Commun. 2014;35:1727–1740. doi: 10.1002/marc.201400225. [DOI] [PubMed] [Google Scholar]
- 40.Constable E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007;36:246–253. doi: 10.1039/B601166G. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto R., Wu K.-H., Matsuoka R., Maeda H., Nishihara H. π-Conjugated bis(terpyridine)metal complex molecular wires. Chem. Soc. Rev. 2015;44:7698–7714. doi: 10.1039/C5CS00081E. [DOI] [PubMed] [Google Scholar]
- 42.Andres P.R., Schubert U.S. New Functional Polymers and Materials Based on 2,2′:6′,2″-Terpyridine Metal Complexes. Adv. Mater. 2004;16:1043–1068. doi: 10.1002/adma.200306518. [DOI] [Google Scholar]
- 43.Winter A., Hoeppener S., Newkome G.R., Schubert U.S. Terpyridine-functionalized surfaces: redox-active, switchable, and electroactive nanoarchitectures. Adv. Mater. 2011;23:3484–3498. doi: 10.1002/adma.201101251. [DOI] [PubMed] [Google Scholar]
- 44.Breivogel A., Kreitner C., Heinze K. Redox and Photochemistry of Bis(terpyridine)ruthenium(II) Amino Acids and Their Amide Conjugates - from Understanding to Applications. Eur. J. Inorg. Chem. 2014;2014:5468–5490. doi: 10.1002/ejic.201402466. [DOI] [Google Scholar]
- 45.Winter A., Gottschaldt M., Newkome G.R., Schubert U.S. Terpyridines and their Complexes with First Row Transition Metal Ions: Cytotoxicity, Nuclease Activity and Self-Assembly of Biomacromolecules. Curr. Top. Med. Chem. 2012;12:158–175. doi: 10.2174/156802612799078919. [DOI] [PubMed] [Google Scholar]
- 46.Winter A., Hager M.D., Newkome G.R., Schubert U.S. The marriage of terpyridines and inorganic nanoparticles: synthetic aspects, characterization techniques, and potential applications. Adv. Mater. 2011;23:5728–5748. doi: 10.1002/adma.201103612. [DOI] [PubMed] [Google Scholar]
- 47.Wild A., Winter A., Schlütter F., Schubert U.S. Advances in the field of π-conjugated 2,2’:6',2"-terpyridines. Chem. Soc. Rev. 2011;40:1459–1511. doi: 10.1039/C0CS00074D. [DOI] [PubMed] [Google Scholar]
- 48.Chelucci G., Thummel R.P. Chiral 2,2‘-Bipyridines, 1,10-Phenanthrolines, and 2,2‘:6‘,2‘‘-Terpyridines: Syntheses and Applications in Asymmetric Homogeneous Catalysis. Chem. Rev. 2002;102:3129–3170. doi: 10.1021/cr0101914. [DOI] [PubMed] [Google Scholar]
- 49.Winter A., Newkome G.R., Schubert U.S. Catalytic Applications of Terpyridines and their Transition Metal Complexes. ChemCatChem. 2011;3:1384–1406. doi: 10.1002/cctc.201100118. [DOI] [Google Scholar]
- 50.Kröhnke F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis. 1976;1:1–24. doi: 10.1055/s-1976-23941. [DOI] [Google Scholar]
- 51.Cave G.W.V., Raston C.L. Efficient synthesis of pyridines via a sequential solventless aldol condensation and Michael addition. J. Chem. Soc. Perkin Trans. 1. 2001:3258–3264. [Google Scholar]
- 52.Suzuki A. Cross-coupling reactions of organoboranes: an easy way to construct C-C bonds (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 2011;50:6722–6737. doi: 10.1002/anie.201101379. [DOI] [PubMed] [Google Scholar]
- 53.Cordovilla C., Bartolomé C., Martínez-Ilarduya J.M., Espinet P. The Stille Reaction, 38 Years Later. ACS Catal. 2015;5:3040–3053. doi: 10.1021/acscatal.5b00448. [DOI] [Google Scholar]
- 54.Nazeeruddin M.K., Pechy P., Renouard T., Zakeeruddin S.M., Humphry-Baker R., Comte P., Liska P., Cevey L., Costa E., Shklover V., Spiccia L., Deacon G.B., Bignozzi C.A., Grätzel M. Engineering of Efficient Panchromatic Sensitizers for Nanocrystalline TiO2-Based Solar Cells. J. Am. Chem. Soc. 2001;123:1613–1624. doi: 10.1021/ja003299u. [DOI] [PubMed] [Google Scholar]
- 55.Dehaudt J., Husson J., Guyard L. A more efficient synthesis of 4,4′,4′′-tricarboxy-2,2′:6′,2′′-terpyridine. Green Chem. 2011;13:3337–3340. doi: 10.1039/c1gc15808b. [DOI] [Google Scholar]
- 56.Husson J., Knorr M. 2,2′:6′,2″-Terpyridines Functionalized with Thienyl Substituents: Synthesis and Applications. J. Heterocycl. Chem. 2012;49:453–478. doi: 10.1002/jhet.813. [DOI] [Google Scholar]
- 57.Kisserwan H., Kamar A., Shoker T., Ghaddar T.H. Photophysical properties of new cyclometalated ruthenium complexes and their use in dye sensitized solar cells. Dalton Trans. 2012;41:10643–10651. doi: 10.1039/c2dt30482a. [DOI] [PubMed] [Google Scholar]
- 58.Raboin J.-C., Kirsch G., Beley M. On the way to unsymmetrical terpyridines carrying carboxylic acids. J. Heterocycl. Chem. 2000;37:1077–1080. doi: 10.1002/jhet.5570370509. [DOI] [Google Scholar]
- 59.Husson J., Beley M., Kirsch G. A novel pathway for the synthesis of a carboxylic acid-functionalised Ru(II) terpyridine complex. Tetrahedron Lett. 2003;44:1767–1770. doi: 10.1016/S0040-4039(03)00123-0. [DOI] [Google Scholar]
