Abstract
The major outer membrane proteins Pgm6 (41 kDa) and Pgm7 (40 kDa) of Porphyromonas gingivalis ATCC 33277 are encoded by open reading frames pg0695 and pg0694, respectively, which form a single operon. Pgm6 and Pgm7 (Pgm6/7) have a high degree of similarity to Escherichia coli OmpA in the C-terminal region and are predicted to form eight-stranded β-barrels in the N-terminal region. By sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Pgm6/7 appear as bands with apparent molecular masses of 40 and 120 kDa, with and without a reducing agent, suggesting a monomer and trimer, respectively. To verify the predicted trimeric structure and function of Pgm6/7, we constructed three mutants with pg0695, pg0694, or both deleted. The double mutant produced no Pgm6/7. The single-deletion mutants appeared to contain less Pgm7 and Pgm6 and to form homotrimers that migrated slightly faster (115 kDa) and slower (130 kDa), respectively, than wild-type Pgm6/7 under nonreducing conditions. N-terminal amino acid sequencing and mass spectrometry analysis of partially digested Pgm6/7 detected only fragments from Pgm6 and Pgm7. Two-dimensional, diagonal electrophoresis and chemical cross-linking experiments with or without a reducing agent clearly showed that Pgm6/7 mainly form stable heterotrimers via intermolecular disulfide bonds. Furthermore, growth retardation and arrest of the three mutants and increased permeability of their outer membranes indicated that Pgm6/7 play an important role in outer membrane integrity. Based on results of liposome swelling experiments, these proteins are likely to function as a stabilizer of the cell wall rather than as a major porin in this organism.
Porphyromonas gingivalis, a gram-negative, asaccharolytic anaerobe, is a major causative agent in the initiation and progression of periodontal disease (22, 23). This organism possesses a variety of virulence factors, including fimbriae, capsular polysaccharide, and hemagglutinins, as well as strong proteolytic enzymes such as Lys- and Arg-gingipains (21-23, 44).
Previously, we identified seven major outer membrane proteins of P. gingivalis strain ATCC 33277 (29). Of these proteins, Pgm6 and Pgm7 (Pgm6/7) have been assigned to the immunoreactive 42-kDa antigen PG33 and immunoreactive 43-kDa antigen PG32 of P. gingivalis W83, respectively (31, 39). Pgm6/7 share a high degree of similarity to Escherichia coli OmpA in the C-terminal region, through which they presumably associate with peptidoglycan (6). E. coli OmpA forms nonspecific diffusion channels that allow the penetration of various solutes (42) and is referred to as “monomeric porin” (34), which plays a structural role in the integrity of the bacterial cell surface (20).
Pgm6/7 appear as a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with apparent molecular masses of 40 and 120 kDa with and without 2-mercaptoethanol (2-ME), respectively (29). This suggests that Pgm6/7 form heterotrimers in the outer membrane, although there is no strong evidence that OmpA or OmpA homologs exist as stable oligomers (34).
In this study, we constructed three mutants by deleting the open reading frames (ORFs) pg0695 and/or pg0694, assigned by The Institute for Genomic Research, which encode Pgm6 and Pgm7, respectively, and then verified the heterotrimeric structure and examined the physiological roles of Pgm6/7 in this organism.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are shown in Table 1. All P. gingivalis strains were grown in Trypticase soy broth (Becton Dickinson and Company, Franklin Lakes, N.J.) supplemented with 2.5 mg of yeast extract/ml, 2.5 μg of hemin/ml, 5 μg of menadione/ml, and 0.1 mg of dithiothreitol (DTT)/ml (sTSB) under anaerobic conditions (10% CO2, 10% H2, and 80% N2). In addition, a modified chemically defined medium (CDM) and Dulbecco's modified Eagle's medium (DMEM), each supplemented with 3 or 1% bovine serum albumin (BSA), fraction V (BSA [Frac. V], Miles Inc., Kankakee, Ill.), were used as synthetic media for growth experiments. CDM with 3% BSA was prepared as previously reported (27) except for omission of hemin and menadione. Two BSA preparations, fraction V and high grade (product no. A0281; Sigma-Aldrich Co., St. Louis, Mo.), were used for DMEM. We also used brucella HK agar (Kyokuto Pharmaceutical Industrial Co., Ltd., Tokyo, Japan) supplemented with 5% laked rabbit blood, 2.5 μg of hemin/ml, 5 μg of menadione/ml, and 0.1 mg of DTT/ml (BHK agar). A Zero Blunt TOPO PCR cloning kit, which included the plasmid vector pCR-Blunt II-TOPO, was purchased from Invitrogen Corporation (Carlsbad, Calif.), and E. coli TOP10 carrying pCR-Blunt II-TOPO was grown in Luria-Bertani medium supplemented with 25 μg of kanamycin/ml. E. coli DH5α carrying pKD260 was grown in Luria-Bertani medium supplemented with 25 μg of chloramphenicol (CHL)/ml as described previously (43).
TABLE 1.
Bacterial strain and plasmid list
| Strain | Plasmid | Genotype or relevant characteristicsa | Reference or source |
|---|---|---|---|
| Porphyromonas gingivalis | |||
| ATCC 33277 | Wild type, type strain | ATCCb | |
| Δ695 | pg0695-deletion mutant from ATCC 33277, Cmr | This study | |
| Δ694 | pg0694-deletion mutant from ATCC 33277, Cmr | This study | |
| Δ695-694 | pg0695- and pg0694-deletion mutant from ATCC 33277, Cmr | This study | |
| abfD mutant | abfD mutant of ATCC 33277 with an ermF-ermAM insertion in abfD, Emr | This study | |
| W83 | Wild type, the genome sequenced | 31 | |
| Escherichia coli | |||
| TOP10 | As chemically competent cells, F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZ ΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen Corporation | |
| DH5α | pKD260 | Derivative of pACYC184 with deletion of a 1.1-kbp HincII fragment, bearing cat gene, Cmr | Gift from K. Nakayamac |
| pCR-Blunt II-TOPO | Cloning vector, linearized with DNA topoisomerase I bound to the 3′ end of each DNA strand, Kmr | Invitrogen Corporation |
Cmr, chloramphenicol resistance; Emr, erythromycin resistance; Kmr, kanamycin resistance.
