Abstract
Burkholderia mallei-specific phage ΦE125 encodes DNA methyltransferases in both the lysogenic and replication modules within its genome. Characterization of DNA methylation in recombinant systems, specifically in ΦE125 lysogenic strains of B. mallei and Burkholderia thailandensis, revealed that, upon induction, cytosine methylation was targeted specifically to the phage episome but not the phage provirus or the host chromosome.
The prototypic DNA methylation model system is prokaryotic restriction and modification. Host-encoded DNA methyltransferase (DNA MTase) enzymes modify a particular consensus sequence, thereby protecting the host genome from the cognate restriction endonuclease's destruction of unmodified invading viral genomes (13). Recently, DNA methylation in prokaryotes has also been implicated in developmental gene regulation and virulence functions of many pathogens (2, 9, 12, 16). In vitro DNA MTases modify all sites. However, in vivo, some sites remain unmodified (8, 12). Similarly, eukaryotic genomes maintain specific methylation patterns, though their characterized MTases display little site specificity in vitro (1). In any model system, little is known about the regulatory decisions governing modification timing or site selection (1, 5).
Burkholderia thailandensis is a nonpathogenic, gram-negative soil bacterium closely related to the human pathogens Burkholderia pseudomallei and Burkholderia mallei (3, 4). In a recent report, we described ΦE125, a phage in the Siphoviridae (λ-supergroup) family, produced by B. thailandensis E125 (22). Of particular interest was the discovery of two DNA MTase genes in both the lysogenic and replication modules of the phage genome. The gene for the DNA MTase located in the lysogenic module (the gp27 gene) encoded an enzyme with homology to MTases that modify the N6 position of adenine (N6-methyladenine [m6A]). The replication module-encoded methylase (gp56) was most similar to enzymes that modify the N4 position of cytosine (N4-methylcytosine [m4C]). Genomic DNA isolated from the B. mallei ΦE125 lysogenic strain BML10, but not B. thailandensis E125, contained both m4C and m6A. The expression of the gp27 gene in Escherichia coli demonstrated that this gene encodes an m6A MTase. However, the putative MTase gp56 did not display activity in the recombinant system. The present report extends these initial observations and provides a more in-depth analysis of ΦE125 methylation. The cytosine methylation present in the ΦE125 genome after replication in B. mallei was restricted to the phage genome. Furthermore, the phage-specific cytosine modification observed was correlated with the presence of cytoplasmic extrachromosomal phage genomes. The association between phage induction and DNA methylation suggests that there may be an epigenetic aspect to ΦE125 replication.
The focus of this work was the determination of the contributions of MTases to the m4C detected in the host and phage genomes (22). Recent work by Jeltsch and coworkers demonstrated that m6A MTases are also able to methylate cytosine bases, generating m4C (10). Our previous analysis of gp27 in a recombinant system indicated that it had no m4C MTase activity (22). Table 1 outlines the strains, plasmids, and oligonucleotides used. Hypothesizing that host factors may be important for gp56 gene activity, we cloned the MTase genes into the broad-host-range expression plasmid pBHR1 and mobilized them into B. mallei ATCC 23344 according to the method described previously by DeShazer et al. (6). A previously described immunoblot analysis (22) was performed with both types of resultant transconjugate clones, and a data set similar to that in our previous report was obtained (data not shown). Recombinant expression of the gp27 gene in B. mallei provided m6A to the B. mallei chromosome, whereas no effect of gp56 gene expression was detected (22). A BLASTX analysis of the B. mallei ATCC 23344 genome indicated that it contained neither DNA m6A MTases nor m4C MTases (data not shown;http://www.tigr.org/tdb/mdb/mdbinprogress.html). Therefore,the only source of these methylases was an integrated B. thailandensis provirus; by methylase convention, gp27 was renamed M.BtaI and gp56 was renamed M.BtaII. Expression of the gene in B. mallei was not sufficient to obtain M.BtaII activity. Perhaps other phage gene functions were also required, as both BML10 and ΦE125 clearly contained m4C and the B. mallei host genome did not.
TABLE 1.