- 60.Husson J., Dehaudt J., Guyard L. Preparation of carboxylate derivatives of terpyridine via the furan pathway. Nat. Protoc. 2014;9:21–26. doi: 10.1038/nprot.2013.162. [DOI] [PubMed] [Google Scholar]
- 61.Hobert S.E., Carney J.T., Cummings S.D. Synthesis and luminescence properties of platinum(II) complexes of 4′-chloro-2,2′:6′,2″-terpyridine and 4,4′,4″-trichloro-2,2′:6′,2″-terpyridine. Inorganica Chim. Acta. 2001;318:89–96. doi: 10.1016/S0020-1693(01)00420-0. [DOI] [Google Scholar]
- 62.Duncan T.V., Ishizuka T., Therien M.J. Molecular engineering of intensely near-infrared absorbing excited states in highly conjugated oligo(porphinato)zinc-(polypyridyl)metal(II) supermolecules. J. Am. Chem. Soc. 2007;129:9691–9703. doi: 10.1021/ja0707512. [DOI] [PubMed] [Google Scholar]
- 63.Potts K.T., Konwar D. Synthesis of 4’-Vinyl-2,2':6',2''-terpyridine. J. Org. Chem. 1991;56:4815–4816. doi: 10.1021/jo00015a050. [DOI] [Google Scholar]
- 64.Husson J., Knorr M. Syntheses and applications of furanyl-functionalised 2,2’:6',2''-Terpyridines. Beilstein J. Org. Chem. 2012;8:379–389. doi: 10.3762/bjoc.8.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodward C.P., Coghlan C.J., Rüther T., Jones T.W., Hebting Y., Cordiner R.L., Dawson R.E., Robinson D.E.J.E., Wilson G.J. Oligopyridine ligands possessing multiple or mixed anchoring functionality for dye-sensitized solar cells. Tetrahedron. 2015;71:5238–5247. doi: 10.1016/j.tet.2015.06.029. [DOI] [Google Scholar]
- 66.Dick G.R., Woerly E.M., Burke M.D. A general solution for the 2-pyridyl problem. Angew. Chem. Int. Ed. Engl. 2012;51:2667–2672. doi: 10.1002/anie.201108608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coluccini C., Manfredi N., Salamone M.M., Ruffo R., Lobello M.G., De Angelis F., Abbotto A. Quaterpyridine ligands for panchromatic Ru(II) dye sensitizers. J. Org. Chem. 2012;77:7945–7956. doi: 10.1021/jo301226z. [DOI] [PubMed] [Google Scholar]
- 68.Barolo C., Yum J.-H., Artuso E., Barbero N., Di Censo D., Lobello M.G., Fantacci S., De Angelis F., Grätzel M., Nazeeruddin M.K., Viscardi G. A simple synthetic route to obtain pure trans-ruthenium(II) complexes for dye-sensitized solar cell applications. ChemSusChem. 2013;6:2170–2180. doi: 10.1002/cssc.201200973. [DOI] [PubMed] [Google Scholar]
- 69.Waser M., Siebenhaar C., Zampese J., Grundler G., Constable E., Height M., Pieles U. Novel grafting procedure of ruthenium 2,2’:6',2''-terpyridine complexes with phosphonate ligands to titania for water splitting applications. Chimia. 2010;64:328–329. doi: 10.2533/chimia.2010.328. [DOI] [PubMed] [Google Scholar]
- 70.Anthonysamy A., Balasubramanian S., Muthuraaman B., Maruthamuthu P. 4’-functionalized 2,2':6',2'' terpyridine ruthenium (II) complex: a nanocrystalline TiO2 based solar cell sensitizer. Nanotechnology. 2007;18:095701/1–095701/5. doi: 10.1088/0957-4484/18/9/095701. [DOI] [Google Scholar]
- 71.Wang Z.-S., Huang C.-H., Huang Y.-Y., Zhang B.-W., Xie P.-H., Hou Y.-J., Ibrahim K., Qian H.-J., Liu F.-Q. Photoelectric behavior of nanocrystalline TiO2 electrode with a novel terpyridyl ruthenium complex. Sol. Energy Mater. Sol. Cells. 2002;71:261–271. doi: 10.1016/S0927-0248(01)00085-X. [DOI] [Google Scholar]
- 72.Funaki T., Yanagida M., Onozawa-Komatsuzaki N., Kawanishi Y., Kasuga K., Sugihara H. Ruthenium (II) complexes with π expanded ligand having phenylene–ethynylene moiety as sensitizers for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells. 2009;93:729–732. doi: 10.1016/j.solmat.2008.09.011. [DOI] [Google Scholar]
- 73.McNamara W.R., Snoeberger III R.C., Li G., Richter C., Allen L.J., Milot R.L., Schmuttenmaer C.A., Crabtree R.H., Brudvig G.W., Batista V.S. Hydroxamate anchors for water-stable attachment to TiO2 nanoparticles. Energy Environ. Sci. 2009;2:1173–1175. doi: 10.1039/b910241h. [DOI] [Google Scholar]
- 74.Vougioukalakis G.C., Stergiopoulos T., Kantonis G., Kontos A.G., Papadopoulos K., Stublla A., Potvin P.G., Falaras P. Terpyridine- and 2,6-dipyrazinylpyridine-coordinated ruthenium(II) complexes: Synthesis, characterization and application in TiO2-based dye-sensitized solar cells. J. Photochem. Photobiol., A. 2010;214:22–32. doi: 10.1016/j.jphotochem.2010.06.001. [DOI] [Google Scholar]
- 75.Manríquez J., Hwang S.-H., Cho T.J., Moorefield C.N., Newkome G.R., Godínez L.A. Sensitized Solar Cells based on Hexagonal Dyes of Terpyridine-Ruthenium(II): Effect of the Electropolymerization of Dyes during their Performance in Solar Cells. ECS Trans. 2006;3:1–5. [Google Scholar]