ATCC, American Type Culture Collection.
Division of Microbiology and Oral Infection, Department of Developmental and Reconstructive Medicine, Nagasaki University Graduate School of Biomedical Sciences.
Construction of deletion mutants.
We applied the PCR-based overlap extension method (14) to construct DNA fragments that allowed the replacement of pg0695, pg0694, or both genes with the cat gene in the P. gingivalis chromosome. The primers and their annealing sites are shown in Table 2 and Fig. 1A, respectively. The cat gene was amplified from the ATG start codon to the TAA stop codon with primers AGU-01 and AGU-02 to generate a 660-bp product from pKD260. For construction of the pg0695-deletion cassette, the flanking sequence upstream of pg0695 was amplified with primers AGU-32 and AGU-36, which have homology to the 5′ end of the cat fragment. The flanking sequence downstream of pg0695 was amplified with AGU-33 and AGU-37, which have homology to the 3′ end of the cat fragment. The cat, pg0695-upstream, and pg0695-downstream fragments were used as templates for overlap extension PCR to generate a deletion cassette in which pg0695 was replaced by cat.
TABLE 2.
Primer lista
| Name | Sequence (5′-3′)b |
|---|---|
| AGU-01 | ATGGAGAAAAAAATCACTGGA |
| AGU-02 | TTACGCCCCGCCCTGCCACTC |
| AGU-32 | GATGAAGTCGCCAAAGGTGTC |
| AGU-33 | CGGAAGTAAACCACATTGTCA |
| AGU-34 | TTTAATTCCATGGGTAGGTGTTG |
| AGU-35 | CTACTGAATTCATTGAGGGACGA |
| AGU-36 | CCAGTGATTTTTTTCTCCATAGTTTTACTTTTCTAAGTGTATTTTTA |
| AGU-37 | GCAGGGCGGGGCGTAATTCATCTGAGACTTTTGTTGGATAATA |
| AGU-38 | CCAGTGATTTTTTTCTCCATAATTCTGTATGTCATTTTATATTATCCAAC |
| AGU-39 | GCAGGGCGGGGCGTAATCTCAAATATCCCCACAAATAAATG |
| AGU-40 | TACGAGCCTAATTCTCCCATG |
| AGU-41 | CTTGTATTCGTCCATCTCTGC |
Primer binding sites are shown in Fig. 1.
Underlines show overlapping regions of 5′ or 3′ end of cat.
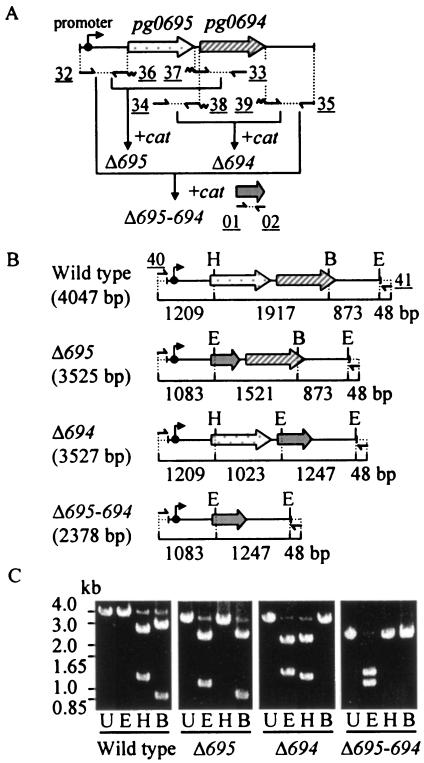
FIG. 1.
Construction of pg0695- and pg0694-deletion mutants from P. gingivalis ATCC 33277. (A) Gene arrangement in the chromosome and procedure for construction of allele-exchange gene deletion and location of primers. (B) Restriction sites and predicted lengths of the DNA regions in each genotype. Sizes of the DNA regions are shown as base pairs under the names and lines. (C) Verification of the mutants by gel electrophoresis of PCR products. After amplification by PCR with primers AGU-40 and AGU-41 shown at the top of panel B as 40 and 41, respectively, the PCR products were digested with the restriction enzymes described below and subjected to electrophoresis. Dotted arrows, pg0695; diagonally striped arrows, pg0694; gray arrows, cat; small arrows, primers; wavy line, overlap regions on cat; underlined numbers, primer designation in a simplified manner (see Table 1 for details); U, undigested; E, digested with EcoRI; H, digested with HpaI; B, digested with BanII.
The deletion cassettes of pg0694 and pg0695/0694 were generated by similar procedures. Each deletion cassette created was ligated into pCR-Blunt II-TOPO, and the resulting recombinant plasmids were transformed into competent cells of E. coli TOP10 according to the manufacturer's directions (Invitrogen Corporation). Constructs to be introduced for making mutants were sequenced before electroporation to rule out unintended base changes. A similar deletion of an adjacent gene downstream from pg0694 (abfD) was also done as described previously (16).
Electroporation of P. gingivalis was performed essentially as described by Fletcher et al. (8). The plasmid constructs were linearized by digestion with endonucleases KpnI and XbaI and introduced into electrocompetent cells of P. gingivalis. After 6 h of anaerobic incubation in sTSB, the pulsed cells were plated on BHK agar supplemented with 12 μg of CHL/ml, and the plates were incubated anaerobically at 37°C for 7 days. Possible recombinants were verified by PCR and restriction enzyme digestion of their PCR products.