Strains, plasmids, and oligonucleotides
| Strain, plasmid, or oligonucleotide | Description | Reference(s) and/or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| TOP10 | Cloning strain | Invitrogen |
| SM10 | Mobilizing, tra (Kmr) | 17 |
| DB24 | Lacks all genomic DNA methylation | 11 |
| B. thailandensis | Nonpathogenic soil saprophyte | |
| E27 | 3, 18, 23 | |
| E125 | ||
| B. mallei | Soleploid and human pathogen | |
| ATCC 23344 | Human disease isolate; Cms Kms | 24; ATCC |
| BML10 | ΦE125-resistant ATCC 23344 | 22 |
| Plasmids | ||
| pCR2.1 | Topoisomerase-mediated cloning, Apr Kmr | Invitrogen |
| pAM1 | pCR2.1 lacZ-gp27 methylase; Apr Kmr | 22 |
| pCM1 | pCR2.1 lacZ-gp56 methylase; Apr Kmr | 22 |
| pBHR1 | Broad-host-range, mobilizing and expression (tra oriT Cmr Kmr) | MoBiTec |
| pAM15 | pBHR1: pcat-gp27; Cms Kmr | This study |
| pAM17 | pBHR1: pcat-gp27; Cms Kmr | This study |
| pCM18 | pBHR1: pcat-gp56; Cms Kmr | This study |
| pCM38 | pBHR1: pcat-gp56; Cms Kmr | This study |
| pDW18 | pBluescript KS containing 12,983-bp HindIII fragment from ΦE125; Apr | 22 |
| Oligonucleotides | ||
| attP F1 | GCACGCTGAAGAGTGAGGATG | This study |
| attP R1 | CATACGAGCCAAGGCCCTAAC | This study |
| 16S 5′ | 3 | |
| 16S 3′ | 3 | |
| B.th_rDNA F1 | CCGGCCTCAACCTGGCAACTGC | This study |
| B.th_rDNA R1 | GAGCCGACATCGAGGTGCCAAACA | This study |
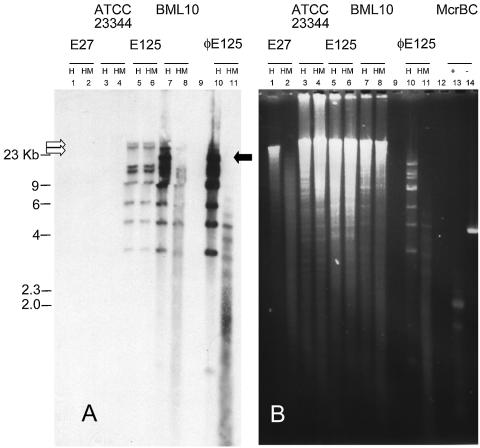
Next, the extent of m4C methylation present in both the phage and lysogenic strain genomes was determined by McrBC restriction analysis. McrBC detects the methylation of cytosine bases at either position 4 or position 5. McrBC cleaves only DNA methylated at PumC dinucleotides, as long as there are two methylated PuC's within 20 bp to 4 kb of each other (7, 14, 15, 19-21). Genomic DNAs from control B. thailandensis isolates E27, E125, ATCC 23344, and BML10 and from ΦE125 plate lysate recovered from B. mallei were digested with HindIII alone or HindIII plus McrBC. Southern blot analysis was performed with labeled plate lysate-recovered ΦE125 as a probe (Fig. 1A). Because B. thailandensis E27 does not harbor ΦE125, and because immunoblot analysis of its genomic DNA detected no modification (m4C or m6A), it was chosen as a negative control (22). However, E27 genomic DNA was further digested by added McrBC (Fig. 1B), indicating that its genome contained m5C (i.e., m4C that was antibody negative and McrBC positive). The phage genome was extensively digested by added McrBC, indicating cytosine methylation (Fig. 1B, lane 11). Interestingly, the results from the E125 and BML10 lysogens were different. The provirus present in the uninduced E125 genome was not methylated (the HindIII digestion pattern matches the digestion pattern of HindIII plus McrBC [Fig. 1B, compare lanes 5 and 6]). Although the phage present in BML10 was modified, it appeared to be less modified than purified phage genomes alone (Fig. 1B, compare lanes 7 and 8 to lanes 10 and 11). Notice that the mode of the ethidium bromide fluorescence appears to suggest that the genomic DNA from E125 was actually overloaded relative to that from BML10 (Fig. 1B, compare lanes 5 and 6 to lanes 7 and 8). Conversely, the intensity of the phage-specific restriction fragment hybridization was more abundant in the sample from BML10 (Fig. 1A, compare lanes 5 and 6 to lanes 7 and 8). Furthermore, it appears that the genomic HindIII junction fragments are of a different stoichiometry than that of phage-derived internal HindIII fragments present in BML10 (Fig. 1A). E125, on the other hand, displayed fragments of similar intensities (compare lanes 5 and 7). The presence of phage episomes in BML10 may explain both the nonstoichiometric ratio of junction fragment hybridization and the increased amount of phage-specific restriction fragment hybridization. To be consistent with this hypothesis, excised cytoplasmic viral genomes would yield HindIII-specific restriction fragments that are shared with purified virus (Fig. 1A, compare lane 5 to lanes 7 and 10). An ∼13-kb HindIII fragment contains the attP integration target (22).
