Abstract
Pyruvate:quinone oxidoreductase catalyzes the oxidative decarboxylation of pyruvate to acetate and CO2 with a quinone as the physiological electron acceptor. So far, this enzyme activity has been found only in Escherichia coli. Using 2,6-dichloroindophenol as an artificial electron acceptor, we detected pyruvate:quinone oxidoreductase activity in cell extracts of the amino acid producer Corynebacterium glutamicum. The activity was highest (0.055 ± 0.005 U/mg of protein) in cells grown on complex medium and about threefold lower when the cells were grown on medium containing glucose, pyruvate, or acetate as the carbon source. From wild-type C. glutamicum, the pyruvate:quinone oxidoreductase was purified about 180-fold to homogeneity in four steps and subjected to biochemical analysis. The enzyme is a flavoprotein, has a molecular mass of about 232 kDa, and consists of four identical subunits of about 62 kDa. It was activated by Triton X-100, phosphatidylglycerol, and dipalmitoyl-phosphatidylglycerol, and the substrates were pyruvate (kcat = 37.8 ± 3 s−1; Km = 30 ± 3 mM) and 2-oxobutyrate (kcat = 33.2 ± 3 s−1; Km = 90 ± 8 mM). Thiamine pyrophosphate (Km = 1 μM) and certain divalent metal ions such as Mg2+ (Km = 29 μM), Mn2+ (Km = 2 μM), and Co2+ (Km = 11 μM) served as cofactors. In addition to several dyes (2,6-dichloroindophenol, p-iodonitrotetrazolium violet, and nitroblue tetrazolium), menadione (Km = 106 μM) was efficiently reduced by the purified pyruvate:quinone oxidoreductase, indicating that a naphthoquinone may be the physiological electron acceptor of this enzyme in C. glutamicum.
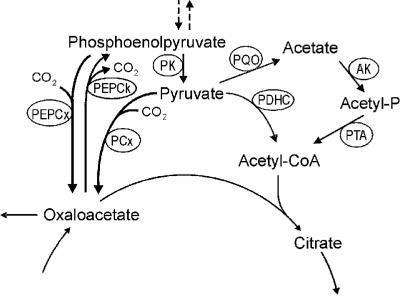
Corynebacterium glutamicum is an aerobic, gram-positive organism that grows on a variety of sugars and organic acids and is widely used in the industrial production of amino acids, particularly l-glutamate and l-lysine (40). Due to its importance for the carbon flux distribution within the metabolism and for the precursor supply for amino acid synthesis, the phosphoenolpyruvate (PEP)-pyruvate node of this organism (see Fig. 1) has been intensively studied and much attention has been focused on some of the enzymes involved, e.g., pyruvate kinase, PEP carboxylase, pyruvate carboxylase, and PEP carboxykinase (24, 33, 34, 51, 52, 55). The oxidative decarboxylation of pyruvate and thus the fueling of the tricarboxylic acid (TCA) cycle with acetyl coenzyme A (acetyl-CoA) in C. glutamicum have been generally attributed to the pyruvate dehydrogenase complex (16, 40, 61).
FIG. 1.
PEP-pyruvate node in C. glutamicum. Abbreviations: AK, acetate kinase; PCx, pyruvate carboxylase; PDHC, pyruvate dehydrogenase complex; PEPCk, PEP carboxykinase; PEPCx, PEP carboxylase; PK, pyruvate kinase; PQO, pyruvate:quinone oxidoreductase; PTA, phosphotransacetylase.
The genome of C. glutamicum has recently been determined and annotated (GenBank accession numbers NC_003450 and BX927147) (35, 63), and an open reading frame (cg2891) with significant identity to the Escherichia coli pyruvate oxidase gene (poxB) has been detected (8, 35). This finding indicated the presence of an additional pyruvate-decarboxylating enzyme at the C. glutamicum PEP-pyruvate node (Fig. 1). The E. coli pyruvate oxidase (EC 1.2.2.2) catalyzes the oxidative decarboxylation of pyruvate to acetate and CO2 (68) and is a nonessential, peripheral membrane protein consisting of four identical subunits each containing tightly bound flavin adenine dinucleotide (FAD) and loosely bound thiamine pyrophosphate (TPP) and Mg2+ (6, 26, 43, 44, 68). As the reaction of the enzyme is oxygen independent and uses ubiquinone as the physiological electron acceptor (9, 19, 38, 42), the pyruvate oxidase enzyme in fact represents a pyruvate:quinone oxidoreductase. The enzyme has been shown to be strongly activated by low concentrations of phospholipids and detergents (5, 11, 20, 21, 29), resulting in an increase of about 20-fold in Vmax and a decrease of about 10-fold in the Km for pyruvate (26, 43). The (phospho)lipid activation by and association with the protein are accompanied by conformational changes and alteration of various properties of the enzyme (13, 14, 67). Expression of the E. coli pyruvate oxidase gene (poxB) was shown to be induced in the stationary growth phase and to be dependent on sigma factor RpoS (10). Grabau and Cronan (27) and recently also Abdel-Hamid et al. (1) showed complementation of the acetate-auxotrophic phenotype of an E. coli pyruvate dehydrogenase complex mutant by introduction of multiple copies of the poxB gene, indicating that a high pyruvate oxidase (i.e., pyruvate:quinone oxidoreductase) activity, together with acetyl-CoA synthetase, can compensate for the pyruvate dehydrogenase complex function. The same authors also demonstrated that a poxB mutant shows lower growth rates, growth yields, and carbon conversion efficiencies than the parental E. coli strain and, thus, that the pyruvate oxidase contributes to the aerobic growth efficiency of E. coli. Very recently, Moreau (46) speculated that pyruvate oxidase in E. coli may protect glucose-metabolizing cells against oxidative stress under aerobic, phosphate starvation conditions.
Aside from the E. coli type of pyruvate oxidase, another type of pyruvate oxidase from two lactic acid bacteria, Lactobacillus delbrueckii (28) and Lactobacillus plantarum (41, 48, 59, 60, 65), has been found and characterized. This type of enzyme (EC 1.2.3.3) catalyzes in a phosphate- and oxygen-dependent reaction the formation of acetyl-phosphate, CO2, and hydrogen peroxide and in fact represents a pyruvate oxidase sensu stricto. This enzyme is also a flavoprotein with tightly bound FAD, TPP, and a divalent metal ion (60) and its synthesis is induced in the early stationary growth phase (41, 59). However, it is a soluble enzyme, and to our knowledge, there are no reports on activation by detergents and/or phospholipids. A recent functional analysis of the poxB gene, which encodes the pyruvate oxidase in Lactobacillus plantarum, revealed a predominant role of the pyruvate oxidase in the control of acetate production by L. plantarum under aerobic conditions (41).
To our knowledge, the presence of pyruvate:quinone oxidoreductase activity has been shown only for E. coli and, so far, nothing is known about this type of enzyme from any other organism. We are interested in the PEP-pyruvate node in C. glutamicum and the biochemistry of all enzymes involved in this node. Therefore, we addressed questions regarding the presence and nature of pyruvate:quinone oxidoreductase in C. glutamicum. In a first approach, we identified and analyzed pyruvate:quinone oxidoreductase activity in C. glutamicum cells, purified the pyruvate:quinone oxidoreductase from C. glutamicum, and performed a biophysical and biochemical characterization of the purified enzyme. Furthermore, we performed some sequence analyses on the pyruvate oxidase-pyruvate:quinone oxidoreductase genes available in the databases to clarify the relationships between the C. glutamicum and E. coli enzymes and other related enzymes.
MATERIALS AND METHODS
Materials.
All biochemicals were purchased from Sigma or Fluka and used without further purification. Triton X-114 was precondensed three times to obtain a more homogeneous preparation as described by Bordier (7) except that 100 mM 2-morpholinoethanesulfonic acid (MES) buffer, pH 6.0, instead of 10 mM Tris-HCl, pH 7.4, was used. For protein purification, a 10% (wt/vol) stock solution of Triton X-114 in 100 mM MES containing 150 mM NaCl was used. Phospholipids were dispersed by sonication.
Microorganisms and growth conditions.
The wild-type (WT) strain of C. glutamicum was employed in this study. The minimal medium used for C. glutamicum has been described previously (23) and contained 1% (wt/vol) acetate, lactate, pyruvate, ribose, maltose, or glucose or 2% (wt/vol) glucose as the carbon and energy source. Tryptone-yeast extract (TY) medium (56) was used as the complex medium. If not stated otherwise, C. glutamicum was grown aerobically at 30°C in 60-ml cultures in 500-ml baffled Erlenmeyer flasks on a rotary shaker at 120 rpm.
