Abstract
AIM
To compare the efficacy and safety of omeprazole-domperidone combination vs omeprazole monotherapy in gastroesophageal reflux disease (GERD).
METHODS
In a comparative, randomized controlled, phase 4 study, outpatients with GERD were randomly allocated to either group 1 (omeprazole 20 mg + domperidone 30 mg) or group 2 (omeprazole 20 mg) in an equal ratio; 2 capsules daily in the morning were administered for 8 weeks.
RESULTS
Sixty patients were enrolled. Esophagitis reversal was observed in 92% patients in group 1 vs 65.2% in group 2. Approximately, 83.3% patients in group 1 vs 43.3% patients in group 2 demonstrated full cupping of reflux symptoms at 8 weeks. Combined therapy resulted in significantly longer period of heartburn-free days (23 vs 12 days on omeprazole). There were no safety concerns.
CONCLUSIONS
Omeprazole-domperidone combination was more effective than omeprazole alone in providing complete cupping of reflux symptoms and healing of esophagitis in patients with GERD. Both the treatments were well tolerated with few reports of adverse events.
TRIAL REGISTRATION
This trial is registered with http://clinicaltrials.gov, number NCT02140073.
Keywords: Omeprazole, domperidone, gastroesophageal reflux, visual analogue pain scale
Introduction
Gastroesophageal reflux disease (GERD) is a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications such as heartburn or acid indigestion.1 It is a common condition with a prevalence of less than 5% in Asia and 10% to 20% in Europe and the United States.2 It has a huge impact on patient’s quality of life.3
The disease severity and the wide symptomatic diversity have led to the need for more individualized therapeutic approach. The diagnosis of GERD is challenging—symptom evaluation and invasive investigations—with endoscopy being the common tool.4
Proton pump inhibitors (PPIs) have been considered as the cornerstone of the GERD treatment as compared with antacids, prokinetics, and H2 receptor blockers as per the American College of Gastroenterology Guidelines, 20135 and World Gastroenterology Organisation Global Guidelines.6 However, there was no effective symptomatic resolution in patients treated alone with PPI as observed in previous studies.2,7 Proton pump inhibitors, as on-demand therapy, might be suitable for long-term management of patients with GERD8; they have a slower onset of action and they do not act on the nonacid component which is also a contributing factor in causing reflux.9
Retention of PPIs in the stomach for longer time due to dysmotility and delayed gastric emptying in patients with GERD may result in an impaired acid suppression. Therefore, their rapid transit to the upper intestine is beneficial. Prokinetic agents act by increasing lower esophageal sphincter pressure, enhancing esophageal peristalsis, gastric emptying, and bowel motility.10
Omeprazole is a highly effective inhibitor of gastric acid secretion; it inhibits the H+/K+-adenosine triphosphatase in the proton pump of gastric parietal cells. Domperidone is a prokinetic which blocks the effects of endogenous dopamine in the gut and speeds up gastrointestinal peristalsis and causes prolactin release.7
Omeprazole and domperidone given in combination did not have any clinically relevant pharmacokinetic interactions.11
A combination therapy comprising a PPI and a prokinetic agent is rational, attractive, and effective treatment modality in patients with GERD. It has been frequently used earlier and is proven to have added therapeutic benefit in patients with GERD.9
Previous clinical trials have suggested increased efficacy of combination therapy in terms of symptomatic and endoscopic responses relative to PPI alone. It may also improve patient’s quality of life.10
The aim of this study was to compare the efficacy and safety of omeprazole-domperidone combination vs omeprazole monotherapy in GERD.
Methods
Participants and study design
The study was an open-label, comparative, parallel, randomized controlled, late-phase study of 12 weeks duration conducted for the first time in the Belarus region. The active drug was administered for 8 weeks. Safety follow-up was done for 4 weeks. It studied the combination of omeprazole (20 mg) enteric coated and domperidone (30 mg) sustained release capsule for treating patients with GERD. Endoscopic evidence of esophagitis healing was an efficacy measure of interest by the physician.
Adult outpatients (aged 18–70 years) with mild to moderate GERD accompanied by heartburn at least twice a week before enrollment and with an endoscopic diagnosis of reflux esophagitis (Los Angeles [LA] grades A-B) were enrolled in the study. The study was conducted in Belarus during the period December 2013 to July 2014. It was performed in accordance with the Declaration of Helsinki and Good Clinical Practice and was approved by the local ethics committee. All patients provided signed informed consent prior to the start of the study.