Membrane preparations.
Separation of whole envelopes and the outer membrane from P. gingivalis strains was performed essentially as described previously (29). Briefly, bacterial cells were washed with 10 mM HEPES-NaOH (pH 7.4) containing 0.15 M NaCl and then resuspended with 10 mM HEPES-NaOH (pH 7.4) containing 0.1 mM N-α-p-tosyl-l-lysine chloromethyl ketone, 0.2 mM phenylmethylsulfonyl fluoride, and 0.1 mM leupeptin (HEPES buffer). The cells were disrupted by sonication, and the remaining undisrupted bacterial cells were removed by centrifugation at 1,000 × g for 10 min. The envelope was collected as a pellet by centrifugation at 100,000 × g for 60 min at 4°C. The pellet was washed once by resuspension in HEPES buffer and recentrifuged. The final pellet was suspended in HEPES buffer, and the outer membrane was obtained from the cell envelopes by the differential detergent extraction method (29). The protein content of the membrane preparation was estimated by the Bradford method (4).
SDS-PAGE and Western blotting.
SDS-PAGE was performed in a 1.0-mm-thick 12% gel as described by Lugtenberg et al. (25). Two-dimensional (2-D), diagonal SDS-PAGE was performed essentially as described previously (19). The samples were usually solubilized in SDS buffer with or without 2-ME at 100°C for 5 min (13). For 2-D, diagonal SDS-PAGE cell envelopes were applied to the 12% slab gel under nonreducing conditions as the first dimension. After electrophoresis the gel strip was cut out, placed at the top of the second gel, and overlaid with the SDS buffer containing 2-ME to provide reducing conditions. The gels were stained with Coomassie brilliant blue R-250 (CBB).
Western blotting was performed as described previously (36). Proteins in an SDS-polyacrylamide gel were electrophoretically transferred to a nitrocellulose membrane. The membrane was blocked with 1% BSA (fraction V) in 20 mM Tris-HCl (pH 7.5) containing 0.5 M NaCl. Then the membrane was reacted with the anti-Pgm6/7 serum followed by incubation with peroxidase-conjugated anti-rabbit immunoglobulin G (MP Biomedicals Inc., Aurora, Ohio). After the membrane was washed, signals of Pgm6/7 were detected with 0.01% 4-chloro-1-naphthol in 20 mM Tris-HCl (pH 7.5) containing 0.5 M NaCl supplemented with hydrogen peroxide.
Reduction of the Pgm6/7 protein trimer.
In SDS-PAGE, a solubilization-reduction mixture containing a high concentration of 2-ME was used for reduction of samples. Although the final concentration of 2-ME was about 300 to 700 mM, this could have an unknown solvent effect on proteins (32). Therefore, Pgm6/7 were also tested for dissociation of trimers to monomers by reduction with various concentrations of DTT. The dissociation of Pgm6/7 trimers in low pH was also attempted in the absence of the reducing agent as described by Luckey et al. (24).
Purification of heterotrimer and homotrimer of Pgm6/7.
The heterotrimer of Pgm6/7 and each homotrimer were electrophoretically purified from bacterial envelopes. Briefly, the envelope fraction was subjected to SDS-PAGE under nonreducing conditions. Protein bands (120, 115, and 130 kDa) stained with CBB were cut out from gels with a clean blade. After gel pieces containing the protein were applied again to another SDS-polyacrylamide gel under reducing or nonreducing conditions, bands that migrated as either a 120- or a 40-kDa protein were excised, and proteins were eluted from gels by using an electrophoretic sample concentrator (Isco, Inc., Lincoln, Nebr.).
Chemical cross-linking.
Whole purified Pgm6/7 proteins (120 kDa) were mixed with 500 μl of 0.1 M triethanolamine-HCl buffer (pH 8.5) containing 0.1% SDS. The cross-linker disuccinimidyl suberate (11-Å length; Sigma-Aldrich Co.) dissolved in dimethyl sulfoxide was added to a final concentration of 50 mM. After incubation of the reaction mixture at room temperature (20°C) for 1 or 2 h, the cross-linking reaction was stopped by the addition of 50 μl of 0.1 M Tris-HCl (pH 8.0). Reaction mixtures were analyzed by SDS-PAGE under nonreducing and reducing conditions.
Protein analyses by N-terminal amino acid sequencing and MS.
The Pgm6/7 protein heterotrimers were partially digested with Staphylococcus aureus V8 protease to analyze the internal sequences as described previously (28). Heterotrimers and homotrimers were also analyzed by matrix-assisted laser-desorption ionization-time-of-flight mass spectrometry (MS). After in-gel trypsin digestion, resulting peptides were extracted, concentrated, and applied to a Voyage-DE STR BioSpectrometry Workstation (Applied Biosystems, Foster City, Calif.) in the reflector mode essentially as described by Fountoulakis and Langen (9). The identities of the proteins were deduced from MS peaks via MS-Fit peptide mass fingerprinting methods in ProteinProspector (http://prospector.ucsf.edu/).
Permeability of the outer membrane in intact cells.
Permeability of the outer membrane was determined by measuring the rate of hydrolysis of p-nitrophenylphosphate by intact cells (49). Alkaline phosphatase is known to be located in the periplasmic space of P. gingivalis (48, 49). Bacterial cells were washed and suspended in 10 mM Tris-HCl (pH 7.4) containing 1 mM MgCl2 and 0.15 M NaCl, and cell suspensions were adjusted to an optical density at 600 nm (OD600) of 1.0. One portion was sonicated to release all alkaline phosphatase activity into the solution, and a part of the other portion was centrifuged to measure enzyme activity that leaked out from intact bacterial cells into the supernatant. The hydrolysis of p-nitrophenylphosphate was determined in a reaction mixture containing 50 mM Tris-HCl buffer (pH 8.0) and 1 mM p-nitrophenylphosphate. After incubation at 37°C for 10 min, the enzymatic reaction was terminated by the addition of NaOH at a final concentration of 0.4 M. The released p-nitrophenol was quantified by measuring the OD400. Experiments were carried out in duplicate and repeated separately three times.
