Abstract
Background
Increased cancer-related inflammation has been associated with unfavorable clinical outcomes. The combination of platelet count and neutrophil–lymphocyte ratio (COP-NLR) has related outcomes in several cancers, except for nasopharyngeal carcinoma (NPC). This study evaluated the prognostic value of COP-NLR in predicting outcome in NPC patients treated with intensity-modulated radiotherapy (IMRT).
Materials and methods
We analyzed the data collected from 232 NPC patients. Pretreatment total platelet counts, neutrophil–lymphocyte ratio (NLR), and COP-NLR score were evaluated as potential predictors. Optimal cutoff values for NLR and platelets were determined using receiver operating curve. Patients with both elevated NLR (>3) and platelet counts (>300×109/L) were assigned a COP-NLR score of 2; those with one elevated or no elevated value were assigned a COP-NLR a score of 1 or 0. Cox proportional hazards model was used to test the association of these factors and relevant 3-year survivals.
Results
Patients (COP-NLR scores 1 and 2=85; score 0=147) were followed up for 55.19 months. Univariate analysis showed no association between pretreatment NLR >2.23 and platelet counts >290.5×109/L and worse outcomes. Multivariate analysis revealed that those with COP-NLR scores of 0 had better 3-year disease-specific survival (P=0.02), overall survival (P=0.024), locoregional relapse-free survival (P=0.004), and distant metastasis-free survival (P=0.046). Further subgrouping by tumor stage also revealed COP-NLR to be an unfavorable prognostic indicator of 3-year failure-free survival (P=0.001) for locally advanced NPC.
Conclusion
COP-NLR score, but not NLR alone or total platelet count alone, predicted survival in NPC patients treated with IMRT-based therapy, especially those with stage III/IVA, B malignancies.
Keywords: nasopharyngeal carcinoma, intensity-modulated radiotherapy, inflammation-based marker, combination of platelet count and neutrophil–lymphocyte ratio, prognosis
Introduction
Nasopharyngeal carcinoma (NPC) differs from other head and neck cancers in its association with the Epstein–Barr virus (EBV) infection, demographic tendencies, clinical behavior, and treatment.1,2 Although recent epidemiologic studies have found it to be declining in incidence in several previous endemic areas, it remains unchanged in southern China, where it has an incidence >20/100,000 person-years.1 Compared with conventional two-dimensional radiotherapy, the combination of newly developed intensity-modulated radiotherapy (IMRT) and chemotherapy has been found to extend survival in patients with non-disseminated NPC.2 The 5-year local control rates provided by IMRT-based treatments has been reported to range from 91.6% to 98.3%, but IMRT has not adequately resolved the problem of distant metastasis which 5.1%–23.1% treated NPC patients develop.2 While studies have attempted to identify factors that can predict outcome in these patients, their findings are limited because they do not distinguish between the diverse radiotherapeutic techniques used.3 In addition, the staging system they use, the American Joint Committee on Cancer (AJCC) TNM classification system, is mostly based on NPC’s anatomy and does not sufficiently reflect its other biologic differences, which may partially explain the large variations in the survivals of NPC populations with same stage disease treated similarly.4 Thus, it is important to identify factors that may more consistently predict likely outcome in NPC patients treated with the increasingly popular IMRT.3,4
Cancer-associated inflammation has a well-established etiologic link with malignancy.5 The dynamic crosstalk among immune cells, inflammatory proteins, and cytokines in the tumor microenvironment and systemic circulations are the important factors contributing to tumorigenesis and cancer’s proliferative and invasive properties.5 The increased inflammatory response that occurs in response to cancer has been found to correlate with negative clinicopathologic predictors in many operable and inoperable neoplasms,5–7 and several retrospective and prospective studies have suggested using different systemic inflammation-based factors as surrogates of cancer’s biology to predict survival.6,7 In non-metastatic NPC, unfavorable outcomes have been associated with elevated total neutrophil counts,8 lymphocyte counts,8 C-reactive protein to albumin ratio,9 lymphocyte-to-monocyte ratio,10 Glasgow prognostic score,11 and decreased prognostic nutritional index.12 Recent studies have reported associations between a new cellular inflammation-based prognostic scoring system, combination of platelet count and neutrophil–lymphocyte ratio (COP-NLR), and survival in some aerodigestive tract cancers, including colorectal cancer13 and gastric cancer,14 esophageal cancer,15 non-small-cell lung cancer,16 and hypopharyngeal cancer.17 The predictive value of this COP-NLR scoring system has not been investigated in the context of NPC. Therefore, in this study, we evaluated the prognostic significance of pretreatment COP-NLR in NPC patients treated with IMRT-based therapy.
Materials and methods
Ethics statement
The Institutional Review Board of Kaohsiung Veterans General Hospital approved the protocol of this study. Usual review board requirement of written informed consent was waived because the study was retrospective in design. Patients’ confidentiality was protected by Cancer Center of Kaohsiung Veterans General hospital.