Patients were excluded from the study if they had refused endoscopic examination, their GERD was accompanied by severe esophagitis (Grade C or D by LA classification)12; those with severe cardiovascular or respiratory failure or other kind of heart arrhythmia, local malignancies, hepatic or renal dysfunction/disease, any other clinically significant medical condition, or a history of alcohol or drug abuse within the previous 6 months, pregnant or breastfeeding women, or women of childbearing age not using effective contraception.
Presence of complications such as ulcer, hemorrhage, perforation, stricture, Barrett esophagus, adenocarcinoma, esophageal mucosa columnar metaplasia extension as assessed by Prague criteria, type 3 pattern of the esophagus as assessed by Toyoda Classification (2004) criteria were the other criteria for the patient exclusion from the trial.13,14
The following drugs were not permitted 1 week prior to the randomization and also during the study (except the study drugs allowed): nonsteroidal anti-inflammatory drugs, aspirin, bisphosphonates, nitrates, calcium antagonists, H2-blockers, prokinetics, antacids (except for those tested in the trial), clopidogrel, and other substances that could affect the relief of symptoms related to acid secretion. Proton pump inhibitors were prohibited 14 days prior to and during the trial.
Randomization and treatment
Patients were administered Gastroesophageal Reflux Disease Questionnaire (GERD-Q) questionnaire and esophagogastroduodenoscopy (EGD) at screening for patient enrollment in the study.
After screening, patients were sequentially randomized to study treatment in an equal ratio of 1:1 using a computerized random number table generated by the WinPepi statistical program.
Patients were randomly assigned to either group 1: omeprazole 20 mg + domperidone 30 mg (Omez-DSR; Dr. Reddy’s Laboratories) or group 2: omeprazole 20 mg; both the treatments were administered as a daily dose of 2 capsules in the morning on an empty stomach 30 minutes before breakfast for 8 weeks. The first dose of the drug was administered in the presence of the investigator.
All randomized patients were dispensed study medications for the period of 8 weeks. Patient diaries were given to record their reflux symptoms. Treatment compliance was assessed by the unused returned drugs and empty packages.
Patients were followed up for 28 days after the end of treatment for monitoring of adverse events (AEs) assessment.
Assessments
Visual analogue scale
Heartburn severity was assessed using a 10-cm visual analogue scale (VAS) with 0 (no symptoms) to 10 (maximum symptom severity). Patients were asked at the beginning and at the end of 8 weeks, to mark the severity of heartburn against the VAS considering their condition for the last week.
GERD-Q questionnaire
Patients were administered with self-assessment GERD-Q questionnaire at screening and at the end of treatment (8 weeks) for recording their reflux symptoms. It comprises 4 positive predictors of GERD: heartburn and regurgitation, sleep disturbance, use of over-the-counter medication in addition to that prescribed, and 2 negative predictors of GERD: epigastric pain and nausea. Scores ranging from 0 to 3 were applied for the positive predictors and from 3 to 0 (reversed order, where 3 = none) for negative predictors. The GERD-Q score was calculated as the sum of these scores (total score: 0–18). Patients with the GERD score 7 or more were subjected to screening procedures and EGD in the study (Supplementary Material).
Esophagogastroduodenoscopy
Reflux esophagitis was diagnosed by EGD during screening and at the end of treatment at 8 weeks. It was graded in accordance with the LA Classification.15 High-resolution video gastroscope (9 mm diameter), the video-signal support HD format, and a capacity to light the mucosa by a narrow wave light for assessing the esophageal mucosa state were used. Each procedure was accompanied by video-recording in the AVI mode.
Partial masking was used for the assessment of EGD done at week 8 of treatment; the specialist conducting endoscopy did not know which therapy was used.
Study end points
Primary end points are as follows:
Incidence and severity of heartburn after 8 weeks of treatment with omeprazole-domperidone fixed dose combination in comparison with omeprazole monotherapy;
Proportion of patients with esophagitis reversed in 8 weeks of therapy among patients suffering from esophagitis at the time of inclusion in the study.
Secondary end points are as follows:
Proportion of patients demonstrating reflux symptoms full cupping;
Number of days lacking heartburn.
Safety evaluation
Physical examination, collection, and monitoring of AEs, serious adverse events, and their relationship to study drug was performed at each visit. The concomitant medications were also reviewed throughout the study.
Statistical analyses
Sample size
The sample size calculation was based on the assumption that the true proportions of responders would be 88% in the test arm and 68% in the reference arm.16,17 Considering 10% dropout, 5% significance level, and 80% power, sample size chosen was 60, with 30 in each arm.
