Abstract
Recombinant ExoU (rExoU) and yeast extract were used to optimize an in vitro phospholipase assay as a basis for identifying the mechanism for enzyme activation and substrate specificity. Our results support a model in which a eukaryotic protein cofactor or complex facilitates the interaction of rExoU with phospholipid substrates.
ExoU expression by Pseudomonas aeruginosa correlates to an acute cytotoxic response and sepsis (1, 6, 9). In Saccharomyces cerevisiae, ExoU expression causes cellular death (15, 16, 17), fatty-acid release, and vacuolar fragmentation (16, 17). ExoU is a member of the patatin family of phospholipases possessing a catalytic dyad and a glycine-rich motif (14, 16, 17). Mutagenesis of either catalytic amino acid, S142 or D344, abolishes ExoU-mediated cytotoxicity in yeast and mammalian systems (14, 17). Recombinant ExoU (rExoU) phospholipase activity is detectable in vitro, but only if cellular extracts are added to the reaction mixture (17). Specific parameters of the enzymatic assay were investigated to develop biochemical tools to identify the factor that activates rExoU phospholipase activity.
Optimization of an in vitro assay.
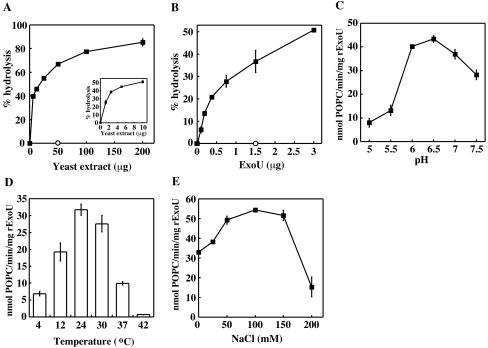
rExoU phospholipase activity was measured as described previously (17). Liposomes consisted of 50% 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), 50% 1-palmitoyl-2-oleoyl-phosphatidylserine (POPS), and 0.54 mol% radiolabeled tracer (1-palmitoyl-2-[1-14C]oleoyl-PC). Hydrolysis of the substrate by 1 μg of rExoU was dose dependent in yeast extract, and 4 μg of yeast proteins demonstrated 50% of the saturated activity (Fig. 1A). The hydrolysis of radiolabeled POPC was also dependent on the amount of rExoU (Fig. 1B). Optimal rExoU activity was observed when the pH of the reaction buffer was between 6.0 and 7.0 and the temperature was between 24 and 30°C in the presence of either yeast or Chinese hamster ovary (CHO) cell extract (Fig. 1C and D and data not shown). The maximal activity of rExoU was observed in buffers containing 50 to 150 mM NaCl (Fig. 1E). Nonionic detergent (Triton X-100 or Tween 20), nondenaturing zwitterionic detergent (CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}), and anionic detergent (sodium dodecyl sulfate) all inhibited rExoU activity at low concentrations (Table 1 and data not shown).
FIG. 1.
Optimization of an in vitro assay. (A) One microgram of rExoU and a range of concentrations of yeast extract from 0 to 200 μg were incubated with radiolabeled liposomes (3.3 mM). After 3 h of incubation at 30°C, phospholipase activity was measured using radiolabeled POPC in POPC-POPS (50:50) liposomes and thin-layer chromatography. Fifty micrograms of yeast extract (open circle) in the absence of rExoU was used as a negative control. (Inset) Assay in the presence of 0 to 10 μg of yeast extract proteins. (B) rExoU was titrated using 4 μg of yeast extract. A noncatalytic mutant, rExoUS142A (open circle), did not hydrolyze the substrate, even in the presence of yeast extract. (C) rExoU (1 μg) was incubated with yeast extract (5 μg) and radiolabeled substrates at various pHs at 30°C for 3 h, and phospholipase activity was measured. (D) The incubation temperature of in vitro assays was varied, and rExoU activity was quantified using 1 μg of rExoU and 5 μg of yeast extract. (E) One microgram of rExoU and 30 μg of yeast extract were incubated in a buffer containing various concentrations of NaCl at 30°C for 3 h.
TABLE 1.
Effect of CaCl2 and detergents on rExoU catalytic activity
| Addition to buffer | Presence of yeast extract | Hydrolysisa (% of that by positive control) | CMCb |
|---|---|---|---|
| None (positive control) | + | 100 | |
| 20 μM Tween 20 | + | 23.1 | 49 μM |
| 40 μM Tween 20 | + | 0.6 | |
| 1.3 mM CHAPS | + | 12.9 | 6.5 mM |
| 3.3 mM CHAPS | + | 0 | |
| 10 mM CaCl2 | − | 0 | |
| 10 mM CaCl2 | + | 61.1 |
One microgram of rExoU and 5 μg of yeast extract were incubated in the presence of CaCl2, Tween 20, or CHAPS at pH 6.5 at 30°C for 3 h. Samples containing rExoU and extract without any additional additives were used as a positive control. Data from a representative experiment of three are shown.