Liposome swelling assay.
The assay was performed essentially as described by Nikaido et al. (35). Liposomes were made from 2.6 μmol of egg phosphatidylcholine (Avanti Polar Lipids, Alabaster, Ala.) and 0.2 μmol of dicetylphosphate (Sigma-Aldrich Co). Envelope fractions (200 μg of protein) from several organisms and strains were used for the reconstitution of proteoliposomes that were resuspended in Dextran T-40 (Amersham Biosciences Corp., Piscataway, N.J.) solution and diluted into an isosmotic solution of arabinose (Mr, 150). The permeability was estimated by measuring the initial rates of the OD400 of the proteoliposome suspension.
Preparation of anti-Pgm6/7 serum.
A mixture of Pgm6/7 proteins purified as described above was emulsified with complete Freund's adjuvant and injected into rabbits subcutaneously four times at 2-week intervals.
CAT assay.
Because there is only one prior report of cat as a resistance marker in P. gingivalis (41), we examined if cat was properly expressed. Even if mutants are successfully selected with CHL, increased resistance might occur due to a decrease in permeability of the outer membrane caused by its being deficient in Pgm6/7 proteins. Bacterial lysates for the CHL acetyltransferase (CAT) assay were prepared by the method described previously (15). CAT activities in the bacterial lysate were measured using a CAT reporter gene activity detection kit (Sigma-Aldrich Co.).
Antimicrobial susceptibility.
Penicillin G was obtained from Meiji Seika Kaisha, Ltd., Tokyo, Japan. Ampicillin and carbenicillin were obtained from Wako Pure Chemical Industries, Ltd. Cefotaxime, cefoxitin, ceftriaxone, cefuroxime, cephalexin, erythromycin, CHL, tetracycline, minocycline, gentamicin, and norfloxacin were purchased from Sigma-Aldrich Co. MIC was evaluated by agar dilution assay, as recommended by the National Committee for Clinical Laboratory Standards (30). Briefly, serial dilutions of the corresponding antibiotics were added to BHK agar. After the bacteria were grown to the late logarithmic phase in sTSB, the bacterial culture (2 μl) was spotted on the antibiotic-containing agar. After 2 days of anaerobic incubation, the susceptibility breakpoints were determined.
Bioinformatics tools.
Database searches for homologs of OmpA and Pgm6/7 were performed with the BLAST program (1). The program's three-dimensional position-specific scoring matrix (3D-PSSM), which can assign homologous proteins based on a structural prediction (18), and three programs for prediction of transmembrane β-sheets were used in this study (2, 17, 46).
Nucleotide sequence accession number.
pg0695, pg0694, and their flanking regions of P. gingivalis ATCC 33277 were sequenced, and the sequences have been deposited in the GenBank database under accession number AB187516.
RESULTS
Pgm6/7 (Pg0695/0694) homologous to E. coli OmpA.
The P. gingivalis genome has six ORFs that share homology with E. coli OmpA but none that are homologous to OmpF and OmpC (13). Among the six ORFs, PgmA (13) and Pgm6/7 (PG33/32) have been identified and inferred to be a protein for fimbriation and candidates for porins, respectively, as OmpA homologs in this organism (29, 45). However, based on BLAST searches, Pgm6/7 have homology to OmpA only in the C-terminal regions that contain a putative peptidoglycan-binding motif (6, 29). Therefore, we performed two analyses to assess whether Pgm6/7 are structural homologs of OmpA or other outer membrane proteins such as lipoproteins (5). The program 3D-PSSM (18) predicted that Pgm6/7 were structurally homologous to OmpA with significant confidence e-values of 8.1 × 10−7 and 5.3 × 10−7, respectively. Both proteins were also predicted to have structural homology to the peptidoglycan-binding lipoproteins as the second highly homologous protein with a less significant e-value of more than 3.0 × 10−2. Since conventional hydropathy methods for predicting membrane topology are useless for strong hydrophilic membrane proteins like porins and OmpA, the structural prediction was performed by the method of Jeanteur et al. (17) and Wimley (46) with the help of H. Nikaido (University of California, Berkeley) and W. C. Wimley (Tulane University Health Sciences Center), as well as by the Bigelow method (2). All results convincingly predicted that Pgm6/7 were similar to OmpA and had eight-stranded β-barrels in the N-terminal domain as does OmpA, although the overall β-barrel score of Pgm6 was low by the Wimley method (data not shown).
The pg0695/0694 locus in ATCC 33277 was sequenced and compared with those in W50 (AF175714.1 and AF175715.1) and W83 (31). The DNA sequence in the locus appeared to be the same in W50 and W83 but was slightly different in ATCC 33277. Pgm6 and Pgm7 in ATCC 33277 carried one (Phe to Leu199 in the middle; numbering from the N-terminal amino acid of mature protein) and four (Gly, Tyr, Met, and Leu to Ser55, Asp126, Ile144, and Met154, respectively) amino acid substitutions in the N-terminal half, respectively.
Construction of deletion mutants.
