Abstract
Although prostate-specific antigen (PSA) testing can identify early-stage prostate cancers, additional biomarkers are needed for risk stratification. In one study, high levels of the actin-bundling protein, fascin-1, were correlated with lethal-phase, hormone-refractory prostate cancer. Analyses of independent samples are needed to establish the value of fascin-1 as a possible biomarker. We examined fascin-1 by immunohistochemistry in tumour specimens from the Wales Cancer Bank in comparison with nuclear-located ETS-related gene (ERG), an emerging marker for aggressive prostate cancer. Fascin-1 was elevated in focal areas of a minority of tumours, yet fascin-1-positivity did not differentiate tumours of low-, intermediate-, or high-risk Gleason scores and did not correlate with PSA status or biochemical relapse after surgery. Stromal fascin-1 correlated with high Gleason score. Nuclear ERG was upregulated in tumours but not in stroma. The complexities of fascin-1 status indicate that fascin-1 is unlikely to provide a suitable biomarker for prediction of aggressive prostate cancers.
Keywords: Fascin, ERG, prostate cancer, tumour progression, PSA, Gleason
Background
The incidence of prostate cancer is increasing and accounts for 25% of cancer diagnosis in men in the United Kingdom1 and 8% of all cancer cases worldwide as of 2012.2 The rising incidence is partly due to an ageing population and is also secondarily related to routine testing for serum prostate-specific antigen (PSA). Although PSA testing can detect prostate cancers at an early stage, some of which will be clinically relevant, the test has the drawback that it cannot accurately differentiate between indolent tumours (the majority of cases) and aggressive disease. Consequently, there is a risk of overdiagnosis and overuse of radical treatments for indolent tumours3 and under-use of strategies to avoid or delay radical treatments, such as active monitoring. There is thus a major clinical need for novel prognostic prostate cancer biomarkers that can discriminate better between early-stage indolent prostate tumours and those that are likely to progress and metastasise.4,5
In general, the ability of carcinomas to spread locally and later establish metastases is linked to an increased functional capacity of the tumour cells to migrate and invade the nearby tissue, lymph nodes, or local blood vessels. These properties relate in large part to altered expression of cell adhesion molecules and cytoskeletal proteins.6 Fascin-1 is an actin-bundling protein which is not expressed in most adult human epithelia but which becomes upregulated in many carcinomas.7–11 To date, fascin-1 has been studied most extensively in carcinomas of the breast, colon, lung, oesophagus, and stomach. In a comprehensive systematic review and meta-analysis, fascin-1 was found to correlate with increased risk of mortality in primary carcinomas of the colon, breast, and oesophagus; with risk of disease progression in breast and colorectal cancers; and with risk of local or distant metastasis in colorectal carcinoma.12 Since the publication of this meta-analysis, fascin-1 has been correlated with pancreatic cancer progression, although no meta-analysis has yet been performed.13,14
The importance of fascin-1 in carcinoma progression has been demonstrated experimentally in several mouse models. Knock-down of the fascin-1 transcript in human colon or prostate carcinoma cell lines led to decreased xenograft tumour growth and metastasis in mice,15,16 whereas specific overexpression of fascin-1 in the intestine promoted colorectal tumour growth.17 Fascin-1 also promoted tumour progression in a mouse genetic model of pancreatic cancer.13 More recently, a small molecule that inhibits actin bundling by fascin-1 was reported to decrease lung metastasis of syngeneic fascin-1–positive breast tumours in mice.18 Therefore, fascin-1 has emerged as a new prospective therapeutic target.19,20
Information on the relevance of fascin-1 to prostate cancer is limited. The first study of human prostate cancer specimens correlated increased fascin-1 protein with localised and hormone refractory prostate cancer compared with uninvolved tissue.16 Studies of prostate cancer cell lines also demonstrated that FSCN1 messenger RNA (mRNA) is a target of miR-145, a microRNA that is downregulated in prostate cancer, thus leading to elevation of FSCN1 mRNA. Silencing of the FSCN1 transcript in these cell lines resulted in decreased cell migration, invasion, and proliferation.21 Similarly, a nanobody that, when introduced into cells, perturbs the binding of fascin-1 to F-actin, inhibited assembly of invadopodia and cell invasion in PC3 prostate cancer cells.22 Although these studies implicate similar functional roles of fascin-1 in prostate carcinoma cells as in colon or breast carcinoma cells, further studies of independent sets of human prostate tumour specimens are crucially needed to evaluate whether fascin-1 might be suitable as a biomarker to distinguish aggressive from indolent prostate carcinomas at an early stage.
