Abstract
Some strains of Streptococcus suis possess a type II restriction-modification (RM) system, whose genes are thought to be inserted into the genome between purH and purD from a foreign source by illegitimate recombination. In this study, we characterized the purHD locus of the S. suis genomes of 28 serotype reference strains by DNA sequencing. Four strains contained the RM genes in the locus, as described before, whereas 11 strains possessed other genetic regions of seven classes. The genetic regions contained a single gene or multiple genes that were either unknown or similar to hypothetical genes of other bacteria. The mutually exclusive localization of the genetic regions with the atypical G+C contents indicated that these regions were also acquired from foreign sources. No transposable element or long-repeat sequence was found in the neighboring regions. An alignment of the nucleotide sequences, including the RM gene regions, suggested that the foreign regions were integrated by illegitimate recombination via short stretches of nucleotide identity. By using a thermosensitive suicide plasmid, the RM genes were experimentally introduced into an S. suis strain that did not contain any foreign genes in that locus. Integration of the plasmid into the S. suis genome did not occur in the purHD locus but occurred at various chromosomal loci, where there were 2 to 10 bp of nucleotide identity between the chromosome and the plasmid. These results suggest that various foreign genes described here were incidentally integrated into the same locus of the S. suis genome.
Streptococcus suis is a gram-positive pathogen that has been identified as the cause of a wide range of clinical disease syndromes in pigs, other animals, and humans (5, 10, 28). Currently, S. suis strains are classified into 35 capsular serotypes (16, 17, 20, 34), and the genomic heterogeneity and the phylogenetic diversity of the strains have been described (9, 19, 32). Previous findings suggest that the heterogeneity of this bacterium is due, at least in part, to the integration of foreign genes (37, 38, 43).
One example of the foreign genes identified is a cluster of the SsuDAT1I genes encoding a type II restriction-modification (RM) system, which was found in S. suis strain DAT1 (37, 38). The RM gene region, comprising 3,503 bp, was thought to be inserted in an intergenic space of a conserved purine biosynthetic gene cluster between purH and purD (37, 38). A sequence comparison between strains with and without the RM genes showed that the borders of the RM region could be clearly recognized as if the exogenous region had simply been inserted. The other example of the foreign genes is the sly gene, which encodes a cholesterol-binding cytolysin (12, 15, 22). With a few exceptions, all S. suis strains have either sly or a hypothetical gene, designated orf102, at the same locus in the genome, and either of the two genes is thought to have been acquired by S. suis from a foreign source and to have replaced the gene that had existed in the locus. In contrast to the borders of the RM gene region, however, the flanking sequences of sly and orf102 show a relatively low degree of similarity (approximately 66% nucleotide identity) to one another, so that the borders of the foreign genes are unclear (43). In both examples, sequences affecting the integration of foreign genes, such as long-repeat sequences or transposable elements, were not found, a result which suggests that these integration events had occurred by illegitimate recombination. However, the structural differences of the borders between the purHD and sly regions lead us to raise the question of whether these foreign genes were integrated via the same mechanism of gene acquisition.
In bacteria, a genetic conversion which joins DNA molecules at sites where they have no or a few identical base pairs is referred to as illegitimate (nonhomologous) recombination (2). Such events were often identified as intramolecular recombination events following double-strand breaks and usually occurred within or near small direct or inverted repeats. These events are exemplified by the formation of deletions in bacterial chromosomes (6) and plasmids (1, 3) and the formation of transducing phages after UV irradiation of lysogenic cells (14, 39). On the other hand, it was shown that naturally transformable bacteria incorporate a foreign DNA that has a region with homology to the resident genome on one side (11, 35). The homologous stretches serve as anchors for homologous recombination and subsequently create a novel joint on the other side, where a short (a few base pairs) stretch of sequence identity exists, by illegitimate recombination. Such foreign DNA acquisition, referred to as homology-facilitated (or homology-directed) illegitimate recombination, is thought to occur widely in many bacterial species (30). It is, therefore, possible that a similar mechanism for genetic conversion was employed to generate the genetic structures as observed in the purHD and sly regions of S. suis. However, the natural transformation has not yet been demonstrated for S. suis, and only two examples of foreign gene regions have so far been examined. Thus, further examples are needed to analyze the mechanism of foreign gene acquisition in S. suis.
We report here that some serotype reference strains of S. suis which were previously shown to lack the RM system possess various genes between purH and purD. Nucleotide sequence comparisons among them and the experimental integrations of a plasmid carrying the RM genes into the S. suis genome suggest that illegitimate integrations of foreign genes in S. suis occurred randomly via a few base pairs of nucleotide identity between resident and foreign DNA.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The S. suis strains used in this study are listed in Table 1. These strains, except DAT1 and 227, are the reference strains of each serotype. The phylogenetic relationships among the strains used have already been described elsewhere (9, 19, 32, 43). The serotype reference strains of S. suis described hereafter are referred to by their serotypes. All of the S. suis strains were grown in Todd-Hewitt (TH) broth or agar medium (Difco Laboratories, Becton Dickinson, Sparks, Md.) at 28 or 37°C under 5% CO2. The Escherichia coli strain used was MC1061 (8), which was cultured in Luria-Bertani broth or agar medium (Difco Laboratories) at 37°C. When necessary, antibiotics were added to culture media at the following concentrations: 50 μg of ampicillin/ml, 10 μg of chloramphenicol (CAM)/ml, and 50 μg of spectinomycin (SPC)/ml for E. coli; and 5 μg of CAM/ml and 100 μg of SPC/ml for S. suis.
TABLE 1.
S. suis strains used in this study
| Strain | Sero- type | Source | Origin | Reference |
|---|---|---|---|---|
| DAT1 | 2 | Diseased pig | Japan | 38 |
| 227 | 2 | Diseased pig | Japan | 38 |
| NCTC10237 | 1 | Diseased pig | The Netherlands | 34 |
| NCTC10234 | 2 | Diseased pig | The Netherlands | 34 |
| 4961 | 3 | Diseased pig | Denmark | 34 |
| 6407 | 4 | Diseased pig | Denmark | 34 |
| 11538 | 5 | Diseased pig | Denmark | 34 |
| 2524 | 6 | Diseased pig | Denmark | 34 |
| 8074 | 7 | Diseased pig | Denmark | 34 |
| 14636 | 8 | Diseased pig | Denmark | 34 |
| 22083 | 9 | Diseased pig | Denmark | 16 |
| 4417 | 10 | Diseased pig | Denmark | 16 |
| 12814 | 11 | Diseased pig | Denmark | 16 |
| 8830 | 12 | Diseased pig | Denmark | 16 |
| 10581 | 13 | Diseased pig | Denmark | 16 |
| 13730 | 14 | Diseased human | The Netherlands | 16 |
| NCTC10446 | 15 | Diseased pig | The Netherlands | 16 |
| 2726 | 16 | Diseased pig | Denmark | 16 |
| 93A | 17 | Clinically healthy pig | Canada | 16 |
| NT77 | 18 | Clinically healthy pig | Canada | 16 |
| 42A | 19 | Clinically healthy pig | Canada | 16 |
| 86-5192 | 20 | Diseased calf | United States | 16 |
| 14A | 21 | Clinically healthy pig | Canada | 16 |
| 88-1861 | 22 | Diseased pig | Canada | 16 |
| 89-2479 | 23 | Diseased pig | Canada | 17 |
| 88-5299A | 24 | Diseased pig | Canada | 17 |
| 89-3576-3 | 25 | Diseased pig | Canada | 17 |
| 89-4109-1 | 26 | Diseased pig | Canada | 17 |
| 89-5259 | 27 | Diseased pig | Canada | 17 |
| 89-590 | 28 | Diseased pig | Canada | 17 |
DNA methods.