DNA preparation and transformation.
The isolation of chromosomal DNA and plasmids from C. glutamicum was performed as described previously (25), and isolation of plasmids from E. coli was carried out according to the method of Birnboim (4). Transfer of DNA into C. glutamicum was performed by electroporation, and the recombinant strains were selected on LBBHIS agar plates containing kanamycin (15 μg ml−1) (66). Electroporation of E. coli DH5α (30) was performed with competent cells according to the method of Dower et al. (22).
PCR techniques.
PCR experiments were performed using a Biometra personal cycler (Biotron). Amplification of DNA was carried out with Vent polymerase (New England Biolabs). Deoxynucleoside triphosphates were purchased from MBI Fermentas, and oligonucleotides (primers) were obtained from MWG Biotech. PCR was conducted as follows: initial denaturation for 5 min at 95°C, 32 identical cycles (1 min at 95°C, 1 min at 56°C, and 2.5 min at 72°C), and a final elongation step of 5 min at 72°C. PCR products were purified from agarose gels by using the Nucleospin extract kit (Macherey-Nagel).
Overexpression of the cg2891 gene in C. glutamicum.
For overexpression, the cg2891 gene was cloned into the expression vector pEKEx2 (25) under control of the tac promoter. The structural gene including the putative ribosomal binding site was amplified from chromosomal DNA of WT C. glutamicum by PCR using the primers pqoover1 (5′-GGGGTACCCCTGAAGTCGCACCAAGTTAGG-3′) and pqoover2 (5′-CGGAATTCCGTCGCGGTCAATGAGAACAGC-3′). The PCR product was digested with KpnI and EcoRI (MBI Fermentas), ligated into KpnI/EcoRI-restricted plasmid pEKEx2, and used to transform E. coli. The recombinant plasmid pEKEx-pqo was isolated from E. coli and introduced into WT C. glutamicum by electroporation. Expression of the plasmid-borne gene was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG).
Enzyme assays.
In order to determine pyruvate:quinone oxidoreductase enzyme activity in cell extracts, C. glutamicum cells were harvested, washed twice in 25 ml of 100 mM MES, pH 6.0, and resuspended in 0.5 ml of the same buffer. The cell suspension was transferred into 2-ml screw cap vials together with 250 mg of glass beads (150 to 212 μm in diameter; Sigma) and subjected to mechanical disruption five times for 30 s with a RiboLyser at 4°C, with intermittent cooling on ice for 2 min. After disruption, the glass beads and the cellular debris were removed by centrifugation (10,000 × g; 4°C; 15 min). The crude extract was then subjected to ultracentrifugation at 180,000 × g for 1.5 h at 4°C, and after addition of 1 mM phenylmethane-sulfonyl fluoride, it was used for assaying pyruvate:quinone oxidoreductase activity. The protein concentration was determined by using a bicinchoninic acid protein assay reagent kit from Pierce with bovine serum albumin as the standard.
Pyruvate:quinone oxidoreductase activity was assayed photometrically by monitoring the reduction of 2,6-dichloroindophenol (DCPIP) upon addition of pyruvate essentially as described by Cunningham and Hager (21). The reaction mixture contained 100 mM MES (pH 6.0), 10 mM MgCl2, 0.1 mM TPP, 1% (wt/vol) Triton X-100, 10% (vol/vol) glycerol, 300 μM (or 1 mM, when indicated) DCPIP, 100 mM potassium pyruvate, and appropriate amounts of cell extract. After preincubation of the reaction mixture at 40°C for 10 min, the reaction was started by addition of pyruvate, and the reduction of DCPIP was monitored by measuring the decrease of absorbance at 600 nm at 40°C. Pyruvate:quinone oxidoreductase activity is expressed in units per milligrams of protein, and 1 U corresponds to 1 μmol of DCPIP reduced per min (ɛ600 = 22 mM−1 cm−1).
Reduction of ferricyanide, p-iodonitrotetrazolium violet (INT), nitroblue tetrazolium (NBT), and horse heart cytochrome c was monitored at 420 nm (ɛ420 = 9.7 mM−1 cm−1), 492 nm (ɛ492 = 15 mM−1 cm−1), 530 nm (ɛ530 = 36 mM−1 cm−1), and 550 nm (ɛ550 = 29.5 mM−1 cm−1), respectively. NAD and NADP reduction was monitored at 340 nm (ɛ340 = 6.2 mM−1 cm−1).
Menadione reductase activity of pyruvate:quinone oxidoreductase was determined in a coupled assay: in a first reaction, menadione was reduced (with pyruvate as the electron donor) to menadiol, which then was oxidized by cytochrome c. The reaction mixture contained 100 mM MES (pH 6.0), 10 mM MgCl2, 0.1 mM TPP, 1% (wt/vol) Triton X-100, 10% (vol/vol) glycerol, 500 μM menadione, 45 μM horse heart cytochrome c, 100 mM potassium pyruvate, and appropriate amounts of cell extract. After preincubation of the reaction mixture at 40°C for 10 min, the reaction was started by addition of pyruvate. The reduction of cytochrome c was determined by monitoring the increase of absorbance at 550 nm (ɛ550 = 29.5 mM−1 cm−1).
Purification of pyruvate:quinone oxidoreductase.
The pyruvate:quinone oxidoreductase enzyme was purified from cell extract of WT C. glutamicum by using Triton X-114 essentially as described by Zhang and Hager (70). For purification, cells were grown overnight in 250 ml of TY complex medium in 1-liter baffled Erlenmeyer flasks, washed twice in 100 mM MES (pH 6.0), and resuspended in 7.5 ml of the same buffer. Disruption of the cells, removal of cell debris, and ultracentrifugation were performed as described above. After addition of 1 mM phenylmethane-sulfonyl fluoride, 2 mM EDTA, and 0.4 M (NH4)2SO4, the cell extract was rapidly heated under vigorous stirring to 65°C in an 80°C water bath, held at approximately 65°C for 3 min, and then rapidly cooled to 4°C in an ice water bath. Heat-precipitated proteins were removed by centrifugation at 100,000 × g for 50 min at 4°C. For the subsequent phase separation, 5 ml of the supernatant was mixed with 7.5 ml of activation buffer (100 mM MES [pH 6.0], 0.67 M sodium pyruvate, 33 mM MgCl2, 0.34 mM TPP) and the reaction mixture was incubated for 10 min at 30°C. The salt concentration was adjusted to 0.15 M NaCl, and 5 ml of 10% (wt/vol) Triton X-114 was added. The reaction mixture was then incubated for 20 min in an ice water bath, and 5-ml aliquots of the resulting clear solution were carefully layered over a cushion of 10 ml of 100 mM MES (pH 6.0)-0.25 M sucrose-0.15 M NaCl-0.06% (wt/vol) Triton X-114 in 50-ml tubes. The mixture was warmed to 30°C for 10 min and then centrifuged for 10 min at 3,000 × g at room temperature. Enriched pyruvate:quinone oxidoreductase was clearly visible by the yellow coloring of the Triton X-114 phase. The upper aqueous phase was discarded, and the Triton X-114 phase was resuspended in 3 ml of 100 mM MES, pH 6.0. After addition of 1.5 volumes of activation buffer, the phase separation (as described above) was repeated twice. To release purified pyruvate:quinone oxidoreductase from Triton X-114, phase separation was then performed three times with 1 ml of buffer containing 100 mM MES (pH 6.0) without pyruvate, TPP, or MgCl2. After the last separation, the yellow aqueous phase containing pyruvate:quinone oxidoreductase was removed, pooled, and stored at 4°C until use.
pH stability of the pyruvate:quinone oxidoreductase.
To test the pH stability, 100 mM potassium acetate, MES, morpholinopropane sulfonic acid, Tris-HCl, and 2-(cyclohexylamino)ethane sulfonic acid buffers were used for incubation of pyruvate:quinone oxidoreductase at pH 4.5 to 5.5, 5.0 to 7.0, 6.5 to 8.0, 7.5 to 9.0, and 8.5 to 9.5, respectively.
Removal of TPP and metal ions from pyruvate:quinone oxidoreductase enzyme.