FIG. 1.
Southern blot (A) and electrophoretic (B) analysis of the M.BtaII-induced methylation of ΦE125. (A) Approximately 50 μg of the indicated genomic DNA and ∼10 μg of ΦE125 purified from plate lysates were subjected to digestion by HindIII (H) according to the NEB-specified conditions. (B) Subsequently, half of each reaction mixture was removed and digested with McrBC (HM) under NEB-specified conditions. The DNAs were blotted by using the alkaline transfer protocol of the TurboBlotter (Schleicher & Schuell, Piscataway, N.J.) and photoimmobilized. The ECL probe (ΦE125::HindIII) was synthesized, hybridized, and detected according to the instructions specified by Amersham. The positive controls for McrBC digestion were E27 genomic DNA (lanes 2) and the NEB-supplied positive control (lane 13). The empty arrows indicate the chromosomal junction fragments and the host chromosome, while the filled arrow indicates the fragment that originates from excised viral genomes.
Figure 2 depicts confirmatory results from the search for episomal phage genomes. Briefly, oligonucleotide primer sets specific to the phage genome, outside the integrating attP region, were designed to amplify the entirety of attP. If the phage present were integrated into the chromosome, the primers would be pointing away from each other and no PCR product would be observed. PCR amplification products were analyzed after electrophoresis. Positive controls for the ∼800-bp amplicon were purified phage genomes (Fig. 2, lane 5) and a recombinant plasmid clone bearing the attP region of the ΦE125 genome (lane 6). Both gave the predicted product, as did BML10 (lane 4), indicating that, in the preparations of genomic DNA, unintegrated viral genomes were present. The cos site of the phage was found to anneal at 37°C, making genomic circles likely in vivo (22). DNA purified from E125 with the same MasterPure (Epicentre, Madison, Wis.) protocol yielded no attP-specific PCR product (lane 2), suggesting that the only copies of the phage present were integrated. These observations, along with the results of the Southern blot analysis shown in Fig. 1, suggest that the PCR product detected in BML10 probably results from extrachromosomal cytoplasmic phage rather than incidentally purified phage from residual culture supernatants. This outcome is probable because uninduced E125 produces spontaneous phage, yet the genomic DNA protocol utilized did not purify enough cytoplasmic phage to foster PCR amplification (Fig. 2, lane 2). The presence of the unintegrated phage genomes (BML10) correlated with the presence of methylated phage genomes (Fig. 1) (22).
FIG. 2.
PCR analysis reveals extrachromosomal phage genomes. Following PCR amplification using 15-s incubations, a 58°C annealing temperature, and 35 cycles, loading dye was added to each 50-μl reaction mixture and 10 μl was loaded and visualized after electrophoresis. Negative controls in this analysis were B. thailandensis E27 and B. mallei ATCC 23344, as they lack ΦE125. Positive controls were the lysate-recovered ΦE125 DNA and a recombinant ΦE125 plasmid subclone (pDW18) that carries the HindIII fragment containing attP. Because these primers lie in the phage genome outside the tRNA gene encoded by attP, the amplicon is phage specific and can detect the presence of only unintegrated phage genomes. Both BML10 and E125 were previously demonstrated to contain integrated ΦE125 at the genomic tRNA homologous to attP (22). Phage genome methylation correlates with the presence of the unintegrated phage genomes.