The following variables were used for the descriptive statistics: N, mean, confidence interval (CI: −95.0% to +95.0%), geometrical mean, sum, quartiles (upper and lower), percentiles, dispersion, standard deviation, standard error. Wilcoxon signed rank test and McNemar test were used for comparison within the groups. For comparing the qualitative data, the Fisher 2-tailed test and Cochran-Mantel-Haenszel test were used.
Data of all patients who were randomized were included for statistical analysis. Efficacy analysis was performed on data of patients treated no less than for 4 weeks (28 ± 2 days after randomization) and present at the end-of-treatment visit 28 ± 2 days after randomization at the earliest.
Safety analysis included data of all patients administered at least 1 dose of the test drug or the comparator.
Results
A total of 60 patients were randomly assigned in 1:1 ratio to receive either omeprazole 20 mg 2 capsules per day or omeprazole 20 mg + domperidone 30 mg (Omez-DSR; Dr. Reddy’s Laboratories) 2 capsules per day in the morning 30 minutes before breakfast for 8 weeks.
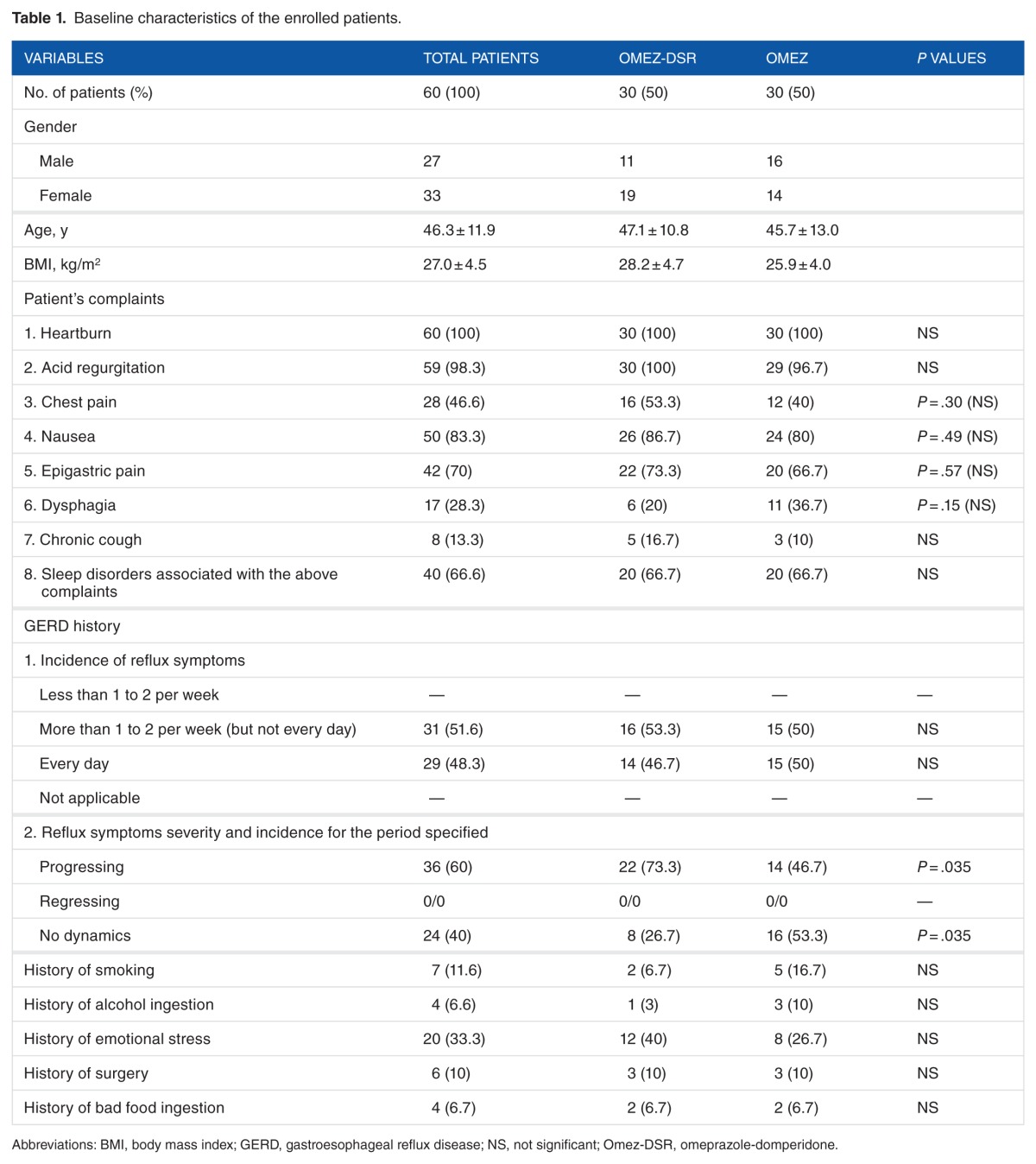
The baseline characteristics of the enrolled patients are shown in Table 1. Both the groups were homogeneous in most of the measured variables.