Patients and data collection
The protocol of this study was approved by the institutional review board of Veterans General Hospital. Two hundred and sixty newly diagnosed NPC patients were identified as being treated with IMRT-based therapy between January 2006 and February 2012 in the cancer registry provided by cancer center of Kaohsiung Veterans General Hospital. Two hundred and fifty-four of these patients were included in this study. They were 18 years old or older, had histologically confirmed nonkeratinized carcinoma or undifferentiated carcinoma, and had received complete blood differential count records within 30 days prior to treatment. Patients were excluded if they had distant metastasis at initial presentation (n=10), had inadequate medical record (n=1), had other treated previous and/or synchronous malignancies (n=6), had any other known autoimmune disorders (n=2) or infectious conditions (n=2), or had received glucosteroid 30 days prior to blood sampling (n=1). In cases in which patients had received more than one serum study prior to treatment, we utilized the most recent one. After exclusion, 232 patients were enrolled in our study.
Diagnosis and staging
All patients had received routine evaluations including detailed medical history, endoscopic examination and biopsy, and pretreatment serum measurements. Their diseases were staged based on the 7th Edition of AJCC Cancer Staging system for NPC accessed by a combination of magnetic resonance imaging, chest radiograph, abdominal ultrasonography, whole-body bone scanning, and/or positron emission tomography–computed tomography.
Radiotherapy, chemotherapy, and follow-up
Patients with stages I–II malignancies were treated by radiotherapy alone or concurrent chemoradiotherapy (CCRT). Patients with stages III–IVB malignancies were treated with CCRT with or without induction/adjuvant chemotherapies. The total cumulative dose of radiation administered to the gross nasopharyngeal tumor ranged from 68 to 76 Gy. Clinical negative nodal regions were treated prophylactically with 50–56 Gy, whereas the positive nodal areas were treated with 60–66 Gy. Our institution recommends induction chemotherapy for those with T4 and/or N3 disease. Adjuvant chemotherapy was administered to subjects of AJCC N3 disease or of multiple lymph node involvement with concomitant AJCC classifications of T3 or T4 disease and/or with one of the lymph nodes being >4 cm in size.18 The regimen for induction and adjuvant chemotherapy included cisplatin (80 mg/m2, on Day 1) and fluorouracil (1,000 mg/m2, 96-hour continuous infusion from Day 2 to Day5) administrated every 3–4 weeks for two to three cycles. The regimen for concurrent chemotherapy was 80–100 mg/m2 of cisplatin on Day 1, Day 22, and Day 43 or 30 mg/m2 of cisplatin every week for six to eight cycles during the period of radiotherapy. After completing treatment, patients were asked to return to our clinic every 3 months for the first 3 years. This follow-up was gradually decreased to every 6 months in the following 2 years and to once annually, thereafter, from the sixth year.
Optimal cutoff values for hematologic measurements
Peripheral blood samples were placed in tubes containing ethylenediaminetetraacetic acid (EDTA) to measure circulating absolute neutrophil, total lymphocytes, and total platelet counts. The NLR was calculated as neutrophil count divided by lymphocyte count. To avoid a predetermined cutoff point, the cutoff values of NLR and platelet counts utilized in this study were determined using receiver operating characteristic (ROC) curve analysis. An NLR value of 2.23 was considered optimal because it was closest to maximum joint sensitivity (0.590) and specificity (0.565) in its prediction of overall survival (OS). Similarly, the cutoff value for platelet count was 290.5×109/L (sensitivity: 0.285; specificity: 0.767). The COP-NLR score we used was obtained from previous studies.13–15,17 Briefly, patients with elevated NLR (>3) and platelet count (>300×109/L) were assigned a COP-NLR score of 2. Those with only one elevated value were assigned a COP-NLR score of 1 and those without abnormal values a score of 0. Because the sample size of patients with COP-NLR score of 2 was small, we grouped these patients along with those with scores of 1 into a high-risk group and those scores of 0 into a low-risk group.
Statistical analyses
All statistical operations were performed using SPSS ver.22 (IBM Corporation, Armonk, NY, USA). Patient characteristics were summarized descriptively as mean ± standard deviation (SD). Categorical variables were presented as numbers and percentages. The differences between categorical variables were analyzed using Pearson’s chi-square tests and those of continuous variables using independent t-test. The differences of patients’ demographics were further assessed by differences in risk groups stratified by COP-NLR score (0 vs 1&2).
The clinical outcomes were 3-year disease-specific survival (DSS), 3-year locoregional relapse-free survival (LRFS), 3-year distant metastasis-free survival (DMFS), 3-year failure-free survival (FFS), and 3-year OS. The events of FFS denote the occurrence of metastasis and/or recurrence. OS was calculated starting on the date on which a pathology-based diagnosis was made. All other end points were calculated starting on the first date of treatment. All the patients were followed up until the event occurrence or until the end of the study period (December 31, 2015), whichever came first. Those who were lost in the cancer registry were censored at their last date of follow-up.
Univariate survival analysis was performed by the Kaplan–Meier log-rank test to assess differences in survival analyzed by age, sex, smoking status, Charlson Comorbidity Index Score (CCIS), AJCC T classification, AJCC N classification, treatment modalities, radiotherapy duration, serum hemoglobin level, total platelet counts, NLR, and the COP-NLR scores. The potential risk variables were further analyzed in a multivariable Cox regression model to determine the independent predictors of DSS, LRFS, DMFS, FFS, and OS with adjustment for other exploratory variables. All data from survival analysis are presented as adjusted hazard ratio (aHR) ±95% confidence interval (95% CI). Two-sided P-values <0.05 were considered significant.