TPP-free protein was prepared by exhaustive dialysis against 100 mM MES, pH 6.0, at 4°C. For the preparation of metal ion-free protein, the same buffer contained 20 mM EDTA. Excess EDTA was removed by dialysis against 100 mM MES, pH 6.0, at 4°C. Initial velocity data were fitted to the Michaelis-Menten equation to obtain dissociation constants for metals and TPP.
Quantification of acetate.
The acetate formed by the decarboxylation of pyruvate with pyruvate:quinone oxidoreductase was quantified enzymatically using the acetic acid determination kit from Roche Diagnostics.
Flavin analysis.
Flavins were analyzed by high-performance liquid chromatography (HPLC) using a Hewlett Packard LC 1100 chromatograph and fluorescence detector (HP G1321A). Flavins were released from purified pyruvate:quinone oxidoreductase by boiling for 5 min and subsequent removal of denatured protein by centrifugation for 10 min at 20,000 × g. FAD, flavin mononucleotide (FMN), and riboflavin were detected by fluorescence (excitation, 450 nm; emission, 520 nm) following separation using a C18 Hypersil ODS column (150 by 3 mm; particle size, 5 μm; Chromatographie Service GmbH). A linear gradient between 100 mM sodium acetate, pH 7.2, containing 25% (vol/vol) methanol and 100 mM sodium acetate, pH 7.2, containing 70% (vol/vol) methanol was used for elution. The initial flow rate of 0.35 ml/min was increased to 0.6 ml/min during elution (total elution time, 25 min). Authentic FMN, FAD, and riboflavin standards obtained from Sigma were used as controls.
Kinetic parameters.
Km and kcat values for pyruvate:quinone oxidoreductase were determined using pyruvate or 2-oxobutyrate as the electron donor and DCPIP or menadione as the electron acceptor in the presence of 10 mM MgCl2, 0.1 mM TPP, and 1% (wt/vol) Triton X-100 at 40°C. Initial velocity data were fitted to the Michaelis-Menten equation by nonlinear least-squares regression.
Pyruvate:quinone oxidoreductase size determination.
The subunit size was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (5% stacking gel, 10% separation gel) as described by Laemmli (39). Commercial protein molecular weight standards (prestained protein marker, broad range; New England Biolabs) were used to generate a plot of log Mr versus distance traveled on the gel.
The size of the native pyruvate:quinone oxidoreductase was estimated by native gradient gel electrophoresis (3) using Novex precast 8 to 16% Tris-glycine gels (Invitrogen Life Technologies). Electrophoresis was conducted following the instructions of the manufacturer. Commercial protein molecular weight standards (high-molecular-weight native marker kit; Amersham Bioscience) were used to generate a plot of log Mr versus distance traveled on the gel.
Computational analysis.
DataBank searches and alignments were carried out by using BLAST and CLUSTALW (2, 64).
RESULTS
Analysis of open reading frame cg2891 of C. glutamicum.
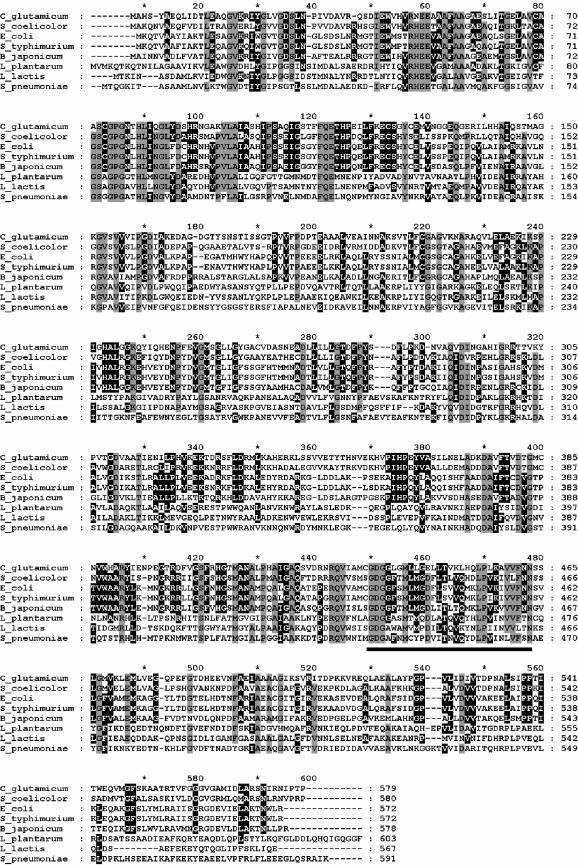
The cg2891 gene of the C. glutamicum genome has recently been annotated as a putative pyruvate oxidase gene (35). According to the nucleotide sequence, the protein encoded by the cg2891 gene consists of 579 amino acids and has a predicted molecular mass of 61,951 Da. DataBank analysis and alignment studies revealed 43% identity and 61% similarity to the functionally analyzed pyruvate oxidase (pyruvate:quinone oxidoreductase; see the introduction) of E. coli and significant levels of identity to putative pyruvate oxidases from other bacteria, e.g., the high-G+C-content gram-positive bacteria C. diphtheriae (70% identity), Streptomyces avermitilis (55%), and Streptomyces coelicolor (54%); the gram-negative bacteria Bradyrhizobium japonicum (43%), Pseudomonas syringae (44%), and Burkholderia cepacia (40%); and several enterobacteria such as Shigella flexneri (44%), Salmonella enterica (43%), Salmonella enterica serovar Typhimurium (43%), and Yersinia pestis (43%). In contrast, the C. glutamicum protein showed much less similarity to the functionally proven acetyl-phosphate- and H2O2-forming pyruvate oxidase of Lactobacillus plantarum (26% identity) and to the putative pyruvate oxidases of other lactic acid bacteria such as Lactococcus lactis (28%) and Streptococcus pyogenes (26%). An alignment of the deduced amino acid sequence of the C. glutamicum cg2891 gene product with (putative) pyruvate oxidase sequences from some of the bacteria listed above is shown in Fig. 2. In summary, the sequence analysis of the cg2891 gene suggests the presence of an E. coli type pyruvate oxidase (pyruvate:quinone oxidoreductase) in C. glutamicum.
FIG. 2.
Sequence alignment of the pyruvate:quinone oxidoreductases or pyruvate oxidases of C. glutamicum (accession number NP_601811), Streptomyces coelicolor (T34668), E. coli (NP_415392), Salmonella enterica serovar Typhimurium (S_typhimurium; NP_459912), Bradyrhizobium japonicum (NP_773926), Lactobacillus plantarum (NP_786788), Lactococcus lactis (NP_26820), and Streptococcus pneumoniae (AAB40976). Amino acids identical in at least seven sequences are shaded in grey, and amino acids identical in at least five sequences are shaded in black. The TPP signature motif is underlined.
Pyruvate:quinone oxidoreductase activity in C. glutamicum.
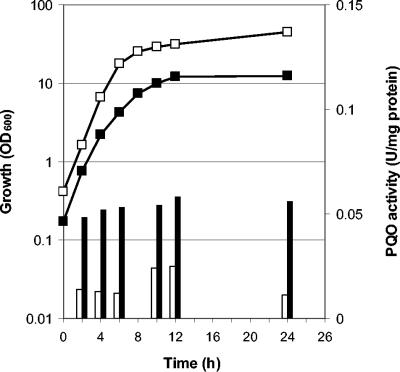
In order to test for the presence of a functional pyruvate:quinone oxidoreductase in C. glutamicum, the specific activity was determined in cell extracts of WT C. glutamicum grown on complex medium or on minimal media containing different carbohydrates or organic acids as carbon and energy sources and cells were harvested at the mid-exponential or the early stationary growth phase, i.e., when the growth rate declined substantially (Table 1). The highest specific activity was found in cells grown on complex medium without any additional substrate. The activity was about two- to fourfold lower when glucose was added to the complex medium or when the cells were grown on minimal medium containing glucose, maltose, ribose, pyruvate, or acetate (Table 1). When the cells were harvested at the early stationary instead of the mid-exponential growth phase, the respective pyruvate:quinone oxidoreductase activities were identical (with ribose, pyruvate, or acetate as carbon sources) or only slightly higher (with glucose or maltose as carbon sources). Representative relationships between the growth phase and the specific activities of pyruvate:quinone oxidoreductase are shown in Fig. 3. These results show that WT C. glutamicum possesses a functional pyruvate:quinone oxidoreductase and indicate that this enzyme is weakly regulated by the carbon source and that it is not or is only weakly regulated by dependence on the growth phase.