Restriction enzymes and DNA-modifying enzymes were purchased from Takara Shuzo Co., Ltd. (Tokyo, Japan) and used according to the manufacturer's recommendations. The vector plasmids used were pSET1 (41) and pSET4s (42). Minipreparations of recombinant plasmids from E. coli and transformations of E. coli were performed by standard procedures (36). Isolation of plasmids from S. suis and transformation of S. suis were carried out by using the methods described previously (40, 41). Genomic Southern hybridization was performed by using the procedures described previously (33). DNA fragments used for probes were labeled with digoxigenin by using a digoxigenin-PCR labeling mixture (Roche Diagnostics GmbH, Manheim, Germany) according to the manufacturer's instructions. The DNA fragments were amplified by PCR with the following primers: SsuDAT1I5′ plus SsuDAT1I3′ for SsuDAT1I genes; 16conF1 plus 16conR3 for serotype 16; 10conF1 plus 10conR2 for serotype 10; 12conF1 plus 12R1 for serotype 12; 13conF plus 13conR for serotype 13; 1conF plus 1conR for serotype 1; 6conF plus 6conR for serotype 6; and 20conF plus 20conR for serotype 20. PCR was performed by using a Perkin-Elmer thermal cycler model 2400 or 9600 (PE Biosystems Japan, Tokyo, Japan) and by using Takara Ex Taq polymerase and a Takara LA PCR kit, as described previously (38, 41).
DNA sequencing and data analysis.
PCR products were directly sequenced by dye terminator chemistry by using an Applied Biosystems model 310 automated DNA sequencer (PE Biosystems). The sequencing was done initially with the primers used for PCR, followed by a series of primer walking reactions with primers designed from the sequenced regions. The sequence data obtained were assembled and analyzed with Sequencher software, version 3.1.1 (Hitachi Software Engineering Co., Ltd., Yokohama, Japan), and GENETYX-MAC software, version 12.1 (GENETYX Corp., Tokyo, Japan). The putative genes were identified on the basis of the adopted criteria that an open reading frame (ORF) consists of at least 40 codons preceded by a potential Shine-Dalgarno sequence at an appropriate distance (6 to 15 bp) from one of the commonly used initiation codons (AUG, UUG, and GUG). The deduced amino acid sequences obtained were searched for similarity with databases by using the BLAST network service available at the National Center for Biotechnology Information, Bethesda, Md. (http://www.ncbi.nlm.nih.gov).
Experimental integration of plasmid. (i) Insertion of a gene cassette into the RM genes.
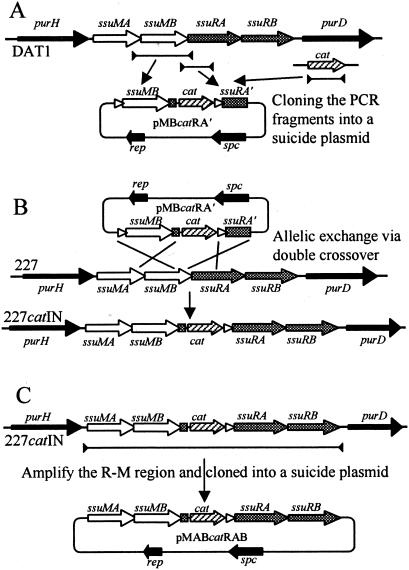
As mentioned above, the recombinant plasmids were constructed in E. coli. Because genes for the SsuDAT1I system overlap each other in a head-to-tail manner and the restriction gene must be accompanied by the cognate modification gene (38), the RM gene region with insertion of a gene cassette was constructed by allelic exchange on the genome as shown in Fig. 1. A 936-bp fragment containing the whole length of the ssuMB gene with a 120-bp upstream region and a 3-bp downstream region, which overlapped the first 23 nucleotides of the ssuRA gene, and a 594-bp fragment containing the first 443 nucleotides of the ssuRA gene, which overlapped the last 171 nucleotides of the ssuMB gene, were amplified separately from the genomic DNA of S. suis DAT1 with primers MBKI5′ plus MBKI3′ and RAKI2-5′ plus RAKI3′, respectively. The two PCR products were digested with EcoRI plus EcoT22I or EcoT22I plus BamHI, fused at the EcoT22I site, and cloned between the EcoRI-BamHI sites of pSET4s, generating pMBRA′. A CAM acetyltransferase (cat) gene cassette containing its promoter region but missing the transcriptional terminator was amplified from pSET1 with primers CT2 plus CT3, digested with EcoT22I, and cloned into a unique EcoT22I site of pMBRA′, generating pMBcatRA′ (Fig. 1A).
FIG. 1.
Strategy for construction of a suicide plasmid carrying a cat gene cassette placed between the ssuMB and ssuRA genes of the SsuDAT1I system. (A) Construction of pMBcatRA′. Lines between the closed arrowheads are PCR fragments amplified from the above regions. The amplified fragments were fused and cloned into pSET4s. (B) Generation of a mutant RM gene containing the cat gene cassette by allelic exchange via double crossover on the chromosome. (C) Construction of pMABcatRAB. The line between the closed arrowheads is a PCR fragment amplified from the genomic DNA of S. suis 227catIN. The amplified fragment was cloned into pSET4s.
(ii) Gene replacement by allelic exchange in S. suis.
One field isolate, strain 227, was selected for the experiment as it did not carry any cryptic plasmids and was shown to possess the same genetic organization with respect to the RM genes as strain DAT1 (38). Procedures for selection of mutants whose genes were replaced by an allelic exchange via double crossover were described previously (33, 42). Briefly, S. suis strain 227 was transformed with pMBcatRA′, and the cells were grown at 28°C in the presence of CAM and SPC. At mid-logarithmic growth phase, the cells were diluted with TH broth containing CAM and grown at 28°C to early logarithmic phase. The cultures were then shifted to 37°C and incubated for 4 h. Subsequently, the cells were spread on TH agar containing CAM and incubated at 37°C. Temperature-resistant CAM-resistant (Cmr) colonies obtained were screened for loss of vector-mediated SPC resistance (Spcr) to detect putative mutants which had exchanged their wild-type allele for a genetic segment containing the cat gene as a consequence of homologous recombination via a double crossover. Finally, the genetic organization of the mutant alleles was examined by Southern hybridization, and the resulting mutant obtained was designated 227catIN (Fig. 1B).
(iii) Selection of integration mutants.
The DNA region containing the whole length of the RM genes with insertion of the cat cassette was amplified with primers SsuDAT1I5′B plus SsuDAT1I3′E from the genomic DNAs of 227catIN. The fragment was digested with BamHI plus EcoRI and cloned into the corresponding sites of pSET4s to generate the plasmids designated pMABcatRAB (Fig. 1C). S. suis serotype 2 (NCTC10234) was transformed with pMABcatRAB and grown on TH agar containing CAM and SPC at 28°C for 40 h. The cells were harvested and suspended in saline solution. Aliquots of appropriately diluted bacterial suspension, which contained approximately 107 cells, were spread on prewarmed TH agar containing CAM and grown at 37°C for 18 h. Bacterial lawn that appeared on the plate was transferred to another TH agar plate containing CAM by replica plating and grown at 37°C for 18 h. Colonies that appeared on the plates were purified by two consecutive single-colony isolations on TH agar containing CAM at 37°C. Two hundred forty-three temperature-resistant Cmr mutants which did not possess an autonomously replicating plasmid (e.g., one that had lost its temperature sensitivity) were selected from 25 independent experiments and used in this study. Twelve of the mutants obtained from 3 of the 25 independent experiments were designated Int1 to Int12. For estimation of the integration frequency, the bacterial suspension of S. suis serotype 2 carrying pMABcatRAB was spread on prewarmed TH agar containing CAM plus SPC and grown at 37°C for 24 h. The numbers of temperature-resistant colonies that appeared on the agar were used for the calculation.
(iv) Nucleotide sequence determination of the junction between the integrated plasmid and genome.