Results
Demographic features
As can be seen in Table 1, 163 patients (70.3%) were male and the average age was 50.7 years (range, 19–80 years). Most had no comorbidities (n=178, 76.7%) and most (n=206, 88.8%) had pathologically confirmed non-keratinized undifferentiated carcinoma. One hundred and sixteen subjects (50%) had advanced AJCC T classifications; 32 (13.8%) had no clinical nodal involvement. Two hundred and four (87.9%) had stages III–IVB malignancies. Median radiotherapy duration was 7.41 weeks. Thirty-nine patients (16.8%) received radiotherapy only, while 91 (39.2%) were treated with concurrent chemoradiotherapy (CCRT). Additional chemotherapies prior to or after the definite radiotherapy were administered to 102 patients (44%). Mean follow-up was 55.19±29.37 months. In our comparison of the two COP-NLR groups, the 85 who were assigned to the high-risk group (COP-NLR 1&2) had significantly higher AJCC T classifications (P=0.002) and lower hemoglobin levels (P=0.043) than the 147 patients assigned to low-risk group (COP-NLR 0) (Table 1).
Table 1.
Patient demographics and disease characteristics within different COP-NLR scores
| Variables | Total (n=232) | COP-NLR =0 | COP-NLR =1&2 | P-value |
|---|---|---|---|---|
| Age (years) | 50.70±11.47 | 50.86±11.84 | 50.44±10.85 | 0.788 |
| Sex | 0.703 | |||
| Male | 163 (70.3) | 102 (69.4) | 61 (71.8) | |
| Female | 69 (29.7) | 45 (30.6) | 24 (28.2) | |
| Smoke | 0.964 | |||
| No | 156 (67.2) | 99 (67.3) | 57 (67.1) | |
| Yes | 76 (32.8) | 48 (32.7) | 28 (32.9) | |
| CCIS | 0.800 | |||
| 0 | 178 (76.7) | 112 (76.2) | 66 (77.6) | |
| ≥1 | 54 (23.3) | 35 (23.8) | 19 (22.4) | |
| Histology type | 0.133 | |||
| NUC | 206 (88.8) | 134 (91.2) | 72 (84.7) | |
| NDC | 26 (11.2) | 13 (8.8) | 13 (15.3) | |
| AJCC T classification | 0.002 | |||
| T1 | 80 (34.5) | 58 (39.5) | 22 (25.9) | |
| T2 | 36 (15.5) | 23 (15.6) | 13 (15.3) | |
| T3 | 76 (32.8) | 51 (34.7) | 25 (29.4) | |
| T4 | 40 (17.2) | 15 (10.2) | 25 (29.4) | |
| AJCC N classification | 0.779 | |||
| N0 | 32 (13.8) | 20 (13.6) | 12 (14.1) | |
| N1 | 41 (17.7) | 28 (19.0) | 13 (15.3) | |
| N2 | 142 (61.2) | 87 (59.2) | 55 (64.7) | |
| N3 | 17 (7.3) | 12 (8.2) | 5 (5.9) | |
| AJCC Stage | 0.109 | |||
| Stage 1 | 10 (4.3) | 5 (3.4) | 5 (5.9) | |
| Stage 2 | 18 (7.8) | 12 (8.2) | 6 (7.0) | |
| Stage 3 | 131 (56.5) | 91 (61.9) | 40 (47.1) | |
| Stage 4 | 73 (31.4) | 39 (26.5) | 34 (40.0) | |
| Treatment arm | 0.299 | |||
| RT alone | 39 (16.8) | 26 (17.7) | 13 (15.3) | |
| CCRT alone | 91 (39.2) | 62 (42.2) | 29 (34.1) | |
| RT/CCRT + CT | 102 (44.0) | 59 (40.1) | 43 (50.6) | |
| RT duration (week) | 7.41±0.84 | 7.39±0.85 | 7.43±0.83 | 0.703 |
| Hemoglobin | 13.86±1.76 | 14.06±1.55 | 13.48±2.06 | 0.043 |
Note: Data presented as N (%) or mean ± standard deviation.
Abbreviations: COP-NLR, combination of platelet count and neutrophil–lymphocyte ratio; SD, standard deviation; CCIS, Charlson Comorbidity Index Score; NUC, nonkeratinizing undifferentiated carcinoma; NDC, nonkeratinizing differentiated carcinoma; AJCC, American Joint Committee on Cancer; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; CT, chemotherapy.
Univariate survival analysis
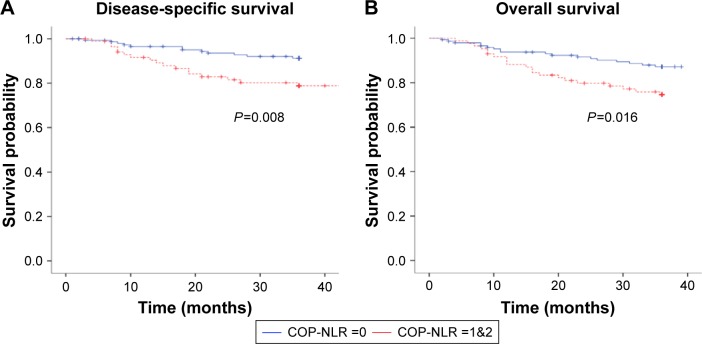
The 3-year DSS, LRFS, DMFS, and OS rates were 86.6%, 90.5%, 87.6%, and 82.5%, respectively. As seen in Table 2, we found no association between higher NLR alone or platelet counts alone with poorer survivals. However, compared with those with COP-NLR scores of 1 or 2, patient with a COP-NLR score of 0 had better LRFS (95.2% vs 82.1%, P=0.001), DMFS (91.4% vs 80.8%, P=0.026), DSS (91.2% vs 78.8%, P=0.008), and OS (87.2% vs 74.6%, P=0.016) (Table 2; Figure 1). Male subjects and those treated with CCRT only had better DMFS (P=0.037 and P=0.017, respectively). Patients with CCIS scores of 1 or higher had worse locoregional recurrence-free survival than their counterparts (77% vs 94.3%, P=0.001).