A question remaining was whether the m4C present in the ΦE125 genome extended to the host chromosome. Figure 1B suggests that the BML10 host chromosome is not appreciably methylated, since the mode of the HindIII digestion ethidium bromide staining was unaffected by McrBC treatment (compare lanes 7 and 8). Replicate Southern blots, with probes specific to large B. mallei chromosomal regions (the rRNA genes), were analyzed in a preliminary follow-up experiment. If the chromosome were methylated, then adding McrBC to any digest would alter the restriction pattern. The restriction analysis and Southern blot analysis both suggested that substantial cytosine modification of the chromosomes did not occur (data not shown).
The observation of methylated extrachromosomal phage DNA from BML10 but not from uninduced E125 suggested that phage replication induction may be connected to the DNA methylation observed. This possibility was explored by using genomic DNA recovered from duplicate E125 cultures grown in parallel that had been either UV induced or uninduced under our previously described induction conditions (22). A control purification of any residual cell-free (or phage) genomic DNA was also attempted by using the culture supernatants as the DNA source (i.e., DNA was purified from cell pellets and supernatants). Following purification, the DNA was subjected to the same attP (extrachromosomal phage)-detecting PCR assay, as described for Fig. 2, with one modification. Approximately 1 μg from each UV-induced genomic DNA preparation was either digested with McrBC or mock digested and then subjected to limited rounds of PCR amplification (20 cycles). In this way, any difference in concentration of the amplification product between treated and untreated samples may be interpreted as indicating methylation content. The use of a sensitive detection system like PCR allowed investigation of the medium supernatant in parallel, affording us the ability to eliminate the possibility that contamination of the cell pellet with residual phage could serve as an explanation for any methylated genomes detected. Representative results are depicted in Fig. 3. Not only did the induction of phage replication from E125 by UV treatment foster the cytoplasmic accumulation of extrachromosomal phage, but those accumulated phage were also methylated, since McrBC treatment suppressed the accumulation of the PCR product by more than 50% (Fig. 3, compare lane 1 to lane 2 and lane 3 to lane 4).
FIG. 3.
Induction of replication triggers methylated extrachromosomal phage accumulation in E125. Depicted is a representative digital gel output from an Agilent 2100 bioanalyzer using the DNA 12000 chip system. One microgram of genomic DNA from each of the indicated genomic DNAs was either digested with McrBC or mock digested under the NEB-suggested conditions. A total of ∼100 ng of each was then subjected to a limited PCR. Following PCR amplification using 15-s incubations, a 58°C annealing temperature, and 20 cycles, 1 μl of each 25-μl PCR mixture was loaded onto an Agilent 2100 bioanalyzer, which can size and quantify reaction products. Negative controls in this analysis were a water control (NTC) (lanes 11 and 12), any genomic DNA purified from the indicated cell supernatants (UV SUP) (lanes 5 and 6), and uninduced B. thailandensis E125 (NO UV) (lanes 7 and 8). The positive amplification control was a plasmid subclone (pDW18) that carries the HindIII fragment containing attP (lanes 9 and 10). Because these primers lie in the phage genome outside the tRNA gene carried by attP, the amplicon is phage specific and can detect the presence of only unintegrated phage genomes. Both BML10 and E125 were previously demonstrated to contain integrated ΦE125 at the genomic tRNA homologous to attP (22). Induced (actively replicating) phage genome methylation correlates with the presence of the unintegrated phage genomes. UV IND, UV induction.
Again, as in the experiment in which BML10 was the host, M.BtaII methylation in the E125 chromosome was apparently excluded, since PCR amplification using primer sets designed for the B. thailandensis 16S rRNA genes, as well as an ∼5-kb segment targeting both the 16S and 23S RNA, was found to not display a difference between McrBC-treated and untreated fractions. Similarly, as predicted by the results shown in Fig. 1B, McrBC treatment had no effect upon genomic restriction fragment distributions obtained from UV-induced E125 (data not shown).
It is currently unclear how the phage selectively modified only its own genome. M.BtaII, either independently or in connection with other phage components, was able to distinguish between the viral genomes (the extrachromosomal phase) and the host chromosome. One simple explanation of the preferential phage episome methylation by M.BtaII would be based on a paucity of chromosomal sites relative to abundant phage sites and a connection of methylase expression with phage induction.