The genomic DNA of the temperature-resistant Cmr mutants was digested with EcoT22I plus PstI, which cut pMABcatRAB at three sites and generated cohesive ends compatible with each other. The digested DNA was self-ligated and used as templates for inverse PCRs with two sets of primers, each of which amplified either end of the junction regions independently. The primers, which were designed from the sequence of pMABcatRAB, were as follows: TS2 plus oriE1-50 and SsuMB3′ plus TS1 for mutants Int1, Int5, Int6, and Int7; TS2 plus PC1 and SsuMB3′ plus TS1 for mutants Int2, Int3, Int9, Int11, and Int12; MA1 plus MA2 and MA3 plus dam-in2 for mutant Int4; SsuRA3′ plus PC1 and SsuMB3′ plus RA3 for mutant Int10. The DNA fragments amplified by the inverse PCR were directly sequenced. On the basis of the sequences of junction regions, 11 primer sets were designed to amplify the target regions where the plasmid was integrated, from the genomic DNA of S. suis serotype 2. The primers used for the amplifications of the target regions of mutants Int1, Int2, Int3, Int4, Int5, Int6, Int7, Int9, Int10, Int11 and Int12 were 1-1FJ plus 1-1Rend, 2-1Fend plus 2-1Rend, 3-1Fend plus 3-1Rend, 3-2Fend plus 3-2Rend, 5FJ plus 5Rend, 9Fend plus 9Rend, 21Fend plus 21Rend, 2-61Fend plus 2-61Rend, 2-69FJ plus 2-69RJ, S-1Fend plus S-1RJ, and S-3Fend plus S-3Rend, respectively.
Oligonucleotides used in PCRs.
The following oligonucleotides were purchased from Hokkaido System Science Co., Ltd. (Sapporo, Japan) and were used as PCR primers (shown in the 5′-to-3′ direction): purH, GCGCTAGCTATTTTGACCAATA; purD, GCAAAGTCTTTTGACCACTCTA; purN-F, GTGTTTGCATCAGGCAACGGCTCCA; purH-R2, GTCTTGGTCACGGACAGAACCACCT; purD-F2, GAGCAGGTCTTTGTTGCTCCTGGAA; purE, GCCGCCTGGCATTTGCACGATA; MBKI5′, CAAGCCCAGTAGTTGAAGAATTGTA; MBKI3′, CTATGAAAAGTTCTTTTTGTCATCT; RAKI2-5′, ATGCATGCTTCAACTCAAGAAGGAGA; RAKI3′, CCGTAAACATAGTCCTGTAAGTTT; RAKI5′, AACTAGATTGGATAAGGAAGATGA; CT2, ATGCATCACCGAACTAGAGCTTGATG; CT3, ATGCATATTATAAAAGCCAGTCATTA; SsuDAT1I5′B, CGGATCCAAGTATAGCACCCCAGCTGGAGAAG; SsuDAT1I3′E, GGAATTCCTTGATTATCTAAACAAATCATGC; SsuDAT1I5′, AAGTATAGCACCCCAGCTGGAGAAG; SsuDAT1I3′, CTTGATTAT CTAAACAAATCATGC; SsuMB3′, CACATCTGGATACTTGTCAC; SsuRA3′, GTAATCTGGCGTTCGATTAG; SsuRB5′, TGGTGAAGGTTGGCAAAGGT; MA1, CCCCTCCTCCAACAAACGGTTCAAA; MA2, TCCGATGTGGAGCGAGCAGCACGCA; MA3, CTGGGCTTGAAGAATTAGAAAGCAT; RA3, TACAGGACTATGTTTACGGTGTAGA; oriE1-50, GCTCACTCATTAGGCACCCCAGGCT; SP3, ACTAGTGTTCGTGAATACATGTTATA; SP4, ACTAGTGTTTTCTAAAATCTGAT; PC1, ACTAGTTATCTACACGACGGGG; LZ3, AGATCTCGGTGATGACGGTGAAAACC; TS1, AGATCTATTAATCGCAACATCAAACC; TS2, ACTAGTTATCGGCATAATCGTT; RAfuse3′, CCGTAAACATAGTCCTGTAAGTTT; dam-in2, CAGTGGGTATGGCCTTAGAA; dam-in4, TGGTCGTAAAAATAGAATGGGT; dam-in7, GGTCTAAGGTTGAACGAGAA; 16conF1, CAAGGAGTGTGCTAAATAACCTTCT; 16conR3, AACTTGCTTTACTTCCTCACATTCA; 10conF1, CAGATAGTGTGAATGAAGTTAAGTT;10conR2, CAATCTACCATTGGTGCAGATACTC; 12conF1, CG AGAAATACAGTATTAAACAGTCC; 12R1, CAATAGCCGATACCATGAAACAAGT;13conF, CGCTTCGTAATGGGGAGGCGAAAGA; 13conR, GCAAGTATTTCGCCTTGTTTAGAGA; 1conF, CTTTTGTGAGAAGGAGGAGTTAGA;1conR, AGAAAAAAGCACCTCTAAGGTGCG; 6conF, GGAGTGGATTGCAGTATGTTTGGA; 6conR, AGAGGGAGGAGAATAACTAGCAAT; 20conF, GTCTAGTCCGTTAAGCTGTTCTCA; 20conR, GTCTCTACTTGCCTAGCCTCCTCT; 1-1FJ, GCCATCCTACCCATTCCAAATAA; 1-1Rend, GTAGATGGGGCCTATCGAATTGGTA; 2-1Fend, TTCAAGTATCGCAGGCTTACTCTCT; 2-1Rend, CAATCTTCTCAGGCTGGACACCCTT; 3-1Fend, AGTCTGCAATTTGGCGACCAATCTT; 3-1Rend, AGCCAAACACGGAGGAAGTTTATCA;3-2Fend, CCTCTATGCC GCGTCGTGAGCTATT; 3-2Rend, CTTCACGACGCATGGCTGCTGTCAT; 5FJ, CTGAG GTGTAGTCTCCCTTTCAATT; 5Rend, ATTGCAGGTTTGGATGCGGCCCGTT; 9Fend, GGACGTGGTACTACCCTACCTGGAA; 9Rend, TGATTATTTTAGAAACTGGTCGTAT; 21Fend, CAACCTCGTCTTTAGAGTATGCTGT; 21Rend, GAGAAAAAAGCATACTTTGATGATA; 2-61Fend, GCATGTATTGCCTCTTTCATTCGAT; 2-61Rend, TGGCGGCAGG TCTGGTAGGAACAAT; 2-69FJ, AGTCATTTCGAGCTGAGAATCTGAC; 2-69RJ, GAAGATCGTGTGAAGAAGCAGGCTT; S-1Fend, TTCTACAAGTGTGATGGCACGCTCA; S-1RJ, GAGCTGAATAAGTATGCTGATCGAA; S-3Fend, AGTGCAGTAGTTGATTGCTCTAGCA; and S-3Rend, TGCCCTAGACAAATCTTATAGGATT.
Nucleotide sequence accession numbers.
The nucleotide sequences for the purHD regions in serotypes 1, 4, 5, 6, 8, 9, 10, 11, 12, 13, 14, 15, 16, 20, 22, 24, 25, 27, and 28 determined in this study have been deposited in the DDBJ/EMBL/GenBank database under accession nos. AB183829, AB183830, AB183831, AB183832, AB183833, AB183834, AB183835, AB183836, AB183837, AB183838, AB183839, AB183840, AB183841, AB183842, AB183843, AB183844, AB183845, AB183846, and AB183847, respectively. Sequences for the purHD regions in serotypes 2, 3, 7, 23, and 26, previously published elsewhere (38, 39), were extended in this study and were assigned accession nos. AB045618, AB058942, AB058943, AB058944, and AB058945, respectively. The nucleotide sequence data of the genomic regions in serotype 2, which were used for the targets of the integration mutants Int1, Int2, Int3, Int4, Int5, Int6, Int7, Int9, Int10, Int11, and Int12, have been deposited in the database under accession nos. AB183474, AB183475, AB183476, AB183477, AB183478, AB183479, AB183480, AB183481, AB183482, AB183483, and AB183484, respectively.
RESULTS
Genes and gene products of the extra regions between purH and purD.
