Abstract
The halophilic bacterium Halomonas elongata accumulates K+, glutamate, and the compatible solute ectoine as osmoprotectants. By functional complementation of Escherichia coli mutants defective in K+ uptake, we cloned three genes that are required for K+ uptake in H. elongata. Two adjacent genes, named trkA (1,374 bp) and trkH (1,449 bp), were identified on an 8.5-kb DNA fragment, while a third gene, called trkI (1,479 bp), located at a different site in the H. elongata chromosome, was found on a second 8.5-kb fragment. The potential protein expressed by trkA is similar to the cytoplasmic NAD+/NADH binding protein TrkA from E. coli, which is required for the activity of the Trk K+ uptake system. The deduced amino acid sequences of trkH and trkI showed significant identity to the transmembrane protein of Trk transporters. K+ transport experiments with ΔtrkH and ΔtrkI mutants of H. elongata revealed that TrkI exhibits a Km value of 1.12 mM, while the TrkH system has a half-saturation constant of 3.36 mM. Strain KB12, relying on TrkH alone, accumulated K+ with a lower Vmax and required a higher K+ concentration for growth in highly saline medium than the wild type. Strain KB15, expressing only TrkI, showed the same phenotype and the same K+ transport kinetics as the wild type, proving that TrkI is the main K+ transport system in H. elongata. In the absence of both transporters TrkH and TrkI, K+ accumulation was not detectable. K+ transport was also abolished in a trkA deletion mutant, indicating that TrkI and TrkH depend on one type of TrkA protein. Reverse transcriptase PCR experiments and Northern hybridization analyses of the trkAH locus revealed cotranscription of trkAH as well as a monocistronic transcript with only trkA.
In response to osmotic stress, Bacteria and Archaea accumulate organic compounds like sugars, amino acids, and/or their derivatives to serve as osmolytes (21, 22, 50). These highly water-soluble molecules, which carry no net charge at physiological pH, do not interfere with the cell's metabolism and are named compatible solutes (5). At a high external salt concentration, the osmotic equilibrium of the adapted cell is maintained mainly by amassing compatible solutes, while inorganic ions like Na+ and Cl− are, to a large extent, kept outside the cell (23, 32). However, after a sudden increase in osmolality, nonhalotolerant and halotolerant (marine) Bacteria take up K+ from the surrounding medium and transiently accumulate K+ as an osmoregulatory solute to achieve an osmotic equilibrium (28, 48, 49). In Escherichia coli most of the additionally accumulated K+ is replaced by synthesized compatible solute trehalose within 60 min following osmotic upshock (7). As shown for Halomonas elongata, halophilic bacteria react in a similar way and increase their cytoplasmic K+ level after osmotic upshock (19). However, in contrast to the case with nonhalophiles, the K+ level remains elevated for a longer period (>120 min) even in the presence of newly synthesized compatible solute.
The uptake of K+ is catalyzed by specific transport systems, which have been studied intensively at the genetic and physiological levels, but only in nonhalophilic and halotolerant Bacteria, such as E. coli (1, 8), Vibrio alginolyticus (25, 26), and recently Bacillus subtilis (17). The major transport systems for K+ accumulation in these organisms are the transporter Kdp (E. coli), the Ktr system (V. alginolyticus and B. subtilis), and the Trk transporter (E. coli and V. alginolyticus). Kdp is an inducible, high-affinity K+-translocating P-type ATPase (Km = 2 μM) encoded by the kdpABC operon. Homologues of Kdp were found in many other Bacteria (9, 47). Furthermore, it is now known that P-type ATPases different from Kdp are involved in K+ uptake in Bacteria as well (36).
The Ktr system consists of two components: a transmembrane-spanning subunit named KtrB, forming the actual pore, and a cytoplasmic membrane-associated KtrA protein containing a NAD+-binding domain. Ktr transporters, identified in many Bacteria and at least one member of the archaeal domain (31), allow for medium- to low-affinity K+ uptake, which is Na+ dependent (41).
The Trk system has an evolutionary relationship to Ktr, is widespread in both Bacteria and Archaea (10), and has a medium to low affinity for its substrate K+. Trk systems are secondary transporters, and the uptake of K+ is thought to be linked to H+ symport (29, 39). Trk consists of a transmembrane protein named TrkH or TrkG, which is the actual K+-translocating subunit, and the cytoplasmic membrane surface protein TrkA, which is a NAD+ binding protein (4, 35). In E. coli the TrkH system requires an ATP-binding protein named TrkE (SapD), which is thought to activate transport (38). TrkE, in E. coli, is expressed by sapD, located in the sapABCDF operon,coding for an ABC transporter of unknown function (16). Not all Trk systems need sapD for activity. It is thought that TrkG and Trk systems in other bacteria can use ATP-binding proteins stemming from ABC transporters different from SapABCDF.