Here, we present the results of an analysis of fascin-1 by immunohistochemistry in prostate carcinoma specimens from the Wales Cancer Bank (WCB). Immunohistochemistry was performed on conventional sections of prostate tumours and on a new tissue microarray (TMA) that comprised 211 tumours that included specimens of low, intermediate, or high Gleason risk scores. We also investigated the relationship between fascin-1 and nuclear-located ETS-related gene (ERG) protein. The TMPRSS2-ERG gene fusion is detected in around 50% of prostate cancers and correlates with aggressive disease progression.23,24 Overexpression of truncated ERG protein in nuclei is detectable by immunohistochemistry and correlates well with occurrence of the gene fusion.25,26 Functional consequences of TMPRSS2-ERG expression include increased cell migration.27 Thus, it was of interest to assess fascin-1 by immunohistochemistry against ERG because the latter is an emerging marker of aggressive prostate cancer progression and because of the possibility that stimulation of cell migration by TMPRSS2-ERG might be a fascin-1–dependent process.
Methods
Patients and surgical specimens
All specimens were obtained as anonymised samples from the WCB (www.walescancerbank.com) as sections prepared from formalin-fixed, paraffin-embedded prostate tumour biopsies. For the conventional sections, 20 specimens were selected at random from the eligible specimens in WCB as examples of low/intermediate (Gleason score 6 [n = 4] or 7 [n = 6]) or high (Gleason score 8 [n = 8], score 9 [n = 1], or score 10 [n = 1]) risk tumours and examined as 4 μm thick sections. Two samples of uninvolved tissues were analysed as controls.
A TMA of prostate tumour samples was constructed by the WCB using a semi-automated TMA Master (3DHISTECH; http://www.3dhistech.com/tma_master) and 0.6 mm diameter cores. The TMA contained 211 prostate samples of low, intermediate, and high Gleason risk scores from 158 patients; for 53 patients, including those with multi-focal disease, multiple samples from different locations had been taken (Supplementary Datafile). In all, 145 samples were from prostatectomy specimens and 66 were from trans-rectal, ultrasound-guided needle core biopsies. The median age of the patients was 65 years (range: 40-86 years) with a 5-year median follow-up. The pathological grading of the samples according to Gleason risk score and available patient data are summarised in Table 1. 19 samples were from 18 patients who subsequently developed biochemical relapse following surgery. Biochemical relapse was defined as a PSA rise of greater than 0.2 ng/mL following surgery. Because only 4 patients died in the follow-up period, no meaningful mortality analysis could be undertaken. Each patient sample was assigned a unique ID number by WCB, and each sample ID included this number and a TMA location number. Sample IDs were subsequently linked to a database of demographic and clinico-pathological data. The Gleason score of each core was quality assured by a consultant urohistopathologist in accordance with the 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of prostate carcinoma.28 These studies were approved under the Human Tissue Act (HTA) (WCB project number 13/014 for the conventional sections and 12/007 for the TMA).
Table 1.
Pathological classification by Gleason risk score and patient cohort information for the prostate tumour specimens on the TMA.
Immunohistochemistry
For specimens examined as conventional sections, slides were de-waxed in Histoclear (National Diagnostics, Atlanta, USA) followed by re-hydration by sequential washes in 100% and 70% ethanol and then water. Antigen retrieval was carried out in hot 10 mM sodium citrate buffer at pH 6.0 for 20 minutes. Immunohistochemistry was performed with a mouse monoclonal antibody to fascin-1 (clone 55k2; Dako, Denmark) at 1:50 dilution for 30 minutes, followed by Vectastain Universal Elite ABC immunohistochemistry kit (with 1:100 dilution of secondary antibody) and ImmPACT DAB peroxidase substrate detection reagent (all from Vector Labs, Peterborough, UK). Slides were then washed in cold running water for 5 minutes and counter-stained in Gill’s hematoxylin (Sigma-Aldrich, Gillingham, UK). A secondary antibody–only control was included in each set of slides stained to assess any background diaminobenzidine tetrahydrochloride (DAB) reactivity. Images were taken under the 4× bright-field objective of a Leica DMI4000B microscope using a Leica DFC410 FX CCD camera controlled by LAS 3.7 software and were exported as tif files. Fascin-1 immunoreactivity of endothelial cells in microvessels provided an internal positive control in each section. The TMA block was sectioned as 4 μm thick sections on SuperFrost Plus slides for experimental immunohistochemistry and hematoxylin-eosin staining. The same method of fascin-1 staining was used for samples on the TMA. ETS-related gene staining of the TMA was performed by UCL Advanced Diagnostics (London, UK). Sections underwent automated dewaxing, followed by automated heat-induced epitope retrieval (HIER) on Leica Bond-III (Leica Biosystems) machines. Heat-induced epitope retrieval was performed with Leica Bond ER2 (pH 9.0, Cat. No. AR9640) for 30 minutes at 100°C. Peroxidase block (part of the Leica Bond Refine detection kit, Cat. No. DS9800) was applied for 5 minutes, followed by ERG antibody (Epitomics, Slough, UK; Clone EP111, Cat. No. AC-0105, diluted 1/150 in Leica Bond diluent, Cat. No. AR9352) for 15 minutes. Sections were then incubated in Leica Bond secondary antibody for 8 minutes, followed by Leica Bond Polymer for 8 minutes. Between each reagent step, sections were rinsed in Leica Bond Wash (Cat. No. AR9590) and deionised water. Bound antibody was visualised by 10-minute incubation with DAB and DAB enhancement with 0.5% copper sulphate for 5 minutes. Sections were counterstained with hematoxylin for 45 seconds and dehydrated through graded alcohol, cleared in xylene, and mounted in DPX media (Sigma). All TMA slides were scanned and photographed with a Zeiss Axio Scan.Z1.