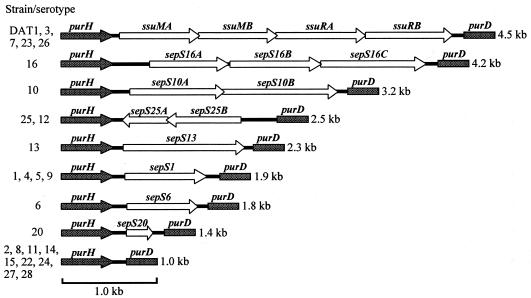
With a set of PCR primers, purH plus purD, which were designed from the internal sequences of purH and purD, respectively, we were able to amplify the purHD regions, ranging from 4.5 to 1.0 kb, of the reference strains excluding serotypes 17, 18, 19, and 21 (data not shown). In the serotypes 17, 18, 19, and 21, however, each of the purH and purD gene regions could be amplified with specific primers, i.e., purN-F plus purH-R2 and purD-F2 plus purE, respectively (data not shown). Therefore, the purH gene was thought to be separated from the purD gene in these serotypes, and the four serotypes above have not been examined further. The amplified purHD fragments above were directly sequenced. The putative genes and their organization found in the sequenced fragments are summarized in Fig. 2. All of the genes identified in this study were located on the same DNA strand as the pur genes except for those found in the serotypes 12 and 25, which were located on the complementary strand. No transposable element or long-repeat sequence was found in the sequenced regions. The average G+C content of each sequence, especially that of the coding region (27 to 35%), was clearly lower than that of conserved pur genes (49%) (38) and of the total genome of S. suis (39 to 41%) (24).
FIG. 2.
Schematic representations of purHD regions. Genetic organizations of representative serotypes (serotypes 16, 10, 25, 13, 1, 6, 20, and 2) are depicted here, and the corresponding serotypes that showed identical or very similar genetic structures are listed on the left. The structures of RM genes were taken from previous reports (37, 38). Sizes of the DNA fragments amplified by PCR are shown on the right. Open arrows, genes identified in these regions; filled arrows, purH; filled boxes, purD.
The 4.5-kb fragments obtained from strain DAT1 and serotypes 3, 7, 23, and 26 contained genes encoding SsuDAT1I or its isoschizomers, as has been previously described (37), whereas no putative gene was found in the intergenic space of the 1.0-kb fragments. The 4.2-kb fragment obtained from serotype 16 contained three putative genes, designated sepS16A (for suis extra gene in pur region of serotype 16), sepS16B, and sepS16C, the latter two overlapped each other in a head-to-tail manner. The 3.2-kb fragment obtained from serotype 10 contained two putative genes, designated sepS10A and sepS10B, which overlapped each other in a head-to-tail manner. The 2.5-kb fragment obtained from serotype 25 contained two overlapping genes, designated sepS25A and sepS25B. The 2.5-kb fragment obtained from serotype 12 was almost identical to that of serotype 25 (97.2% nucleotide identity); however, an ORF corresponding to sepS25B in serotype 12 contains an 8-bp duplication that causes a frameshift mutation, resulting in a 180-nucleotide truncation at the 5′ terminus. On the basis of the 16S rRNA sequences (9, 19, 43), serotype 12 is not related to serotype 25. The 1.9-kb fragments obtained from serotypes 1, 4, 5, and 9 were very similar to one another (95.9 to 99.9% nucleotide identity). They each contained a single gene; that which was found in the fragment of serotype 1 was designated sepS1. Although serotypes 4 and 5 are closely related in the 16S rRNA-based tree, they are genetically distant from serotypes 1 and 9, which are also unrelated to each other (9, 19, 43). The 2.3-, 1.8-, and 1.4-kb fragments obtained from serotypes 13, 6, and 20, respectively, each contained a single gene, designated sepS13, sepS6, and sepS20, respectively. Thus, the genetic regions containing the novel genes were categorized into seven classes.
The deduced translational products of the genes found in the extra genetic regions are summarized in Table 2. The deduced SepS16A, SepS16B, and SepS16C proteins showed amino acid similarity with Staphylococcus aureus hypothetical proteins. As with S. suis, the genes showing similarity on the S. aureus genome are also contiguous, although they are not positioned between the S. aureus purH and purD genes. The deduced SepS10A protein showed a low degree of amino acid similarity to a Staphylococcus epidermidis hypothetical protein. The deduced SepS25A and SepS25B proteins showed amino acid similarity to Streptococcus pyogenes hypothetical proteins Spy0970 and Spy0968, whose genes were also located in tandem but were separated from the pur genes on the S. pyogenes genome. The rest of proteins encoded in the extra regions showed no significant similarity to proteins in the database. The extra regions of the seven classes, together with the RM genes, showed no similarity with one another at either the DNA or deduced amino acid sequence levels.
TABLE 2.
Predicted gene products and the similarity to amino acid sequences in the database
| Gene | Product size
|
Similaritya
|
Description of closest relativec | ||
|---|---|---|---|---|---|
| No. of amino acids | kDa | BLAST E value | Amino acid identityb | ||
| sepS16A | 275 | 33.0 | 9e-53 | 39/275 | Hypothetical protein of S. aureus [AC025591] |
| sepS16B | 315 | 36.1 | 3e-46 | 35/314 | Hypothetical protein of S. aureus [AC025591] |
| sepS16C | 363 | 42.1 | 7e-37 | 28/379 | Hypothetical protein of S. aureus [AC025591] |
| sepS10A | 319 | 36.4 | 1e-14 | 26/276 | Hypothetical protein of S. epidermidis [AF270077] |
| sepS10B | 390 | 46.0 | No similarity | ||
| sepS25A | 150 | 16.9 | 9e-18 | 37/127 | Hypothetical protein (Spy0970) of S. pyogenes SF370 [AE006544] |
| sepS25B | 311 | 36.8 | 8e-67 | 47/263 | Hypothetical protein (Spy0968) of S. pyogenes SF370 [AE006544] |
| sepS13 | 418 | 48.5 | No similarity | ||
| sepS1 | 283 | 32.6 | No similarity | ||
| sepS6 | 248 | 28.9 | No similarity | ||
| sepS20 | 93 | 10.5 | No similarity | ||
Values do not appear in the respective sections for the putative proteins that showed no similarity to the proteins in the database.
Percent amino acid identity/number of amino acids evaluated.
Numbers in brackets are accession numbers from the DDBJ/EMBL/GenBank database.
Distribution of the extra genes among S. suis.
The internal regions of the PCR fragments which contained the putative gene(s) were amplified with specific primers from the purHD fragments obtained above. The fragments were labeled and used as hybridization probes against genomic DNAs from S. suis strains that had been digested with HindIII and separated by agarose gel electrophoresis. A DNA probe obtained from serotype 16 showed a hybridizing fragment of 18 kb only in the digested DNA of serotype 16, whereas no hybridizing fragments appeared in those of other strains (data not shown). A DNA probe prepared from serotype 1 showed a hybridizing fragment of either 2.8 or 9.5 kb in the digested DNAs of serotypes 1, 4, 5, and 9, whereas no hybridizing fragments appeared in those of other strains (data not shown). Similarly, probes derived from the internal regions of gene segments between purH and purD in other serotypes hybridized only with DNA from serotypes, yielding a similar class of PCR amplicon between purH and purD as the strain from which the probe was derived.
Nucleotide sequence comparison of the purHD regions.