Although K+ accumulation is essential for halophilic Bacteria, which thrive over a wide range of salinity (12, 45), the K+ transport systems in these organisms have not been analyzed at present. Here we report on our investigation of K+ transport systems in a halophilic bacterium, namely H. elongata, a proteobacterium of the γ subdivision (3, 46). Previous studies on H. elongata by Kraegeloh and Kunte (19) determined the affinity of whole cells for K+ and concluded that H. elongata accumulates K+ by a medium-affinity transporter of unknown design (6, 19). In the present study, we carried out molecular and physiological experiments on K+ uptake and found that H. elongata expresses two Trk-like K+ transporters, named TrkH and TrkI. TrkH exhibits only a low affinity for K+, while TrkI exhibits medium affinity for K+ and is the main K+ transporter in osmotically adapted cells of H. elongata.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains, vectors, and recombinant plasmids used for this study are listed in Table 1. For DNA isolation, H. elongata was cultured in nutrient broth with 0.51 M NaCl at 30°C and harvested in the late exponential growth phase. For physiological characterization, H. elongata was grown either aerobically in 100 ml of saline Na-MM63 liquid medium in 250-ml flasks with side arms for turbidity measurements at 30°C or on Na-MM63 agar medium (19). The NaCl concentrations varied from 0.51 to 2.1 M; KCl was supplemented to final concentrations of 5 to 200 mM.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or descriptiona | Source or reference |
|---|---|---|
| H. elongata | ||
| DSM 2581T | Type strain | DSMZb |
| KB12 | ΔtrkI | This study |
| KB12.2 | ΔtrkI ΔtrkH | This study |
| KB14 | ΔtrkA | This study |
| KB15 | ΔtrkH | This study |
| KB16 | ΔtrkAH ΔtrkI | This study |
| E. coli | ||
| TK2420 | trkA trkD1 Δ(kdpFAB)5 trkG82 trkH1 | W. Epstein, University of Chicago |
| TK2691 | trkH trkG trkD1 Δ(kdpFAB) Δ(trkA) | W. Epstein, University of Chicago |
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF) U169 recA1 hsdR17 (rK− mK+) supE44 Δthi-1 gyrA relA1 | 15 |
| S17-1 | thi pro hsdR hsdM+ recA; Tpr Smr | 36 |
| Plasmids | ||
| pHSG575 | lacZ′, Cmr | 39 |
| pKH4 | pHSG575::8.5-kb chromosomal Sau3A-hydrolyzed DNA from H. elongata carrying trkI, Cmr | This study |
| pKA2-30 | pHSG575::8.5-kb chromosomal Sau3A-hydrolyzed DNA from H. elongata carrying trkAH, Cmr | This study |
| pK18mobsacB | Kmrmob sacB | 33 |
| pAKB7 | 2,172-bp PCR-fragment in pK18mobsacB for trkI deletion containing 1,071-bp upstream and 1,101 bp downstream of trkI; Kmr | This study |
| pAKB8 | 2,229-bp PCR-fragment in pK18mobsacB for trkA deletion containing 1,108 bp upstream and 1,121 bp downstream of trkA; Kmr | This study |
| pAKB9 | 2,323-bp PCR-fragment in pK18mobsacB for trkH deletion containing 1,162 bp upstream and 1,161 bp downstream of trkH; Kmr | This study |
| pAKB10 | 2,355-bp PCR fragment in pK18mobsacB for trkH deletion in ΔtrkA strain KB14, contains 1,194 bp upstream and 1,161 bp downstream of trkH; Kmr | This study |
Abbreviations of antibiotics: Cm, chloramphenicol; Km, kanamycin; Sm, streptomycin.
DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany.
E. coli strains were grown aerobically at 37°C in Luria-Bertani (24) medium with chloramphenicol (50 μg ml−1), kanamycin (50 μg ml−1), or streptomycin (200 μg ml−1).
DNA isolation and manipulation.
Total DNA from H. elongata was isolated using the genomic kit QIAGEN tip 100. Routine manipulation of DNA, plasmid isolation, construction of recombinant plasmids, electrophoresis of DNA, and transformation were carried out according to standard procedures. DNA sequencing, based on the method of Sanger et al. (33), was carried out by SequiServe (Vaterstetten, Germany).
Construction of a plasmid-encoded genomic library of H. elongata and complementation of transport-defective E. coli mutants.
Genomic DNA of H. elongata was partially digested using restriction enzyme Sau3A. The chromosomal Sau3A fragments were ligated into low-copy-number plasmid pHSG575 (40), and the ligation products were transformed into E. coli DH5α. The resulting colonies were pooled; plasmids were isolated from the DH5α cells and transferred into the E. coli strains TK2420 and TK2691, defective in K+ uptake (8). E. coli clones with the gene library of H. elongata were selected for K+ uptake on K-Na-minimal medium (27), which contained chloramphenicol (50 μg/ml), thiamine (1 mg/ml), nicotinic acid (1 mg/ml), and tryptophan (100 μM) and was supplemented with K+ to final concentrations of 0.1, 0.3, 1, or 3 mM. E. coli strains able to grow on these selection media were isolated and analyzed further.
Generation of deletion mutants.
DNA sequences upstream and downstream from the desired gene were joined together by applying the splicing-by-overlap-extension PCR technique (18). The resulting PCR fragments were ligated into the shuttle vector pK18mobsacB (34) and transferred into H. elongata by E. coli S17-1-mediated conjugation (20), and resultant deletion mutants were selected as described previously (13).
RNA isolation.
For total RNA isolation, H. elongata was grown in Na-MM63 minimal medium (19) containing 200 mM KCl or 510 mM NaCl. One hundred milliliters of exponentially growing cells (optical density at 600 nm [OD600] of 0.6 to 0.7) were harvested by centrifugation, and approximately 100 mg of the pellet was resuspended in 4 ml of buffer A (50 mM Na acetate, 10 mM EDTA). 0.5 ml sodium dodecyl sulfate (10% [wt/vol]) was added to lyse the cells, followed by 4 ml of hot phenol (65°C). After 4 min of incubation at 65°C, the mixture was frozen in liquid nitrogen for 2 min and thawed at 37°C in a water bath. To enhance phase separation, the sample was centrifuged for 10 min (2,700 × g), and the aqueous top layer (400 μl) was mixed with 400 μl of phenol-chloroform-isoamyl alcohol and centrifuged at 4°C. The RNA in the aqueous top layer was precipitated with 40 μl of Na acetate solution (3 M) and 400 μl of ethanol (100%) at −70°C. Prior to use for Northern hybridization or reverse transcriptase PCR (RT-PCR), RNA was further purified by using the RNeasy mini-kit (QIAGEN) according to the manufacturer's instructions.
Synthesis of DIG-labeled RNA probes and Northern hybridization experiments.
To prepare RNA probes, the corresponding sequences of trkA and trkH were amplified by PCR using the reverse primers T7-trkA (5′-GGATCCTAATACGACTCACTATAGGACGACCTTGGAGGACTGCGTAT-3′) and T7-trkH (5′-GGATCCTAATACGACTCACTATAGGGGAAGTGCAGGCTGAAGCTGAA-3′) containing a viral T7 promoter (underlined). The PCR products from trkA (606 bp) and trkH (772 bp) with a T7 promoter in the 3′ position were purified by using the QIAquick PCR purification kit (QIAGEN) and diluted to 50 ng of DNA μl−1. Two hundred nanograms of the purified DNA was used for the RNA labeling reaction by the T7 polymerase. After incubation in 20 μl (total volume) of denaturation buffer (4 μl of glyoxal [40% {wt/vol}], 10 μl of dimethyl sulfoxide [100%], 2 μl of KiPO4 buffer [0.1 M, pH 6.8]) for 1 h at 50°C, total RNA (5 μg) was separated by agarose electrophoresis and transferred onto a nylon membrane (Nytran SuPerCharge; Schleicher & Schuell, Dassel, Germany). RNA-RNA hybridization was performed at 68°C with 10 ng of digoxigenin (DIG)-labeled RNA probe per ml of hybridization solution (DIG easy-hyb; Roche Diagnostics). After washing and antibody incubation, 10 μl of CDP-Star chemiluminescent reagent in 1 ml of buffer (0.1 M TRIS-HCl, 0.1 M NaCl [pH 9.5]) was pipetted onto the membrane and incubated for 5 min. Light emission was detected by using Kodak x-omat film (exposure time, 0.5 to 5 min).