Analysis and scoring of immunohistochemical staining
Fascin-1 positivity of tumour cells was defined as cytoplasmic staining. Conventional sections were viewed independently, blinded to Gleason grade, by 2 observers and scored according to the extent of the area of fascin-1 staining within the tumour and also the staining intensity relative to uninvolved tissue. Fascin-1 positivity of the tumour stroma was assessed in relation to the stroma of uninvolved tissues. For the TMA, scoring for fascin-1 was blinded to Gleason grade and ERG status and was based on the proportion of positively stained cells within the tumour on a scale from 0 to 3: 0 = no staining; 1 = staining of 1% to 25% of cells; 2 = staining of 25% to 50% of cells; 3 = staining of >50% of cells. Staining of stromal cells was assessed visually. Blinded scoring of TMA samples for ERG was based on a combined evaluation of intensity (scale 0-3) and proportion of tumour cells with nuclear ERG (scale 0-6), giving a total scoring range of 0 to 18. Statistical analysis was carried out with GraphPad Prism Version 5.0b. The distribution of the data was checked visually using frequency distribution graphs (histograms). Both fascin-1 and ERG datasets were not normally distributed, so median and interquartile ranges (IQR) were computed and are presented as box and whisker plots. The Mann-Whitney U test was used to compare data between each test group (eg, fascin-1 score between normal tissue and cancer samples).
Results and Discussion
Fascin-1 is elevated in prostate carcinomas and their adjacent stroma and has focal and variable localisation patterns
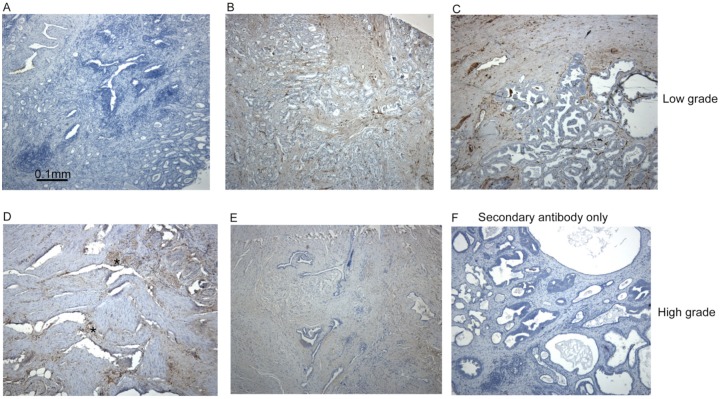
Immunohistochemistry of conventional sections for fascin-1 demonstrated limited staining of stromal cells or microvascular endothelium in samples of uninvolved tissue; these served as a positive control for the specificity of the antibody (Figure 1A). In low/intermediate-grade (Gleason score 6 or 7) tumour specimens, weak staining for fascin-1 was apparent in stromal cells in 8 of the 10 specimens examined (Figure 1B, C). Only a minority of the low/intermediate-grade tumours examined (2 of 10 cases) contained fascin-1–positive tumour cells, and these corresponded to <10% of the tumour cells (Figure 1C). High-grade (Gleason score 8-10) tumours showed wide variability in the extent and intensity of fascin-1 staining. In all cases (10 of 10 samples), there was widespread stromal staining (Figure 1D, E). Three tumours also showed fascin-1 staining in focal groups of tumour cells (Figure 1D, examples of small areas of fascin-1–positive tumour cells are marked with asterisks). No staining was detected in the absence of the primary antibody (Figure 1F).
Figure 1.
Fascin-1 staining patterns in human prostate carcinomas. Examples of fascin-1 staining in conventional sections of prostate carcinomas. (A) Uninvolved tissue; (B, C), low/intermediate grade, Gleason score 7 tumours; (D)-(F), high grade, Gleason score 9 tumours. Focal areas of fascin-1–positive tumour cells are indicated by asterisks in (D). All sections were counter-stained with hematoxylin-eosin.