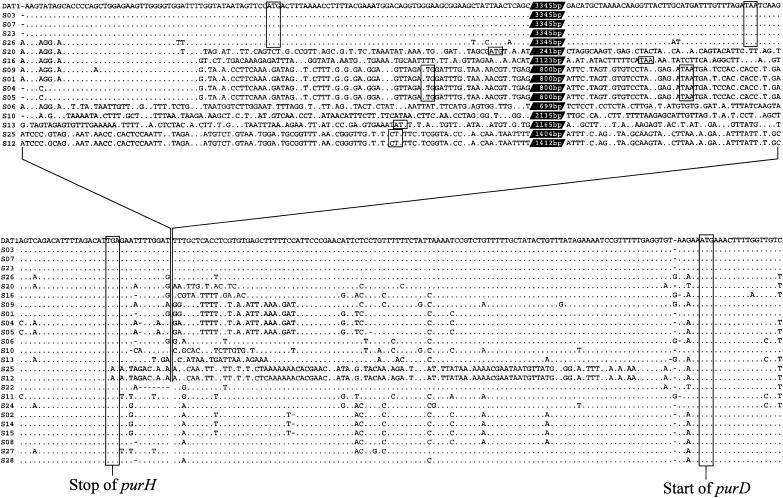
The nucleotide sequences determined were aligned bidirectionally from the purH and purD sides. As shown in Fig. 3, the genetic regions expressed to yield PurH and PurD were almost completely identical to one another in all of the strains examined. The intergenic sequences of serotypes 22, 11, 24, 2, 14, 15, 8, 27, and 28, which did not possess any genes between purH and purD, were very similar, except that a 7-bp deletion was noted in serotype 22 (Fig. 3, lower alignment). Although the RM gene regions (Fig. 3, upper alignment, lines 1 to 5 from the top) have been thought simply to be inserted 11 bp downstream of the stop codon of purH, as described before (38), the alignment including other sequences determined in this study revealed different putative borders which discriminate the extra genetic regions from the conserved pur genes. As shown in the lower alignment of Fig. 3, serotypes 25 and 12 showed nucleotide mismatches further upstream from the putative insertion site of the RM genes, whereas serotypes 20, 16, 9, 1, 4, 5, 10, 13, 25, and 12 showed nucleotide mismatches further downstream. These results indicated that the extra regions of these serotypes were not simply inserted between purH and purD, but that either of these genes was integrated in this position and replaced the intergenic sequences. Moreover, serotypes 20, 16, 9, 1, 4, and 5 showed regions of 37 to 40 bp homologous to the corresponding regions of DAT1 and serotypes 3, 7, 23, and 26 (5′ regions of the upper alignment in Fig. 3). The homologous regions are apparently parts of the extra regions and a few nucleotide mismatches common to serotypes 26, 20, 16, 9, and 1 also appear within the homologous regions, indicating that either of these extra genes that had existed at this position was further replaced by another gene.
FIG. 3.
Alignment of the partial sequences of the purHD regions from S. suis reference strains and DAT1. The sequences are aligned bidirectionally from the purH and purD regions (lower sequences), and the extra portions are shown in the upper alignment and are connected by the lines with the lower sequences. Strains are indicated to the left of the sequences; reference strains are referred to as S plus the serotype number. Numbers of nucleotides not appearing in the upper alignment are indicated in white type in black parallelograms. Dots, nucleotides identical to those of the aligned sequence of DAT1; dashes, gaps in the aligned sequences; boxes, stop and start codons of the genes appearing in the sequences.
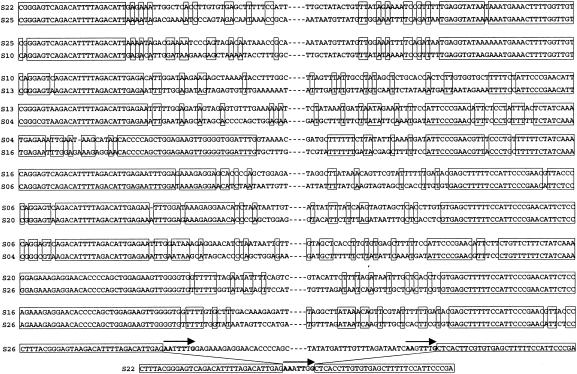
One-to-one comparison of the sequences more obviously showed the borders of the junction regions where the recombination events to incorporate the extra regions may have occurred; some representatives are shown in Fig. 4. Next to the highly conserved pur regions, sequences with a low degree of similarity, where a few base pairs of identity appeared contiguously, were seen at one or both ends of the border regions. The relatively low degree of sequence similarity shown in the junction regions resembles that observed in the flanking regions of sly and orf102 (43). The putative borders appeared at the same positions in a few comparisons (e.g., a comparison among serotypes 22, 25, and 10); however, most of them appeared at different positions from one another. Moreover, the comparison between serotype 26 (or other strains possessing the RM genes) and serotype 22 revealed that serotype 26 (and other strains possessing the RM genes) had small imperfect direct repeats consisting of 7 bp at the putative borders. A very similar 7-bp sequence was also present in serotype 22 (Fig. 4), a result which suggested that the extra region of serotype 26 (or other strains possessing the RM genes) had been deleted at these short repeats.
FIG. 4.
One-to-one sequence comparisons of the border regions of some serotypes. Serotypes compared are indicated to the left of the sequences as S plus serotype number. Boxes, identical nucleotides; arrows, short direct repeats.
Integration of a plasmid carrying RM genes.
We examined whether the purHD region is a hot spot for illegitimate recombination by using SsuDAT1I genes as the model. The SSuDAT1I genes with an insertion of the cat gene were cloned into a suicide plasmid, and the recombinant plasmid was introduced into S. suis serotype 2, for which the purHD region did not possess any foreign genes (38). Since temperature-resistant and Cmr mutants obtained from the bacterial culture were expected to carry the RM genes being integrated into the genome, we selected 243 such mutants. All of the mutants were still Spcr, indicating that the mutants contained the vector region as well as the RM gene region. Genomic DNAs were isolated from the mutants and used for PCR with primers purN-F plus purE, which encompassed the purHD region. All of the samples produced amplicons of the same size (4.0 kb) as that of the parent strain (data not shown), indicating that the genetic organization of the amplified regions was not affected by the integration, i.e., none of the integration events had occurred at the purHD locus.
Structures of integrated plasmids.
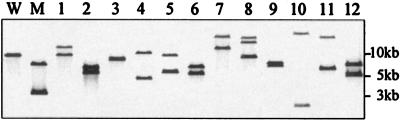
To analyze the genetic structures of the integrated plasmids, we further examined 12 temperature-resistant Cmr mutants, named Int1 to Int12. The genomic DNAs of the mutants were digested with StuI, which cut the pMABcatRAB at one site (see Fig. 6). The digested DNAs were separated by agarose gel electrophoresis and examined by Southern hybridization with an SsuDAT1I probe. The digested DNAs of S. suis strain 227, which possesses the RM genes, and its derivative 227catIN provided one and two fragments that hybridized with the probe, respectively, whereas those of the mutants, except Int8, provided two hybridizing fragments of different sizes (Fig. 5), demonstrating that pMABcatRAB was integrated into the S. suis genome at different positions. The digested DNA of the mutant Int8 provided three hybridizing fragments, one of which was the same size as the whole length of pMABcatRAB, implying that the plasmid was duplicated in tandem.
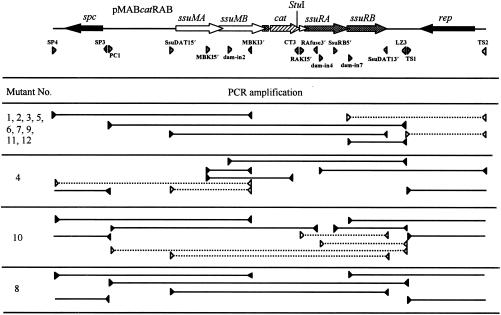
FIG. 6.
Schematic representations of the results of systematic PCR analyses of temperature-resistant Cmr mutants generated by integration of pMABcatRAB into the genome. Positions of primers relative to the genetic organization of pMABcatRAB are indicated at the top by gray arrowheads. Solid lines between the filled arrowheads, regions amplified by PCR; dashed lines between the open arrowheads, regions not amplified by PCR.
FIG. 5.
Genomic Southern hybridization of temperature-resistant Cmr S. suis mutants. Genomic DNAs were digested with StuI, separated by agarose gel electrophoresis, and probed with labeled SsuDAT1I genes. Molecular sizes are indicated on the right. Lane numbers correspond to the numbers in the designations of mutants Int1 to Int12. W, wild-type strain 227; M, mutant strain 227catIN.