- 76.Kanniyambatti Lourdusamy V.J., Anthonysamy A., Easwaramoorthi R., Shinde D.V., Ganapathy V., Karthikeyan S., Lee J., Park T., Rhee S.-W., Kim K.S., Kim J.K. Cyanoacetic acid tethered thiophene for well-matched LUMO level in Ru(II)-terpyridine dye sensitized solar cells. Dyes Pigm. 2016;126:270–278. [Google Scholar]
- 77.Numata Y., Singh S.P., Islam A., Iwamura M., Imai A., Nozaki K., Han L. Enhanced Light-Harvesting Capability of a Panchromatic Ru(II) Sensitizer Based on π-Extended Terpyridine with a 4-Methylstylryl Group for Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2013;23:1817–1823. doi: 10.1002/adfm.201202504. [DOI] [Google Scholar]
- 78.Yang S.-H., Wu K.-L., Chi Y., Cheng Y.-M., Chou P.-T. Tris(thiocyanate) ruthenium(II) sensitizers with functionalized dicarboxyterpyridine for dye-sensitized solar cells. Angew. Chem. Int. Ed. Engl. 2011;50:8270–8274. doi: 10.1002/anie.201103515. [DOI] [PubMed] [Google Scholar]
- 79.Kimura M., Masuo J., Tohata Y., Obuchi K., Masaki N., Murakami T.N., Koumura N., Hara K., Fukui A., Yamanaka R., Mori S. Improvement of TiO2/dye/electrolyte interface conditions by positional change of alkyl chains in modified panchromatic Ru complex dyes. Chem. - Eur. J. 2013;19:1028–1034. doi: 10.1002/chem.201202709. [DOI] [PubMed] [Google Scholar]
- 80.Dehaudt J., Husson J., Guyard L., Oswald F., Martineau D. A simple access to “Black-Dye” analogs with good efficiencies in dye-sensitized solar cells. Renew. Energy. 2014;66:588–595. doi: 10.1016/j.renene.2013.12.033. [DOI] [Google Scholar]
- 81.Koyyada G., Botla V., Thogiti S., Wu G., Li J., Fang X., Kong F., Dai S., Surukonti N., Kotamarthi B., Malapaka C. Novel 4’-functionalized 4,4''-dicarboxyterpyridine ligands for ruthenium complexes: near-IR sensitization in dye sensitized solar cells. Dalton Trans. 2014;43:14992–15003. doi: 10.1039/C4DT01598C. [DOI] [PubMed] [Google Scholar]
- 82.Ozawa H., Fukushima K., Sugiura T., Urayama A., Arakawa H. Ruthenium sensitizers having an ortho-dicarboxyl group as an anchoring unit for dye-sensitized solar cells: synthesis, photo- and electrochemical properties, and adsorption behavior to the TiO2 surface. Dalton Trans. 2014;43:13208–13218. doi: 10.1039/C4DT01450B. [DOI] [PubMed] [Google Scholar]
- 83.Ozawa H., Sugiura T., Shimizu R., Arakawa H. Novel ruthenium sensitizers having different numbers of carboxyl groups for dye-sensitized solar cells: effects of the adsorption manner at the TiO2 surface on the solar cell performance. Inorg. Chem. 2014;53:9375–9384. doi: 10.1021/ic501479p. [DOI] [PubMed] [Google Scholar]
- 84.Ozawa H., Yamamoto Y., Kawaguchi H., Shimizu R., Arakawa H. Ruthenium sensitizers with a hexylthiophene-modified terpyridine ligand for dye-sensitized solar cells: synthesis, photo- and electrochemical properties, and adsorption behavior to the TiO2 surface. ACS Appl. Mater. Interfaces. 2015;7:3152–3161. doi: 10.1021/am507442s. [DOI] [PubMed] [Google Scholar]
- 85.Ozawa H., Fukushima K., Urayama A., Arakawa H. Efficient ruthenium sensitizer with an extended π-conjugated terpyridine ligand for dye-sensitized solar cells. Inorg. Chem. 2015;54:8887–8889. doi: 10.1021/acs.inorgchem.5b01640. [DOI] [PubMed] [Google Scholar]
- 86.Ozawa H., Yamamoto Y., Fukushima K., Yamashita S., Arakawa H. Synthesis and Characterization of a Novel Ruthenium Sensitizer with a Hexylthiophene-functionalized Terpyridine Ligand for Dye-sensitized Solar Cells. Chem. Lett. 2013;42:897–899. doi: 10.1246/cl.130217. [DOI] [Google Scholar]
- 87.Ozawa H., Kuroda T., Harada S., Arakawa H. Efficient Ruthenium Sensitizer with a Terpyridine Ligand Having a Hexylthiophene Unit for Dye-Sensitized Solar Cells: Effects of the Substituent Position on the Solar Cell Performance. Eur. J. Inorg. Chem. 2014;2014:4734–4739. doi: 10.1002/ejic.201402530. [DOI] [Google Scholar]
- 88.Nazeeruddin M.K., Zakeeruddin S.M., Humphry-Baker R., Gorelsky S.I., Lever A.B.P., Grätzel M. Synthesis, spectroscopic and a ZINDO study of cis- and trans-(X2)bis(4,4′-dicarboxylic acid-2,2′-bipyridine)ruthenium(II) complexes (X=Cl−, H2O, NCS−) Coord. Chem. Rev. 2000;208:213–225. doi: 10.1016/S0010-8545(00)00338-6. [DOI] [Google Scholar]
- 89.Renouard T., Grätzel M. Functionalized tetradentate ligands for Ru-sensitized solar cells. Tetrahedron. 2001;57:8145–8150. doi: 10.1016/S0040-4020(01)00801-8. [DOI] [Google Scholar]
- 90.Renouard T., Fallahpour R.-A., Nazeeruddin M.K., Humphry-Baker R., Gorelsky S.I., Lever A.B.P., Grätzel M. Novel Ruthenium Sensitizers Containing Functionalized Hybrid Tetradentate Ligands: Synthesis, Characterization, and INDO/S Analysis. Inorg. Chem. 2002;41:367–378. doi: 10.1021/ic010512u. [DOI] [PubMed] [Google Scholar]