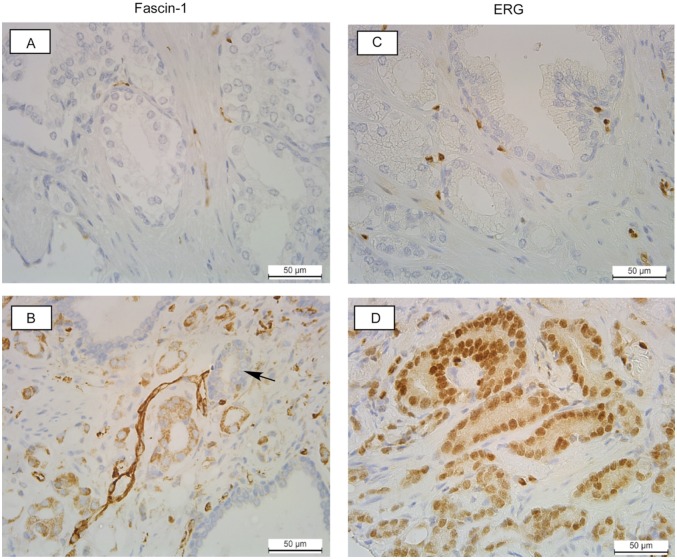
Within the TMA specimen set, fascin-1 staining patterns were in agreement with those observed on the conventional sections and were for the most part patchy and focal. On the TMA, 62% of the samples (n = 131) had fascin-1 staining in stromal cells as well as in the epithelial cells, and stromal staining was frequently more intense than that in the epithelial cells (Figure 2B and Supplementary Datafile). As observed in the conventional sections, areas of fascin-1–positive glands in tumours were often sharply limited, such that a fascin-negative gland was surrounded by fascin-positive glands (Figure 2B). We conclude that fascin-1 staining of prostate tumours is very heterogeneous and includes elevation of fascin-1 in stromal cells more commonly than in tumour cells.
Figure 2.
Examples of fascin-1 and ETS-related gene (ERG) staining patterns in human prostate carcinomas from the tissue microarray. (A, B) Staining for fascin-1; (C, D), staining for ERG. (A) and (C) are from Gleason grade 6 tumours with fascin-1 (A) or ERG (C), staining that is indistinguishable from the normal tissue. (B) Example of a Gleason grade 8 tumour with elevated fascin-1 in the stroma and de-differentiated glands. Arrow indicates a predominantly fascin-1–negative gland adjacent to 2 fascin-1–positive glands. (D) Example of a Gleason grade 7 tumour with elevated nuclear ERG in most cells.
Relationship of fascin-1–positive tumour cells or stroma to clinico-pathological characteristics of the TMA study set
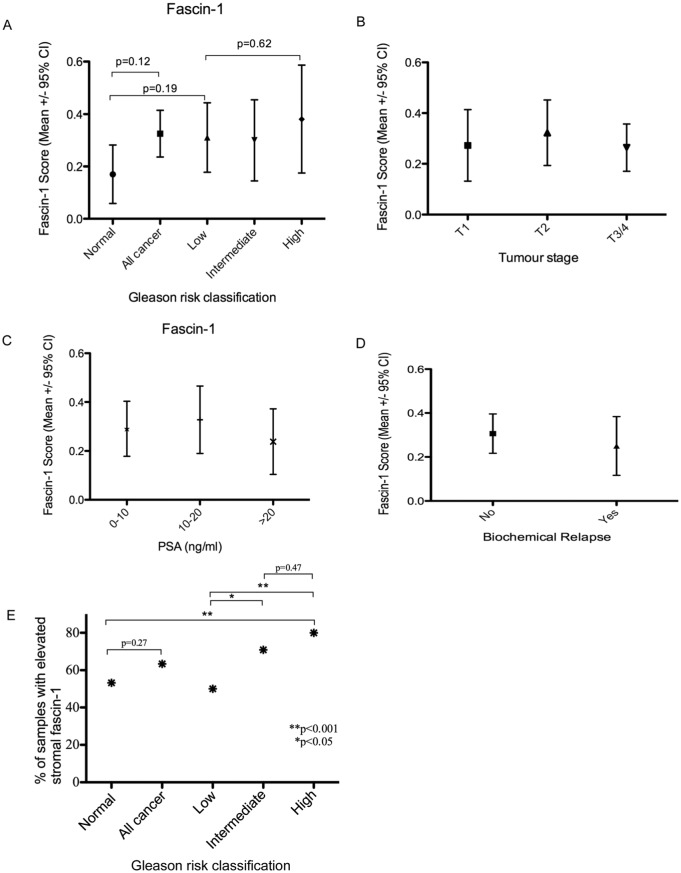
The scoring data for fascin-1 (see ‘Methods’ section) from the TMA were analysed in relation to the anonymous clinico-pathological characteristics of the patients. The fascin-1 score for tumour cells tended to be elevated in prostate tumour samples relative to the normal prostate tissue, but this did not reach statistical significance (median score = 0, IQR 0-1, range, 0-3 in the tumour samples, vs median score = 0, IQR 0-0, range 0-1 in normal prostate tissue, P = .12; Figure 3A). A notable limiting factor in the analysis was that relatively few tumours (15 of 211 samples on the TMA) included more than 10% of tumour cells that were positive for fascin-1. When tumour samples were analysed according to Gleason risk score (Table 1), elevation of fascin-1 in comparison with the normal tissue was not apparent (normal, fascin median score = 0, IQR 0-0, range 0-1; low risk: fascin median score = 0, IQR 0-1, range 0-3; intermediate risk: fascin median score = 0, IQR 0-1, range 0-2; high risk: fascin median score = 0, IQR 0-1, range 0-3; Figure 3A). Furthermore, there was no trend of fascin-1 score with tumour stage (Figure 3B) or with patient PSA status at the time of biopsy (Figure 3C). Fascin-1 score was also examined in relation to biochemical relapse after surgery (18 of 145 patients); no relationship was apparent (Figure 3D). Fascin-1 staining was seen more commonly in the tumour-associated stroma, with a trend towards a higher incidence of positivity seen in high-risk tumours. Thus, 53.2% (n = 25) of normal samples, 50.0% (n = 37) of low-risk tumours, 70.9% (n = 39) of intermediate-risk tumours, and 80.0% (n = 28) of