The 12 mutants were then analyzed by PCR by using various combinations of primers designed from the sequence of pMABcatRAB. The strategy of using PCRs for mapping the structure of the integrated plasmids assumed a Campbell-type recombination event. If the plasmid integrates by a single recombination event, then PCR amplicons would be obtained from internal segments of the integrated plasmid whereas plasmid-derived priming sites at the junction of the recombination event would be separated and would not yield amplicons. As shown in Fig. 6, the genomic DNAs of the mutants Int1, Int2, Int3, Int5, Int6, Int7, Int9, Int11, and Int12 produced PCR fragments for all of the primer sets except those encompassing the replication region (rep) of the plasmid, indicating that the whole plasmid was integrated into the host genome via the rep region. Similarly, the genomic DNAs of the mutants Int4 and Int10 did not produce a PCR fragment with the primer sets encompassing ssuMA and ssuRA, respectively, indicating that these mutants formed cointegrates via these regions. However, the genomic DNA of the mutant Int8 produced PCR fragments with all of the primer sets examined. This result, together with the above results, indicates that at least two copies of the plasmid were present in tandem in the genome due to tandem duplication.
Selection of the mutants by CAM did not allow us to estimate the integration frequency, as many false-positive colonies appeared on the selective agar. However, the results described above indicated that almost all of the mutants were generated by Campbell-type recombination. Therefore, we selected the temperature-resistant mutants on TH agar containing CAM and SPC in order to calculate the integration frequency. The frequency was estimated to be 5.6 × 10−6 to 1.5 × 10−7, depending on the experiment.
Nucleotide sequences of junctions between plasmid and chromosome.
As the integration sites of the plasmid in the mutants except Int8 could be estimated by PCR analyses described above, the junction regions were then amplified by inverse PCR and directly sequenced. The sequence information enabled us to amplify the target in the original host chromosome of serotype 2, and the amplified segments were directly sequenced. None of the integration target sites were localized in purHD; rather, all were in the vicinity of or within other miscellaneous genes, which were apparently different from purine biosynthetic genes (Table 3).
TABLE 3.
Predicted proteins which exhibited similarity to the putative translational products of the target regions in the mutants
| Mutant | Description or function of closest relativea | Similarity
|
|
|---|---|---|---|
| BLAST E value | Amino acid identityb | ||
| Int1 | Putative prolyl-tRNA synthetase of Streptococcus pneumoniae [AE007339; AAK74442] (7) | 5e-43 | 81/107/119 |
| Int2 | Surface immunogenic protein of Streptococcus agalactiae (7) [AF151362; AAG18478] | 6e-23 | 52/98/118 |
| Int3 | Putative glutathione reductase of S. pneumoniae [AE008446; AAK99496] | 1e-121 | 71/305/305 |
| Int4 | Putative glycosyl hydrolase family 3 of S. agalactiae [AE014223; AAM99592] | 0.0 | 74/594/596 |
| Int5 | Putative lacI family regulator YvdE of Bacillus subtilis [Z99121; CAB15468] | 3e-23 | 42/144/146 |
| Int6 | Putative 23S rRNA methyltransferase of S. pneumoniae [AE008555; AAL00715] | 2e-63 | 50/244/249 |
| Int7 | Extracellular protein of S. suis [AY341262; AAQ19848] | 5e-82 | 84/194/195 |
| Int9 | Putative ABC transporter permease protein of Lactococcus lactis [AE006342; AAK05202] | 3e-77 | 41/403/397 |
| Int10 | Putative N-acetylneuramic acid synthetase NeuB of S. agalactiae [AF355776; AEAAK43615] | 1e-158 | 80/338/338 |
| Int11 | Putative ABC transporter permease of S. pneumoniae [AE008562; AAL00778] | 1e-113 | 82/233/234 |
| Int12 | Putative ribosomal protein L11 methyltransferase of S. agalactiae [AE014280; AAN00829] | 1e-133 | 85/275/275 |
Numbers in brackets are accession numbers and protein identification numbers from the GenBank/DDBJ/EMBL database.
Percent amino acid identity/number of amino acids evaluated/number of amino acids queried.
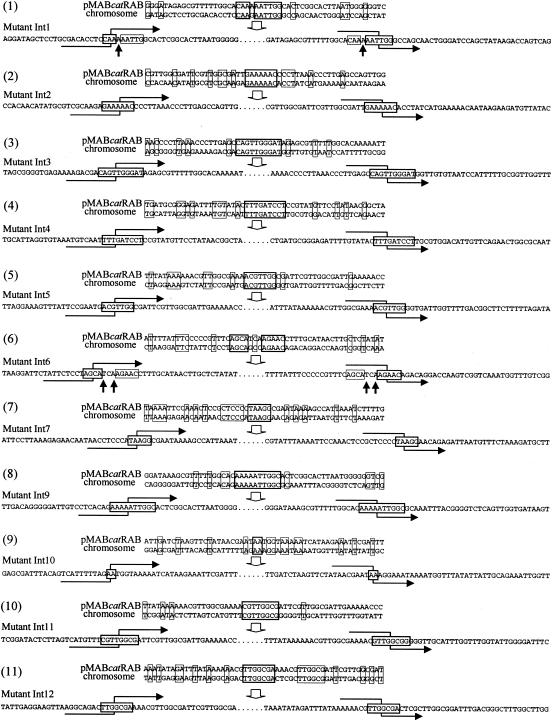
As shown in Fig. 7, the sequences of the junction points between the plasmid and the host chromosome revealed small regions of identity between the two molecules (Fig. 7). These ranged from 2 to 10 nucleotides in length. Additional short regions of identity were also present around the junction points, as was seen in the border regions in the purHD locus. There was no consensus sequence discernible among the junction points, although six of them (Int1, Int3, Int5, Int9, Int11, and Int12) contained the tetranucleotide TTGG. The recognition sequence of SsuDAT1I (5′-GATC-3′) was seen only in the junction point of Int4. Of the 11 mutants examined, 9 of them, i.e., Int2, Int3, Int4, Int5, Int7, Int9, Int10, Int11, and Int12, showed complete identity in the junction sequences. However, the other two, Int1 and Int6, showed one or two nucleotide mismatches in the junction points when the plasmid and chromosome were compared. The two mutants had nucleotides that originated from the plasmid in both the junction points (Fig. 7, indicated by upward-pointing arrows), which indicated that strand crossover had taken place at different positions.
FIG. 7.
Sequences of the junction points between pMABcatRAB and the S. suis genome. The sequences of pMABcatRAB and the corresponding S. suis genomic sequences are shown in the upper part of each panel. The sequence of the mutant is shown in the lower part of each panel. Stretches of sequence identity that are presumed to be the junction points are boxed with thick lines. Other positions with nucleotide identity are boxed with thin lines. Hooked arrows intercrossing the junction points of the mutants indicate possible positions of crossover, showing the recombination flow starting from the chromosome and shifting to the plasmid (left) and from the plasmid to the chromosome (right). Nucleotide mismatches found in the junction regions of Int1 and Int6 are indicated by upward-pointing arrows.
DISCUSSION
We have demonstrated here the presence of novel extra genetic regions in the purHD locus of some serotype reference strains of S. suis. The extra genetic regions, which were categorized into seven classes, showed no similarity with those of other classes and thus were unique to the strain(s) of each group. The RM genes are also present uniquely in the purH-purD regions of some strains (37), and constitute an eighth class of sequences there; it is thus evident that the eight classes of extra regions are mutually exclusive. The conserved genetic organization of the pur gene cluster (38), as has been observed with other gram-positive bacteria (4, 13, 44), together with the low G+C contents of the extra genetic regions compared with the pur genes, support the notion that the novel genetic regions found in this study were also transferred from foreign sources. Moreover, the fact that some of the phylogenetically unrelated serotypes share common genes indicates that the foreign genes have also spread among the S. suis strains, as has been described for the RM genes and sly regions (37, 38, 43).