A lack of chromosomal M.BtaII sites in the B. mallei and B. thailandensis regions explored by blotting and PCR assays is not a likely explanation for the observation for several reasons. First, these genomes are enormous (∼6 to 7 Mbp) and have a high G+C content. Importantly, the G+C contents of the phage and Burkholderia sp. genomes are only modestly different, and both have abundant acceptor cytosines (over 60% G+C) (22). Second, McrBC restriction analysis suggests that the M.BtaII site occurs frequently in the phage genome. There was no 5-kb or larger segment of the phage genome that fully survived cleavage by McrBC (Fig. 1). The mode of restriction fragments obtained from the viral genome following McrBC treatment was approximately 1 kb (Fig. 1). Given that McrBC requires two methylated RC motifs to observe cleavage, this result suggests that M.BtaII has a frequently occurring recognition sequence, perhaps 4 bp or less, because nearly every HindIII fragment was eliminated with McrBC treatment. In order for the HindIII restriction pattern obtained from the BML10 genomic DNA not to be affected by the McrBC treatment, the cooccurrence of M.BtaII sites with HindIII sites in both chromosomes would have to be less frequent than one event every 23 kb (the limit of gel resolution), such that no chromosomal small HindIII fragments were cooccurring within M.BtaII restriction sites, implying a >8-bp recognition site for M.BtaII. No described 7- to 18-bp C-containing palindromic sites exist in the phage genome at the density necessary to support this hypothesis (∼2/kb) (data not shown).
Our group previously reported that induced E125 produced about twice as many phage as BML10. One way to unify this observation with these data, which suggest that BML10 produces more phage episomes, is to posit that BML10 may make as many phage as UV-induced strain E125, but for some reason BML10 is less capable of exporting virions, which leads to their cytoplasmic accumulation.
The necessity of the methylation modification must be governed by the interaction of the virus with its host, as the uninduced B. thailandensis E125 provirus was unmethylated yet still produced infectious phage (22). In certain hosts, molecularly differentiating between the proviral and any extrachromosomal phage genomes must be important for some aspect of the phage life cycle and likely connected with the rate of virus replication. Methylation of infectious phage may confer protection against other host restriction systems or modify the expression of viral genes for expression in other hosts. The connections among DNA methylation, extrachromosomal phage replication, and the partitioning of target sequences suggest that this system shares features with not only prokaryotic but also eukaryotic DNA methylation systems. Future studies would likely be aimed at understanding the phage's selective cytosine methylation-targeting mechanism, directly demonstrating the enzymatic activity of M.BtaII, and exploring the potentially epigenetic aspect to extrachromosomal phage replication.
Acknowledgments
R. Roberts (New England BioLabs [NEB], Beverly, Mass.) provided technical insight and experimental reagents. J. Ordway (Orion Genomics), E. J. Richards, C. Pikaard (Washington University, St. Louis, Mo.), D. DeShazer, and R. Ulrich (USAMRIID, Fort Detrick, Md.) provided critical analysis.
This work was funded by the U.S. Army Medical Research and Material Command.