Table 2.
Univariate analysis of risk factors for 3-year LRFS, DMFS, DSS, and OS rates
| Variables | LRFS (%) | P-value | DMFS (%) | P-value | DSS (%) | P-value | OS (%) | P-value |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.288 | 0.962 | 0.421 | 0.107 | ||||
| <50 | 92.8 | 87.9 | 88.7 | 86.9 | ||||
| ≥50 | 88.3 | 87.3 | 84.5 | 78.5 | ||||
| Sex | 0.577 | 0.037 | 0.210 | 0.974 | ||||
| Male | 91.5 | 90.7 | 88.7 | 82.5 | ||||
| Female | 88.4 | 80.6 | 82.1 | 82.5 | ||||
| Smoke | 0.608 | 0.266 | 0.190 | 0.603 | ||||
| No | 91.2 | 85.9 | 84.6 | 81.6 | ||||
| Yes | 89.1 | 91.2 | 91.0 | 84.5 | ||||
| CCIS | 0.001 | 0.695 | 0.401 | 0.157 | ||||
| 0 | 94.3 | 87.0 | 87.5 | 84.0 | ||||
| ≥1 | 77.0 | 89.8 | 83.4 | 77.5 | ||||
| T classification | 0.162 | 0.984 | 0.259 | 0.309 | ||||
| T1/T2 | 93.2 | 87.3 | 89.2 | 85.0 | ||||
| T3/T4 | 87.7 | 88.0 | 83.8 | 79.9 | ||||
| N classification | 0.531 | 0.159 | 0.109 | 0.208 | ||||
| N0/N1 | 92.6 | 92.9 | 93.0 | 88.0 | ||||
| N2/N3 | 89.7 | 85.7 | 84.3 | 80.5 | ||||
| Treatment | 0.611 | 0.017 | 0.260 | 0.937 | ||||
| RT alone | 94.1 | 86.7 | 89.4 | 84.6 | ||||
| CCRT alone | 91.7 | 95.1 | 90.5 | 83.0 | ||||
| RT/CCRT + CT | 87.8 | 81.3 | 82.1 | 81.2 | ||||
| RT duration (weeks) | 0.195 | 0.626 | 0.903 | 0.750 | ||||
| ≤7.4 | 93.0 | 88.5 | 86.8 | 81.8 | ||||
| >7.4 | 87.3 | 86.4 | 86.3 | 83.4 | ||||
| Hemoglobin | 0.583 | 0.698 | 0.705 | 0.764 | ||||
| ≤13.5 | 89.1 | 86.7 | 85.4 | 81.9 | ||||
| >13.5 | 91.6 | 88.2 | 87.4 | 83.0 | ||||
| Platelet count (×103) | 0.219 | 0.891 | 0.103 | 0.053 | ||||
| ≤290.5 | 95.5 | 88.4 | 89.5 | 85.3 | ||||
| >290.5 | 86.5 | 87.3 | 80.3 | 74.8 | ||||
| NLR | 0.084 | 0.054 | 0.056 | 0.069 | ||||
| ≤2.23 | 93.6 | 91.6 | 90.5 | 86.5 | ||||
| >2.23 | 86.8 | 82.7 | 82.1 | 77.9 | ||||
| COP-NLR | 0.001 | 0.026 | 0.008 | 0.016 | ||||
| 0 | 95.2 | 91.4 | 91.2 | 87.2 | ||||
| 1&2 | 82.1 | 80.8 | 78.8 | 74.6 |
Abbreviations: LRFS, locoregional relapse-free survival; DMFS, distant metastasis-free survival; DSS, disease-specific survival; OS, overall survival; CCIS, Charlson Comorbidity Index Score; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; CT, chemotherapy; NLR, neutrophil–lymphocyte ratio; COP-NLR, combination of platelet count and neutrophil–lymphocyte ratio (patients with both an elevated platelet count [>300×103/mL] and an elevated NLR [>3] were allocated a score of 2, and patients showing one or neither were allocated a score of 1 or 0).
Figure 1.
Kaplan–Meier curves illustrate the DSS and OS for 232 NPC patients.
Notes: (A) Patients with COP-NLR 1&2 had worse DSS (P=0.008). (B) The difference of OS among two different COP-NLR groups was statistically significant (P=0.016).
Abbreviations: DSS, disease-specific survival; OS, overall survival; NPC, nasopharyngeal carcinoma; COP-NLR, combination of platelet count and neutrophil–lymphocyte ratio.