We constructed three deletion mutants from P. gingivalis wild-type strain ATCC 33277 by the PCR-based overlap extension method, in which ORFs pg0695 and/or pg0694 were deleted and replaced by cat, the gene encoding CAT. The overall design for construction of the mutants is shown in Fig. 1A. To confirm the replacement of ORFs pg0695 and/or pg0694 by cat, the cloning sites were amplified from flanking regions by PCR with the use of each mutant chromosome as a template with primers AGU-40 and AGU-41 as shown in Fig. 1B. Amplified PCR products were then digested with selected restriction enzymes. Since EcoRI cuts within cat (as well as 48 bp from the 3′ flanking end), HpaI cuts within pg0695, and BanII cuts within pg0694, these enzymes were used to verify the genotypes of the putative mutants. As shown in Fig. 1C, gel electrophoresis of digested DNA yielded the predicted fragments.
SDS-PAGE, Western blotting, and protein analyses.
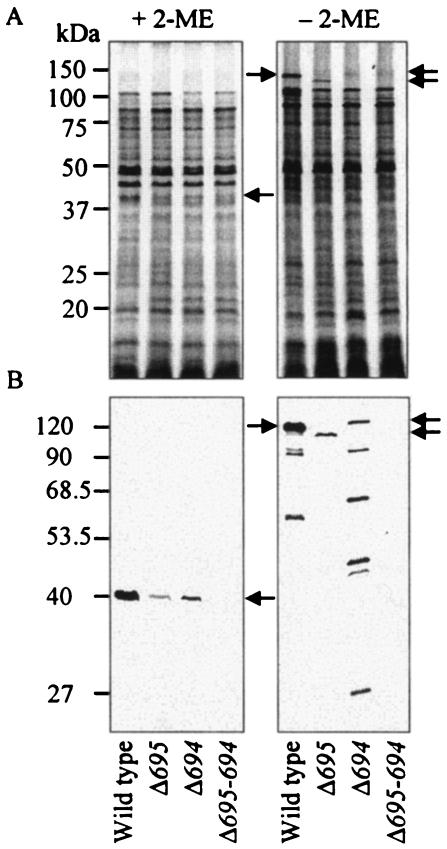
Bacterial envelopes denatured in SDS were subjected to SDS-PAGE. As shown previously (29), Pgm6/7 formed 40- and 120-kDa bands with and without 2-ME that were interpreted as Pgm6/7 monomers and trimers, respectively (Fig. 2A). No such bands were seen for the mutants.
FIG. 2.
Typical protein patterns and Western blots of envelope fractions. Bacterial envelopes were denatured in SDS with or without 2-ME at 100°C for 5 min and then loaded onto SDS-polyacrylamide gels. The gels were stained with CBB (A) and subjected to Western blot analysis with anti-Pgm6/7 serum (B). Each 50-μg protein was applied to each lane of the gel. Arrows show the predicted monomers and trimers that consisted of Pg0695 (Pgm6 monomer, 41 kDa) and/or Pg0694 (Pgm7 monomer, 40 kDa). Monomers of Pgm6 and Pgm7 were not discriminated from each other in SDS-PAGE assays.
To verify these assignments, a specific antiserum to Pgm6/7 was used for Western blot analysis (Fig. 2B). No immunoreactive bands appeared in the double mutant, confirming that Pgm6/7 are products of pg0695/0694. When samples were reduced with 2-ME, a heavy band at the 40-kDa position was detected in the wild type, and weak bands at the same position appeared in Δ695 and Δ694. No other band was detected. Under nonreducing conditions, a strong band at the 120-kDa position and two minor bands were observed in the wild type. Bands slightly lower (115 kDa) and higher (130 kDa) than the 120-kDa protein band and with lower intensities were detected in Δ695 and Δ694, respectively, and several minor bands were detected especially in Δ694 (the right panel of Fig. 2B). Interestingly, the most slowly migrating bands in Δ695 and Δ694 appeared to be homotrimers of Pgm7 and Pgm6, respectively. Different sizes of monomers of Pgm7 (40 kDa) and Pgm6 (41 kDa), based on hypothetical translation of DNA sequences, could explain the differences in trimer mobility. The immunoreactive bands with molecular masses lower than the 120- and 130-kDa bands in the wild type and Δ694 could be partially dissociated (or degradation) products of Pgm6/7 and Pgm6 trimers, respectively.
The 120-kDa band disappeared upon treatment with 1 mM DTT, regardless of whether it was from envelopes or from pure protein preparations, and full conversion to monomers occurred in the presence of 5 mM DTT. The dissociated protein (40 kDa) partially returned to the 120-kDa position upon dialysis against distilled water, indicating that the dissociation was due to reduction, not to an unknown solvent effect of 2-ME (32), and the conversion was partially reversible (data not shown). In the absence of the reducing agent, the dissociation did not occur at low pH (data not shown).