Table 1.
Baseline characteristics of the enrolled patients.
Nausea was a typical GERD symptom and was observed in more than 80% of cases with the incidence of 2 to 3 days a week or oftener in 56.6% and 76.7% of the patients in groups 1 and 2, respectively. Progressing heartburn and laryngitis were more common in group 1 patients.
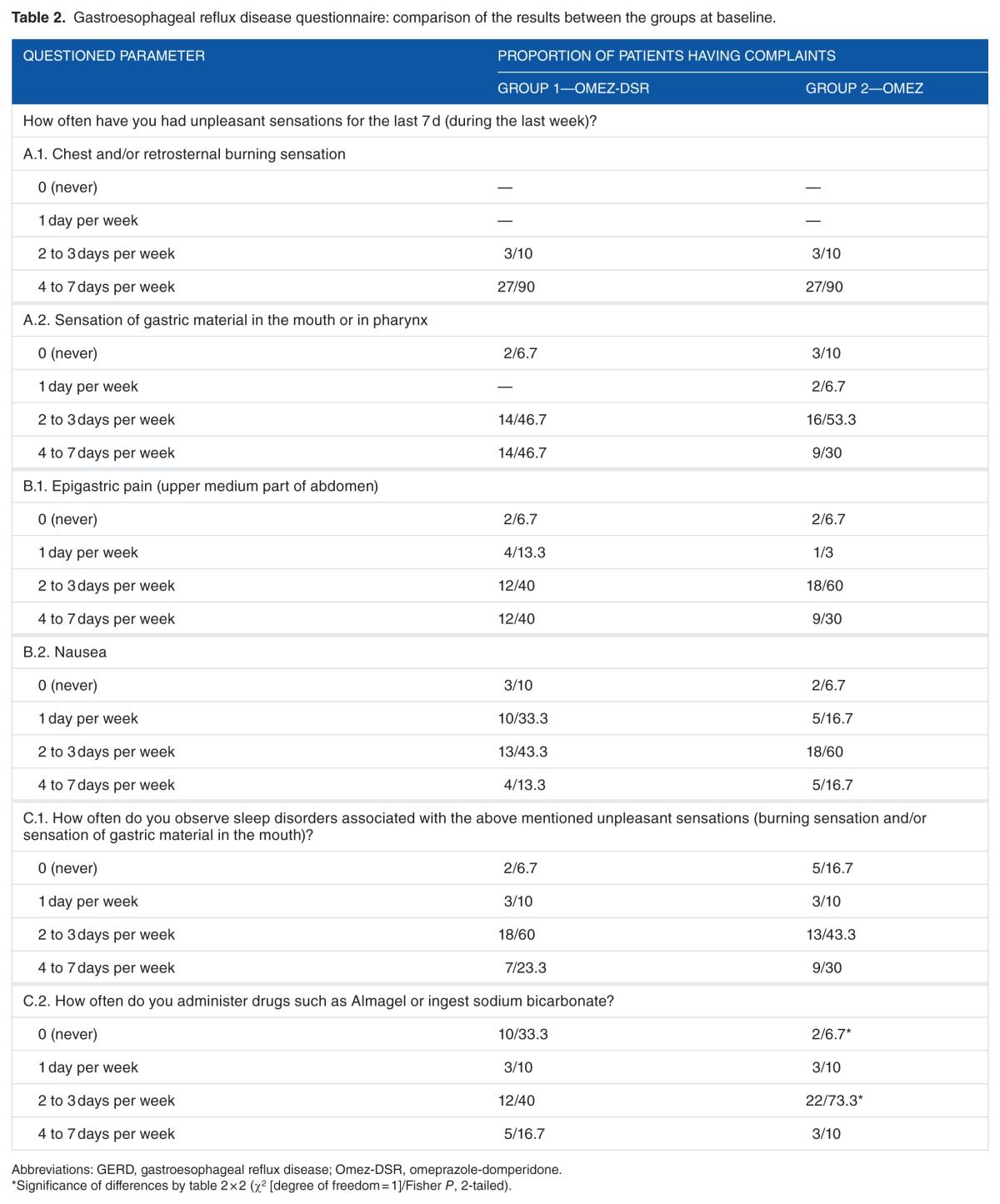
Drugs such as aluminum and magnesium hydroxide gel or sodium bicarbonate were ingested more often by the patients on omeprazole than patients on omeprazole + domperidone fixed dose combination (Table 2).