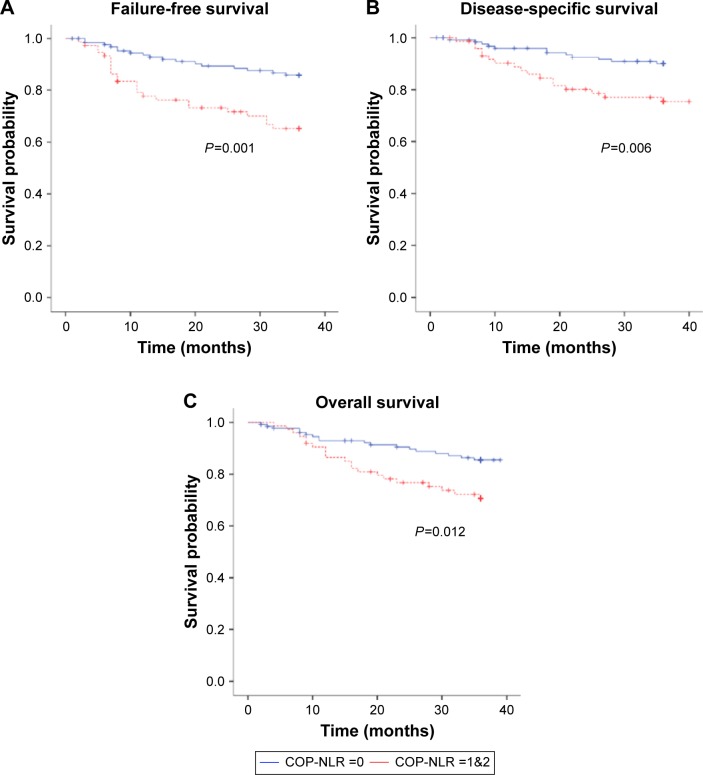
Further subgroup analysis based on AJCC stage found COP-NLR to also predict survival for patients with locally advanced NPC (AJCC stage III/IVA, B). Of the 204 with advanced-stage NPC, 130 had a COP-NLR score of 0 and 74 had either a COP-NLR score of 1 or 2. We found advanced-stage patients with COP-NLR of 0 to have better 3-year FFS (85.9% vs 65.2%; P=0.001), 3-year DSS (90.0% vs 75.4%; P=0.006), and 3-year OS (85.5% vs 70.6%; P=0.012), respectively (Figure 2).
Figure 2.
Comparison of (A) FFS, (B) DSS, and (C) OS according to COP-NLR scores for stages III and IVA/IVB malignancies.
Abbreviations: FFS, failure-free survival; DSS, disease-specific survival; OS, overall survival; COP-NLR, combination of platelet count and neutrophil–lymphocyte ratio.
Multivariate survival analysis for LRFS and DMFS
Multivariate analysis revealed a significant difference in 3-year LRFS between patients with a CCIS of one or higher compared to those without comorbidities (aHR: 4.934, 95% CI: 1.951–12.481, P=0.001) and between patients with COP-NLR scores 1 or 2 and those with a COP-NLR score of 0 (aHR: 4.401, 95% CI: 1.598–12.117, P=0.004), after adjusting for other potential risk variables (Table 3). Male patients and those with a COP-NLR score of 0 had better 3-year DMFS (aHR: 2.237, 95% CI: 1.048–4.775, P=0.037 and aHR: 2.188, 95% CI: 1.013–4.723, P=0.046, respectively) (Table 3). Patients treated with CCRT alone also had fewer events for distant failure than those treated with CCRT with additional chemotherapies (aHR: 3.415, 95% CI: 1.118–10.430). However, there was no statistical significance between CCRT alone and DMFS, compared with radiotherapy alone (aHR: 2.605, 95% CI: 0.693–9.792).
Table 3.
Multivariate analysis for 3-year LRFS and DMFS for all patients
| Variables | Comparison | LRFS
|
DMFS
|
||
|---|---|---|---|---|---|
| P-value | aHR (95% CI) | P-value | aHR (95% CI) | ||
| Sex | Female vs male | 0.227 | 1.819 (0.689–4.803) | 0.037 | 2.237 (1.048–4.775) |
| CCIS | ≥1 vs 0 | 0.001 | 4.934 (1.951–12.481) | 0.888 | 0.933 (0.353–2.468) |
| T classification | T3/T4 vs T1/T2 | 0.334 | 1.621 (0.609–4.314) | 0.586 | 0.804 (0.368–1.759) |
| N classification | N2/N3 vs N0/N1 | 0.967 | 0.974 (0.287–3.306) | 0.395 | 1.621 (0.533–4.928) |
| Treatment | CCRT vs RT | 0.465 | 0.549 (0.110–2.741) | 0.156 | 2.605 (0.693–9.792) |
| CCRT vs RT/CCRT + CT | 0.985 | 0.990 (0.348–2.820) | 0.031 | 3.415 (1.118–10.430) | |
| COP-NLR score | 1&2 vs 0 | 0.004 | 4.401 (1.598–12.117) | 0.046 | 2.188 (1.013–4.723) |
Abbreviations: LRFS, locoregional relapse-free survival; DMFS, distant metastasis-free survival; aHR, adjusted hazard ratio; CI, confidence interval; CCIS, Charlson Comorbidity Index Score; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; CT, chemotherapy; COP-NLR, combination of platelet count and neutrophil–lymphocyte ratio.