Nearly half of the deduced translational products encoded by the novel foreign genes showed no similarity to proteins in the database; however, it is noteworthy that genes of other bacteria encoding the proteins which showed similarity to those of serotypes 16 and 25 are also contiguous in the genomes of the counterparts, suggesting that the sepS16ABC and sepS25AB gene clusters were both incorporated in a block. Moreover, the deduced SepS16ABC proteins showed similarity to three hypothetical proteins described for at least two S. aureus strains; however, the three proteins of S. suis showed no similarity to any proteins of other S. aureus strains, especially of those whose complete genome sequences have been determined (our unpublished observation), suggesting that the S. aureus genes encoding the hypothetical proteins were also acquired from foreign sources. As discussed below, these extra genetic regions were not only inserted independently into the genome of different strains but also replaced by a newcomer. This finding suggests that some of the foreign genes were successively transferred from one bacterial genome to the other.
The RM gene region has been considered simply to be inserted between purH and purD on the basis of the comparison between only two classes of purHD regions, i.e., one possessing the RM genes and the other lacking the genes, such as serotype 2 (37, 38). In the present study, however, we obtained information on seven additional classes of foreign genes that showed striking sequence variation even in the borders between the exogenous regions and the pur genes. Genetic borders of each exogenous region as estimated from the alignment of all of these sequences indicated that these genes were incorporated into different nucleotide positions of the same locus. Furthermore, some of them were likely to have been substituted for predecessors that had been inserted previously. One-to-one comparison of the sequences clearly showed differences in the border positions where the recombination may have occurred. Nucleotides with a few base pairs of identity scattered throughout the junction regions were observed next to the highly conserved pur genes. The identical sequences ranged from 1 to at least 6 nucleotides in length, which is reminiscent of illegitimate recombination sites described in other studies (1, 11, 23, 26, 29-31, 35, 39). The low degree of sequence similarity in the junction regions was also observed in the flanking sequences of sly and orf102 in S. suis (43). From these observations, together with the fact that no vestige of sequences affecting their integration, such as long-repeat sequences or remnants of transposable elements, were found in the close vicinity, we can consider that the foreign genes that have thus far been found in S. suis were integrated by the same mechanism by using illegitimate recombination.
Once a foreign gene is integrated in the genome, it can be transferred among S. suis by homologous recombination en bloc along with the conserved flanking regions (37, 43) or by means of homology-facilitated illegitimate recombination (11, 30, 35), and accumulation of the foreign genes may contribute to the genetic heterogeneity in S. suis. However, in the original insertion, the foreign genes found in this study must have been integrated by illegitimate recombination. Therefore, one can pose the following question: why are there so many different foreign genes at the same chromosomal locus? One possible explanation is that the intergenic space of purHD involves a hot spot for the type of recombination that is induced by these foreign genes. Indeed, at least one class of the foreign genes, RM genes, is proposed to comprise mobile genetic elements that can migrate among bacteria of different genera (25). The R enzyme can elicit a double-strand breakage, and this breakage may induce illegitimate recombination when the two genetic segments have a few base pairs of nucleotide identity (27).
To assess the validity of the above explanation, we constructed a thermosensitive suicide plasmid that carried the SsuDAT1I genes together with a cat gene, and we obtained S. suis mutants in which the plasmid and the host genome formed cointegrates. None of the 243 mutants examined formed cointegrates via the purHD region. If the integration events occurred uniformly all over the genome, one should be able to find a certain integration site among 500 mutants by a PCR spanning 4 kb, since the genome size of this bacterium is estimated to be 1,800 to 2,000 kb (13, 44). On the other hand, if a particular region, e.g., the purH-purD region, is the hot spot for the recombination and if the integration events occur five times more frequently there than at other regions, one should be able to find the integration site among 100 mutants by PCR. From this rationale, our results indicated that even if the purHD region was the hot spot for recombination, the frequency of the event was not high (less than 2.1 times higher than at other regions). Therefore, the purHD region was not likely to be the hot spot for recombination.
Although a limited number of integration mutants were sequenced, no mutant that had obviously integrated the plasmid via mobile genetic elements, such as a transposon, insertion sequence, integron, or intron, was obtained. This finding implies that the integration had occurred by illegitimate recombination irrespective of the mobile genetic elements. Integration sites localized on the plasmid pMABcatRAB appeared clearly nonrandom, as 9 out of 12 mutants formed cointegrates via the rep region of the plasmid. The rep region of the plasmid usually forms a single strand as a replication intermediate so that this region is likely to form a Holliday junction between the plasmid and the genome, leading to the recombination event, or, alternatively, the rep region of the plasmid shares more sequence similarity with the genome than other segments of the plasmid. However, the plasmid was integrated into the genome of the 12 sequenced mutants at apparently different regions. Although we cannot rule out the possibility that there was something different about the events that occurred during the evolution of the different serotypes and the experimental integration described in this study, our results imply that the purHD region did not show any preference for integrating particular foreign genes, i.e., the foreign genes found in this study were incidentally integrated into the same locus of the genome among the different strains by illegitimate recombination, presumably via a few base pairs of nucleotide identity.
The structures of the eight classes of foreign genes identified here may have represented the results of several consecutive recombination events, and therefore it is not possible to define the extra genetic segments as putative plasmids, phages, or conjugative transposons or to predict the exact limits of these inserted elements. Nevertheless, the present results together with previous findings (37, 38, 43) suggest that foreign gene acquisitions may have occurred by the same mechanism at various chromosomal loci. The fact that different foreign genes were incidentally integrated into the same locus of the S. suis genome and/or exchanged among the species by legitimate or illegitimate recombination leads us to propose a hypothesis to explain the presence of various foreign genes in the same locus, i.e., a high rate of gene acquisition in this bacterium. Actually, the integration frequency determined in this study was a little higher than those observed elsewhere (18, 21). However, the frequency may have been overestimated by elimination of plasmid-cured cells caused by postsegregational killing (25). If the foreign gene did not induce postsegregational killing, the illegitimate recombination may have occurred randomly at a frequency as low as 10−7 to 10−9 (18, 21). Therefore, it is conceivable that S. suis has the ability to take a foreign DNA into the genome through some mechanism, such as natural competence. S. suis is not known to enter a state of competence in vitro; however, it is possible that some isolates may, under certain growth conditions, be competent and/or that the ancestors of the S. suis populations were competent.
Whichever mechanisms are employed for transporting the DNA into the cytoplasm, the present results indicate that a seemingly large number of integration events have occurred in the purHD region, perhaps by an intrinsic recombination mechanism of the host bacteria. Most of the foreign genes found in the purHD locus are functionally unknown, and not all of the genes may contribute directly to the host survival. Integrated genes that are not deleterious will become fixed within the bacterial population and may contribute to the evolution of this bacterium.
Acknowledgments
We thank Toshio Fujisawa for preparing photographs and Sachiho Manabe and Mitoyo Takahashi for technical assistance.
This work was supported in part by a Grant-in-Aid for the Pioneer Research Project to T.S. from the Ministry of Agriculture, Forestry, and Fisheries (MAFF), Japan, and by a Grant-in-Aid for the Zoonosis Research Project from MAFF.
REFERENCES
- 1.Albertini, A. M., M. Hofer, M. P. Calos, and J. H. Miller. 1982. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell 29:319-328. [DOI] [PubMed] [Google Scholar]
- 2.Allgood, N. D., and T. J. Shilhavy. 1988. Illegitimate recombination in bacteria, p. 309-330. In R. Kucherlapati and G. R. Smith (ed.), Genetic recombination. American Society for Microbiology, Washington, D.C.
- 3.Allgood, N. D., and T. J. Shilhavy. 1991. Escherichia coli xonA (sbcB) mutants enhance illegitimate recombination. Genetics 127:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostopoulos, C., P. J. Piggot, and J. A. Hoch. 1993. The genetic map of Bacillus subtilis. p. 425-461. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. American Society for Microbiology, Washington D.C.