Table 2.
Gastroesophageal reflux disease questionnaire: comparison of the results between the groups at baseline.
All patients completed the study.
Primary Efficacy Analysis
Esophagitis reversal
The proportion of patients with esophagitis was comparatively lesser in group 2 than in group 1, with 25 patients (17 patients of Grade A and 8 patients of Grade B) having esophagitis at baseline in group 1 and 23 patients (18 patients of Grade A and 5 patients of Grade B) having esophagitis at baseline in group 2.
After treatment, at 8 weeks, 2 patients had Grade A esophagitis, whereas it was absent in 92.0% of patients with initial esophagitis in the group 1. In group 2, 8 patients had Grade A esophagitis, whereas it was absent in 65.2% of all patients with initial esophagitis in group 2.
Heartburn incidence and severity at the end of treatment
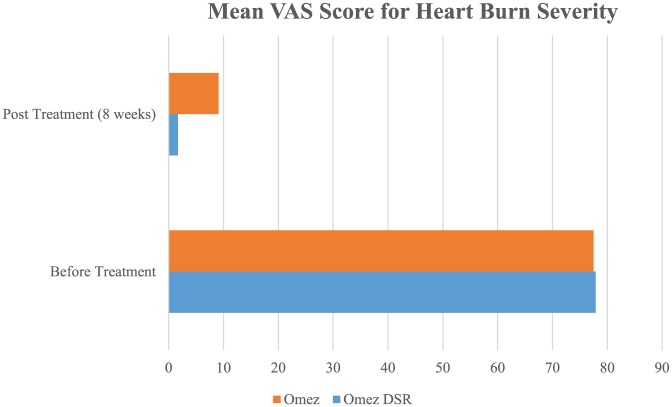
The reduction in heartburn severity as assessed by VAS was observed in all (100%) patients by the end of treatment in both the groups. Before treatment, mean VAS score for heartburn severity was 77.9 ± 11.7 and 77.5 ± 12.7 (95% CI of mean: 72.69–83.1 and 72.70–82.2; P = .898) in Omez-DSR and Omez groups, respectively. After treatment, at 8 weeks, mean VAS score for heartburn severity was 1.7 ± 3.30 and 9.1 ± 6.48 (95% CI of mean: 0.432–2.9 and 6.21–11.96; P = .000) for Omez-DSR and Omez groups, respectively (Figure 1).
Figure 1.
Mean VAS score for heartburn severity.
The incidence of heartburn was also observed equally often in both the groups. At baseline, 10% of patients complained of heartburn 2 to 3 days per week and 90% of patients complained for 4 to 7 days per week in both the groups.
Secondary Efficacy Analysis
Reflux symptoms relief
Proportion of patients demonstrating reflux symptoms complete cupping in 8 weeks of treatment was comparatively higher in group 1 as compared with group 2 (25 patients [83.3%] [95% CI = exact 95% CI (Fisher) = 65.3–94.4] in group 1 vs 13 patients [43.3%] [95% CI = exact 95% CI (Fisher) = 25.5–62.6] in group 2). The odds ratio of the symptoms cupping was 6.54 (P = .003), ie, symptom relief was prognostically 6 times higher when omeprazole was administered in combination with domperidone.
In group 1, baseline GERD mean score was 7.7 ± 0.7; 95% CI = 7.5 to 7.9. After treatment, the mean score was 5.0 ± 0.5; 95% CI = 3.0 to 7.0. In group 2, baseline GERD mean score was 7.8 ± 0.7; 95% CI = 7.5 to 8.0. After treatment, the mean score was 5.8 ± 0.6; 95% CI = 3.6 to 8.0.
Number of days without heartburn
The omeprazole combined with domperidone therapy for 8 weeks was accompanied by a significantly longer period of heartburn-free days: 22.8 ± 3.0 days (95% CI = 21.6–23.9 days) vs 11.8 ± 4.3 days (95% CI = 10.2–13.4 days) on omeprazole (P = .00).