Multivariate survival analysis for DSS and OS
As can be seen in Table 4, patients with higher COP-NLR scores had increased hazard of DSS (aHR: 2.445, 95% CI: 1.148–5.206, P=0.02) and OS (aHR: 2.106, 95% CI: 1.102–4.025, P=0.024), respectively. There were no other variables significantly associated with survival.
Table 4.
Multivariate analysis for 3-year OS and DSS for all patients
| Variables | Comparison | DSS
|
OS
|
||
|---|---|---|---|---|---|
| P-value | aHR (95% CI) | P-value | aHR (95% CI) | ||
| Sex | Female vs male | 0.154 | 1.721 (0.816–3.627) | 0.772 | 1.107 (0.557–2.197) |
| CCIS | ≥1 vs 0 | 0.278 | 1.574 (0.694–3.572) | 0.141 | 1.672 (0.843–3.318) |
| T classification | T3/T4 vs T1/T2 | 0.596 | 1.231 (0.571–2.651) | 0.547 | 1.226 (0.632–2.378) |
| N classification | N2/N3 vs N0/N1 | 0.245 | 1.928 (0.638–5.827) | 0.243 | 1.668 (0.706–3.938) |
| Treatment | CCRT vs RT | 0.870 | 1.107 (0.328–3.734) | 0.910 | 0.946 (0.360–2.483) |
| CCRT vs RT/CCRT + CT | 0.466 | 1.387 (0.575–3.342) | 0.683 | 0.861 (0.419–1.769) | |
| COP-NLR score | 1&2 vs 0 | 0.020 | 2.445 (1.148–5.206) | 0.024 | 2.106 (1.102–4.025) |
Abbreviations: DSS, disease-specific survival; OS, overall survival; aHR, adjusted hazard ratio; CI, confidence interval; CCIS, Charlson Comorbidity Index Score; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; CT, chemotherapy; COP-NLR, combination of platelet count and neutrophil–lymphocyte ratio.
Discussion
This study found pretreatment COP-NLR but not the baseline platelet count alone or NLR alone to be predictive of survival in this patient population. To the best of our knowledge, this study may be the first to find pretreatment COP-NLR to be a simple and useful predictor of survival in NPC patients receiving IMRT as the only radiotherapy technique in their treatment program.
Cumulative evidence has demonstrated that inflammation is a hallmark of cancer and that this cancer-related inflammation can promote cancer and affect host immunity and tumor response to treatment.5 The resultant changes in circulating hematologic components associated with this inflammation make several indices involving immune cells at the systemic level useful prognosticators for some malignancies.6,7 However, the mechanisms underlying their prognostic ability are multifaceted and have not been clarified. There is consistent evidence that interleukin (IL)-6 is overexpressed in EBV-infected nasopharyngeal epithelial cancer cells.19 The enhanced circulating IL-6 not only contributes to NPC’s proliferative properties by activating IL-6-associated transcription factors NF-kappa B and STAT319,20 but also mediates platelet generation.21 In addition to these activated platelets providing protection of circulating cancer cells via their influence on the function of natural killer cells enabling tumor metastasis,22 they also secrete numerous growth factors, including tumor growth factor-beta, platelet-derived growth factor, and vascular endothelial growth factor, to promote the progression of cancer and neovasculization.23 Chemokines simultaneously released along with the IL-1 beta and platelet-activating factor have also been found to be involved in modulating hosts’ immune responses to cancer cells.24 These mechanisms make peripheral thrombocystosis an attractive possible indicator of cancer-secreted cytokines that might be used to predict prognosis in NPC subjects. In Chen et al’s study25 investigating 2,626 NPC patients, those with platelet counts >300×109/L had a poorer prognosis than their counterparts, even after stratifying by different TNM classifications. Gao et al26 also demonstrated that their patients with pretreatment thrombocytosis had more metastatic events than those without. This study, however, did not find such an inverse correlation in NPC patients treated using the IMRT technique with a uniform cutoff value of 290.5×109/L.
The use of NLR to predict cancer survival has been more extensively discussed than the use of total platelet count.7 The overproduction of IL-6 by tumors causes the proliferation of neutrophils,19 and these neutrophils along with tumor-associated macrophages27 enhance the seeding of cancer into systemic circulation through angiogenesis28 and extracellular matrix degradation.29 Furthermore, neutrophil-mediated T lymphocyte deficiency and disrupted cytotoxicity render individuals more susceptible to carcinogenesis and future cancer progression.30 Thus, a higher NLR can serve as a negative prognosticator for various cancers, including NPC. Chang et al31 found a 5-year difference in survival between NPC populations with high NLR (>2.5) and low NLR (≤2.5). In that study, they further divided their patients into development and validation cohorts to test the prognostic value of NLR in patients receiving IMRT or non-IMRT-based treatment. They found NLR’s ability to predict survival to be inconsistent. In a more recent prospective study investigating 393 advanced-stage NPC patients treated with IMRT or two-dimensional radiotherapy-based treatments, Chua et al32 found a pretreatment NLR ≥3 to be associated with increased AJCC T classification, AJCC N classification, overall tumor stage, and pretreatment EBV DNA titer but not survival regardless of whether the induction or adjuvant chemotherapy was administered. Similarly, Zeng et al,33 who conducted a retrospective study including 675 patients treated with uniformly IMRT, did not find evidence that NLR had prognostic value. Likewise, our study did not find a pretreatment NLR cutoff value of 2.23 to predict survival.