- 5.Arends, J. P., and H. C. Zanen. 1988. Meningitis caused by Streptococcus suis in humans. Rev. Infect. Dis. 10:131-137. [DOI] [PubMed] [Google Scholar]
- 6.Brake, A. J., A. V. Fowler, I. Zabin, J. Kania, and B. Müller-Hill. 1978. β-Galactosidase chimeras: primary structure of a lac repressor-β-galactosidase protein. Proc. Natl. Acad. Sci. USA 75:4824-4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodeur, B. R., M. Boyer, I. Charlebois, J. Hamel, F. Couture, C. R. Rioux, and D. Martin. 2000. Identification of group B streptococcal Sip protein, which elicits cross-protective immunity. Infect. Immun. 68:5610-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 9.Chatellier, S., J. Harel, Y. Zhang, M. Gottschalk, R. Higgins, L. A. Devriese, and R. Brousseau. 1998. Phylogenetic diversity of Streptococcus suis strains of various serotypes as revealed by 16S rRNA gene sequence comparison. Int. J. Sys. Bacteriol. 48:581-589. [DOI] [PubMed] [Google Scholar]
- 10.Clifton-Hadley, F. A. 1983. Streptococcus suis type 2 infections. Br. Vet. J. 139:1-5. [DOI] [PubMed] [Google Scholar]
- 11.de Vries, J., and W. Wackernagel. 2002. Integration of foreign DNA during natural transformation of Acinetobacter sp. by homology-facilitated illegitimate recombination. Proc. Natl. Acad. Sci. USA 99:2094-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feder, I., M. M. Chengappa, B. Fenwick, M. Rider, and J. Staats. 1994. Partial characterization of Streptococcus suis type 2 hemolysin. J. Clin. Microbiol. 32:1256-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin, N. C. 1971. Illegitimate recombination, p. 175-194. In A. D. Hershey (ed.), The bacteriophage lambda. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Gottschalk, M., A. Lebrun, H. Wisselink, J. D. Dubreuil, H. Smith, and U. Vecht. 1998. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 62:75-79. [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschalk, M., R. Higgins, M. Jacques, K. R. Mittal, and J. Henrichsen. 1989. Description of 14 new capsular types of Streptococcus suis. J. Clin. Microbiol. 27:2633-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottschalk, M., R. Higgins, M. Jacques, M. Beaudoin, and J. Henrichsen. 1991. Characterization of six new capsular types (23 through 28) of Streptococcus suis. J. Clin. Microbiol. 29:2590-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanada, K., T. Ukita, Y. Kohno, K. Saito, J. Kato, and H. Ikeda. 1997. RecQ DNA helicase is a suppressor of illegitimate recombination in Escherichia coli. Proc. Natl. Acad. Sci. USA 94:3860-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harel, J., R. Higgins, M. Gottschalk, and M. Bigras-Poulin. 1994. Genomic relatedness among reference strains of different Streptococcus suis serotypes. Can. J. Vet. Res. 58:259-262. [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins, R., M. Gottschalk, M. Boudreau, A. Lebrun, and J. Henrichsen. 1995. Description of six new capsular types (29-34) of Streptococcus suis. J. Vet. Diagn. Investig. 7:405-406. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda, H., K. Moriya, and T. Matsumoto. 1981. In vitro study of illegitimate recombination: involvement of DNA gyrase. Cold Spring Harbor Symp. Quant. Biol. 45:399-408. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs, A. A. C., P. L. W. Loeffen, A. J. G. van den Berg, and P. K. Storm. 1994. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect. Immun. 62:1742-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, I. M., S. B. Primrose, and S. D. Ehrlich. 1982. Recombination between short direct repeats in a recA host. Mol. Gen. Genet. 188:486-489. [DOI] [PubMed] [Google Scholar]
- 24.Kilpper-Bälz, R., and K. H. Schleifer. 1987. Streptococcus suis sp. nov., nom. rev. Int. J. Sys. Bacteriol. 37:160-162. [Google Scholar]
- 25.Kobayashi, I. 2001. Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution. Nucleic Acids Res. 29:3742-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumagai, M., and H. Ikeda. 1991. Molecular analysis of the recombination junctions of λbio transducing phages. Mol. Gen. Genet. 230:60-64. [DOI] [PubMed] [Google Scholar]
- 27.Kusano, K., K. Sakagami, T. Yokochi, T. Naito, Y. Tokunaga, E. Ueda, and I. Kobayashi. 1997. A new type of illegitimate recombination is dependent on restriction and homologous interaction. J. Bacteriol. 179:5380-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutticken, R., N. Temme, G. Hahn, and E. W. Bartelheimer. 1986. Meningitis caused by Streptococcus suis: case report and review of the literature. Infection 14:181-185. [DOI] [PubMed] [Google Scholar]
- 29.Marvo, S. L., S. R. King, and S. R. Jaskunas. 1983. Role of short regions of homology in intermolecular illegitimate recombination events. Proc. Natl. Acad. Sci. USA 80:2452-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meier, P., and W. Wackernagel. 2003. Mechanisms of homology-facilitated illegitimate recombination for foreign DNA acquisition in transformable Pseudomonas stutzeri. Mol. Microbiol. 48:1107-1118. [DOI] [PubMed] [Google Scholar]
- 31.Meima, R., B. J. Haijema, H. Dijkstra, G.-J. Haan, G. Venema, and S. Bron. 1997. Role of enzymes of homologous recombination in illegitimate plasmid recombination in Bacillus subtilis. J. Bacteriol. 179:1219-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okwumabua, O., J. Staats, and M. M. Chengappa. 1995. Detection of genomic heterogeneity in Streptococcus suis isolates by DNA restriction fragment length polymorphisms of rRNA genes (ribotyping). J. Clin. Microbiol. 33:968-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osaki, M., D. Takamatsu, Y. Shimoji, and T. Sekizaki. 2002. Characterization of Streptococcus suis genes encoding proteins homologous to sortase of gram-positive bacteria. J. Bacteriol. 184:971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perch, B., K. B. Pedersen, and J. Henrichsen. 1983. Serology of capsulated streptococci pathogenic for pigs: six new serotypes of Streptococcus suis. J. Clin. Microbiol. 17:993-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prudhomme, M., V. Libante, and J.-P. Claverys. 2002. Homologous recombination at the border: insertion-deletions and the trapping of foreign DNA in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 99:2100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Sekizaki, T., M. Osaki, D. Takamatsu, and Y. Shimoji. 2001. Distribution of the SsuDAT1I restriction-modification system among different serotypes of Streptococcus suis. J. Bacteriol. 183:5436-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekizaki, T., Y. Otani, M. Osaki, D. Takamatsu, and Y. Shimoji. 2001. Evidence for horizontal transfer of SsuDAT1I restriction-modification genes to the Streptococcus suis genome. J. Bacteriol. 183:500-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu, H., H. Yamaguchi, Y. Ashizawa, Y. Kohno, M. Asami, J. Kato, and H. Ikeda. 1997. Short-homology-independent illegitimate recombination in Escherichia coli: distinct mechanism from short-homology-dependent illegitimate recombination. J. Mol. Biol. 266:297-305. [DOI] [PubMed] [Google Scholar]
- 40.Takamatsu, D., M. Osaki, and T. Sekizaki. 2000. Sequence analysis of a small cryptic plasmid isolated from Streptococcus suis serotype 2. Curr. Microbiol. 40:61-66. [DOI] [PubMed] [Google Scholar]
- 41.Takamatsu, D., M. Osaki, and T. Sekizaki. 2001. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid 45:101-113. [DOI] [PubMed] [Google Scholar]
- 42.Takamatsu, D., M. Osaki, and T. Sekizaki. 2001. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 46:140-148. [DOI] [PubMed] [Google Scholar]
- 43.Takamatsu, D., M. Osaki, and T. Sekizaki. 2002. Evidence for lateral transfer of the suilysin gene region of Streptococcus suis. J. Bacteriol. 184:2050-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K., Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]