CMC, critical micelle concentration.
rExoU substrate specificity.
rExoU substrate specificity was quantified by using honeybee phospholipase A2 (PLA2) as a positive control (19, 21). rExoU hydrolyzed radiolabeled POPC at a level equivalent to 141% of that hydrolyzed by honeybee PLA2 (Table 2). When 1,2-dioleoyl-l-3-phosphatidyl-l-[3-14C]serine (DOPS) was used to label POPC-POPS (50:50) liposomes, hydrolysis of DOPS was undetectable (Table 2). rExoU, after incubation with liposomes containing 1,2-di[1-14C]palmitoyl-l-3-PC (DPPC) or l-α-dipalmitoyl-[glycerol-U-14C]-phosphatidic acid (DPPA), demonstrated reduced activity (Table 2). To simulate bacterial membranes, liposomes composed of 80% 1-palmitoyl-2-oleoyl-phosphatidylethanolamine (POPE) and 20% 1-palmitoyl-2-oleoyl-phosphatidylglycerol (POPG) labeled with 1-palmitoyl-2-[1-14C]linoleoyl-PE (PLPE) were tested as substrates. rExoU hydrolyzed PLPE in these liposomes at a level equivalent to 86.6% of that hydrolyzed by the positive control (Table 2). The head groups of PC and PE are neutral (zwitterionic), whereas PS and PA carry a net negative charge. We conclude that phospholipids with saturated fatty acids and/or acidic head groups are less suitable substrates for rExoU.
TABLE 2.
Substrate specificities of rExoU
| 14C-labeled phospholipid | Liposome composition | Hydrolysisa (% of that by positive control) |
|---|---|---|
| POPC | PC-PS (50:50) | 141.0 |
| DOPS | PC-PS (50:50) | 0 |
| DPPC | PC-PS (50:50) | 22.1 |
| DPPA | PC-PS (50:50) | 7.9 |
| PLPE | PE-PG (80:20) | 86.6 |
rExoU (34.4 pmol) and 5 μg of yeast extract were incubated with liposomes with various 14C-labeled tracers at pH 6.5 and 30°C for 3 h. Phospholipase activity is shown as a percentage of the activity of a positive control of identical liposomes treated with honeybee venom PLA2 (17.2 pmol). Data from a representative experiment of three are shown.
rExoU activity on lyso-palmitoyl-1-[14C]PC (lysoPC) in POPC-POPS (50:50) liposomes was estimated based on comparing the specific activity of lysoPC to that of POPC. The specific activity of rExoU for lysoPC was approximately twofold lower than that observed for POPC (data not shown).
rExoU requires a eukaryotic proteinaceous cofactor.
rExoU exhibits in vitro PLA2-like activity in the presence of yeast or CHO cell extracts. Bacterial soluble extracts did not activate rExoU (data not shown). Human cytosolic PLA2 (cPLA2) requires calcium ions (3, 5, 13). The addition of CaCl2 and other cations, namely, Mg2+ and Zn2+, did not activate rExoU (Table 1 and data not shown). The mechanism of ExoU activation may involve a eukaryotic cofactor or a modification of the protein. To differentiate between these two models, rExoU was preincubated with yeast extract and then subjected to the addition of substrate. Samples both with and without preincubation demonstrated similar kinetics and levels of hydrolysis (data not shown). In an additional experiment, His6-tagged rExoU was preincubated with yeast extract, and pretreated rExoU was removed from the extract by use of cobalt Sepharose beads. The isolated rExoU after preincubation was inactive, yet phospholipase activity was regained upon the addition of fresh yeast extract (data not shown).
Incubating yeast extract at 56 or 100°C decreased or completely abolished, respectively, its ability to activate rExoU (Fig. 2A). When yeast extract was treated with chymotrypsin before its addition to the assay, rExoU activity was reduced (Fig. 2B) or abolished upon longer protease treatment (data not shown). Size exclusion filtration with a spin column indicated that the activator possessed a molecular mass of over 100 kDa (data not shown). We concluded that the cofactor in yeast could be a large protein or a protein complex of over 100 kDa.
FIG. 2.
The activity of the rExoU cofactor is susceptible to heat and protease treatment. (A) Yeast extract (10 μg) was incubated at 30 or 56°C for 30 min or boiled for 5 min, followed by the addition of 1 μg of rExoU and radiolabeled liposomes. (B) Twenty micrograms of yeast extract was digested with chymotrypsin at 30°C for 15 min. After the proteolysis reaction was stopped by the addition of protease inhibitors, the treated extract was added to 1 μg of rExoU and substrate liposomes.