high-risk tumours had elevated stromal fascin-1 staining (Supplementary Datafile). There were a greater number of samples with positive stromal staining when comparing low Gleason risk versus intermediate-risk tumours (P = .036), low-risk versus high-risk tumours (P = .009), or normal tissue versus high-risk tumours (P = .035) (Figure 3E). When accounting for Bonferroni correction for these 3 sub-analysis (the statistically significant P-value using Bonferroni correction = 0.05/3 = 0.017), only the stromal fascin-1 staining in high-risk tumours compared with low-risk tumours reached statistical significance (P = .009). In the colon, elevated fascin-1 has been associated with inflammatory states29; it is unknown whether elevated stromal fascin-1 in prostate tumours might result from inflammatory cues.
Figure 3.
Assessment of fascin-1 status against clinico-pathological characteristics of human prostate carcinomas from the tissue microarray. (A) Relationship between fascin-1 score and Gleason risk classification of the TMA tumour specimens. (B) Relationship between fascin-1 score and tumour stage (stage T1 = 12 tumours, T2 = 93 tumours, T3/4 = 106 tumours). (C) Relationship between fascin-1 score and serum PSA levels of the patients prior to prostate biopsy. Serum PSA was between 0 and 10 ng/mL for 108 patients; between 10 and 20 ng/mL for 61 patients, and >20 ng/mL for 42 patients. (D) Relationship between fascin-1 score and occurrence of biochemical relapse after surgery. The data set includes 19 samples from 18 patients with biochemical relapse and 160 from patients who did not undergo biochemical relapse. (E) Relationship between elevated stromal fascin-1 (scored as yes or no, see Supplementary Datafile) and Gleason risk classification. Data in (A) to (D) are presented as box and whisker plots, and statistical analyses were carried out by Mann-Whitney U test. CI indicates confidence interval; PSA, prostate-specific antigen; TMA, tissue microarray.
Relationship of fascin-1 positivity to nuclear-located ERG
We next compared the data obtained for fascin-1 in tumour cells with an emerging predictive immunohistochemical marker of prostate cancer aggressiveness, nuclear ERG. TMPRSS2-ERG expression has been related to prostate cancer progression and, in mouse models, to increased tumour growth and metastasis to bone.30,31 In view that ERG activity in prostate cancer cell lines results in increased migratory capacity,27 it was also of interest to consider whether fascin-1 might be upregulated in a TMPRSS2-ERG-dependent pathway. From staining of the TMA for ERG, 183 of the 211 specimen cores were informative for ERG staining. Nuclear ERG reports on the truncated ERG protein expressed as a result of the TMPRSS2-ERG gene fusion that is present in approximately 50% of prostate cancers.23–26 In uninvolved tissue, nuclear ERG was detected in only a small number of cells and appeared random with regard to cell type and tissue organisation (Figure 2C). In contrast, nuclear ERG was clearly elevated in the tumour cells of 44% (n = 81) of tumour specimens (Supplementary Datafile). As expected, nuclear ERG was restricted to the carcinoma cells and was not detected in stromal cells (Figure 2D).