TABLE 1.
Specific activity of pyruvate:quinone oxidoreductase in cell extracts of WT C. glutamicuma
| Medium | Sp act (U/mg of protein)b at:
|
|
|---|---|---|
| Exponential growth phase | Stationary growth phase | |
| TY | 0.052 | 0.058 |
| TY + glucose | 0.013 | 0.024 |
| MM + glucose | 0.016 | 0.030 |
| MM + maltose | 0.013 | 0.023 |
| MM + ribose | 0.025 | 0.020 |
| MM + pyruvate | 0.018 | 0.019 |
| MM + acetate | 0.024 | 0.018 |
WT C. glutamicum was grown on TY medium without and with 4% (wt/vol) glucose or on minimal medium (MM) containing 1% (wt/vol) glucose, maltose, ribose, pyruvate, or acetate as the carbon source, and cells were harvested in the mid-exponential or early stationary growth phase.
The values are means obtained from at least two independent cultivations and two determinations per experiment. The standard deviations were in all cases below 10%.
FIG. 3.
Growth (squares) and pyruvate:quinone oxidoreductase (PQO) activities (bars) of WT C. glutamicum grown on TY medium without glucose (closed symbols) or with 4% glucose (open symbols). The standard deviations were in all cases below 10%.
In order to test whether the cg2891gene codes for the pyruvate:quinone oxidoreductase, the cg2891 gene was amplified from the genome of WT C. glutamicum, ligated into the expression vector pEKEx2, and used to transform WT C. glutamicum and the resulting strain was tested for pyruvate:quinone oxidoreductase activity. C. glutamicum(pEKEx-pqo) showed the same growth behavior (growth rate and final optical density) as the original host strain; however, it had 15-fold higher pyruvate:quinone oxidoreductase activity (0.870 ± 0.08 versus 0.058 ± 0.004 U/mg of protein). This result shows that cg2891 in fact codes for an enzyme mediating pyruvate:quinone oxidoreductase activity in C. glutamicum.
Purification of the pyruvate:quinone oxidoreductase.
To investigate the cellular distribution of pyruvate:quinone oxidoreductase, C. glutamicum WT cells were disrupted by mechanical lysis and the cell extract was separated into particulate and supernatant fractions by ultracentrifugation. More than 90% of pyruvate:quinone oxidoreductase activity was found in the supernatant fraction. Thus, pyruvate:quinone oxidoreductase may be only loosely bound to the cytoplasmic membrane and readily removed from membranes by mechanical cell disruption.
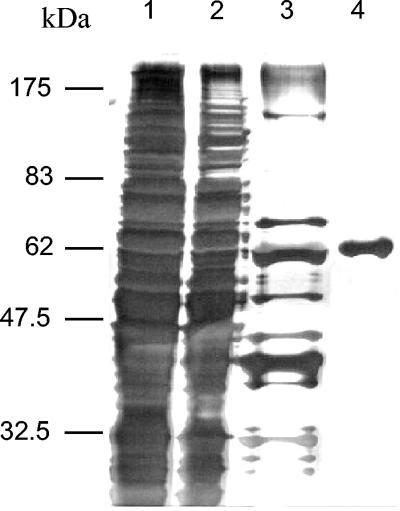
From cell extracts of WT C. glutamicum, the pyruvate:quinone oxidoreductase was purified to homogeneity by applying the protocol outlined in Table 2. The enzyme was enriched about 180-fold with an overall recovery of about 8%, indicating that pyruvate:quinone oxidoreductase represents about 0.5% of the soluble cytoplasmic protein fraction of WT C. glutamicum. After separation by SDS-PAGE and subsequent silver staining of the last fraction, only one protein band was detected, confirming the high (>99%) purity of the pyruvate:quinone oxidoreductase protein (Fig. 4).
TABLE 2.
Purification of pyruvate:quinone oxidoreductase from C. glutamicum
| Purification step | Total amt (mg) of protein | Total activitya (U) | Sp act (U/mg) | Recovery of activity (%) | Purification (fold) |
|---|---|---|---|---|---|
| Harvesting of cell extract | 309 | 13 | 0.042 | 100 | 1 |
| Ultracentrifugation | 195.8 | 10.77 | 0.055 | 82 | 1.3 |
| Heat precipitation | 36.1 | 7.04 | 0.195 | 54 | 4.6 |
| Triton X-114 extraction | 0.13 | 1.02 | 7.64 | 8 | 182 |
One unit of enzyme activity is defined as the amount of enzyme required to reduce 1 μmol of DCPIP per min.
FIG. 4.
SDS-PAGE of pyruvate:quinone oxidoreductase from C. glutamicum after each step of the purification procedure. Lane 1, crude extract; lane 2, extract after ultracentrifugation; lane 3, extract after heat denaturation; lane 4, purified pyruvate:quinone oxidoreductase. Proteins were visualized by silver staining.
The last step of the pyruvate:quinone oxidoreductase purification, Triton X-114 extraction, was completely dependent upon activation with pyruvate, TPP, and Mg2+. The enzyme was not recovered in the Triton X-114 phase when these compounds were omitted from the activation buffer (see Materials and Methods). The binding of pyruvate:quinone oxidoreductase to the detergent was reversible since Triton X-114-bound enzyme was readily removed from the detergent by washing with buffer devoid of pyruvate.
Molecular mass and subunit structure.
The size of the C. glutamicum pyruvate:quinone oxidoreductase subunits as determined by SDS-PAGE analysis was about 62 kDa (Fig. 4), which corresponds well with the molecular mass deduced from the cg2891 nucleotide sequence (see above). After native gradient gel electrophoresis of pyruvate:quinone oxidoreductase, only one major band was detected by silver staining. The size of native pyruvate:quinone oxidoreductase corresponded to about 232 kDa as estimated from the standard curve obtained from native gradient gel electrophoresis of molecular weight standards. These results indicate that the quaternary structure of the C. glutamicum pyruvate:quinone oxidoreductase is homotetrameric.
Temperature and pH stability.
Purified C. glutamicum pyruvate:quinone oxidoreductase was stable for at least 1 week when stored in 0.1 M MES, pH 6.0, containing 20% glycerol at −20°C. The thermostability of pyruvate:quinone oxidoreductase was investigated by incubation of reaction mixtures without substrates at temperatures from 40 to 70°C for 15 min prior to carrying out of the assay at 40°C. The enzyme retained its full activity upon heating at 55°C for 15 min, but activity decreased with further increases in temperature. After 15 min of preincubation at 65 and 70°C, only 62 and 20%, respectively, of the initial activity was detected.
The influence of pH on pyruvate:quinone oxidoreductase stability was evaluated by incubation of the enzyme in buffers with various pHs at 40°C for 15 min prior to the assay at 40°C at a pH of 6. The enzyme retained its full activity upon incubation between pH 5.5 and 8.5, but activity significantly decreased at lower or higher pHs (incubation at pH 4.5 and 9.5 resulted in 10 and 37% of the maximal activity, respectively).
Activation by Triton X-100, phosphatidylglycerol, and dipalmitoyl-phosphatidylglycerol.
According to the pyruvate:quinone oxidoreductase determination methods given in the literature (e.g., references 11 and 15), we generally added Triton X-100 to the reaction mixture when assaying pyruvate:quinone oxidoreductase in cell extracts or as a pure enzyme. In order to test for the actual requirement of Triton X-100 for maximal pyruvate:quinone oxidoreductase activity, we performed the assay without adding this detergent. Under this condition, the purified enzyme showed only sluggish activity: the progress curves displayed a considerable lag phase, the slopes increased gradually over the course of the reaction (about 2 min), and maximal pyruvate:quinone oxidoreductase activity was four- to fivefold lower than in the presence of Triton X-100. This result indicates that the C. glutamicum pyruvate:quinone oxidoreductase is activated by detergents.
Phosphatidylglycerol and dipalmitoyl-phosphatidylglycerol {1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] } were tested as activators of pyruvate:quinone oxidoreductase at concentrations of up to 200 μg/ml. In the absence of Triton X-100, both phospholipids were found to stimulate the activity approximately five- to sixfold, i.e., to about the same maximal activity as that observed in the presence of Triton X-100. Maximum activation occurred at about 50 μg of phosphatidylglycerol or dipalmitoyl-phosphatidylglycerol per ml. As was the case when Triton X-100 was added, no significant lag phase of the enzymatic reaction was observed. These results indicate that pyruvate:quinone oxidoreductase is activated by components of the C. glutamicum membrane and support the notion that the enzyme interacts in vivo with the inner surface of the membrane.