Safety
Both the treatments were well tolerated. In total, 5 side effects (SEs) were reported which were mild in severity—2 SEs (galactorrhea and headache in 1 patient each) were reported in group 1 (6.7%; 95% CI = 0.8%–22.1%) and 3 SEs (breast swelling in 1 patient and headache in 2 patients) were reported in group 2 (10%; 95% CI = 2.1%–26.5%).
“Good” and “Very good tolerance” was observed in 25 and 5 patients, respectively (total 30 patients or 100%) in group 1 and in 19 and 10 patients, respectively (total 29 patients or 96.7%) in group 2.
Discussion
This study has tried to address whether prokinetic added to the PPI has any advantage of healing rate or symptom relief effects in patients with GERD. This was the first study conducted in Belarus region in patients with GERD. The study used the high-resolution video gastroscope (9 mm) for endoscopic measurement of esophagitis reversal.
Proton pump inhibitors, widely prescribed for GERD management, despite their known advantages over other treatment drugs, have certain limitations. They have a comparatively slower onset of action and they need several doses to achieve maximum acid suppression; thus, on-demand therapy for symptom relief is challenging with the use of PPIs.18
Prokinetic agents increase lower esophageal sphincter pressure and enhance esophageal peristalsis and bowel motility; this helps in rapid transit of PPIs to the upper intestine, which is essential to prevent retention of PPIs in the stomach and thus preventing impaired acid suppression.10,11
Therefore, it is beneficial to add a prokinetic to a PPI therapy in the treatment of GERD.
In our study, we had compared omeprazole-domperidone fixed dose combination with the omeprazole monotherapy.
Domperidone had been chosen as a prokinetic drug. It does not cross the blood-brain barrier as metoclopramide does, has a fewer SEs,19 and a lower cardiovascular risk while having a good clinical efficacy.20
Both erythromycin and domperidone were effective in improving symptom score as compared with other prokinetics in patients with gastroparesis.21
Cisapride is another prokinetic drug used earlier; however, it is known to cause QT prolongation and has a higher cardiovascular risk.
It has been indicated earlier that omeprazole 40 mg is significantly superior to omeprazole 20 mg in the control of intragastric pH. Thus, we have selected 40-mg dose of omeprazole for the treatment of patients with GERD.5
As per our study results, combination of omeprazole and domperidone (group 1) seemed to be more effective than omeprazole alone (group 2) in providing symptomatic relief to patients with mild or moderate GERD. Oesophagitis reversal was also comparatively better in group 1 patients. The response to omeprazole alone was lower than those reported in other studies.
The superiority of omeprazole combined with domperidone as compared with omeprazole alone was proven in a study by Suzanna; the improvement in frequency scale for symptoms of GERD score was higher in a combined group than in omeprazole alone (7.5 ± 5.9 vs 4.6 ± 3.3; P = .02).10
In a prospective study, no statistically significant difference was observed in symptomatic responses (69.7% in pantoprazole group vs 89.2% in combined therapy; P = .11). Mean symptom score was significantly lower in combined therapy after 8 weeks (3.78 ± 3.62 vs 1.67 ± 2.09; P = .009). In erosive esophagitis, endoscopic healing of esophagitis occurred equally with either regimen (54.5% vs 70.5% in combined therapy; P = .44).22
Mosapride addition to pantoprazole conferred a therapeutic benefit in patients with erosive GERD. This could not be compared in our study as we have not done subgroup analysis and enrolled patients belonged to LA grades A and B. Nevertheless, combination therapy led to a greater therapeutic benefit in our study.
On the contrary, in a retrospective meta-analysis, no added advantage of prokinetic to a PPI was found in terms of symptomatic relief or mucosal healing; however, the combined therapy suggested improved patient’s reported symptom score and quality of life.6
To corroborate our finding, further large-scale, randomized controlled trials on prokinetic-PPI combination therapy are warranted.
Omeprazole used in our study is a good choice; various PPIs used for GERD treatment are comparable regarding symptom relief and overall healing.
Our study demonstrated a relatively higher usage of antacids drugs when omeprazole was administered alone.