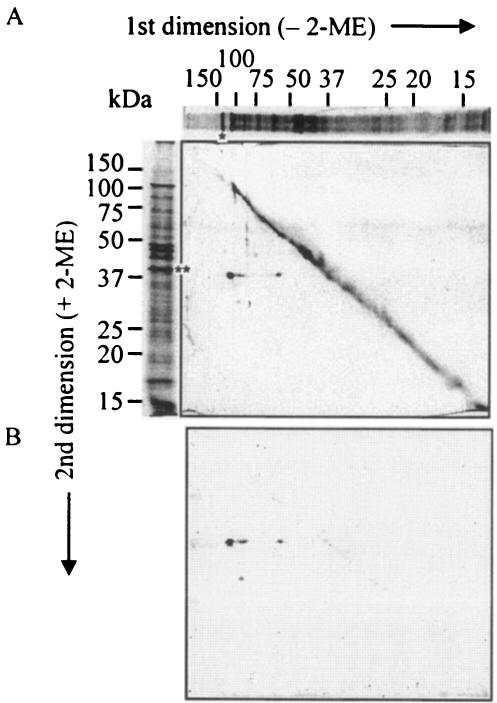
To confirm the composition of the proposed Pgm6/7 heterotrimers and of Pgm6 or Pgm7 homotrimers, the envelope fraction of the wild-type strain was subjected to 2-D diagonal electrophoresis with the first dimension under nonreducing conditions and the second dimension under reducing conditions. If a protein complex contains disulfide (SS) bonds, its components will appear as spots below the diagonal that reflect their individual molecular weights. Both a CBB-stained gel and a Western blot with the anti-Pgm6/7 serum showed a major 40-kDa spot and several minor spots at the off-diagonal position (Fig. 3). The main 40-kDa spot appeared to come from the major trimer band (120 kDa), and two minor 40-kDa spots were presumably derived from the dimer (90 kDa) and monomer (55 kDa) in the nonreducing dimension. The specific antibody reacted with these three spots and a smaller spot of lower molecular weight, indicating that all spots at the off-diagonal position came from proteins with modified SS bonds. Next, 120-kDa heterotrimers and 115- and 130-kDa homotrimers were purified from SDS-polyacrylamide gels, and the N-terminal amino acids of partially digested peptides were sequenced and analyzed by MS. Five major peptides derived from 120-kDa heterotrimers with V8 protease were sequenced. The sequences of two peptides were identified as internal peptides of Pg0694 and Pg0695, and three peptides produced no sequence, presumably because they were blocked by an N-terminal pyroglutamyl residue (29, 45). In MS, peptides from heterotrimers were assigned with very high probability to Pgm6 (10 peaks of mass values were matched [matched peaks] and the matched peptides covered 47% of the protein [coverage]) and Pgm7 (42% coverage from nine matched peaks). Those from 115- and 130-kDa homotrimers derived from the single-deletion mutants were assigned to Pgm7 (42% coverage from 11 matched peaks) and Pgm6 (42% coverage from nine matched peaks), respectively. No other peptides were assigned to any protein or possible ORF in the P. gingivalis genome. This confirmed that Pgm6/7 were mainly present as heterotrimers in the outer membrane of the wild type, and the 115- and 130-kDa proteins from the single mutants were presumably homotrimers, based on their subtle differences in mobility.
FIG. 3.
2-D, diagonal SDS-PAGE and Western blotting of P. gingivalis wild-type envelope fraction. Envelopes (100 μg) were denatured in SDS at 100°C for 5 min under nonreducing conditions before the first-dimension electrophoresis. A gel strip was cut out from the first gel and placed on a well of the second gel. Then the strip in the well was overlaid with a buffer containing 2-ME. After the second electrophoresis, the gel was stained with CBB (A) or subjected to Western blotting with the ant-Pgm6/7 serum (B). The top and left parts of the figure show protein patterns in one dimension of the sample treated without and with 2-ME, respectively, to more easily elucidate the result in the central panel. The asterisk and double asterisks show the 120 (predicted trimer)- and 40 (monomer)-kDa bands, respectively.
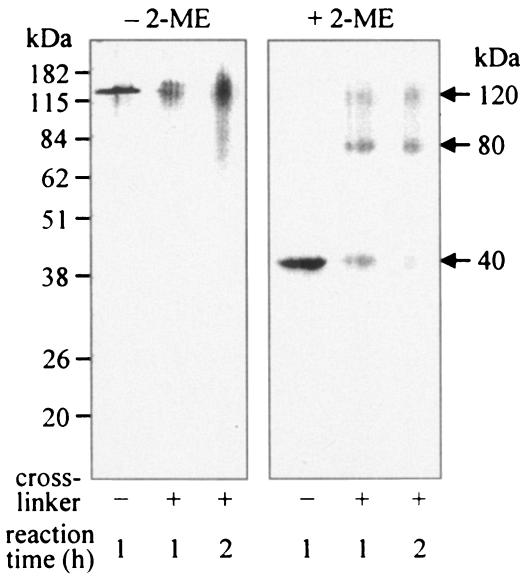
Cross-linking experiment.
Purified Pgm6/7 proteins of the wild-type strain were treated with the cross-linker disuccinimidyl suberate and electrophoresed under nonreducing conditions (Fig. 4, left panel). A diffuse band migrated at the position of 120 kDa, suggesting that the cross-linker indeed bound to the 120-kDa protein. When electrophoresed under reducing conditions (Fig. 4, right panel), most Pgm6/7 migrated at the positions assigned to trimers (120 kDa), dimers (80 kDa), and monomers (40 kDa). In Western blot assays, the anti-Pgm6/7 serum reacted with all three bands, further supporting the trimeric structure of Pgm6/7 (data not shown).
FIG. 4.
Cross-linking of P. gingivalis wild-type Pgm6/7 proteins. The samples were treated with disuccinimidyl suberate as a cross-linker for 1 or 2 h. The cross-linked samples, denatured in SDS with or without 2-ME, were loaded onto SDS-polyacrylamide gels, and gels were stained with CBB.
Growth in rich and synthetic media.