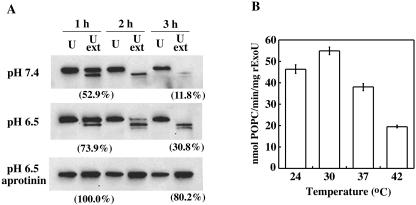
Although cellular extracts are necessary to reconstitute rExoU phospholipase activity, endogenous proteases present in the extracts may degrade rExoU, the cofactor, or both molecules. Loss of the full-length protein and generation of proteolytic products were observed after 1 h (Fig. 3A). The disappearance of rExoU was more extensive at pH 7.4 than at pH 6.5. Higher rExoU activity was retained at pH 6.5 (Fig. 3A). rExoU degradation was decreased, and activity was preserved, by the addition of aprotinin (Fig. 3A). To minimize the protein degradation, rExoU phospholipase activity was measured in the presence of aprotinin at various temperatures after a 15-min incubation. Optimal rExoU activity was observed at 24 to 30°C, and relatively high phospholipase activity was retained at 37°C (Fig. 3B). Our results suggest that the detection of the poor phospholipase activity of rExoU (18) may result from a combination of inappropriate substrates and reaction conditions that induce the proteolysis of rExoU and the cofactor.
FIG. 3.
Incubation conditions affecting the stability of rExoU and phospholipase activity. (A) The degradation of rExoU (1 μg) during incubation at pH 7.4 or 6.5 at 30°C in the presence (U ext) or absence (U) of yeast extract (5 μg) was monitored by Western blotting. Aprotinin (2.3 μg) was added to the incubation mixture at pH 6.5 to minimize rExoU degradation. The samples were used after 1 or 3 h of incubation for the in vitro assay. For positive controls, 1 μg of rExoU or 5 μg of extract was incubated individually, and fresh extract or rExoU was added immediately prior to the assay. Percentages indicate levels of rExoU activity relative to those exhibited by positive controls. (B) The incubation temperature of in vitro assays was varied, and rExoU activity was quantified in the presence of a protease inhibitor. Samples containing 5 μg of rExoU, 5 μg of yeast extract proteins, and 6 μg of aprotinin were incubated with liposomes for 15 min.
Conformation of rExoU.
Circular dichroism was used to determine if rExoU activity is influenced by a conformational change of the protein. In the absence of NaCl at pH 6.5, a circular-dichroism spectrum of rExoU predominantly demonstrated an α-helical protein. Neither addition of liposomes nor alterations in pH and NaCl concentration resulted in a detectable change in the overall secondary-structure content of rExoU (data not shown).
Overview.
Parameters that influenced rExoU activity included the type of substrate liposomes, time, temperature, pH, the presence of NaCl and detergents, the amount of cofactor and enzyme, and the inclusion of protease inhibitors. Overall, conditions were established such that the contribution of endogenous proteases from yeast extract was minimized and rExoU activity was maximized.
The optimized assay was used to examine the substrate specificity of rExoU. In a recent report, rExoU exhibited activity that was about 10-fold greater for lysoPC than for PC (18). Unfortunately, in those studies, lysoPC was presented to rExoU as micelles, while the PC substrate was contained in DPPC gel-phase vesicles (11). Most PLA2 enzymes have high activity in bilayers around the melting temperatures of substrates and only weakly hydrolyze phospholipids from gel-phase bilayers (8, 19), contributing to the minimal phospholipase activities reported. The ability of rExoU to hydrolyze lysoPC is not surprising, given that cPLA2 hydrolyzes 1-palmitoyl-2-lysoPC and patatin cleaves monoacylglycerol and monoacylglycolphosphocholines (2, 10, 13). Degradation of bilayer membrane phospholipids, however, would be expected to have more-significant consequences for cell viability than lysoPC hydrolysis.
The selectivity of ExoU for eukaryotic cells is not based on phospholipid substrate specificity but is related to a proteinaceous cofactor, consistent with the biochemical properties of all other P. aeruginosa type III effectors, ExoS, ExoT, and ExoY (4, 7, 20). The activator for ExoU must be present during substrate hydrolysis, i.e., it must be a cofactor; this process contrasts with one in which ExoU is modified or preactivated. For cPLA2 activity, phosphorylation and interfacial activation are necessary, as are calcium ions (3, 12, 13). It is feasible that rExoU may also require modification or interfacial activation as well as the cofactor for its catalytic activity.
The overall secondary structure of rExoU is not altered in the presence of liposomes. This result suggests that interaction with phospholipid substrates does not alter rExoU conformation or that rExoU may not bind a substrate in the absence of the cofactor.