- 91.Barolo C., Nazeeruddin M.K., Fantacci S., Di Censo D., Comte P., Liska P., Viscardi G., Quagliotto P., De Angelis F., Ito S., Grätzel M. Synthesis, characterization, and DFT-TDDFT computational study of a ruthenium complex containing a functionalized tetradentate ligand. Inorg. Chem. 2006;45:4642–4653. doi: 10.1021/ic051970w. [DOI] [PubMed] [Google Scholar]
- 92.Abbotto A., Sauvage F., Barolo C., De Angelis F., Fantacci S., Grätzel M., Manfredi N., Marinzi C., Nazeeruddin M.K. Panchromatic ruthenium sensitizer based on electron-rich heteroarylvinylene π-conjugated quaterpyridine for dye-sensitized solar cells. Dalton Trans. 2011;40:234–242. doi: 10.1039/C0DT01190H. [DOI] [PubMed] [Google Scholar]
- 93.Kalyanasundaram K., editor. Dye-sensitized Solar Cells. CRC Press; Boca Raton, Florida, US: 2010. [Google Scholar]
- 94.Nazeeruddin M.K., Grätzel M. Separation of linkage isomers of trithiocyanato (4,4′,4″-tricarboxy-2,2′,6,2″-terpyridine)ruthenium(II) by pH-titration method and their application in nanocrystalline TiO2-based solar cells. J. Photochem. Photobiol., A. 2001;145:79–86. doi: 10.1016/S1010-6030(01)00572-X. [DOI] [Google Scholar]
- 95.Pal A.K., Hanan G.S. Design, synthesis and excited-state properties of mononuclear Ru(II) complexes of tridentate heterocyclic ligands. Chem. Soc. Rev. 2014;43:6184–6197. doi: 10.1039/C4CS00123K. [DOI] [PubMed] [Google Scholar]
- 96.Medlycott E.A., Hanan G.S. Designing tridentate ligands for ruthenium(II) complexes with prolonged room temperature luminescence lifetimes. Chem. Soc. Rev. 2005;34:133–142. doi: 10.1039/b316486c. [DOI] [PubMed] [Google Scholar]
- 97.Hammarström L., Johansson O. Expanded bite angles in tridentate ligands. Improving the photophysical properties in bistridentate Ru (II) polypyridine complexes. Coord. Chem. Rev. 2010;254:2546–2559. doi: 10.1016/j.ccr.2010.01.006. [DOI] [Google Scholar]
- 98.Chandrasekharam M., Rajkumar G., Rao C.S., Suresh T., Soujanya Y., Reddy P.Y. High molar extinction coefficient Ru(II)-mixed ligand polypyridyl complexes for dye sensitized solar cell application. Adv. Optoelectron. 2011;2011:1–12. doi: 10.1155/2011/432803. [DOI] [Google Scholar]
- 99.Giribabu L., Singh V.K., Srinivasu M., Kumar C.V., Reddy V.G., Soujnya Y., Reddy P.Y. Synthesis and photoelectrochemical characterization of a high molar extinction coefficient heteroleptic ruthenium(II) complex. J. Chem. Sci. 2011;123:371–378. doi: 10.1007/s12039-011-0096-1. [DOI] [Google Scholar]
- 100.Koyyada G., Pavan Kumar CH., Salvatori P., Marotta G., Lobello M.G., Bizzarri O., De Angelis F., Malapaka C. New terpyridine-based ruthenium complexes for dye sensitized solar cells applications. Inorganica Chim. Acta. 2016;442:158–166. doi: 10.1016/j.ica.2015.11.031. [DOI] [Google Scholar]
- 101.Pavan Kumar C.H., Anusha V., Narayanaswamy K., Bhanuprakash K., Islam A., Han L., Singh S.P., Chandrasekharam M. New ruthenium complexes (Ru[3+2+1]) bearing π-extended 4-methylstyryl terpyridine and unsymmetrical bipyridine ligands for DSSC applications. Inorganica Chim. Acta. 2015;435:46–52. [Google Scholar]
- 102.Giribabu L., Bessho T., Srinivasu M., Vijaykumar C., Soujanya Y., Reddy V.G., Reddy P.Y., Yum J.-H., Grätzel M., Nazeeruddin M.K. A new family of heteroleptic ruthenium(II) polypyridyl complexes for sensitization of nanocrystalline TiO2 films. Dalton Trans. 2011;40:4497–4504. doi: 10.1039/c0dt01417f. [DOI] [PubMed] [Google Scholar]
- 103.Mosurkal R., Kim Y., Kumar J., Li L., Walker J., Samuelson L.A. Mono- and Dinuclear Ruthenium Complexes for Nanocrystalline TiO2 Based Dye-Sensitized Photovoltaics. J. Macromol. Sci. Part A. 2003;40:1317–1325. doi: 10.1081/MA-120025311. [DOI] [Google Scholar]
- 104.Erten-Ela S., Sogut S., Ocakoglu K. Synthesis of novel ruthenium II phenanthroline complex and its application to TiO2 and ZnO nanoparticles on the electrode of dye sensitized solar cells. Mater. Sci. Semicond. Process. 2014;23:159–166. doi: 10.1016/j.mssp.2014.02.034. [DOI] [Google Scholar]
- 105.Mongal B.N., Pal A., Mandal T.K., Datta J., Naskar S. Synthesis, characterisation, electrochemical study and photovoltaic measurements of a new terpyridine and pyridine-quinoline based mixed chelate ruthenium dye. Polyhedron. 2015;102:615–626. doi: 10.1016/j.poly.2015.10.040. [DOI] [Google Scholar]
- 106.Stergiopoulos T., Arabatzis I.M., Kalbac M., Lukes I., Falaras P. Incorporation of innovative compounds in nanostructured photoelectrochemical cells. J. Mater. Process. Technol. 2005;161:107–112. doi: 10.1016/j.jmatprotec.2004.07.014. [DOI] [Google Scholar]