In 2014, the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee has recommended use of domperidone-containing medicines to relieve symptoms of nausea and vomiting and reduce dose and duration of treatment as key to minimize its risks, especially in children.23
Frequency scale for symptoms of GERD questionnaire had been used earlier to predict the necessity of adding prokinetics to PPI prior to the treatment.24 We used GERD-Q questionnaire in our study for screening and assessing the treatment response; it had been validated and could prove to be a better gatekeeper than endoscopy for proper diagnostic workup and medical treatment.25
The reduction in heartburn severity is the most apparent objective tool of measurement for the patients with GERD. We used VAS for assessing heartburn severity, as its reproducibility and responsiveness are very well established in the upper gastrointestinal symptoms.26
Open-label design, small sample size, and administration of domperidone for longer duration are few limitations of this study. The recommended dose for domperidone is 10 mg up to 3 times a day with a maximum dose of 30 mg per day.27
In a study by Ortiz et al, high-dose domperidone therapy with doses ranging from 40 to 120 mg/d compared with the standard dose of 40 mg has been explored in patients with nausea and vomiting. Mean duration of therapy was 8 months. Domperidone was found to have a low risk of adverse cardiovascular events and good clinical efficacy.28
Symptom improvement, reflux esophagitis healing, and prevention of complications are the main goals of GERD therapy; symptom relief is the most important criterion for treatment success and has been associated with patient satisfaction and better health–related quality of life.29 Symptom relief is highly predictive of endoscopic healing. This has been proven in our study.
The study drug was very well tolerated and was found to have a good safety profile.
Patient’s quality of life can be highly affected by the GERD. It is associated with a high morbidity in elderly patients or patients with diabetes.
Conclusions
The combination therapy of omeprazole and domperidone may be a therapy of choice in such population groups; further studies are required for confirming safety and efficacy. It might also be beneficial in patients in whom on-demand therapy with PPI remains ineffective.
To conclude, omeprazole-domperidone combined therapy demonstrates significantly greater efficacy (reflux symptoms relief and esophagitis reversal) than omeprazole alone in the treatment of patients with GERD. The study drugs were safe and well tolerated.
Acknowledgments
The authors thank the Medical Writer for editorial assistance.
Footnotes
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1377 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the funds provided by the sponsoring agency (Dr. Reddy’s Laboratories Ltd.).
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
All authors read and approved the final manuscript.
Disclosures and Ethics
The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material.
REFERENCES
- 1.Vakil N, Van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus Group The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren LH, Chen WX, Qian LJ, Li S, Gu M, Shi RH. Addition of prokinetics to PPI therapy in gastroesophageal reflux disease: a meta-analysis. World J Gastroenterol. 2014;20:2412–2419. doi: 10.3748/wjg.v20.i9.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiyama T, Yoshihara M, Tanaka S, Haruma K, Chayama K. Strategy for treatment of nonerosive reflux disease in Asia. World J Gastroenterol. 2008;14:3123–3128. doi: 10.3748/wjg.14.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 6.World Gastroenterology Organisation Global Guidelines Global Perspective on Gastroesophageal Reflux Disease (GERD) http://www.worldgastroenterology.org/guidelines/global-guidelines/gastroesophageal-reflux-disease/gastroesoph-ageal-reflux-disease-english. Update October 2015. [DOI] [PubMed]
- 7.Lowe RC. Medical management of gastroesophageal reflux disease. PART 1 Oral cavity, pharynx and esophagus—review [published online ahead of print May 16, 2006] GI Motility Online. doi: 10.1038/gimo54. [DOI] [Google Scholar]
- 8.Leodolter A, Penagini R. On-demand therapy is a valid strategy in GERD patients: pros and cons. Dig Dis. 2007;25:175–178. doi: 10.1159/000103880. [DOI] [PubMed] [Google Scholar]
- 9.Abbasinazaria M, Panahib Y, Mortazavic SA, et al. Effect of a combination of omeprazole plus sustained release Baclofen versus omeprazole alone on symptoms of patients with gastroesophageal reflux disease (GERD) Iran J Pharm Res. 2014;13:1221–1226. [PMC free article] [PubMed] [Google Scholar]