A rapidly increasing number of studies are looking at the possibility of using pretreatment blood parameters to predict survival. Some researchers have found these parameters to be associated with therapeutic resistance to (chemo) radiotherapy.34,35 The mechanism underlying this negative correlation may have been partially driven by proliferations of myeloid-derived suppressor cells (MDSCs) potentiated by granulocyte colony-stimulating factor-producing cancer cells.34 In a study by Mabuchi et al34 investigating uterine cervical cancer, the authors proposed that the expansions of MDSCs were responsible for enhanced tumor progression and, interestingly, could have been involved in the diminished effectiveness of radiotherapy for cancer cells displaying peripheral leukocytosis. By abrogating the MDSCs in an animal model, they succeeded in enhancing cancers’ radiosensitivity and hampering tumor growth.34 Further clinical evidence provided by Mizunuma et al35 of 56 patients with stages IB1–IV uterine cervical cancers, it was observed that those with higher pretreatment NLR (≥2.5) were more likely to be resistant to radiotherapy compared with those with lower NLR (<2.5). With regard to NPC, Li et al36 have shown that MDSC expansions promote NPC metastasis by activating the β-catenin/TCF4 pathway. However, the clinical implication of peripheral hematologic parameters on (chemo)radio-resistance in NPC population has not yet been investigated. Further study is warranted to determine whether certain combination of blood components can be considered a distinct clinical entity conferring (chemo)radio-resistance in NPC patients, affecting their survival.
Combining the two markers into a scoring system might provide a score better able to reflect the intensity of cancer-associated inflammation. This scoring system may better reflect the cancer’s anatomic and biologic status. Previous studies have found significant differences in conventional tumor-related factors and other inflammation-based markers among cancer patients categorized into different COP-NLR groups.13–17 Additionally, a higher COP-NLR has consistently translated into poorer prognoses in a variety of cancers.13–17 COP-NLR may be better applied to the estimation of cancer survival than other inflammation-based markers because it is cost efficient and reproducible, it is readily estimated prior to treatment, and it can be used to stratify cancer patients into different risk subgroups by scoring the two separate parameters.13–17 In this study, NPC patients categorized as higher risk subgroup by their having COP-NLR scores of 1 or 2 had worse survival outcomes than those with scores of 0 when using cutoff values of 3 for NLR and 300×109/L for total platelet count, respectively. Moreover, we found that locally advanced NPC patients in the higher risk subgroup to be at greater risk of locoregional and distant failures. These findings suggest that COP-NLR can also reflect NPC’s capacity for both lymphatic and hematogenous metastasis in patients treated with IMRT.
This study has some limitations. One limitation is its small sample collected from a single institute. This made further validation analysis by separating patients into training and validation cohorts difficult. Another limitation was that we did not assess cancer-related cytokines and chemokines in the NPC specimens before therapy, so it was not possible to determine to what extent the inflammatory mediator levels in the local tissue or in the systemic circulation correspond with peripheral COP-NLR in our study. We did not routinely measure plasma EBV DNA level, either. EBV is known to contribute to the development of NPC. Still another limitation is that because we did not take serial blood samplings during and after treatment period, we could not determine whether COP-NLR’s interval changes predicted treatment response and survival or establish its possible role in post-treatment surveillance. In addition, it is worth noting that, although blood parameters are of clinical value in predicting disease severities of the associated complications of common disorders such as diabetes mellitus37 and hypertension,38 we did not exclude patients with these comorbid conditions as our sample size was already limited and we lacked detailed information regarding their disease severity. Nonetheless, the confounding impact of these comorbidities should be minimized as CCIS was one of risks we factored into our survival analysis.
Conclusion
Despite the aforementioned limitations, our findings suggest that COP-NLR can better predict survival either in NLR alone or total platelet count in patients with NPC treated with IMRT-based therapy. Cost-effective and easily assessable, COP-NLR can help physicians identify NPC patients at higher risk of survival failure prior to the treatment and help them provide their patients with the most optimal treatment and surveillance programs.