RT-PCR.
Five micrograms of purified total RNA of H. elongata was transcribed into DNA by using reverse primer trk-cDNA (5′-TGATGCGTGGTGTCAGCTTGGAA-3′), using the SuperScript First Strand synthesis kit (Invitrogen) according to the instructions of the manufacturer. The following PCR, using the newly synthesized cDNA as a template, was carried out with the forward primer 2trk2 (5′-CATCGTCAACGTGCACTCGCT-3′) and the reverse primer trkAup (5′-TCCAGAACAGAGCCGCGATCAGA-3′).
Transport measurements.
H. elongata strains were grown overnight at 30°C in Na-MM63 mineral salt medium (19) containing 0.51 M NaCl. Minimal medium was supplemented with KCl to a final concentration of 5 mM for growth of H. elongata DSM 2581T, strain KB12, and strain KB15 and to a final concentration of 200 mM for growth of strains KB12.2, KB14 and KB16. Cultures were diluted in fresh minimal medium containing 0.51 M NaCl, and the OD600 was adjusted to 0.5. After incubation at 30°C, exponentially growing cells (OD600 of 1.0) were harvested from a 400-ml culture. Cells were washed and incubated (10 min) twice at 25°C in DEA buffer (50 mM diethanol amine HCl [pH 8.5], 0.5 M NaCl). Cells were then washed and incubated (10 min) twice in Tricine buffer (50 mM Tricine NaOH [pH 8.5], 0.5 M NaCl). The cell pellets were resuspended in 8 ml of K+-free Na-MM63 medium. One milliliter of the cell suspension was added to 8.9 ml of K+-free Na-MM63, and cells were incubated on a shaker for 10 min. One hundred microliters of KCl solution of suitable concentration was added to adjust the K+ concentrations in the medium to 0.5, 1, 2, 5, 10, and 20 mM. Fifteen seconds after the K+ addition, samples were removed over a time period of 8 min 45 s and immediately centrifuged through 250 μl of silicone oil (14,000 × g). Pellets were resuspended in 1 ml of trichloroacetic acid (5%) and frozen at −25°C. After thawing, 3 ml of CsCl solution (0.1%) was added to the trichloroacetic acid solution and heated at 90°C for 10 min, and denatured protein was removed by centrifugation (10 min, 2,710 × g). The supernatants were diluted (in CsCl), and samples were analyzed by atomic absorption spectroscopy (Shimadzu AA660). All transport measurements for each strain and K+ concentration were done at least three times.
Computer methods.
Protein and translated nucleotide databases were screened to find proteins similar to TrkA, TrkH, and TrkI using the BLAST program (2).
Nucleotide sequence accession number.
The nucleotide sequences of trkAH and trkI were submitted to GenBank and assigned accession numbers AY437838 and AY437839, respectively.
RESULTS
trk genes of H. elongata restore K+ transport activity in E. coli mutant strains.
Three genes coding for K+ transporter proteins from H. elongata were identified and isolated by functional complementation of two E. coli mutants, TK2420 and TK2691, defective in K+ uptake. Strain TK2420 carries a mutation in trkA, while strain TK2691 lacks both functional transmembrane domains (trkH and trkG). TK2420 and TK2691 need approximately 50 mM K+ to achieve growth rates like those of E. coli strains with functional Trk systems. Both strains were transformed with a plasmid-encoded gene bank of H. elongata, and the resulting transformants were selected for colonies that grew on medium containing 0.1 to 3 mM K+. Growth indicates transport of K+ via a plasmid-encoded transporter from H. elongata. Plasmid pKH4 with an 8.5-kb DNA fragment of H. elongata and also a 4.4-kb fragment subcloned from pKH4 restored K+ uptake in the E. coli mutant TK2691 (trkH trkG). Plasmid pKA2-30 carrying a different 8.5-kb fragment of H. elongata DNA allowed E. coli TK2420 (trkA) to grow on low-K+ medium. Transfer of pKA2-30 also restored growth in the E. coli mutant TK2691 (trkH trkI), indicating that the 8.5-kb insert encodes proteins to compensate the loss of the NAD+ binding protein TrkA and the K+-translocating membrane protein TrkH.
After restriction analyses of the 8.5-kb insert of pKA2-30 and subcloning, a 2.7-kb fragment which restored growth in TK2691 was sequenced. Sequence analyses of pKA2-30 revealed that the chromosome of H. elongata carries two adjacent open reading frames (ORF) of 1,374 and 1,449 bp, whose deduced amino acid sequences revealed high similarity to proteins of the Trk transporter family. The putative gene product encoded by the 1,374-bp ORF showed significant similarity to the NAD+/NADH binding protein of the Trk transport systems and was therefore named TrkA. The putative TrkA protein of H. elongata has a calculated molecular mass of 50 kDa (457 amino acids) and shares 65% identical amino acids with the putative TrkA protein of Vibrio alginolyticus and 63% identical amino acids with TrkA of E. coli. The 1,449-bp ORF named trkH, which begins 43 bp downstream of trkA (Fig. 1), encodes a protein of 52 kDa (482 amino acids) and has the closest similarity to transmembrane proteins of Trk transporters. TrkH of H. elongata shows the highest comparison score with the transmembrane protein TrkH of E. coli, having 55% identical amino acids, and still shares 39% identical amino acids with TrkG, the second Trk transporter of E. coli.
FIG. 1.