Quantified scoring of nuclear ERG positivity demonstrated that nuclear ERG is upregulated in prostate tumours (ERG median score = 3, IQR 0-10, range 0-18) compared with normal prostate epithelium (ERG median score = 0, IQR 0-3, range 0-12, P < .001; Figure 4A). Further analyses of the tumour samples as a function of Gleason score demonstrated that the mean nuclear ERG score was higher in intermediate-risk tumours (ERG median score = 5, IQR 0-12, range 0-18) than in low-risk tumours (ERG median score = 3, IQR 0-8, range 0-18, P < .05; Figure 4A). Although the high-risk tumours had greater nuclear ERG scores (ERG median score = 4, IQR 0-11, range 0-18) than normal tissue (ERG median score = 0, IQR 0-3, range 0-12, P > .001) or low-risk tumours (ERG median score = 3, IQR 0-8, range 0-18), the nuclear ERG score was not significantly increased between low- and high-risk tumours (Figure 4A). The level of nuclear ERG in high-risk tumours was diminished relative to intermediate-risk tumours (high risk, ERG median score = 4, IQR 0-11, range 0-18 vs intermediate risk, ERG median score = 5, IQR 0-12, range 0-18; Figure 4A). These observations are in line with previous studies of ERG in prostate tumours.25,26
Figure 4.
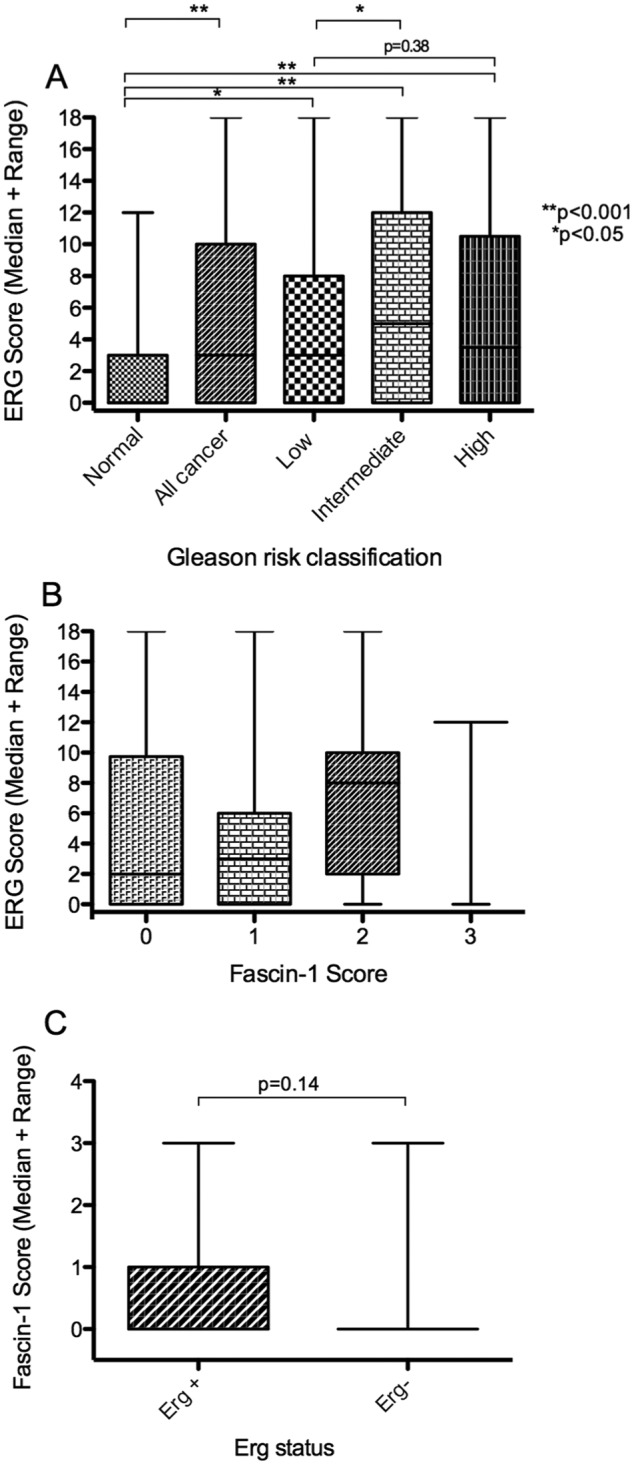
Analysis of fascin-1 in relation to nuclear ERG status in human prostate carcinomas from the tissue microarray. (A) Correlation of increased nuclear ERG score with Gleason risk classification. (B) Relationship between fascin-1 score and ERG score. (C) Relationship between fascin-1 score and ERG-positive or ERG-negative status, where ERG negativity is taken as tumours containing <10% of cells with nuclear ERG. On the TMA, 183 samples were informative for ERG staining, of which 81 samples were ERG positive. Statistical analyses were carried out by Mann-Whitney U test. ERG indicates ETS-related gene; TMA, tissue microarray.