Acknowledgments
Expert technical assistance was provided by M. Casey.
This work was supported by funds from the National Institutes of Health (grant AI049577).
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, D. L., B. Beames, M. D. Summers, and W. D. Park. 1988. Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochem. J. 252:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark, J. D., L. L. Lin, R. W. Kriz, C. S. Ramesha, L. A. Sultzman, A. Y. Lin, N. Milona, and J. L. Knopf. 1991. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 65:1043-1051. [DOI] [PubMed] [Google Scholar]
- 4.Coburn, J., A. V. Kane, L. Feig, and D. M. Gill. 1991. Pseudomonas aeruginosa exoenzyme S requires a eukaryotic protein for ADP-ribosyltransferase activity. J. Biol. Chem. 266:6438-6446. [PubMed] [Google Scholar]
- 5.Dessen, A., J. Tang, H. Schmidt, M. Stahl, J. D. Clark, J. Seehra, and W. S. Somers. 1999. Crystal structure of human cytosolic phospholipase A(2) reveals a novel topology and catalytic mechanism. Cell 97:349-360. [DOI] [PubMed] [Google Scholar]
- 6.Finck-Barbançon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 7.Fu, H., J. Coburn, and R. J. Collier. 1993. The eukaryotic host factor that activates exoenzyme S of Pseudomonas aeruginosa is a member of the 14-3-3 protein family. Proc. Natl. Acad. Sci. USA 90:2320-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabriel, N. E., N. V. Agman, and M. F. Roberts. 1987. Enzymatic hydrolysis of short-chain lecithin/long-chain phospholipid unilamellar vesicles: sensitivity of phospholipases to matrix phase state. Biochemistry 26:7409-7418. [DOI] [PubMed] [Google Scholar]
- 9.Hauser, A. R., P. J. Kang, and J. N. Engel. 1998. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807-818. [DOI] [PubMed] [Google Scholar]
- 10.Hirschberg, H., J. Simons, N. Dekker, and M. R. Egmond. 2001. Cloning, expression, purification and characterization of patatin, a novel phospholipase A. Eur. J. Biochem. 268:5037-5044. [DOI] [PubMed] [Google Scholar]
- 11.Lee, B. S., S. A. Mabry, A. Jonas, and J. Jonas. 1995. High-pressure proton NMR study of lateral self-diffusion of phosphatidylcholines in sonicated unilamellar vesicles. Chem. Phys. Lipids 78:103-117. [DOI] [PubMed] [Google Scholar]
- 12.Lin, L. L., M. Wartmann, A. Y. Lin, J. L. Knopf, A. Seth, and R. J. Davis. 1993. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72:269-278. [DOI] [PubMed] [Google Scholar]
- 13.Nalefski, E. A., L. A. Sultzman, D. M. Martin, R. W. Kriz, P. S. Towler, J. L. Knopf, and J. D. Clark. 1994. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca(2+)-dependent lipid-binding domain and a Ca(2+)-independent catalytic domain. J. Biol. Chem. 269:18239-18249. [PubMed] [Google Scholar]
- 14.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A(2) inhibitors. J. Biol. Chem. 278:41326-41332. [DOI] [PubMed] [Google Scholar]
- 15.Rabin, S. D. P., and A. R. Hauser. 2003. Pseudomonas aeruginosa ExoU, a toxin transported by the type III secretion system, kills Saccharomyces cerevisiae. Infect. Immun. 71:4144-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato, H., and D. W. Frank. 2004. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 53:1279-1290. [DOI] [PubMed] [Google Scholar]
- 17.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbançon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura, M., T. Ajayi, L. R. Allmond, K. Moriyama, J. P. Wiener-Kronish, and T. Sawa. 2004. Lysophospholipase A activity of Pseudomonas aeruginosa type III secretory toxin ExoU. Biochem. Biophys. Res. Commun. 316:323-331. [DOI] [PubMed] [Google Scholar]
- 19.Upreti, G. C., and M. K. Jain. 1980. Action of phospholipase A2 on unmodified phosphatidylcholine bilayers: organizational defects are preferred sites of action. J. Membr. Biol. 55:113-121. [DOI] [PubMed] [Google Scholar]
- 20.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu, B. Z., F. Ghomashchi, Y. Cajal, R. R. Annand, O. G. Berg, M. H. Gelb, and M. K. Jain. 1997. Use of an imperfect neutral diluent and outer vesicle layer scooting mode hydrolysis to analyze the interfacial kinetics, inhibition, and substrate preferences of bee venom phospholipase A2. Biochemistry 36:3870-3881. [DOI] [PubMed] [Google Scholar]