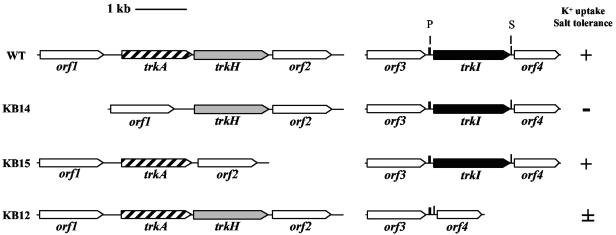
Gene organization at the trkAH and trkI loci of H. elongata. The relevance of the trk genes for K+ uptake was determined by K+ transport experiments (see Fig. 4) and growth experiments with minimal medium under K+ limitation (see Fig. 3). +, K+ transport and growth like that of the wild type; ±, reduced K+ uptake and growth; −, no K+ uptake via TrkH and TrkI, no growth under K+ limitation in mineral salt medium. Open reading frame orf1 upstream of trkAH encodes a potential RNA methyltransferase; orf2 downstream of trkH as well as orf3 and orf4 adjacent to trkI are of unknown function. P, putative promoter sequence; S, putative stem-loop sequence. Sequence analysis did not reveal promoter or stem-loop sequences for trkAH.
The 4.4-kb DNA fragment derived from pKH4 carries a 1,479-bp ORF, which is preceded by a potential σ70-dependent promoter sequence and a likely ribosome binding site and is followed by a potential stem-loop structure (Fig. 1). The ORF, which we refer to as trkI, encodes a putative transmembrane protein of the TrkH type. Comparison of the potential TrkI protein, which has a calculated molecular mass of 53 kDa (492 amino acids), revealed a high degree of identity to the TrkH protein of V. alginolyticus (48% identical amino acids) but only 32 and 29% identity, respectively, to the E. coli transporters TrkH and TrkG. TrkI and its counterpart, the TrkH transmembrane protein of H. elongata, contain 36% identical amino acids.
trk deletion mutants of H. elongata display different salt tolerances under potassium limitation.
To test the role of the proteins encoded by trkH and trkI for K+ uptake in H. elongata, we constructed deletion mutants of the corresponding genes. Strains KB12 (ΔtrkI), KB14 (ΔtrkA), KB15 (ΔtrkH), KB12.2 (ΔtrkH ΔtrkI), and KB16 (ΔtrkA ΔtrkH ΔtrkI) were used for growth experiments on agar medium (Fig. 2) and in liquid medium with different K+ concentrations and various osmolarities. At a K+ concentration of 5 mM, deletion strain KB12 (ΔtrkI) showed growth behavior similar to that of the wild type even at elevated salinity of 2.05 M NaCl. However, growth of ΔtrkI mutant KB12 was greatly diminished compared to that of the wild type at a K+ concentration of 0.1 mM. With increasing salinity, the growth inhibition was even more pronounced, and strain KB12 failed to grow in 0.1 mM K+ medium at a salinity of 2.05 M NaCl (Fig. 2). Additional deletion of trkH created the double mutant KB12.2 (ΔtrkI ΔtrkH), which could grow only in high-potassium medium of 100 mM or higher (Fig. 2) and showed a phenotype similar to that of the triple mutant KB16 (ΔtrkA ΔtrkH ΔtrkI). This indicates that the trkH-encoded transporter is involved in K+ uptake and allows for the reduced growth of strain KB12 (ΔtrkI) in low-potassium medium. In contrast, the ΔtrkH mutant KB15 displayed the same phenotype as the wild-type strain at all salt and K+ concentrations. Strain KB14, missing the potential NAD+/NADH binding protein TrkA, also failed to grow in medium with low K+ concentration (Fig. 2).
FIG. 2.
(A) Growth of H. elongata wild-type (WT) and trk deletion mutants KB12 (ΔtrkI) and KB15 (ΔtrkH) on minimal medium at different salinities (0.51, 1.03, and 2.05 M NaCl) and low (0.1 mM) and high (5 mM) K+ concentrations. Growth was scored after 3 days (0.51 and 1.03 M NaCl) and 4 days (2.05 M NaCl) of incubation at 30°C. Mutant KB15 grew similarly to the wild type in low and high K+ at all salinities, while growth of KB12 accumulating K+ only via TrkH was hampered in low-K+ medium, especially at high salinity. (B) Growth of H. elongata wild type, trkA deletion mutant KB14, and KB12.2 (ΔtrkH ΔtrkI) on minimal medium at 1.03 M NaCl and different K+ concentrations (0.25 and 200 mM). For (B), growth was scored after 3 days' incubation at 30°C.
The growth experiments revealed that TrkI and TrkH are involved in potassium uptake in H. elongata. As judged by the growth behavior of the trk mutants, TrkI appears to have a higher affinity for its substrate K+ than does TrkH and can compensate the knockout of trkH at least under the conditions tested. Furthermore, the knockout of trkA appears to effect both transporter TrkI and TrkH.
TrkH and TrkI are K+ transporters with different substrate affinities and transport kinetics.
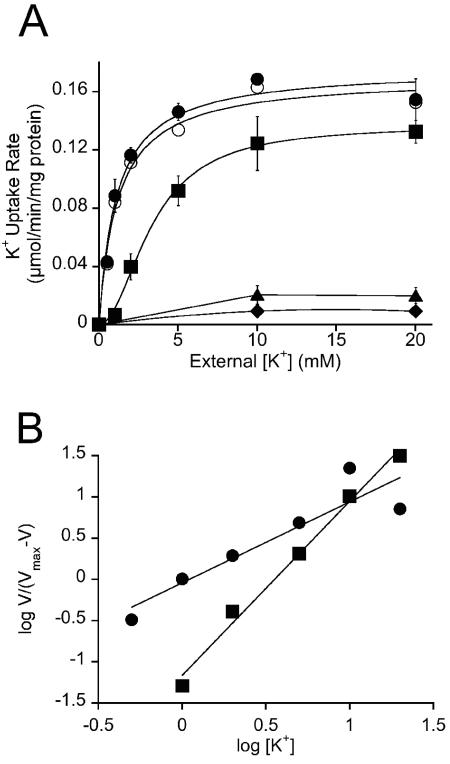
To investigate the role of both the TrkI and TrkH transporters in more detail, transport experiments were carried out and the kinetic parameters of K+ uptake for the two trk systems were determined. To carry out K+ uptake experiments, H. elongata wild type and strains KB12, KB12.2, and KB15 were grown in mineral salt medium containing 0.51 M NaCl and 5 mM K+. The cells were depleted of more than 70% of their cytoplasmic K+ content by washing them twice in DEA buffer and Tricine buffer according to the method described by Tokuda (42, 43). The washing procedure had no deleterious effect on K+ transport, and all cells were able to accumulate about 1.2 μmol of K+ per mg of cell protein, which corresponds to the natural K+ content found in exponentially growing cells of H. elongata (19). After washing, the K+ uptake in K+- depleted cells was initiated by adding K+ to the cells, and the initial rates for K+ uptake were determined in 4 min at different K+ concentrations. The initial rate for K+ uptake increased with increasing external K+ (data not shown). K+ uptake rates of TrkI determined with strain KB15 (ΔtrkH) were fitted by nonlinear regression, and the uptake kinetics were best fit to the Michaelis-Menten model (Fig. 3A). Analysis of the transport kinetics of TrkI revealed a Km value of 1.12 mM and a Vmax of 176 nmol of K+ taken up min−1 mg of protein−1.