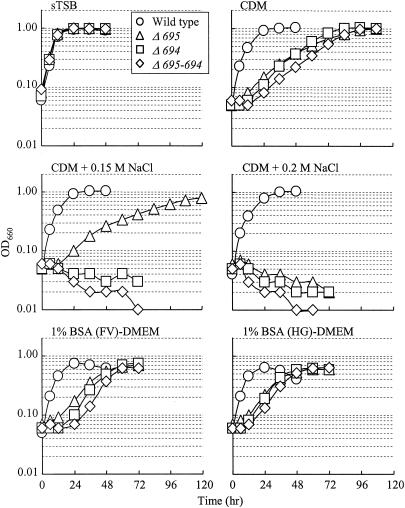
An early-stationary-phase culture in sTSB was inoculated at a 1:20 ratio into rich and synthetic media. Growth experiments were repeated at least twice, and typical results are shown in Fig. 5. In sTSB, a rich medium, the three mutants showed growth rates similar to that of the wild type and reached the stationary phase within 24 h. In CDM, the wild type reached the stationary phase by 36 h, while all mutants grew more slowly and reached the same level as the wild type at about 96 h. In CDM supplemented with NaCl, the growth rate of the wild type did not change dramatically, whereas the three mutants grew more slowly (0.15 M) or did not grow (0.2 M) (Fig. 5). Similar growth inhibition by NaCl (or KCl) was obtained with sTSB (data not shown). We found that P. gingivalis could also grow in DMEM, a cell culture medium, as long as 1% BSA, a lower concentration than that in CDM, was added. As shown in the bottom panels of Fig. 5, no significant differences were observed between growth in pure and crude BSA preparations, suggesting that growth of P. gingivalis and growth retardation of the mutants were not influenced by contaminants in BSA. The wild type and mutants substantially digested BSA during growth (data not shown).
FIG. 5.
Growth curves in various media. P. gingivalis ATCC 33277 (wild type), Δ695, Δ694, and Δ695-694 were cultured in sTSB, CDM, CDM supplemented with 0.15 M NaCl or 0.2 M NaCl, and DMEM supplemented with 1% BSA (FV, fraction V) or 1% BSA (HG, high grade purified).
Outer membrane permeability.
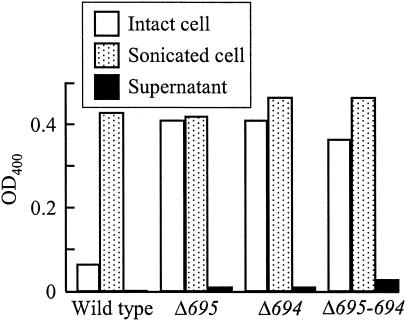
The permeability of the outer membrane in the wild type, as measured by alkaline phosphatase activity with whole intact cells and cell lysates, was much lower than those of the mutants (Fig. 6). Intact cells of the mutants showed high alkaline phosphatase activities almost equal to the total enzyme activities in sonicated cells. Negligible levels of leaked-out enzyme activity were found in both single-deletion strains, and a low level of activity was found in the double mutant. A different substrate, thymolphthalein monophosphate (Mr, 509), showed similar results (data not shown). Outer membranes lacking either or both Pgm6/7 seemed to be freely permeated by low-molecular-weight substances such as p-nitrophenylphosphate (Mr, 217) but not alkaline phosphatase (a high-molecular-weight periplasmic enzyme). These experiments were independently done several times, and typical results are shown in Fig. 6.
FIG. 6.
Permeability of outer membranes in P. gingivalis wild type and the mutants. Permeability of the outer membranes was estimated by hydrolysis of p-nitrophenylphosphate with alkaline phosphatase. The released p-nitrophenol was determined by measuring the OD400. Values are means of duplicate determinations. Similar results were obtained in three independent assays. Open bar, intact cell suspension; dotted bar, sonicated broken cells; closed bar, supernatant obtained from intact cell suspension.
Pore-forming activity.
Channel-forming activities of various protein preparations were determined following reconstitution into liposomes. The liposome swelling rates of envelopes from wild-type P. gingivalis were measurable but 10- to 100-fold lower than that from E. coli and even lower than that from Pseudomonas aeruginosa when the same amount of envelopes was incorporated (data not shown) (50). The swelling rates obtained using envelopes of wild-type P. gingivalis and the three mutants showed no significant differences. In addition, the swelling rate produced by the incorporation of a given amount of purified Pgm6/7 (120 kDa) protein was much lower than what was produced by the incorporation of envelopes containing an equivalent amount of the protein. Moreover, an interesting observation on a transposon-insertion mutant of P. gingivalis having an unrelated mutation in the pg0695/0694 locus was made during liposome experiments. Envelopes from this uncharacterized mutant, containing the same amount of Pgm6/7 as the wild type, had a much lower swelling rate than those of strains including the wild type, or the single and double mutants, indicating that another protein(s) lost in the mutant may form major channels (data not shown). This is under further investigation.
Antimicrobial susceptibility.
There was no significant difference of MICs between the wild type and mutants. Strong CAT activities were detected only in the mutants where cat had been introduced (data not shown). MICs of CHL for P. gingivalis wild-type strains ATCC 33277 and W83 were 3 μg/ml, while those for the three mutants ranged from 33 to 39 μg/ml, presumably due to the strong CAT expression. However, MICs of other antibiotics did not differ significantly between the wild-type strains and the three mutants (Table 3). Most MIC tests were repeated three times.
TABLE 3.
MICs of various antibiotics for P. gingivalis wild-type strains and mutants
| Strain | MIC (μg/ml)a
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CHL | PEN | AMP | CAR | LEX | FOX | CRO | CTX | TET | MIN | GEN | |
| ATCC 33277 | 3.0 | 0.038 | 0.125 | 0.50 | 2.0 | 1.0 | 0.250 | 0.125 | 0.125 | 0.031 | >512 |
| W83 | 3.0 | 0.038 | 0.063 | 0.125 | 1.0 | 0.50 | 0.031 | 0.031 | 0.125 | 0.031 | 256 |
| Δ695 | 39.0 | 0.075 | 0.063 | 0.250 | 2.0 | 0.50 | 0.125 | 0.063 | 0.250 | 0.031 | >512 |
| Δ694 | 39.0 | 0.038 | 0.125 | 0.125 | 2.0 | 0.50 | 0.125 | 0.063 | 0.250 | 0.063 | >512 |
| Δ695-694 | 33.0 | 0.038 | 0.125 | 0.250 | 1.0 | 0.50 | 0.125 | 0.063 | 0.125 | 0.063 | >512 |
The MICs of cefuroxime, erythromycin, and norfloxacin were 0.5, 0.5, and 4 μg/ml, respectively, for all the strains tested. Abbreviations: CHL, chloramphenicol; PEN, penicillin G; AMP, ampicillin; CAR, carbenicillin; LEX, cephalexin; FOX, cefoxitin; CRO, ceftriaxone; CTX, cefotaxime; TET, tetracycline; MIN, minocycline; GEN, gentamicin.