- 107.Houarner C., Blart E., Buvat P., Odobel F. Ruthenium bis-terpyridine complexes connected to an oligothiophene unit for dry dye-sensitised solar cells. Photochem. Photobiol. Sci. 2005;4:200–204. doi: 10.1039/b414031a. [DOI] [PubMed] [Google Scholar]
- 108.Houarner-Rassin C., Blart E., Buvat P., Odobel F. Improved efficiency of a thiophene linked ruthenium polypyridine complex for dry dye-sensitized solar cells. J. Photochem. Photobiol., A. 2007;186:135–142. doi: 10.1016/j.jphotochem.2006.07.022. [DOI] [Google Scholar]
- 109.Houarner-Rassin C., Chaignon F., She C., Stockwell D., Blart E., Buvat P., Lian T., Odobel F. Synthesis and photoelectrochemical properties of ruthenium bisterpyridine sensitizers functionalized with a thienyl phosphonic acid moiety. J. Photochem. Photobiol., A. 2007;192:56–65. doi: 10.1016/j.jphotochem.2007.05.004. [DOI] [Google Scholar]
- 110.Duprez V., Biancardo M., Krebs F.C. Characterisation and application of new carboxylic acid-functionalised ruthenium complexes as dye-sensitisers for solar cells. Sol. Energy Mater. Sol. Cells. 2007;91:230–237. doi: 10.1016/j.solmat.2006.08.007. [DOI] [Google Scholar]
- 111.Duprez V., Krebs F.C. New carboxy-functionalized terpyridines as precursors for zwitterionic ruthenium complexes for polymer-based solar cells. Tetrahedron Lett. 2006;47:3785–3789. doi: 10.1016/j.tetlet.2006.03.098. [DOI] [Google Scholar]
- 112.Duprez V., Biancardo M., Spanggaard H., Krebs F.C. Synthesis of Conjugated Polymers Containing Terpyridine−Ruthenium Complexes: Photovoltaic Applications. Macromolecules. 2005;38:10436–10448. doi: 10.1021/ma051274f. [DOI] [Google Scholar]
- 113.Krebs F.C., Biancardo M. Dye sensitized photovoltaic cells: Attaching conjugated polymers to zwitterionic ruthenium dyes. Sol. Energy Mater. Sol. Cells. 2006;90:142–165. doi: 10.1016/j.solmat.2005.02.006. [DOI] [Google Scholar]
- 114.Chan H.T., Mak C.S.K., Djurišić A.B., Chan W.K. Synthesis of Ruthenium Complex Containing Conjugated Polymers and Their Applications in Dye-Sensitized Solar Cells. Macromol. Chem. Phys. 2011;212:774–784. doi: 10.1002/macp.201000589. [DOI] [Google Scholar]
- 115.Caramori S., Husson J., Beley M., Bignozzi C. A., Argazzi R., Gros P.C. Combination of cobalt and iron polypyridine complexes for improving the charge separation and collection in Ru(terpyridine)(2)-sensitised solar cells. Chem. - Eur. J. 2010;16:2611–2618. doi: 10.1002/chem.200902761. [DOI] [PubMed] [Google Scholar]
- 116.Funaki T., Funakoshi H., Kitao O., Onozawa-Komatsuzaki N., Kasuga K., Sayama K., Sugihara H. Cyclometalated ruthenium(II) complexes as near-IR sensitizers for high efficiency dye-sensitized solar cells. Angew. Chem. Int. Ed. Engl. 2012;51:7528–7531. doi: 10.1002/anie.201108738. [DOI] [PubMed] [Google Scholar]
- 117.Kusama H., Funaki T., Sayama K. Theoretical study of cyclometalated Ru(II) dyes: Implications on the open-circuit voltage of dye-sensitized solar cells. J. Photochem. Photobiol., A. 2013;272:80–89. doi: 10.1016/j.jphotochem.2013.09.001. [DOI] [Google Scholar]
- 118.Funaki T., Yanagida M., Onozawa-Komatsuzaki N., Kasuga K., Kawanishi Y., Kurashige M., Sayama K., Sugihara H. Synthesis of a new class of cyclometallated ruthenium(II) complexes and their application in dye-sensitized solar cells. Inorg. Chem. Commun. 2009;12:842–845. doi: 10.1016/j.inoche.2009.06.030. [DOI] [Google Scholar]
- 119.Islam A., Singh S.P., Han L. Synthesis and application of new ruthenium complexes containing β-diketonato ligands as sensitizers for nanocrystalline TiO2 solar cells. Int. J. Photoenergy. 2011;2011:204639. doi: 10.1155/2011/204639. [DOI] [Google Scholar]
- 120.Han L., Islam A. High efficient dye-sensitized solar cells. MRS Online Proc. Libr. 2011;1327 doi: 10.1557/opl.2011.1121. Symposium G - Complex Oxide Materials for Emerging Energy Technologies. [DOI] [Google Scholar]
- 121.Islam A., Singh S.P., Han L. Thiocyanate-free, panchromatic ruthenium (II) terpyridine sensitizer having a tridentate diethylenetriamine ligand for Near-IR sensitization of nanocrystaline TiO2. Funct. Mater. Lett. 2011;04:21–24. doi: 10.1142/S1793604711001555. [DOI] [Google Scholar]
- 122.Islam A., Singh S.P., Yanagida M., Karim M.R., Han L. Amphiphilic ruthenium(II) terpyridine sensitizers with long alkyl chain substituted beta-diketonato ligands: An efficient coadsorbent-free dye-sensitized solar cells. Int. J. Photoenergy. 2011. 757421. [DOI]
- 123.Islam A., Chowdhury F.A., Chiba Y., Komiya R., Fuke N., Ikeda N., Nozaki K., Han L. Synthesis and Characterization of New Efficient Tricarboxyterpyridyl (β-diketonato) Ruthenium(II) Sensitizers and Their Applications in Dye-Sensitized Solar Cells. Chem. Mater. 2006;18:5178–5185. doi: 10.1021/cm0602141. [DOI] [Google Scholar]