FIG. 3.
Kinetic analysis of K+ transport via TrkH and TrkI in H. elongata wild type and strains KB12 (ΔtrkI), KB15 (ΔtrkH), KB12.2 (ΔtrkH ΔtrkI) and KB14 (ΔtrkA). Osmolarity of growth medium and transport buffer was adjusted by 0.51 M NaCl. Transport was started by adding potassium to the assay. (A) Lowering the K+ concentration from 20 mM to 500 μM led to a decreasing transport rate. The K+ uptake data were fitted by nonlinear regression. The curve for K+ transport via TrkI into KB15 (•) and transport by the wild type (○) were fitted best by the Michaelis-Menten model and showed Km values of 1.12 and 1.18 mM K+, respectively. The transport data for TrkH in strain KB12 (▪) showed a sigmoidal dependence of transport rate versus K+ concentration. The half-saturation constant was determined to be 3.36 mM. Deletion of trkI and trkH abolished transport activity in strain KB12.2 (▴), proving that transporters TrkH and TrkI are responsible for the observed uptake of K+. No transport activity was measured for the ΔtrkA deletion mutant KB14 (♦), proving that trkA encodes the potential NAD binding protein for both TrkH and TrkI. Error bars indicate standard deviations. (B) Hill plot based on transport data from TrkI (•, KB15) and TrkH (▪, KB12) to evaluate the Hill coefficient h, which was estimated to be 0.98 for TrkI and 2.1 for TrkH. Values of h greater than 1 indicate a deviation of the Michaelis-Menten kinetics and can be used as an index of enzyme cooperativity (see Discussion). Symbols are as defined in the legend to panel A.
In contrast, the kinetic parameters for TrkH determined by transport experiments with strain KB12 (ΔtrkI) were different from those for TrkI (Fig. 3A), showing a lower affinity for the substrate (half-saturation constant of 3.36 mM K+) and a lower transport velocity (Vmax of 137 nmol K+ min−1 mg of protein−1). Furthermore, the K+ uptake did not follow the Michaelis-Menten kinetics but was best described by the following equation:
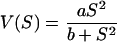 |
in which V is the transport rate, S is the substrate concentration, a is the limiting rate (maximum velocity), and the square root of b is the substrate concentration at which V = 0.5a (rate is half-limiting). The difference in kinetics of TrkH for K+ uptake compared to those for TrkI was confirmed by the Hill plot (Fig. 3B), where the Hill coefficient was estimated to be 0.98 for TrkI and 2.1 for TrkH.
The transport data for the wild-type strain were similar to those for strain KB15 (ΔtrkH), having a Km value of 1.18 mM and a Vmax of 170 nmol of K+ min−1 mg of protein−1. Although the wild type is equipped with both Trk systems, TrkH and TrkI, the Vmax was not additive, and K+ uptake followed the Michaelis-Menten kinetics as described for strain KB15, where K+ was accumulated only via the single transporter TrkI (Fig. 3).
Taken together, these findings suggest that TrkI is the dominant K+ uptake system in the H. elongata wild type, while TrkH does not contribute to K+ uptake at least under the conditions tested, where salt-adapted cells were used.
TrkA is the putative NAD binding protein for both transporters TrkH and TrkI.
K+ uptake mediated by Trk transport systems requires a TrkA NAD+/NADH binding protein (35). By complementation of E. coli TK2420 (trkA), only one trkA gene could be identified from the genome of H. elongata, which is located adjacent to trkH separated only by an intergenic region of 43 bp (Fig. 1). To clarify the role of the trkA gene product for the transport of K+ through TrkH and TrkI, trkA was deleted in the wild type of H. elongata. The resulting mutant, KB14, showed a growth behavior and salt sensitivity similar to those of the triple knockout mutant KB16 (ΔtrkAH ΔtrkI). Transport experiments revealed that the loss of trkA abolished any K+ uptake activity via TrkH and TrkI (Fig. 3A). These results show that there is only one type of TrkA protein in H. elongata expressed from the trkAH locus, on which both TrkH and TrkI rely for transport of K+.
Transcriptional organization of the trkAH and trkI loci in H. elongata.
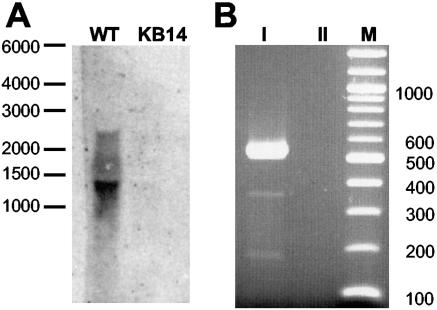
Complementation experiments, sequence analysis of trkA, and transport experiments with the trkA deletion mutant KB14 revealed that trkA encodes the putative NAD binding protein required for active TrkH and TrkI transport systems. To investigate how the transcriptional organization of the trkAH genes allows for sufficient synthesis of the TrkA protein for both transport systems, Northern hybridization experiments were performed. To analyze the total RNA of the H. elongata wild type and strain KB14 (ΔtrkA), single-stranded DIG-labeled antisense RNA probes directed against trkA and trkH, respectively, were used in Northern blots. The presented results show (Fig. 4A) that the trkA probe hybridized to mRNA of approximately 1.3 kb in size, which is in close agreement with the size of the calculated trkA mRNA, indicating that trkA can be transcribed separately from trkH. However, a single trkH mRNA was not detectable using the DIG-labeled trkH probe. Also, a common trkAH transcript was not found using either RNA probe. Since the trkAH mRNA was estimated to have a size of approximately 2.8 kb, such a transcript would be most likely covered by 23S rRNA. To overcome the limitations of the Northern hybridization technique, RT-PCR experiments were carried out. cDNA was synthesized with a reverse primer located in trkH and amplified by PCR with a primer pair binding in trkA and trkH. A DNA fragment of 550 bp was synthesized (Fig. 4B) matching the calculated size of the trkAH PCR product (554 bp). The amplified DNA was digested by different enzymes, and the resulting restriction pattern analyzed by gel electrophoresis was identical to the restriction fragments of trkAH (data not shown). The Northern hybridization experiments and RT-PCR analysis revealed a polycistronic transcription of trkAH as well as the existence of a single trkA transcript.