Acknowledgments
This manuscript is original, and it, or any part of it, has not been previously published nor is it under consideration for publication elsewhere.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Zhang MX, Li J, Shen GP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51(17):2587–2595. doi: 10.1016/j.ejca.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2015;108(1):djv291. doi: 10.1093/jnci/djv291. [DOI] [PubMed] [Google Scholar]
- 4.Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122(4):546–558. doi: 10.1002/cncr.29795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 6.McMillan DC. The systemic inflammation-based Glasgow prognosis score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 8.He JR, Shen GP, Ren ZF, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck. 2012;34(12):1769–1776. doi: 10.1002/hed.22008. [DOI] [PubMed] [Google Scholar]
- 9.Tao CJ, Chen YY, Jiang F, et al. The C-reactive protein/albumin ratio is an independent prognostic factor for overall survival in patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy. J Cancer. 2016;7(14):2005–2011. doi: 10.7150/jca.16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Jiang R, Liu WS, et al. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One. 2013;8(12):e83069. doi: 10.1371/journal.pone.0083069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XH, Chang H, Xu BQ, et al. An inflammatory biomarker-based nomogram to predict prognosis of patients with nasopharyngeal carcinoma: an analysis of a prospective study. Cancer Med. 2017;6(1):310–319. doi: 10.1002/cam4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Xia L, Wang Y, et al. Low prognostic nutritional index (PNI) predicts unfavorable distant metastasis-free survival in nasopharyngeal carcinoma: a propensity score-matched analysis. PLoS One. 2016;11(7):e0158853. doi: 10.1371/journal.pone.0158853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109(2):401–407. doi: 10.1038/bjc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishizuka M, Oyama Y, Abe A, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. J Surg Oncol. 2014;110(8):935–941. doi: 10.1002/jso.23753. [DOI] [PubMed] [Google Scholar]
- 15.Feng JF, Huang Y, Chen QX. The combination of platelet count and neutrophil lymphocyte ratio is a predictive factor in patients with esophageal squamous cell carcinoma. Transl Oncol. 2014;7(5):632–637. doi: 10.1016/j.tranon.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Zhang L, Zhu K, et al. Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: based on a large cohort study. PLoS One. 2015;10(5):e0126496. doi: 10.1371/journal.pone.0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakahira M, Sugasawa M, Matsumura S, et al. Prognostic role of the combination of platelet count and neutrophil-lymphocyte ratio in patients with hypopharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2016;273(11):3863–3867. doi: 10.1007/s00405-016-3996-3. [DOI] [PubMed] [Google Scholar]
- 18.Lin JC, Jan JS, Hsu CY, Jiang RS, Wang WY. Outpatient weekly neoadjuvant chemotherapy followed by radiotherapy for advanced nasopharyngeal carcinoma: high complete response and low toxicity rates. Br J Cancer. 2003;88(2):187–194. doi: 10.1038/sj.bjc.6600716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G, Tsang CM, Deng W, et al. Enhanced IL-6/IL-6R signaling promotes growth and malignant properties in EBV-infected premalignant and cancerous nasopharyngeal epithelial cells. PLoS One. 2013;8(5):e62284. doi: 10.1371/journal.pone.0062284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zergoun AA, Zebboudj A, Sellam SL, et al. IL-6/NOS2 inflammatory signals regulate MMP-9 and MMP-2 activity and disease outcome in nasopharyngeal carcinoma patients. Tumour Biol. 2016;37(3):3505–3514. doi: 10.1007/s13277-015-4186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 22.Placke T, Kopp HG, Salih HR. Modulation of natural killer cell anti-tumor reactivity by platelets. J Innate Immun. 2011;3(4):374–382. doi: 10.1159/000323936. [DOI] [PubMed] [Google Scholar]
- 23.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YP, Zhao BC, Chen C, et al. Pretreatment platelet count improves the prognostic performance of the TNM staging system and aids in planning therapeutic regimens for nasopharyngeal carcinoma: a single-institutional study of 2,626 patients. Chin J Cancer. 2015;34(3):137–146. doi: 10.1186/s40880-015-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Zhang HY, Xia YF. Increased platelet count is an indicator of metastasis in patients with nasopharyngeal carcinoma. Tumour Biol. 2013;34(1):39–45. doi: 10.1007/s13277-012-0508-y. [DOI] [PubMed] [Google Scholar]
- 27.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105(7):2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10(15):4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- 30.Salazar-Onfray F, López MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007;18(1–2):171–182. doi: 10.1016/j.cytogfr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Chang H, Gao J, Xu BQ, et al. Haemoglobin, neutrophil to lymphocyte ratio and platelet count improve prognosis prediction of the TNM staging system in nasopharyngeal carcinoma: development and validation in 3,237 patients from a single institution. Clin Oncol (R Coll Radiol) 2013;25(11):639–646. doi: 10.1016/j.clon.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Chua ML, Tan SH, Kusumawidjaja G, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in locally advanced nasopharyngeal carcinoma: a pooled analysis of two randomised controlled trials. Eur J Cancer. 2016;67:119–129. doi: 10.1016/j.ejca.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Zeng L, Guo P, Li JG, et al. Prognostic score models for survival of nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy and chemotherapy. Oncotarget. 2015;6(36):39373–39383. doi: 10.18632/oncotarget.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mabuchi S, Matsumoto Y, Kawano M, et al. Uterine cervical cancer displaying tumor-related leukocytosis: a distinct clinical entity with radioresistant feature. J Natl Cancer Inst. 2014;106(7):dju147. doi: 10.1093/jnci/dju147. [DOI] [PubMed] [Google Scholar]
- 35.Mizunuma M, Yokoyama Y, Futagami M, Aoki M, Takai Y, Mizunuma H. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Int J Clin Oncol. 2015;20(5):989–996. doi: 10.1007/s10147-015-0807-6. [DOI] [PubMed] [Google Scholar]
- 36.Li ZL, Ye SB, OuYang LY, et al. COX-2 promotes metastasis in nasopharyngeal carcinoma by mediating interactions between cancer cells and myeloid-derived suppressor cells. Oncoimmunology. 2015;4(11):e1044712. doi: 10.1080/2162402X.2015.1044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulu SM, Dogan M, Ahsen A, et al. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther. 2013;15(11):942–947. doi: 10.1089/dia.2013.0097. [DOI] [PubMed] [Google Scholar]
- 38.Kim BJ, Cho KI, Choi JH, et al. Epicardial fat thickness and neutrophil to lymphocyte ratio are increased in non-dipper hypertensive patients. J Cardiovasc Ultrasound. 2016;24(4):294–302. doi: 10.4250/jcu.2016.24.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]