- 124.Islam A., Chowdhury F.A., Chiba Y., Komiya R., Fuke N., Ikeda N., Han L. Ruthenium(II) tricarboxyterpyridyl complex with a fluorine-substituted β-diketonato ligand for highly efficient dye-sensitized solar cells. Chem. Lett. 2005;34:344–345. doi: 10.1246/cl.2005.344. [DOI] [Google Scholar]
- 125.Islam A., Sugihara H., Yanagida M., Hara K., Fujihashi G., Tachibana Y., Katoh R., Murata S., Arakawa H. Efficient panchromatic sensitization of nanocrystalline TiO2 films by beta-diketonato ruthenium polypyridyl complexes. New J. Chem. 2002;26:966–968. doi: 10.1039/b202392j. [DOI] [Google Scholar]
- 126.Jiang X., Marinado T., Gabrielsson E., Hagberg D.P., Sun L., Hagfeldt A. Structural Modification of Organic Dyes for Efficient Coadsorbent-Free Dye-Sensitized Solar Cells. J. Phys. Chem. C. 2010;114:2799–2805. doi: 10.1021/jp908552t. [DOI] [Google Scholar]
- 127.Chen B.-S., Chen K., Hong Y.-H., Liu W.-H., Li T.-H., Lai C.-H., Chou P.-T., Chi Y., Lee G.-H. Neutral, panchromatic Ru(II) terpyridine sensitizers bearing pyridine pyrazolate chelates with superior DSSC performance. Chem. Commun. 2009:5844–5846. doi: 10.1039/b914197a. [DOI] [PubMed] [Google Scholar]
- 128.Chou C.-C., Wu K.-L., Chi Y., Hu W.-P., Yu S.J., Lee G.-H., Lin C.-L., Chou P.-T. Ruthenium(II) sensitizers with heteroleptic tridentate chelates for dye-sensitized solar cells. Angew. Chem. Int. Ed. Engl. 2011;50:2054–2058. doi: 10.1002/anie.201006629. [DOI] [PubMed] [Google Scholar]
- 129.Wu K.-L., Li C.-H., Chi Y., Clifford J.N., Cabau L., Palomares E., Cheng Y.-M., Pan H.-A., Chou P.-T. Dye molecular structure device open-circuit voltage correlation in Ru(II) sensitizers with heteroleptic tridentate chelates for dye-sensitized solar cells. J. Am. Chem. Soc. 2012;134:7488–7496. doi: 10.1021/ja300828f. [DOI] [PubMed] [Google Scholar]
- 130.Chou C.-C., Hu F.-C., Yeh H.-H., Wu H.-P., Chi Y., Clifford J. N., Palomares E., Liu S.-H., Chou P.-T., Lee G.-H. Highly efficient dye-sensitized solar cells based on panchromatic ruthenium sensitizers with quinolinylbipyridine anchors. Angew. Chem. Int. Ed. Engl. 2014;53:178–183. doi: 10.1002/anie.201305975. [DOI] [PubMed] [Google Scholar]
- 131.Chang T.-K., Li H., Chen K.-T., Tsai Y.-C., Chi Y., Hsiao T.-Y., Kai J.-J. Substituent effect of Ru(II)-based sensitizers bearing a terpyridine anchor and a pyridyl azolate ancillary for dye sensitized solar cells. J. Mater. Chem. A. 2015;3:18422–18431. doi: 10.1039/C5TA04934B. [DOI] [Google Scholar]
- 132.Wadman S.H., Kroon J.M., Bakker K., Lutz M., Spek A.L., van Klink G.P.M., van Koten G. Cyclometalated ruthenium complexes for sensitizing nanocrystalline TiO2 solar cells. Chem. Commun. 2007:1907–1909. doi: 10.1039/b703636a. [DOI] [PubMed] [Google Scholar]
- 133.Wadman S.H., Kroon J.M., Bakker K., Havenith R.W.A., van Klink G.P.M., van Koten G. Cyclometalated Organoruthenium Complexes for Application in Dye-Sensitized Solar Cells. Organometallics. 2010;29:1569–1579. doi: 10.1021/om900481g. [DOI] [Google Scholar]
- 134.Wadman S.H., van Leeuwen Y.M., Havenith R.W.A., van Klink G.P.M., van Koten G. A Redox Asymmetric, Cyclometalated Ruthenium Dimer: Toward Upconversion Dyes in Dye-Sensitized TiO2 Solar Cells. Organometallics. 2010;29:5635–5645. doi: 10.1021/om100865k. [DOI] [Google Scholar]
- 135.Kisserwan H., Ghaddar T.H. Enhancement of photocurrent in dye sensitized solar cells incorporating a cyclometalated ruthenium complex with cuprous iodide as an electrolyte additive. Dalton Trans. 2011;40:3877–3884. doi: 10.1039/c0dt01554g. [DOI] [PubMed] [Google Scholar]
- 136.Robson K.C.D., Koivisto B.D., Yella A., Sporinova B., Nazeeruddin M.K., Baumgartner T., Grätzel M., Berlinguette C.P. Design and development of functionalized cyclometalated ruthenium chromophores for light-harvesting applications. Inorg. Chem. 2011;50:5494–5508. doi: 10.1021/ic200011m. [DOI] [PubMed] [Google Scholar]
- 137.Al-mutlaq F.A., Potvin P.G., Philippopoulos A.I., Falaras P. Catechol-Bearing Dipyrazinylpyridine Complexes of Ruthenium(II) Eur. J. Inorg. Chem. 2007;2007:2121–2128. doi: 10.1002/ejic.200600772. [DOI] [Google Scholar]
- 138.Sepehrifard A., Chen S., Stublla A., Potvin P.G., Morin S. Effects of ligand LUMO levels, anchoring groups and spacers in Ru(II)-based terpyridine and dipyrazinylpyridine complexes on adsorption and photoconversion efficiency in DSSCs. Electrochim. Acta. 2013;87:236–244. doi: 10.1016/j.electacta.2012.09.025. [DOI] [Google Scholar]