FIG. 4.
Analysis of the transcriptional organization of the trkAH gene cluster by Northern hybridization experiments and RT-PCR. (A) Northern blot analysis of total RNA from H. elongata wild-type (WT) and the trkA deletion mutant KB14 using a trkA-specific RNA probe. Total RNA was isolated from cells grown in minimal medium containing 510 mM NaCl and 200 mM KCl. RNA was electrophoretically separated on agarose gels, blotted onto a nylon membrane, and hybridized with a DIG-labeled RNA probe. A single transcript of approximately 1.4 kb corresponding to the calculated size of a trkA transcript was detected by hybridization with the WT RNA, while no hybridization signal was detectable with the RNA of control strain KB14 (ΔtrkA). There were no transcripts detectable using a trkH-specific probe in similar experiments. (B) RT-PCR analysis of the trkAH locus proving the existence of a common trkAH transcript. cDNA was synthesized by using a reverse primer binding to trkH mRNA. trkAH cDNA was amplified by PCR with the help of forward primer 2trk2 binding at the 3′ end of trkA and reverse primer trkAup binding at the 5′ region of trkH. A 550-bp PCR product was separated by agarose gel electrophoresis (I), which matched the size of the calculated trkAH PCR product (554 bp). No DNA was amplified from purified total RNA prior to cDNA synthesis, proving that the trkAH product was indeed amplified from cDNA and not from contaminating chromosomal DNA (II). M, DNA size marker (bp).
DISCUSSION
The data show that H. elongata possesses two active Trk transport systems, TrkAH and TrkAI, which are the major K+ transport systems in this organism. TrkAH and TrkAI differ in their substrate affinity and transport velocity, and it was shown that mainly TrkI transports K+ under the conditions tested. It is surprising that two closely related transporters of the same design sharing the same components (putative NAD+ binding protein TrkA) not only differ in substrate affinity and transport velocity but also in their overall transport kinetics. While TrkI transport was best described by the Michaelis-Menten model, the uptake via TrkH showed a sigmoidal dependence. The equation
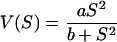 |
best fits the TrkH transport data. More complex models do not improve the nonlinear regression of the data. Based on transport kinetics revealed in this study and the unknown transport mechanism(s) for K+ uptake through Trk transporters, we offer the following hypotheses to explain the different transport behaviors of TrkAH and TrkAI: (i) TrkAH but not TrkAI could be a cooperative transport enzyme, which typically show sigmoidal rate kinetics as a function of substrate concentration and a Hill coefficient greater than 1; (ii) the differences in kinetics could also be explained by random-order binding of the substrates K+ and H+ to TrkH, which would also lead to a sigmoid curve and a different Hill plot, respectively, in contrast to the compulsory-order mechanism (37).
The different transport kinetics of TrkI and TrkH also help to explain the reduced growth of strain KB12 (ΔtrkI) in low-potassium medium compared to that of strain KB15 (ΔtrkH). The transport velocities of TrkI and TrkH differed only by a factor of 1.3 when the transport systems were saturated. However, at low K+ concentrations of 100 to 250 μM, the transport rates of TrkH were estimated to be at least 40 times lower than the rates of TrkI. The large differences in growth of strain KB12 and KB15 were observed only at these low concentrations, while at K+ levels of 5 mM or higher both mutants were phenotypically identical.
H. elongata is one of the few prokaryotes found in which the trkA and trkH genes are clustered together. A similar organization of trkAH exists in V. alginolyticus with the same position of the corresponding genes in the trkAH cluster (26). Not only is the trkAH cluster similar in structure and sequence, but also the genes adjacent to trkAH are similar in H. elongata and V. alginolyticus. orf1 upstream of trkAH in H. elongata codes for a potential protein containing a tRNA/rRNA-m5C-methyltransferase domain. Enzymes that function as RNA-methyltransferases are encoded by open reading frames named fmu and fmv, respectively (14, 44). Upstream of the trkAH cluster in V. alginolyticus, three open reading frames are located, named fmt, fmu, and fmv (26). Interestingly, similarities in this respect were also found in E. coli, where fmt and fmu precede the trkA gene as well (35). In all three organisms the arrangement of the genes fmu/fmv and trkA is similar. However, in contrast to H. elongata and V. alginolyticus, the trkA gene in E. coli is located separately from trkH and the second system of this type, trkG. For trkAH of V. alginolyticus, it is assumed that the gene cluster is organized as an operon and, since V. alginolyticus encodes only one Trk system, a coordinated transcription of trkAH would be advantageous (26). Although trkA is linked to trkH in H. elongata as well and can be transcribed along with trkH, a single trkA transcript was detected, which can arise either by partial termination of the transcription after trkA or from processing of the RNA to separate trkA and trkH.