There was no relationship between fascin-1 positivity and ERG score in 183 tumours from the TMA (Figure 4B). However, the data are very limited because only 3 tumours had a fascin-1 score of 3. Because only a small number of the specimens examined contained more than 10% fascin-1–positive tumour cells, the data were also examined by grouping the ERG scores into 2 categories of ERG positive (>10% of cells with nuclear ERG) and ERG negative. Some ERG-positive tumours also had a high fascin-1 score, but this did not reach statistical significance (ERG-negative tumours had a fascin score median of 0, IQR of 0-0, and range of 0-3, whereas ERG-positive tumours had a fascin-1 score median of 0, IQR of 0-1, and range of 0-3, P = .14; Figure 4C). Thus, the presence of fascin-1 in tumour cells does not correlate with nuclear ERG.
Conclusions
In this set of prostate carcinomas specimens from WCB, only 8% of the tumours on the TMA contained >10% fascin-1–positive carcinoma cells which were typically detected in variable, focal patches, adding to the complexity of the scoring. Fascin-1 was more commonly elevated in cells of the tumour stroma, and stromal fascin-1 was significantly elevated in high Gleason score versus low Gleason score tumours. The fascin-1 status of tumour cells did not correlate with Gleason score, tumour stage, serum PSA levels, or biochemical relapse following surgery. In contrast, nuclear ERG was clearly elevated in tumour cells and correlated well with Gleason score. Fascin-1 status did not correlate with nuclear ERG status. Overall, these results indicate that, unlike in breast and colon carcinomas, fascin-1 immunohistochemistry is unlikely to provide a robust biomarker for early prediction of aggressive prostate cancers.
Supplementary Materials
Acknowledgments
The authors thank Wales Cancer Bank for making available the tumour specimens for this project and UCL Advanced Diagnostics for conducting ERG staining of the TMA.
Footnotes
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totalled 445 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a University of Bristol Cancer Research Fund grant to R.M.M. and a Prostate Cancer UK grant to A.R.C. (PG12-16). R.M.M. is Joint Principal Investigator on a Cancer Research UK (C18281/A19169) Programme Grant (the Integrative Cancer Epidemiology Programme). R.M.M. is supported by the National Institute for Health Research (NIHR) Bristol Nutritional Biomedical Research Unit based at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The funding bodies were not involved in the design of the study or collection, analysis, and interpretation of data or in writing the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
RMM, ARC, and JCA designed the study. MTJ and CSP carried out experiments. CSP, JCA, MTJ, and RMM analysed data. CSP, MTJ, and JCA prepared figures. All authors wrote the paper and approved the final version.
Availability of Data and Material
The data set(s) supporting the results of this article are included within the article and in the supplementary file.
Ethical Approval and Consent to Participate
Wales Cancer Bank holds ethics approval from the Wales REC 3 to give deemed ethics to projects involving anonymised samples. The studies were approved under the HTA as Wales Cancer Bank project number 13/014 for the conventional sections and 12/007 for the tissue microarray.
REFERENCES
- 1. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/survival.
- 2. http://www.wcrf.org/int/cancer-facts-figures/worldwide-data.
- 3.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of screening for prostate cancer. J Natl Cancer Inst. 2003;95:868–878. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 4.Stephan C, Ralla B, Jung K. Prostate-specific antigen and other serum and urine markers in prostate cancer. Biochim Biophys Acta. 2014;1846:99–112. doi: 10.1016/j.bbcan.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Romero OJ, Garcia GB, Campos JF, Touijer KA. Prostate cancer biomarkers: an update. Urol Oncol. 2014;32:252–260. doi: 10.1016/j.urolonc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Fife CM, McCarroll JA, Kavallaris M. Movers and shakers: cell cytoskeleton in cancer metastasis. Br J Pharmacol. 2014;171:5507–5523. doi: 10.1111/bph.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto Y, Kim DJ, Adams JC. The role of fascins in health and disease. J Pathol. 2011;224:289–300. doi: 10.1002/path.2894. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto Y, Skacel M, Mukherjee A, Lavery I, Casey G, Adams JC. Prognostic significance of fascin expression in advanced colorectal cancer: an immunohistochemical study of colorectal adenomas and adenocarcinomas. BMC Cancer. 2006;6:241. doi: 10.1186/1471-2407-6-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoder BJ, Tso E, Skacel M, et al. The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin Can Res. 2005;11:186–192. [PubMed] [Google Scholar]