- 10.Ndraha S. Combination of PPI with a Prokinetic drug in gastroesophageal reflux disease. Acta Med Indones. 2011;43:233–236. [PubMed] [Google Scholar]
- 11.Zhang YF, Chen XY, Dai XJ, et al. Influence of omeprazole on pharmacokinetics of domperidone given as free base and maleate salt in healthy Chinese patients. Acta Pharmacol Sin. 2007;28:1243–1246. doi: 10.1111/j.1745-7254.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- 12.Lundell L, Dent J, Bennet JR, et al. Endoscopic assessment of oesophagitis-clinical and functional and functional correlates and further validation of the Los Angeles Classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong D. Review article: towards consistency in the diagnostic in the endoscopic diagnosis of Barrett’s oesophagus and columnar metaplasia. Aliment Pharmacol Ther. 2004 Oct;20:40–47. doi: 10.1111/j.1365-2036.2004.02132.x. [DOI] [PubMed] [Google Scholar]
- 14.Toyoda H, Rubio C, Befritis R, et al. Detection of intestinal metaplasia in distal esophagus and esophagogastric junction by enhanced-magnification endoscopy. Gastrointest Endosc. 2004;59:15–21. doi: 10.1016/s0016-5107(03)02527-6. [DOI] [PubMed] [Google Scholar]
- 15.ASGE Standards of Practice Committee. Muthusamy VR, Lightdale JR, et al. The role of endoscopy in the management of GERD. Gastrointest Endosc. 2015;81:1305–1310. doi: 10.1016/j.gie.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev. 2007;2:CD003244. doi: 10.1002/14651858.CD003244.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Mandel KG, Daggy BP, Brodie DA, Jacoby HI. Review article: alginate-raft formulations in the treatment of heartburn and acid reflux. Aliment Pharmacol Ther. 2000;14:669–690. doi: 10.1046/j.1365-2036.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 18.Tytgat GN. Shortcomings of the first-generation proton pump inhibitors. Eur J Gastroenterol Hepatol. 2001 Jun;13:S29–S33. [PubMed] [Google Scholar]
- 19.Champion MC, Hartnett M, Yen M. Domperidone, a new dopamine antagonist. CMAJ. 1986;135:457–461. [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz A, Cooper CJ, Alvarez A, Gomez Y, Sarosiek I, McCallum RW. Cardiovascular safety profile and clinical experience with high-dose domperidone therapy for nausea and vomiting. Am J Med Sci. 2015;349:421–424. doi: 10.1097/MAJ.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturm A, Holtmann G, Goebell H, Gerken G. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422–427. doi: 10.1159/000007687. [DOI] [PubMed] [Google Scholar]
- 22.Madan K, Ahuja V, Kashyap PC, Sharma MP. Comparison of efficacy of pantoprazole alone versus pantoprazole plus mosapride in therapy of gastroesophageal reflux disease: a randomized trial. Dis Esophagus. 2004;17:274–278. doi: 10.1111/j.1442-2050.2004.00424.x. [DOI] [PubMed] [Google Scholar]
- 23.The European Medicine Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) report. Mar 7, 2014. [Accessed January 3, 2017]. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Domperidone_31/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500162559.pdf.
- 24.Miyamoto M, Haruma K, Takeuchi K, Kuwabara M. Frequency scale for symptoms of gastroesophageal reflux disease predicts the need for addition of prokinetics to proton pump inhibitor therapy. J Gastroenterol Hepatol. 2008;23:746–751. doi: 10.1111/j.1440-1746.2007.05218.x. [DOI] [PubMed] [Google Scholar]
- 25.Jonasson C, Moum B, Bang C, Andersen KR, Hatlebakk JG. Randomised clinical trial: a comparison between a GerdQ-based algorithm and an endoscopy-based approach for the diagnosis and initial treatment of GERD. Aliment Pharmacol Ther. 2012;35:1290–1300. doi: 10.1111/j.1365-2036.2012.05092.x. [DOI] [PubMed] [Google Scholar]
- 26.Miwa H, Inoue K, Ashida K, et al. Randomised clinical trial: efficacy of the addition of a prokinetic, mosapride citrate, to omeprazole in the treatment of patients with non-erosive reflux disease—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:323–332. doi: 10.1111/j.1365-2036.2010.04517.x. [DOI] [PubMed] [Google Scholar]
- 27.Summary of product characteristics Domperidone 10mg Film-coated tablets. https://www.medicines.org.uk/emc/medicine/18865#POSOLOGY.
- 28.Ortiz A, Cooper CJ, Alvarez A, Gomez Y, Sarosiek I, McCallum RW. Cardiovascular safety profile and clinical experience with high-dose domperidone therapy for nausea and vomiting. Am J Med Sci. 2015;349:421–425. doi: 10.1097/MAJ.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moraes-Filho JP, Pedroso M, Quigley EMM, PAMES Study Group Randomised clinical trial: daily pantoprazole magnesium 40 mg vs. esomeprazole 40 mg for gastro-oesophageal reflux disease, assessed by endoscopy and symptoms. Aliment Pharmacol Ther. 2014;39:47–56. doi: 10.1111/apt.12540. [DOI] [PubMed] [Google Scholar]