Still to be resolved is why H. elongata has two Trk uptake systems, of which one, TrkH, shows lower transport rates and less affinity for the substrate and does not contribute significantly to K+ accumulation in adapted cells of H. elongata. In E. coli K12 strains, often two Trk systems are found, of which the gene trkG encoding the second system was most likely acquired through phage insertion. However, not all E. coli strains are equipped with two Trk transporters. The combination of a constitutively expressed Trk uptake system (11, 30) transporting K+ at a high rate and an inducible high-affinity Kdp system (Km = 2 μM) allows E. coli osmoregulated K+ uptake even in low-K+ medium. Such high-affinity transporters, such as Kdp from E. coli, are absent in organisms from soil and marine environments. This can be explained by the abundance of K+, at least in the marine and saline environment where K+ is found in concentrations of 10 mM or higher. Similar to the case with H. elongata, a couple of low- to medium-affinity transport systems are found in V. alginolyticus (TrkAH and KtrAB; Km = 50 μM) and B. subtilis (KtrAB and KtrCD). In the case of H. elongata, the different transport kinetics of the two K+ transporters might help to explain the requirement for a second Trk system. The importance of TrkI for K+ accumulation was clearly shown in this study. However, one has to keep in mind that enzymes following the Michaelis-Menten kinetics, like TrkI, can only be regulated by comparatively large environmental changes (e.g., substrate concentration). In contrast, enzymes with sigmoidal kinetics can react to small changes in their environment and are often known as well-regulated enzymes. To describe the two K+ uptake systems TrkI and TrkH in even more detail, their behavior during environmental changes like osmotic shifts has to be examined, and such studies might reveal additional information about the role and importance of these two transporters for adaptation and osmoregulation of H. elongata.
Acknowledgments
We are grateful to Wolfgang Epstein (University of Chicago) for generously providing us with Escherichia coli strains TK2420 and TK2691. We thank Udo Hölker for access to equipment, Katrin Grammann for assistance with the Northern hybridization experiments, Jörg Severin for helpful discussion, and Sharon Taylor for critical reading of the manuscript.
We thank the Graduiertenkolleg for providing A.K. with a fellowship.
REFERENCES
- 1.Altendorf, K., M. Gaβel, W. Puppe, T. Möllenkamp, A. Zeeck, C. Boddien, K. Fendler, E. Bamberg, and S. Dröse. 1998. Structure and function of the Kdp-ATPase of Escherichia coli. Acta Physiol. Scand. 163(Suppl.):137-146. [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäfer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arahal, D. R., W. Ludwig, K. H. Schleifer, and A. Ventosa. 2002. Phylogeny of the family Halomonadaceae based on 23S and 165 rDNA sequence analyses. Int. J. Syst. Evol. Microbiol. 52:241-249. [DOI] [PubMed] [Google Scholar]
- 4.Bossemeyer, D., A. Borchard, D. C. Dosch, G. C. Helmer, W. Epstein, I. R. Booth, and E. P. Bakker. 1989. K+-transport protein TrkA of Escherichia coli is a peripheral membrane protein that requires other trk gene products for attachment to the cytoplasmic membrane. J. Biol. Chem. 264:16403-16410. [PubMed] [Google Scholar]
- 5.Brown, A. D. 1976. Microbial water stress. Bacteriol. Rev. 40:803-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings, S. P., M. P. Williamson, and D. J. Gilmour. 1993. Turgor regulation in a novel Halomonas species. Arch. Microbiol. 160:319-323. [Google Scholar]
- 7.Dinnbier, U., E. Limpinsel, R. Schmid, and E. P. Bakker. 1988. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch. Microbiol. 150:348-357. [DOI] [PubMed] [Google Scholar]
- 8.Dosch, D. C., G. L. Helmer, S. H. Sutton, F. F. Salvacion, and W. Epstein. 1991. Genetic analysis of potassium transport loci in Escherichia coli: evidence for three constitutive systems mediating uptake of potassium. J. Bacteriol. 173:687-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durell, S. R., E. P. Bakker, and H. R. Guy. 2000. Does the KdpA subunit from the high affinity K+-translocating P-type KDP-ATPase have a structure similar to that of K+ channels? Biophys. J. 78:188-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durell, S. R., Y. Hao, T. Nakamura, E. P. Bakker, and H. R. Guy. 1999. Evolutionary relationships between K+ channels and symporters. Biophys. J. 77:775-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein, W., and B. S. Kim. 1971. Potassium transport loci in Escherichia coli K12. J. Bacteriol. 108:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galinski, E. A. 1995. Osmoadaptation in Bacteria. Adv. Microbiol. Physiol. 37:273-328. [PubMed] [Google Scholar]
- 13.Grammann, K., A. Volke, and H. J. Kunte. 2002. New type of osmoregulated solute transporter identified in halophilic members of the Bacteria domain: TRAP transporter TeaABC mediates the uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581T. J. Bacteriol. 184:3078-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, X. R., C. Gustafsson, J. Ku, M. Yu, and D. V. Santi. 1999. Identification of the 16S rRNA m5C967 methyltransferase from Escherichia coli. Biochemistry 38:4053-4057. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 16.Harms, C., Y. Domoto, C. Celik, E. Rahe, S. Stumpe, R. Schmid, T. Nakamura, and E. P. Bakker. 2001. Identification of the ABC protein SapD as the subunit that confers ATP dependence to the K+-uptake systems TrkH and TrkG from Escherichia coli K-12. Microbiology 147:2991-3003. [DOI] [PubMed] [Google Scholar]
- 17.Holtmann, G., E. P. Bakker, N. Uozumi, and E. Bremer. 2003. KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J. Bacteriol. 185:1289-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 19.Kraegeloh, A., and H. J. Kunte. 2002. Novel insights into the role of potassium for osmoregulation in Halomonas elongata. Extremophiles 6:453-462. [DOI] [PubMed] [Google Scholar]
- 20.Kunte, H. J., and E. A. Galinski. 1995. Transposon mutagenesis in halophilic eubacteria: conjugal transfer and insertion of transposon Tn5 and Tn1732 in Halomonas elongata. FEMS Microbiol. Lett. 128:293-299. [DOI] [PubMed] [Google Scholar]
- 21.Lai, M. C., K. R. Sowers, D. E. Robertson, M. F. Roberts, and R. P. Gunsalus. 1991. Distribution of compatible solutes in the halophilic methanogenic archaebacteria. J. Bacteriol. 173:5352-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay, M. A., R. S. Norton, and L. J. Borowitzka. 1984. Organic osmoregulatory solutes in cyanobacteria. J. Gen. Microbiol. 130:2177-2191. [Google Scholar]
- 23.Matheson, A. T., G. D. Sprott, I. J. McDonald, and H. Tessier. 1976. Some properties of an unidentified halophile: growth characteristics, internal salt concentration, and morphology. Can. J. Microbiol. 22:780-786. [DOI] [PubMed] [Google Scholar]
- 24.Miller, H. J. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, N.Y.