REFERENCES
- 1.Bestor, T. H. 1996. DNA methyltransferases in mammalian development and genome defense, p. 61-76. In V. E. Russo, R. A. Martiensen, and A. D. Riggs (ed.), Epigenetic mechanisms of gene regulation, vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 2.Braaten, B. A., X. Nou, L. S. Kaltenbach, and D. A. Low. 1994. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell 76:577-588. [DOI] [PubMed] [Google Scholar]
- 3.Brett, P., D. DeShazer, and D. E. Woods. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48:317-320. [DOI] [PubMed] [Google Scholar]
- 4.Brett, P. J., D. DeShazer, and D. E. Woods. 1997. Characterization of Burkholderia pseudomallei and Burkholderia pseudomallei-like strains. Epidemiol. Infect. 118:137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadesus, J., and J. Torreblanca. 1996. Methylation-related epigenetic signals in bacterial DNA, p. 141-154. In V. E. Russo, R. A. Martiensen, and A. D. Riggs (ed.), Epigenetic mechanisms of gene regulation, vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 6.DeShazer, D., P. J. Brett, R. Carlyon, and D. E. Woods. 1997. Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: isolation of motility mutants and molecular characterization of the flagellin structural gene. J. Bacteriol. 179:2116-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gast, F. U., T. Brinkmann, U. Pieper, T. Kruger, M. Noyer-Weidner, and A. Pingoud. 1997. The recognition of methylated DNA by the GTP-dependent restriction endonuclease McrBC resides in the N-terminal domain of McrB. Biol. Chem. 378:975-982. [DOI] [PubMed] [Google Scholar]
- 8.Hale, W. B., M. W. van der Woude, and D. A. Low. 1994. Analysis of nonmethylated GATC sites in the Escherichia coli chromosome and identification of sites that are differentially methylated in response to environmental stimuli. J. Bacteriol. 176:3438-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284:967-970. [DOI] [PubMed] [Google Scholar]
- 10.Jeltsch, A., F. Christ, M. Fatemi, and M. Roth. 1999. On the substrate specificity of DNA methyltransferases: adenine-N6 DNA methyltransferases also modify cytosine residues at position N4. J. Biol. Chem. 274:19538-19544. [DOI] [PubMed] [Google Scholar]
- 11.Kong, H., L. F. Lin, N. Porter, S. Stickel, D. Byrd, J. Posfai, and R. J. Roberts. 2000. Functional analysis of putative restriction-modification system genes in the Helicobacter pylori J99 genome. Nucleic Acids Res. 28:3216-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahan, M. J., and D. A. Low. 2001. DNA methylation regulates bacterial gene expression and virulence. ASM News 67:356-361. [Google Scholar]
- 13.Murray, N. E. 2002. Immigration control of DNA in bacteria: self versus non-self. Microbiology 148:3-20. [DOI] [PubMed] [Google Scholar]
- 14.Panne, D., S. A. Muller, S. Wirtz, A. Engel, and T. A. Bickle. 2001. The McrBC restriction endonuclease assembles into a ring structure in the presence of G nucleotides. EMBO J. 20:3210-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panne, D., E. A. Raleigh, and T. A. Bickle. 1999. The McrBC endonuclease translocates DNA in a reaction dependent on GTP hydrolysis. J. Mol. Biol. 290:49-60. [DOI] [PubMed] [Google Scholar]
- 16.Robertson, G. T., A. Reisenauer, R. Wright, R. B. Jensen, A. Jensen, L. Shapiro, and R. M. Roop II. 2000. The Brucella abortus CcrM DNA methyltransferase is essential for viability, and its overexpression attenuates intracellular replication in murine macrophages. J. Bacteriol. 182:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 18.Smith, M. D., V. Wuthiekanun, A. L. Walsh, and N. J. White. 1995. Quantitative recovery of Burkholderia pseudomallei from soil in Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:488-490. [DOI] [PubMed] [Google Scholar]
- 19.Stewart, F. J., D. Panne, T. A. Bickle, and E. A. Raleigh. 2000. Methyl-specific DNA binding by McrBC, a modification-dependent restriction enzyme. J. Mol. Biol. 298:611-622. [DOI] [PubMed] [Google Scholar]
- 20.Stewart, F. J., and E. A. Raleigh. 1998. Dependence of McrBC cleavage on distance between recognition elements. Biol. Chem. 379:611-616. [PubMed] [Google Scholar]
- 21.Sutherland, E., L. Coe, and E. A. Raleigh. 1992. McrBC: a multisubunit GTP-dependent restriction endonuclease. J. Mol. Biol. 225:327-348. [DOI] [PubMed] [Google Scholar]
- 22.Woods, D. E., J. A. Jeddeloh, D. L. Fritz, and D. DeShazer. 2002. Burkholderia thailandensis E125 harbors a temperate bacteriophage specific for Burkholderia mallei. J. Bacteriol. 184:4003-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wuthiekanun, V., M. D. Smith, D. A. B. Dance, and N. J. White. 1995. Isolation of Pseudomonas pseudomallei from soil in north-eastern Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:41-43. [DOI] [PubMed] [Google Scholar]
- 24.Yabuuchi, E., Y. Kosako, H. Oyaizu, I. Yano, T. Ezaki, and M. Arakawa. 1992. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 36:1251-1275. [DOI] [PubMed] [Google Scholar]