Substrate and electron acceptor specificity.
The ability of the C. glutamicum pyruvate:quinone oxidoreductase to catalyze the decarboxylation of different 2-oxoacids was examined by carrying out the DCPIP reductase assay with various 2-oxoacids as substrates (each at a final concentration of 100 mM). The enzyme catalyzed the decarboxylation of pyruvate as the most preferred substrate; however, 2-oxobutyrate also supported pyruvate:quinone oxidoreductase activity, resulting in about half of the activity observed with pyruvate as the substrate. 3-Methyl-2-oxobutyrate, 3-methyl-2-oxovalerate, 2-oxoglutarate, glyoxylate, and 4-methyl-2-oxovalerate did not serve as substrates for the purified enzyme (they yielded less than 5% of the activity observed with pyruvate).
The electron acceptor specificity was examined, and several artificial electron acceptors were found to be directly reduced by pyruvate:quinone oxidoreductase (Table 3). Highest activities were observed using DCPIP as the electron acceptor, whereas ferricyanide was only poorly reduced by the enzyme. Cytochrome c, NAD, and NADP were inert as electron acceptors. In order to test for pyruvate:quinone oxidoreductase-dependent reduction of quinones, we tested menadione (2-methyl-1,4-naphthoquinone) as an electron acceptor. As shown in Table 3, pyruvate:quinone oxidoreductase was found to directly reduce menadione to menadiol in a cytochrome c-coupled assay, showing 42% of the maximal activity observed with DCPIP as the electron acceptor. This result proves that quinones can serve as electron acceptors for the C. glutamicum pyruvate:quinone oxidoreductase.
TABLE 3.
Electron acceptor specificity of pyruvate:quinone oxidoreductase
| Acceptor | Concentration(s) (mM) | Relative activity (%) |
|---|---|---|
| DCPIP | 0.3 | 100 |
| INT | 0.3 | 28 |
| NBT | 0.3 | 9 |
| Ferricyanide | 1 | 5 |
| NAD | 3 | 0 |
| NADP | 3 | 0 |
| Cytochrome c | 0.045 | 0 |
| Menadione-cytochrome c | 0.5 and 0.045 | 42 |
Kinetic parameters.
The steady-state kinetic constants for pyruvate:quinone oxidoreductase-catalyzed decarboxylation of pyruvate were as follows: kcat, 37.8 ± 3 s−1; Km, 30 ± 3 mM; and kcat/Km, 1260 s−1 M−1 as determined using the DCPIP reductase assay in the presence of 10 mM Mg2+, 0.1 mM TPP, and 1% (wt/vol) Triton X-100 at 40°C. The Km value for menadione as determined from initial velocity data of the menadione reductase assay was 106 ± 11 μM. The kinetic constants for the reaction with 2-oxobutyrate as the substrate were as follows: kcat, 33.2 ± 3 s−1; Km, 90 ± 8 mM; and kcat/Km, 369 s−1 M−1.
Identification of the reaction product.
The reaction product of the pyruvate decarboxylation catalyzed by pyruvate:quinone oxidoreductase was postulated to be acetate. Enzyme assays using 1 mM DCPIP as the electron acceptor were carried out, and the reaction mixture was incubated until the electron acceptor was completely reduced (as indicated by the disappearance of the blue coloring of DCPIP). After completion of the reaction, the assay mixture was tested enzymatically for acetate production and found to contain 0.96 ± 0.05 mM acetate, indicating that one molecule of acetate was formed per molecule of DCPIP reduced in the reaction. Thus, the product of pyruvate:quinone oxidoreductase-dependent decarboxylation of pyruvate was identified to be acetate.
Metal ion and TPP activation.
Metal-free pyruvate:quinone oxidoreductase, prepared by dialysis, was inactive. The metal ions Mg2+, Mn2+, Ca2+, Co2+, Ni2+, Zn2+, and Na+ were tested (at a concentration of 10 mM each) as activators of the metal-free enzyme in the presence of 0.1 mM TPP and 1% (wt/vol) Triton X-100 by using the standard DCPIP reductase assay. Besides Mg2+, Mn2+, Co2+, and Ca2+ were found to be activators of pyruvate:quinone oxidoreductase, yielding 92, 87, and 26%, respectively, of the activity observed with 10 mM Mg2+. Ni2+, Zn2+, and Na+ were not able to support significant pyruvate:quinone oxidoreductase activity. The Km values (corresponding to the Kd for metal ion dissociation from the enzyme-TPP-pyruvate-Mg2+ complex) and the kcat values were determined for Mg2+, Co2+, and Mn2+ and are listed in Table 4. The kcat values for Mg2+-, Mn2+-, and Co2+-activated pyruvate:quinone oxidoreductase were similar; the Km for Mn2+, however, was found to be significantly lower than the Km values for Mg2+ and Co2+.
TABLE 4.
Steady-state kinetic constants of pyruvate:quinone oxidoreductase for Mg2+, Mn2+, and Co2+
| Metal activator | Activator Km (μM)a | kcat (s−1) |
|---|---|---|
| MgCl2 | 28.7 | 34.9 |
| MnCl2 | 2.2 | 29.3 |
| CoCl2 | 11.4 | 32.6 |
The values are means obtained from at least two independent purification batches and two determinations per experiment. Standard deviations were in all cases below 10%.
TPP was removed from purified pyruvate:quinone oxidoreductase by dialysis, showing that this cofactor is bound only loosely to the enzyme. The Km for TPP activation was determined using the standard DCPIP reductase assay by measuring the initial velocity of pyruvate decarboxylation in the presence of 10 mM Mg2+ and 1% (wt/vol) Triton X-100. Initial velocity data defined the kcat as 38.5 s−1 ± 2.0 and the TPP Km as 1 ± 0.05 μM.
Flavin cofactor analysis.
The UV-visible absorbance spectrum of purified pyruvate:quinone oxidoreductase was typical for a flavin-containing protein, showing maxima at 380 and 450 nm and a minimum at 415 nm. Oxidized pyruvate:quinone oxidoreductase, however, was completely converted into its reduced (colorless) form upon incubation with dithionite or pyruvate plus TPP plus Mg2+, as evidenced by the decrease of absorbance of the oxidized flavin at the absorbance maximum of 450 nm. The flavin compound was not released from the enzyme by extensive dialysis but was released upon heat denaturation, indicating that the cofactor is tightly, but not covalently, bound to the protein. A comparison of the high-performance liquid chromatography elution time of the released flavin (4.99 ± 0.025 min) with those of FAD, FMN, and riboflavin unequivocally identified the flavin cofactor as FAD. The proportion of the FAD released from the purified pyruvate:quinone oxidoreductase was 0.73 mol of FAD per mol of 62-kDa subunit, indicating that each subunit contains one molecule of FAD.
DISCUSSION
This study shows for the first time the presence of pyruvate:quinone oxidoreductase activity in a prokaryotic organism apart from E. coli and describes the isolation and biochemical analysis of the pyruvate:quinone oxidoreductase enzyme from C. glutamicum. This enzyme is a homotetrameric flavoprotein consisting of 62-kDa subunits with tightly bound FAD, and it requires TPP and a divalent metal cation such as Mg2+, Mn2+, and Co2+ for enzymatic activity. With these characteristics, the C. glutamicum enzyme closely resembles the E. coli pyruvate oxidase. As an artificial electron acceptor, the C. glutamicum enzyme accepts several dyes, such as DCPIP, INT, and NBT. In contrast, ferricyanide, which is routinely used for assaying the E. coli enzyme (see, e.g., references 1, 12, and 68), is only poorly reduced by the C. glutamicum pyruvate:quinone oxidoreductase and thus is not suitable for testing this enzyme. The physiological electron acceptor of the E. coli pyruvate oxidase has been shown to be coenzyme Q8 (ubiquinone-8) (9, 19, 38, 42). However, ubiquinones are not present in C. glutamicum, and instead this organism possesses menaquinones, predominantly menaquinone-9 (17, 18, 36), which belongs to the naphtoquinones. As we found menadione (2-methyl-1,4-naphtoquinone) to be directly reduced by the C. glutamicum pyruvate:quinone oxidoreductase, menaquinones most probably represent the physiological electron acceptors for this enzyme.