- 139.Sepehrifard A., Stublla A., Haftchenary S., Chen S., Potvin P.G., Morin S. Effects of carboxyl and ester anchoring groups on solar conversion efficiencies of TiO2 dye-sensitized solar cells. J. New. Mat. Electrochem. Syst. 2008;11:281–285. [Google Scholar]
- 140.Schulze B., Brown D.G., Robson K.C.D., Friebe C., Jäger M., Birckner E., Berlinguette C.P., Schubert U.S. Cyclometalated ruthenium(II) complexes featuring tridentate click-derived ligands for dye-sensitized solar cell applications. Chem. - Eur. J. 2013;19:14171–14180. doi: 10.1002/chem.201301440. [DOI] [PubMed] [Google Scholar]
- 141.Sinn S., Schulze B., Friebe C., Brown D.G., Jäger M., Kübel J., Dietzek B., Berlinguette C.P., Schubert U.S. A heteroleptic bis(tridentate) ruthenium(II) platform featuring an anionic 1,2,3-triazolate-based ligand for application in the dye-sensitized solar cell. Inorg. Chem. 2014;53:1637–1645. doi: 10.1021/ic402701v. [DOI] [PubMed] [Google Scholar]
- 142.Park H.-J., Kim K.H., Choi S.Y., Kim H.-M., Lee W.I., Kang Y.K., Chung Y.K. Unsymmetric Ru(II) Complexes with N-Heterocyclic Carbene and/or Terpyridine Ligands: Synthesis, Characterization, Ground- and Excited-State Electronic Structures and Their Application for DSSC Sensitizers. Inorg. Chem. 2010;49:7340–7352. doi: 10.1021/ic100325c. [DOI] [PubMed] [Google Scholar]
- 143.Bonacin J.A., Toma S.H., Freitas J.N., Nogueira A.F., Toma H.E. On the behavior of the carboxyphenylterpyridine(8-quinolinolate) thiocyanatoruthenium(II) complex as a new black dye in TiO2 solar cells modified with carboxymethyl-beta-cyclodextrin. Inorg. Chem. Commun. 2013;36:35–38. doi: 10.1016/j.inoche.2013.08.007. [DOI] [Google Scholar]
- 144.Kinoshita T., Dy J.T., Uchida S., Kubo T., Segawa H. Wideband dye-sensitized solar cells employing a phosphine-coordinated ruthenium sensitizer. Nat. Photonics. 2013;7:535–539. doi: 10.1038/nphoton.2013.136. [DOI] [Google Scholar]
- 145.Kinoshita T., Nonomura K., Joong Jeon N., Giordano F., Abate A., Uchida S., Kubo T., Seok S.I., Nazeeruddin M.K., Hagfeldt A., et al. Spectral splitting photovoltaics using perovskite and wideband dye-sensitized solar cells. Nat. Commun. 2015;6:8834–8842. doi: 10.1038/ncomms9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li G., Yella A., Brown D.G., Gorelsky S.I., Nazeeruddin M.K., Grätzel M., Berlinguette C.P., Shatruk M. Near-IR photoresponse of ruthenium dipyrrinate terpyridine sensitizers in the dye-sensitized solar cells. Inorg. Chem. 2014;53:5417–5419. doi: 10.1021/ic5006538. [DOI] [PubMed] [Google Scholar]
- 147.Swetha T., Niveditha S., Bhanuprakash K., Islam A., Han L., Bedja I.M., Fallahpour R., Singh S.P. New heteroleptic benzimidazole functionalized Ru-sensitizer showing the highest efficiency for dye-sensitized solar cells. Inorg. Chem. Commun. 2015;51:61–65. doi: 10.1016/j.inoche.2014.10.033. [DOI] [Google Scholar]
- 148.Argazzi R., Larramona G., Contado C., Bignozzi C.A. Preparation and photoelectrochemical characterization of a red sensitive osmium complex containing 4,4′,4′′-tricarboxy-2,2′:6′,2′′-terpyridine and cyanide ligands. J. Photochem. Photobiol., A. 2004;164:15–21. doi: 10.1016/j.jphotochem.2003.12.016. [DOI] [Google Scholar]
- 149.Argazzi R., Murakami Iha N.Y., Zabri H., Odobel F., Bignozzi C.A. Design of molecular dyes for application in photoelectrochemical and electrochromic devices based on nanocrystalline metal oxide semiconductors. Coord. Chem. Rev. 2004;248:1299–1316. doi: 10.1016/j.ccr.2004.03.026. [DOI] [Google Scholar]
- 150.Altobello S., Argazzi R., Caramori S., Contado C., Da Fré S., Rubino P., Choné C., Larramona G., Bignozzi C.A. Sensitization of nanocrystalline TiO2 with black absorbers based on Os and Ru polypyridine complexes. J. Am. Chem. Soc. 2005;127:15342–15343. doi: 10.1021/ja053438d. [DOI] [PubMed] [Google Scholar]
- 151.Lapides A.M., Ashford D.L., Hanson K., Torelli D.A., Templeton J.L., Meyer T.J. Stabilization of a ruthenium(II) polypyridyl dye on nanocrystalline TiO2 by an electropolymerized overlayer. J. Am. Chem. Soc. 2013;135:15450–15458. doi: 10.1021/ja4055977. [DOI] [PubMed] [Google Scholar]
- 152.Duchanois T., Etienne T., Cebrián C., Liu L., Monari A., Beley M., Assfeld X., Haacke S., Gros P.C. An Iron-Based Photosensitizer with Extended Excited-State Lifetime: Photophysical and Photovoltaic Properties. Eur. J. Inorg. Chem. 2015;2015:2469–2477. doi: 10.1002/ejic.201500142. [DOI] [Google Scholar]
- 153.Kwok E.C.-H., Chan M.-Y., Wong K.M.-C., Lam W.H., Yam V.W.-W. Functionalized alkynylplatinum(II) polypyridyl complexes for use as sensitizers in dye-sensitized solar cells. Chem. - Eur. J. 2010;16:12244–12254. doi: 10.1002/chem.201001424. [DOI] [PubMed] [Google Scholar]