- 25.Nakamura, T., R. Yuda, T. Unemoto, and E. P. Bakker. 1998. KtrAB, a new type of bacterial K+-uptake system from Vibrio alginolyticus. J. Bacteriol. 180:3491-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura, T., N. Yamamuro, S. Stumpe, T. Unemoto, and E. P. Bakker. 1998. Cloning of the trkAH gene cluster and characterization of the Trk K-uptake system of Vibrio alginolyticus. Microbiology 144:2281-2289. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura, T., Y. Katoh, Y. Shimizu, Y. Matsuba, and T. Unemoto. 1996. Cloning and sequencing of novel genes from Vibrio alginolyticus that support the growth of K+ uptake-deficient mutant of Escherichia coli. Biochim. Biophys. Acta 1277:201-208. [DOI] [PubMed] [Google Scholar]
- 28.Reed, R. H., S. R. C. Warr, D. L. Richardson, D. J. Moore, and W. D. P. Stewart. 1985. Multiphasic osmotic adjustment in a euryhaline cyanobacterium. FEMS Microbiol. Lett. 28:225-229. [Google Scholar]
- 29.Rhoads, D. B., and W. Epstein. 1977. Energy coupling to net K+ transport in Escherichia coli K-12. J. Biol. Chem. 252:1394-1401. [PubMed] [Google Scholar]
- 30.Rhoads, D. B., F. B. Waters, W. Epstein. 1976. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J. Gen. Physiol. 67:325-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roosild, T. P., S. Miller, I. R. Both, and S. Choe. 2002. A mechanism of regulating transmembrane potassium flux through a ligand-mediated conformational switch. Cell 109:781-791. [DOI] [PubMed] [Google Scholar]
- 32.Sadler, M., M. McAninch, R. Alico, and L. I. Hochstein. 1980. The intracellular Na+ and K+ composition of the moderately halophilic bacterium, Paracoccus halodenitrificans. Can. J. Microbiol. 26:496-502. [DOI] [PubMed] [Google Scholar]
- 33.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 35.Schlösser, A., A. Hamann, D. Bossemeyer, and E. P. Bakker. 1993. NAD+ binding to the Escherichia coli K+-uptake protein TrkA and sequence similarity between TrkA and domains of a family of dehydrogenases suggest a role for NAD+ in bacterial transport. Mol. Microbiol. 9:533-543. [DOI] [PubMed] [Google Scholar]
- 36.Sebestian, J., Z. Petrmichlova, S. Sebastianova, J. Naprstek, and J. Svoboda. 2001. Osmoregulation in Bacillus subtilis under potassium limitation: a new inducible K+-stimulated, VO43− inhibited ATPase. Can. J. Microbiol. 47:1116-1125. [PubMed] [Google Scholar]
- 37.Segel, I. H. 1975. Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. John-Wiley & Sons, New York, N.Y.
- 38.Stewart, L. M. D., E. P. Bakker, and I. R. Booth. 1985. Energy coupling to K+ uptake via the Trk system in Escherichia coli: the role of ATP. J. Gen. Microbiol. 131:77-85. [DOI] [PubMed] [Google Scholar]
- 39.Stumpe, S., A. Schlösser, M. Schleyer, and E. P. Bakker. 1996. K+ circulation across the prokaryotic cell membrane: K+-uptake systems, p. 473-499. In W. N. Konings, H. R. Kaback, and J. S. Lolkema (ed.), Handbook of biological physics, vol. 2. Elsevier Science BV, Amsterdam, The Netherlands.
- 40.Takeshita, S., M. Sato, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63-74. [DOI] [PubMed] [Google Scholar]
- 41.Tholema, N., E. P. Bakker, A. Suzuki, and T. Nakamura. 1999. Change to alanine of one out of four selectivity filters in KtrB causes a two orders of magnitude decrease in the affinities for both K+ and Na+ dependent K+ uptake system KtrAB from Vibrio alginolyticus. FEBS Lett. 450:217-220. [DOI] [PubMed] [Google Scholar]
- 42.Tokuda, H. 1986. Sodium translocation by NADH oxidase of Vibrio alginolyticus: isolation and characterization of the sodium pump-defective mutants. Methods Enzymol. 125:520-530. [DOI] [PubMed] [Google Scholar]
- 43.Tokuda, H. 1993. The Na+ cycle in Vibrio alginolyticus, p. 125-138. In E. P. Bakker (ed.), Alkali transport systems in prokaryotes. CRC Press, Boca Raton, Fla.
- 44.Tscherne, J. S., K. Nurse, P. Popienick, H. Michel, M. Sochacki, and J. Ofengand. 1999. Purification, cloning, and characterization of the 16S RNA m5C967 methyltransferase from Escherichia coli. Biochemistry 38:1884-1892. [DOI] [PubMed] [Google Scholar]
- 45.Ventosa, A., J. J. Nieto, A. Oren. 1998. Biology of aerobic moderately halophilic bacteria. Microbiol. Mol. Biol. Rev. 62:504-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vreeland, R. J., C. D. Litchfield, E. L. Martin, and E. Elliot. 1980. Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int. J. Syst. Bacteriol. 30:485-495. [Google Scholar]
- 47.Walderhaug, M. O., E. D. Litwack, and W. Epstein. 1989. Wide distribution of homologs of Escherichia coli Kdp K+-ATPase among gram-negative bacteria. J. Bacteriol. 171:1192-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welsh, D. T., and R. A. Herbert. 1993. Osmoadaptation of Thiocapsa roseopersicina OP-1 in batch and continuous culture: accumulation of K+ and sucrose in response to osmotic stress. FEMS Microbiol. Ecol. 13:151-158. [Google Scholar]
- 49.Whatmore, A. M., J. A. Chudek, and R. H. Reed. 1990. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J. Gen. Microbiol. 136:2527-2535. [DOI] [PubMed] [Google Scholar]
- 50.Wohlfarth, A., J. Severin, and E. A. Galinski. 1990. The spectrum of compatible solutes in heterotrophic halophilic eubacteria of the family Halomonadaceae. J. Gen. Microbiol. 136:705-712. [Google Scholar]