We purified the C. glutamicum pyruvate:quinone oxidoreductase from cell extracts and found most of the activity in the soluble cytoplasmic fraction. Although there is no direct proof for a membrane association of the C. glutamicum pyruvate:quinone oxidoreductase, there are three indications in favor of an in vivo location and function of this enzyme at the cytoplasmic membrane. First, the pyruvate:quinone oxidoreductase was shown to react with menadione but not with NAD or NADP. Due to the physiological location of the quinones within the membrane, an interaction in vivo should be possible only at the membrane. Second, the pyruvate:quinone oxidoreductase was shown to be activated by the detergent Triton X-100 and by the phospholipids phosphatidylglycerol and dipalmitoyl-phosphatidylglycerol. A variety of membrane-associated enzymes have been shown to require detergents or phospholipids for their catalytic activity (57), among them pyruvate oxidase and membrane-bound l-lactate dehydrogenase from E. coli (5, 37) and the FAD-dependent malate dehydrogenases (malate:quinone oxidoreductases; EC 1.1.99.16) from E. coli (49), Mycobacterium smegmatis (32), and C. glutamicum (45). Phosphatidylglycerol and the fatty acids palmitic and oleic acid were found to be the main constituents of the C. glutamicum membrane (31), and thus it seems reasonable to assume that the pyruvate:quinone oxidoreductase enzyme is much more active when in contact with the inner surface of the corynebacterial membrane than when located within the cytoplasm. Third, similar to the case with other membrane proteins (7, 62), the C. glutamicum pyruvate:quinone oxidoreductase was found to bind to the detergent Triton X-114. The binding to Triton X-114 was completely reversible and dependent on the presence of the substrate pyruvate and the cofactors TPP and Mg2+. These data suggest that, analogous to the situation shown for the E. coli pyruvate oxidase (14, 44, 53, 58), the C. glutamicum pyruvate:quinone oxidoreductase may undergo a conformational change upon incubation with a substrate and cofactors, resulting in the exposure of an enzyme domain that enables in vitro the binding of pyruvate:quinone oxidoreductase to the hydrophobic detergent Triton X-114 or in vivo the binding of the enzyme to the membrane. The E. coli pyruvate oxidase is thought to shuttle between the cytosol and the inner membrane, depending on the intracellular pyruvate concentration (11, 15). When pyruvate is absent or present at only low concentrations, the enzyme is inactive and located within the cytosol; at higher pyruvate concentrations, the conformation of the enzyme changes to allow the binding to the membrane, thus giving access to the physiological electron acceptor ubiquinone and to the activating membrane components. However, whether the C. glutamicum pyruvate:quinone oxidoreductase also reacts with a conformational change to pyruvate availability and whether such a conformational change is responsible for the membrane attachment and for a physiological function of the enzyme at the membrane have to be experimentally verified.
The pyruvate:quinone oxidoreductase of C. glutamicum represents a further enzyme at the PEP-pyruvate node (Fig. 1), hitherto not known to be present, which increases the complexity of the carbon flux at the link between glycolysis and the TCA cycle in C. glutamicum. The pyruvate dehydrogenase complex, catalyzing the oxidative decarboxylation of pyruvate into acetyl-CoA with NAD as the electron acceptor, is assumed to be the main enzyme responsible for providing acetyl-CoA for the TCA cycle. Together with acetate kinase and phosphotransacetylase, which are responsible for the activation of acetate to form acetyl-CoA in C. glutamicum (54), the pyruvate:quinone oxidoreductase may bypass the pyruvate dehydrogenase complex. However, energywise this bypass would be unfavorable for the cells since the acetate kinase reaction requires ATP. The fact that both an acetate kinase mutant and a phosphotransacetylase mutant show growth behavior identical to that of the C. glutamicum WT strain (54) indicates that such a bypass of the pyruvate dehydrogenase complex is at least not essential for growth of C. glutamicum on minimal medium containing glucose. Moreover, compared to the pyruvate dehydrogenase complex, the pyruvate:quinone oxidoreductase has very low affinity for pyruvate (Km values of 0.8 and 30 mM, respectively) (61; also this work), and regarding the intracellular concentration of 0.5 to 0.8 mM pyruvate (50), it seems unlikely that pyruvate:quinone oxidoreductase significantly contributes to the oxidative pyruvate decarboxylation under the conditions in which C. glutamicum generally is cultivated. It may well be that a hitherto unknown intracellular factor (a so-far-unidentified metabolite or internal equilibria) positively affects the affinity of pyruvate:quinone oxidoreductase for pyruvate and/or that the enzyme can substitute for pyruvate dehydrogenase complex activity under certain conditions, e.g., when pyruvate dehydrogenase activity is low. However, the true physiological function of this enzyme remains to be elucidated.
By using overexpression studies, we showed that cg2891 conferred pyruvate:quinone oxidoreductase activity. Homology analysis of the amino acid sequence deduced from the cg2891 gene demonstrated a relatively high degree of identity to the E. coli type pyruvate oxidase (pyruvate:quinone oxidoreductase) and much less similarity to the acetyl-phosphate- and H2O2-forming pyruvate oxidase of Lactobacillus plantarum. This and the facts that the C. glutamicum pyruvate:quinone oxidoreductase reaction is phosphate independent and that the product of the C. glutamicum pyruvate:quinone oxidoreductase reaction is acetate instead of acetyl-phosphate argue against a close relationship between the C. glutamicum pyruvate:quinone oxidoreductase and the lactobacterial pyruvate oxidases. However, despite the low overall identities between the primary structures of the pyruvate:quinone oxidoreductases from C. glutamicum and E. coli on the one hand and the lactobacterial pyruvate oxidases on the other, the alignment studies (Fig. 2) revealed some common features in the E. coli type and lactobacterium type pyruvate oxidases. All enzymes aligned contain a TPP-binding motif (residues 435 to 463 in the C. glutamicum sequence) with highly conserved Asp436 and Asn463 involved in the binding of the metal cofactor (69, 47). An aspartate residue involved in the binding of the FAD cofactor (47) and several other residues thought to be important for catalysis of the pyruvate oxidase of Lactobacillus plantarum (47) are also highly conserved (Asp291 and Glu49, His79, Phe111, and Gln112, respectively, in the C. glutamicum sequence). In contrast, an arginine (Arg264 in the Lactobacillus plantarum sequence), also proposed to be functionally important for catalysis of the pyruvate oxidase of Lactobacillus plantarum, occurs only in the lactobacterial pyruvate oxidases. Furthermore, residue Gly35 in the Lactobacillus plantarum sequence, suggested to be a possible binding site for inorganic phosphate (47), is also unique to the lactobacterial pyruvate oxidases and is replaced by aspartate residues in the phosphate-independent pyruvate:quinone oxidoreductase enzymes. In contrast, a proline residue (Pro536) shown to be essential for lipid activation of the E. coli pyruvate oxidase (12) is conserved in all but the lactobacterial enzymes shown in Fig. 2. Thus, the common and also the different biochemical properties of the E. coli-C. glutamicum type and the lactobacterium type pyruvate oxidases are clearly reflected by communities and differences at the amino acid sequence level.
Our DataBank analyses revealed that numerous gram-positive and gram-negative bacteria possess genes with significant identities (>40%) to the E. coli and C. glutamicum pyruvate:quinone oxidoreductase genes. This suggests that several if not many other bacteria also possess a functional pyruvate:quinone oxidoreductase as a pyruvate-decarboxylating enzyme and thus that the enzyme may have broad significance in nature and may be physiologically relevant in at least some bacteria.
Acknowledgments
We thank Joy Schreiner for critically reading the manuscript.
The support of the EC (grant VALPAN, QLK3-2000-00497) and of the Degussa AG is gratefully acknowledged.