- 10.Tsai WC, Jin JS, Chang WK, et al. Association of cortactin and fascin-1 expression in gastric adenocarcinoma: correlation with clinicopathological parameters. J Histochem Cytochem. 2007;55:955–962. doi: 10.1369/jhc.7A7235.2007. [DOI] [PubMed] [Google Scholar]
- 11.Nese N, Kandiloglu AR, Simsek G, et al. Comparison of the desmoplastic reaction and invading ability in invasive ductal carcinoma of the breast and prostatic adenocarcinoma based on the expression of heat shock protein 47 and fascin. Anal Quant Cytol Histol. 2010;32:90–101. [PubMed] [Google Scholar]
- 12.Tan VY, Lewis SJ, Adams JC, Martin RM. Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: a systematic review and meta-analysis. BMC Med. 2013;11:52. doi: 10.1186/1741-7015-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton Li A, Ma JPY, et al. Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology. 2014;146:1386.e1-17–1396.e1-17. doi: 10.1053/j.gastro.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X, Gao S, Ren H, et al. Hypoxia-inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-bundling protein fascin. Cancer Res. 2014;74:2455–2464. doi: 10.1158/0008-5472.CAN-13-3009. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto Y, Parsons M, Adams JC. Dual actin-bundling and protein kinase C-binding activities of fascin regulate carcinoma cell migration downstream of Rac and contribute to metastasis. Mol Biol Cell. 2007;18:4591–4502. doi: 10.1091/mbc.E07-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darnel AD, Behmoaram E, Vollmer RT, et al. Fascin regulates prostate cancer cell invasion and is associated with metastasis and biochemical failure in prostate cancer. Clin Can Res. 2009;15:1376–1383. doi: 10.1158/1078-0432.CCR-08-1789. [DOI] [PubMed] [Google Scholar]
- 17.Schoumacher M, El-Marjou F, Laé M, et al. Conditional expression of fascin increases tumor progression in a mouse model of intestinal cancer. Eur J Cell Biol. 2014;93:388–395. doi: 10.1016/j.ejcb.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Huang FK, Han S, Xing B, et al. Targeted inhibition of fascin function blocks tumour invasion and metastatic colonization. Nat Commun. 2015;6:7465. doi: 10.1038/ncomms8465. [DOI] [PubMed] [Google Scholar]
- 19.Adams JC. Fascin-1 as a biomarker and prospective therapeutic target in colorectal cancer. Expert Rev Mol Diagn. 2015;15:41–48. doi: 10.1586/14737159.2015.976557. [DOI] [PubMed] [Google Scholar]
- 20.Han S, Huang J, Liu B, et al. Improving fascin inhibitors to block tumor cell migration and metastasis. Mol Oncol. 2016;10:966–980. doi: 10.1016/j.molonc.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuse M, Nohata N, Kojima S, et al. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol. 2011;38:1093–1101. doi: 10.3892/ijo.2011.919. [DOI] [PubMed] [Google Scholar]
- 22.Van Audenhove I, Boucherie C, Pieters L, et al. Stratifying fascin and cortactin function in invadopodium formation using inhibitory nanobodies and targeted subcellular delocalization. FASEB J. 2014;28:1805–1818. doi: 10.1096/fj.13-242537. [DOI] [PubMed] [Google Scholar]
- 23.Chevarie-Davis Xu B, Chevalier MS, et al. The prognostic role of ERG immunopositivity in prostatic acinar adenocarcinoma: a study including 454 cases and review of the literature. Hum Pathol. 2014;45:488–497. doi: 10.1016/j.humpath.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Tomlins SA, Bjartell A, Chinnaiyan AM, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56:275–286. doi: 10.1016/j.eururo.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Berg KD, Brasso K, Thomsen FB, et al. ERG protein expression over time: from diagnostic biopsies to radical prostatectomy specimens in clinically localised prostate cancer. J Clin Pathol. 2015;68:788–794. doi: 10.1136/jclinpath-2015-202894. [DOI] [PubMed] [Google Scholar]
- 26.Font-Tello A, Juanpere N, de Muga S, et al. Association of ERG and TMPRSS2-ERG with grade, stage, and prognosis of prostate cancer is dependent on their expression levels. Prostate. 2015;75:1216–1226. doi: 10.1002/pros.23004. [DOI] [PubMed] [Google Scholar]
- 27.Tian TV, Tomavo N, Huot L, et al. Identification of novel TMPRSS2:ERG mechanisms in prostate cancer metastasis: involvement of MMP9 and LXNA2. Oncogene. 2014;33:2204–2214. doi: 10.1038/onc.2013.176. [DOI] [PubMed] [Google Scholar]
- 28.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading Committee The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 29.Qualtrough D, Smallwood K, Littlejohns D, Pignatelli M. The actin-bundling protein fascin is overexpressed in inflammatory bowel disease and may be important in tissue repair. BMC Gastroenterol. 2011;11:14. doi: 10.1186/1471-230X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carver BS, Tran J, Chen Z, et al. ETS rearrangements and prostate cancer initiation. Nature. 2009;457:E1. doi: 10.1038/nature07738. discussion E2–E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deplus R, Delliaux C, Marchand N, et al. TMPRSS2-ERG fusion promotes prostate cancer metastases in bone. Oncotarget. 2017;8:11827–11840. doi: 10.18632/oncotarget.14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.