- 154.Shinpuku Y., Inui F., Nakai M., Nakabayashi Y. Synthesis and characterization of novel cyclometalated iridium(III) complexes for nanocrystalline TiO2-based dye-sensitized solar cells. J. Photochem. Photobiol., A. 2011;222:203–209. doi: 10.1016/j.jphotochem.2011.05.023. [DOI] [Google Scholar]
- 155.Bozic-Weber B., Constable E.C., Hostettler N., Housecroft C.E., Schmitt R., Schönhofer E. The d10 route to dye-sensitized solar cells: step-wise assembly of zinc(II) photosensitizers on TiO2 surfaces. Chem. Commun. 2012;48:5727–5729. doi: 10.1039/c2cc31729j. [DOI] [PubMed] [Google Scholar]
- 156.Hostettler N., Fürer S.O., Bozic-Weber B., Constable E.C., Housecroft C.E. Alkyl chain-functionalized hole-transporting domains in zinc(II) dye-sensitized solar cells. Dyes Pigm. 2015;116:124–130. doi: 10.1016/j.dyepig.2015.01.008. [DOI] [Google Scholar]
- 157.Hostettler N., Wright I.A., Bozic-Weber B., Constable E.C., Housecroft C.E. Dye-sensitized solar cells with hole-stabilizing surfaces: “inorganic” versus “organic” strategies. RSC Adv. 2015;5:37906–37915. doi: 10.1039/C5RA05630F. [DOI] [Google Scholar]
- 158.Housecroft C.E., Constable E.C. The emergence of copper(I)-based dye sensitized solar cells. Chem. Soc. Rev. 2015;44:8386–8398. doi: 10.1039/C5CS00215J. [DOI] [PubMed] [Google Scholar]
- 159.Bozic-Weber B., Constable E.C., Housecroft C.E. Light harvesting with Earth abundant d-block metals: Development of sensitizers in dye-sensitized solar cells (DSCs) Coord. Chem. Rev. 2013;257:3089–3106. doi: 10.1016/j.ccr.2013.05.019. [DOI] [Google Scholar]
- 160.Odobel F., Pellegrin Y. Recent Advances in the Sensitization of Wide-Band-Gap Nanostructured p-Type Semiconductors. Photovoltaic and Photocatalytic Applications. J. Phys. Chem. Lett. 2013;4:2551–2564. doi: 10.1021/jz400861v. [DOI] [Google Scholar]
- 161.Ji Z., Wu Y. Photoinduced Electron Transfer Dynamics of Cyclometalated Ruthenium (II)–Naphthalenediimide Dyad at NiO Photocathode. J. Phys. Chem. C. 2013;117:18315–18324. doi: 10.1021/jp405659m. [DOI] [Google Scholar]
- 162.Sariola-Leikas E., Ahmed Z., Vivo P., Ojanperä A., Lahtonen K., Saari J., Valden M., Lemmetyinen H., Efimov A. Color Bricks: Building Highly Organized and Strongly Absorbing Multicomponent Arrays of Terpyridyl Perylenes on Metal Oxide Surfaces. Chem.-Eur. J. 2015;22:1501–1510. doi: 10.1002/chem.201503738. [DOI] [PubMed] [Google Scholar]
- 163.Constable E.C., Housecroft C.E., Šmídková M., Zampese J.A. Phosphonate-functionalized heteroleptic ruthenium(II) bis(2,2′:6′,2″-terpyridine) complexes. Can. J. Chem. 2014;92:724–730. doi: 10.1139/cjc-2014-0065. [DOI] [Google Scholar]
- 164.Wood C.J., Robson K.C.D., Elliott P.I.P., Berlinguette C.P., Gibson E.A. Novel triphenylamine-modified ruthenium(II) terpyridine complexes for nickel oxide-based cathodic dye-sensitized solar cells. RSC Adv. 2014;4:5782–5791. doi: 10.1039/c3ra44690e. [DOI] [Google Scholar]
- 165.Ogura R.Y., Nakane S., Morooka M., Orihashi M., Suzuki Y., Noda K. High-performance dye-sensitized solar cell with a multiple dye system. Appl. Phys. Lett. 2009;94:073308. doi: 10.1063/1.3086891. [DOI] [Google Scholar]
- 166.Ozawa H., Shimizu R., Arakawa H. Significant improvement in the conversion efficiency of black-dye-based dye-sensitized solar cells by cosensitization with organic dye. RSC Adv. 2012;2:3198–3200. doi: 10.1039/c2ra01257j. [DOI] [Google Scholar]
- 167.Sharma G.D., Daphnomili D., Gupta K.S.V., Gayathri T., Singh S.P., Angaridis P.A., Kitsopoulos T.N., Tasis D., Coutsolelos A.G. Enhancement of power conversion efficiency of dye-sensitized solar cells by co-sensitization of zinc-porphyrin and thiocyanate-free ruthenium(II)-terpyridine dyes and graphene modified TiO2 photoanode. RSC Adv. 2013;3:22412–22420. doi: 10.1039/c3ra42537a. [DOI] [Google Scholar]
- 168.Bahreman A., Cuello-Garibo J.-A., Bonnet S. Yellow-light sensitization of a ligand photosubstitution reaction in a ruthenium polypyridyl complex covalently bound to a rhodamine dye. Dalton Trans. 2014;43:4494–4505. doi: 10.1039/c3dt52643g. [DOI] [PubMed] [Google Scholar]
- 169.Koussi-Daoud S., Schaming D., Fillaud L., Trippé-Allard G., Lafolet F., Polanski E., Nonomura K., Vlachopoulos N., Hagfeldt A., Lacroix J.-C. 3,4-Ethylenedioxythiophene-based cobalt complex: an efficient co-mediator in dye-sensitized solar cells with poly(3,4-ethylenedioxythiophene) counter-electrode. Electrochim. Acta. 2015;179:237–240. doi: 10.1016/j.electacta.2015.04.173. [DOI] [Google Scholar]