REFERENCES
- 1.Abdel-Hamid, A. M., M. M. Attwood, and J. R. Guest. 2001. Pyruvate oxidase contributes to the aerobic growth efficiency of Escherichia coli. Microbiology 147:1483-1498. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, L., H. Borg, and M. Mikaelsson. 1972. Molecular weight estimations of proteins by electrophoresis in polyacrylamide gels of graded porosity. FEBS Lett. 20:199-202. [DOI] [PubMed] [Google Scholar]
- 4.Birnboim, H. C. 1983. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 100:243-255. [DOI] [PubMed] [Google Scholar]
- 5.Blake, R., and L. P. Hager. 1978. Activation of pyruvate oxidase by monomeric and micellar amphiphiles. J. Biol. Chem. 253:1963-1971. [PubMed] [Google Scholar]
- 6.Blake, R. I. I., T. A. O'Brien, R. B. Gennis, and L. P. Hager. 1982. Role of the divalent metal cation in the pyruvate oxidase reaction. J. Biol. Chem. 257:9605-9611. [PubMed] [Google Scholar]
- 7.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. [PubMed] [Google Scholar]
- 8.Bott, M., and A. Niebisch. 2003. The respiratory chain of Corynebacterium glutamicum. J. Biotechnol. 104:129-153. [DOI] [PubMed] [Google Scholar]
- 9.Carter, K., and R. B. Gennis. 1985. Reconstitution of the ubiquinone-dependent pyruvate oxidase system of Escherichia coli with the cytochrome o terminal oxidase complex. J. Biol. Chem. 260:10986-10990. [PubMed] [Google Scholar]
- 10.Chang, Y. Y., A. Y. Wang, and J. E. Cronan, Jr. 1994. Expression of Escherichia coli pyruvate oxidase (PoxB) depends on the sigma factor encoded by the rpoS(katF) gene. Mol. Microbiol. 11:1019-1028. [DOI] [PubMed] [Google Scholar]
- 11.Chang, Y. Y., and J. E. Cronan, Jr. 1984. An Escherichia coli mutant in pyruvate oxidase due to altered phospholipid activation of the enzyme. Proc. Natl. Acad. Sci. USA 81:4348-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, Y. Y., and J. E. Cronan, Jr. 1986. Molecular cloning, DNA sequencing, and enzymatic analyses of two Escherichia coli pyruvate oxidase mutants defective in activation by lipids. J. Bacteriol. 167:312-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, Y. Y., and J. E. Cronan, Jr. 1995. Detection by site-specific disulfide cross-linking of a conformational change in binding of Escherichia coli pyruvate oxidase to lipid bilayers. J. Biol. Chem. 270:7896-7901. [DOI] [PubMed] [Google Scholar]
- 14.Chang, Y. Y., and J. E. Cronan, Jr. 1997. Sulfhydryl chemistry detects three conformations of the lipid binding region of Escherichia coli pyruvate oxidase. Biochemistry 36:11564-11573. [DOI] [PubMed] [Google Scholar]
- 15.Chang, Y. Y., and J. E. Cronan, Jr. 2000. Conversion of Escherichia coli pyruvate oxidase to an ‘alpha-ketobutyrate oxidase’. Biochem. J. 352:717-724. [PMC free article] [PubMed] [Google Scholar]
- 16.Cocaign-Bousquet, M., and N. D. Lindley. 1995. Pyruvate overflow and carbon flux within the central metabolic pathways of Corynebacterium glutamicum during growth on lactate. Enzyme Microb. Technol. 17:260-267. [Google Scholar]
- 17.Collins, M. D., M. Goodfellow, and D. E. Minnikin. 1979. Isoprenoid quinones in the classification of coryneform and related bacteria. J. Gen. Microbiol. 110:127-136. [DOI] [PubMed] [Google Scholar]
- 18.Collins, M. D., T. Pirouz, M. Goodfellow, and D. E. Minnikin. 1977. Distribution of menaquinones in actinomycetes and corynebacteria. J. Gen. Microbiol. 100:221-230. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham, C. C., and L. P. Hager. 1975. Reactivation of the lipid-depleted pyruvate oxidase system from Escherichia coli with cell envelope neutral lipids. J. Biol. Chem. 250:7139-7146. [PubMed] [Google Scholar]
- 20.Cunningham, C. C., and L. P. Hager. 1971. Crystalline pyruvate oxidase from Escherichia coli. II. Activation by phospholipids. J. Biol. Chem. 246:1575-1582. [PubMed] [Google Scholar]
- 21.Cunningham, C. C., and L. P. Hager. 1971. Crystalline pyruvate oxidase from Escherichia coli. 3. Phospholipid as an allosteric effector for the enzyme. J. Biol. Chem. 246:1583-1589. [PubMed] [Google Scholar]
- 22.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eikmanns, B. J., M. Metzger, D. Reinscheid, M. Kircher, and H. Sahm. 1991. Amplification of three threonine biosynthesis genes in Corynebacterium glutamicum and its influence on carbon flux in different strains. Appl. Microbiol. Biotechnol. 34:617-622. [DOI] [PubMed] [Google Scholar]
- 24.Eikmanns, B. J., M. T. Follettie, M. U. Griot, and A. J. Sinskey. 1989. The phosphoenolpyruvate carboxylase gene of Corynebacterium glutamicum: molecular cloning, nucleotide sequence, and expression. Mol. Gen. Genet. 218:330-339. [DOI] [PubMed] [Google Scholar]
- 25.Eikmanns, B. J., N. Thum-Schmitz, L. Eggeling, K. U. Ludtke, and H. Sahm. 1994. Nucleotide sequence, expression and transcriptional analysis of the Corynebacterium glutamicum gltA gene encoding citrate synthase. Microbiology 140:1817-1828. [DOI] [PubMed] [Google Scholar]
- 26.Gennis, R. B., and L. P. Hager. 1976. Pyruvate oxidase, p. 493-504. In A. Martonosi (ed.), The enzymes of biological membranes, vol. 2. Plenum, New York, N.Y.
- 27.Grabau, C., and J. E. Cronan, Jr. 1984. Molecular cloning of the gene (poxB) encoding the pyruvate oxidase of Escherichia coli, a lipid-activated enzyme. J. Bacteriol. 160:1088-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hager, L. P., D. M. Geller, and F. Lipmann. 1954. Flavoprotein-catalyzed pyruvate oxidation in Lactobacillus delbrueckii. Fed. Proc. 13:734-738. [PubMed] [Google Scholar]
- 29.Hamilton, S. E., M. Recny, and L. P. Hager. 1986. Identification of the high-affinity lipid binding site in Escherichia coli pyruvate oxidase. Biochemistry 25:8178-8183. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan, D. 1985. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 31.Hoischen, C., and R. Krämer. 1990. Membrane alteration is necessary but not sufficient for effective glutamate secretion in Corynebacterium glutamicum. J. Bacteriol. 172:3409-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai, T., Y. Kageyama, and J. Tobari. 1995. Mycobacterium smegmatis malate dehydrogenase: activation of the lipid-depleted enzyme by anionic phospholipids and phosphatidylethanolamine. Biochim. Biophys. Acta 1246:189-196. [DOI] [PubMed] [Google Scholar]
- 33.Jetten, M. S., M. E. Gubler, S. H. Lee, and A. J. Sinskey. 1994. Structural and functional analysis of pyruvate kinase from Corynebacterium glutamicum. Appl. Environ. Microbiol. 60:2501-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jetten, M. S. M., and A. J. Sinskey. 1993. Characterization of phosphoenolpyruvate carboxykinase from Corynebacterium glutamicum. FEMS Microbiol. Lett. 111:183-188. [Google Scholar]
- 35.Kalinowski, J., B. Bathe, D. Bartels, N. Bischoff, M. Bott, A. Burkovski, N. Dusch, L. Eggeling, B. J. Eikmanns, L. Gaigalat, A. Goesmann, M. Hartmann, K. Huthmacher, R. Kramer, B. Linke, A. C. McHardy, F. Meyer, B. Mockel, W. Pfefferle, A. Puhler, D. A. Rey, C. Ruckert, O. Rupp, H. Sahm, V. F. Wendisch, I. Wiegrabe, and A. Tauch. 2003. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J. Biotechnol. 104:5-25. [DOI] [PubMed] [Google Scholar]
- 36.Kanzaki, T., Y. Sugiyama, K. Kitano, Y. Ashida, and I. Imada. 1974. Quinones of Brevibacterium. Biochim. Biophys. Acta 348:162-165. [DOI] [PubMed] [Google Scholar]
- 37.Kimura, H., and M. Futai. 1978. Effects of phospholipids on L-lactate dehydrogenase from membranes of Escherichia coli. Activation and stabilization of the enzyme with phospholipids. J. Biol. Chem. 253:1095-1110. [PubMed] [Google Scholar]
- 38.Koland, J. G., M. J. Miller, and R. B. Gennis. 1984. Reconstitution of the membrane-bound, ubiquinone-dependent pyruvate oxidase respiratory chain of Escherichia coli with the cytochrome d terminal oxidase. Biochemistry 23:445-453. [DOI] [PubMed] [Google Scholar]
- 39.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 40.Liebl, W. 1991. The genus Corynebacterium—nonmedical, p. 1157-1171. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H Schleifer (ed.), The procaryotes, vol. 2. Springer, New York, N.Y.
- 41.Lorquet, F., P. Goffin, L. Muscariello, J. B. Baudry, V. Ladero, M. Sacco, M. Kleerebezem, and P. Hols. 2004. Characterization and functional analysis of the poxB gene, which encodes pyruvate oxidase in Lactobacillus plantarum. J. Bacteriol. 186:3749-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchal, D., J. Pantigny, J. M. Laval, J. Moiroux, and C. Bourdillon. 2001. Rate constants in two dimensions of electron transfer between pyruvate oxidase, a membrane enzyme, and ubiquinone (coenzyme Q8), its water-insoluble electron carrier. Biochemistry 40:1248-1256. [DOI] [PubMed] [Google Scholar]
- 43.Mather, M., L. M. Schopfer, V. Massey, and R. B. Gennis. 1982. Studies of the flavin adenine dinucleotide binding region in Escherichia coli pyruvate oxidase. J. Biol. Chem. 257:12887-12892. [PubMed] [Google Scholar]
- 44.Mather, M. W., and R. B. Gennis. 1985. Spectroscopic studies of pyruvate oxidase flavoprotein from Escherichia coli trapped in the lipid-activated form by cross-linking. J. Biol. Chem. 260:10395-10397. [PubMed] [Google Scholar]
- 45.Molenaar, D., M. E. van der Rest, and S. Petrovic. 1998. Biochemical and genetic characterization of the membrane-associated malate dehydrogenase (acceptor) from Corynebacterium glutamicum. Eur. J. Biochem. 254:395-403. [DOI] [PubMed] [Google Scholar]
- 46.Moreau, P. L. 2004. Diversion of the metabolic flux from pyruvate dehydrogenase to pyruvate oxidase decreases oxidative stress during glucose metabolism in nongrowing Escherichia coli cells incubated under aerobic phosphate starvation conditions. J. Bacteriol. 186:7364-7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller, Y. A., and G. E. Schulz. 1993. Structure of the thiamine- and flavin-dependent enzyme pyruvate oxidase. Science 259:965-967. [DOI] [PubMed] [Google Scholar]
- 48.Muller, Y. A., G. Schumacher, R. Rudolph, and G. E. Schulz. 1994. The refined structures of a stabilized mutant and of wild-type pyruvate oxidase from Lactobacillus plantarum. J. Mol. Biol. 237:315-335. [DOI] [PubMed] [Google Scholar]
- 49.Narindrasorasak, S., A. H. Goldie, and B. D. Sanwal. 1979. Characteristics and regulation of a phospholipid-activated malate oxidase from Escherichia coli. J. Biol. Chem. 254:1540-1545. [PubMed] [Google Scholar]
- 50.Petersen, S., C. Mack, A. A. de Graaf, C. Riedel, B. J. Eikmanns, and H. Sahm. 2001. Metabolic consequences of altered phosphoenolpyruvate carboxykinase activity in Corynebacterium glutamicum reveal anaplerotic regulation mechanisms in vivo. Metab. Eng. 3:344-361. [DOI] [PubMed] [Google Scholar]
- 51.Peters-Wendisch, P. G., C. Kreutzer, J. Kalinowski, M. Patek, H. Sahm, and B. J. Eikmanns. 1998. Pyruvate carboxylase from Corynebacterium glutamicum: characterization, expression and inactivation of the pyc gene. Microbiology 144:915-927. [DOI] [PubMed] [Google Scholar]
- 52.Peters-Wendisch, P. G., V. F. Wendisch, S. Paul, B. J. Eikmanns, and H. Sahm. 1997. Pyruvate carboxylase as an anaplerotic enzyme in Corynebacterium glutamicum. Microbiology 143:1095-1103. [DOI] [PubMed] [Google Scholar]
- 53.Recny, M. A., and L. P. Hager. 1983. Isolation and characterization of the protease-activated form of pyruvate oxidase. Evidence for a conformational change in the environment of the flavin prosthetic group. J. Biol. Chem. 258:5189-5195. [PubMed] [Google Scholar]
- 54.Reinscheid, D. J., S. Schnicke, D. Rittmann, U. Zahnow, H. Sahm, and B. J. Eikmanns. 1999. Cloning, sequence analysis, expression and inactivation of the Corynebacterium glutamicum pta-ack operon encoding phosphotransacetylase and acetate kinase. Microbiology 145:503-513. [DOI] [PubMed] [Google Scholar]
- 55.Riedel, C., D. Rittmann, P. Dangel, B. Mockel, S. Petersen, H. Sahm, and B. J. Eikmanns. 2001. Characterization of the phosphoenolpyruvate carboxykinase gene from Corynebacterium glutamicum and significance of the enzyme for growth and amino acid production. J. Mol. Microbiol. Biotechnol. 3:573-583. [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 57.Sandermann, H., Jr. 1978. Regulation of membrane enzymes by lipids. Biochim. Biophys. Acta 515:209-237. [DOI] [PubMed] [Google Scholar]
- 58.Schrock, H. L., and R. B. Gennis. 1977. High affinity lipid binding sites on the peripheral membrane enzyme pyruvate oxidase. Specific ligand effects on detergent binding. J. Biol. Chem. 252:5990-5995. [PubMed] [Google Scholar]
- 59.Sedewitz, B., K. H. Schleifer, and F. Götz. 1984. Physiological role of pyruvate oxidase in the aerobic metabolism of Lactobacillus plantarum. J. Bacteriol. 160:462-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sedewitz, B., K. H. Schleifer, and F. Götz. 1984. Purification and biochemical characterization of pyruvate oxidase from Lactobacillus plantarum. J. Bacteriol. 160:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiio, I., Y. Toride, and S. Sugimoto. 1984. Production of lysine by pyruvate dehydrogenase mutants of Brevibacterium flavum. Agric. Biol. Chem. 48:3091-3098. [Google Scholar]
- 62.Tani, H., T. Kamidate, and H. Watanabe. 1998. Aqueous micellar two-phase systems for protein separation. Anal. Sci. 14:875-888. [Google Scholar]
- 63.Tauch, A., I. Homann, S. Mormann, S. Ruberg, A. Billault, B. Bathe, S. Brand, O. Brockmann-Gretza, C. Ruckert, N. Schischka, C. Wrenger, J. Hoheisel, B. Mockel, K. Huthmacher, W. Pfefferle, A. Puhler, and J. Kalinowski. 2002. Strategy to sequence the genome of Corynebacterium glutamicum ATCC 13032: use of a cosmid and a bacterial artificial chromosome library. J. Biotechnol. 95:25-38. [DOI] [PubMed] [Google Scholar]
- 64.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tittmann, K., R. Golbik, S. Ghisla, and G. Hubner. 2000. Mechanism of elementary catalytic steps of pyruvate oxidase from Lactobacillus plantarum. Biochemistry 39:10747-10754. [DOI] [PubMed] [Google Scholar]
- 66.Van der Rest, M. E., C. Lange, and D. Molenaar. 1999. A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogenic plasmid DNA. Appl. Microbiol. Biotechnol. 52:541-545. [DOI] [PubMed] [Google Scholar]
- 67.Wang, A. Y., Y. Y. Chang, and J. E. Cronan, Jr. 1991. Role of the tetrameric structure of Escherichia coli pyruvate oxidase in enzyme activation and lipid binding. J. Biol. Chem. 266:10959-10966. [PubMed] [Google Scholar]
- 68.Williams, F. R., and L. P. Hager. 1966. Crystalline flavin pyruvate oxidase from Escherichia coli. I. Isolation and properties of the flavoprotein. Arch. Biochem. Biophys. 116:168-176. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, G., J. Dai, Z. Lu, and D. Dunaway-Mariano. 2003. The phosphonopyruvate decarboxylase from Bacteroides fragilis. J. Biol. Chem. 278:41302-41308. [DOI] [PubMed] [Google Scholar]
- 70.Zhang, T. F., and L. P. Hager. 1987. A single-step large-scale purification of pyruvate oxidase. Arch. Biochem. Biophys. 257:485-487. [DOI] [PubMed] [Google Scholar]