DISCUSSION
This study was conducted to further define the structures and functions of Pgm6/7, which are major outer membrane proteins of P. gingivalis. Pgm6/7 were predicted to be OmpA homologs. We constructed three disruption mutants, Δ695, Δ694, and Δ695-694, in which ORFs encoding Pgm6, Pgm7, and both, respectively, were deleted and replaced with cat (Fig. 1). The amount of Pgm6 protein produced by Δ694 seemed to be greater than the amount of Pgm7 protein produced by Δ695, based on the intensity of the immunoreactive 40-kDa band (Fig. 2B). However, an exact comparison of Pgm6 and Pgm7 production in the single mutants is difficult because the anti-Pgm6/7 antibody may have a different affinity for each protein. It is possible that the insertion of cat between the promoter and pg0694 in Δ695 reduced the expression of the downstream pg0694. However, the defects seen in the mutants were probably not due to a polar effect on a downstream gene(s) because a mutant having ermF-ermAM inserted into a gene downstream of pg0694 (abfD) (7) did not have any apparent effect on the Pgm6/7 production or on growth (data not shown).
The proposed heterotrimers (120-kDa protein) and homotrimers (115- and 130-kDa proteins) were analyzed by MS and N-terminal amino acid sequencing of peptides derived from digested proteins. All results indicated that the 120-kDa protein was composed of Pgm6 (41 kDa) and Pgm7 (40 kDa), and the 115- and 130-kDa proteins were of Pgm7 and Pgm6, respectively. No other protein was detected in these analyses. The heterotrimer band (120 kDa) was estimated to contain equal amounts of Pgm6 and Pgm7, based on detection of amino acid residues at nearly the same concentration from two peptides, during the N-terminal amino acid sequencing in a previous report (29). This result suggested that there was no observable preference for the formation of either 2:1 or 1:2 heterotrimers of Pgm6-Pgm7 since such a preference would skew the relative abundance of the two proteins in the sample. This has to be examined further, because a mechanism producing such a random assortment of heterotrimers is interesting, as discussed below.
It has been claimed that Omp40/41 in strain W50, which correspond to Pgm6/7, form heterodimers, based on observation of 34- to 35-kDa (monomer) and 70-kDa (dimer) bands (40, 45). Although we detected a minor 90-kDa protein in Western blot assays of one-dimensional (see the right panel in Fig. 2B) and 2-D gels (Fig. 3B), we observed no 35- or 70-kDa bands. However, it may be pertinent to emphasize that we found unusual resistance to dissociation of outer membrane proteins in the sample buffer for SDS-PAGE and encountered difficulties in control of their degradation by an intrinsic strong proteolytic activity in P. gingivalis (29). These unique properties intrinsic to this organism may be clues to overcoming the differences between the two groups in the future.
In this work, Western blot assays of an SDS-solubilized and boiled 120-kDa protein complex that dissociated upon treatment with reducing agents revealed that the Pgm6/7 heterotrimer was stabilized by SS bonds. A prior report also implicated SS-bond formation in the creation of the Omp40/41 (70-kDa) heterodimer (45). Indeed, Pgm6/7 both have a conserved sequence, RPVSCPECPE, with two closely arranged cysteine residues in the C-terminal region. No other cysteine residue exists in mature Pgm6/7 proteins. However, they could form a stable intramolecular SS bond as in LamB in E. coli, where Cys22 and Cys38 form an SS bond that contributes to trimer stability (24). Dissociation of the LamB trimer was shown to be reversible by low pH in the absence of the reducing agent, but we found that Pgm6/7 did not dissociate into dimers or monomers under these conditions. Thus, it seems that the Cys residues of Pgm6/7 form two intermolecular SS bonds that covalently link monomers into heterotrimers, although intermediate heterodimers may partially exist. Given the high proximity of the two Cys residues within each monomer, it was unusual to find that an outer membrane protein formed a covalent trimer via disulfide linkages in the soluble periplasmic domain. Furthermore, due to the addition of DTT to the medium, the periplasm was predicted to have a reducing potential that would disrupt oxidized SS bonds. Therefore, a mechanism must exist in P. gingivalis to facilitate intermolecular SS-bond formation in reducing conditions. Such an oxidation mechanism in the periplasmic space has also been proposed for another anaerobe, Fibrobacter succinogenes (26). As described in Results and below, because physiological phenotypes of the single mutants losing either protein were almost the same as those of the null mutant, the heterotrimer is likely to be a more stable and functional form than the homotrimer (or homo-oligomers) in the outer membrane for an unknown reason. We tentatively think that the formation of a heterodimer, an intermediate, would occur via two intermolecular SS bonds as the first step and that then either the subunit of Pgm6 or that of Pgm7 would associate with the dimer with an equal chance to finally form a heterotrimer by three intermolecular SS bonds through partial reduction of the SS bond and reoxidation among three molecules as the second step. If heterodimers formed as intermediates are assumed to be a transiently stable state in the whole heterotrimer population, some heterodimers would be preferentially detected under certain experimental conditions as reported previously (45). More work is needed to explore the possible mechanism of the heterotrimer formation, what components are involved in this mechanism, and where it occurs.
Growth and permeability experiments with intact cells suggested that the three mutants had a somewhat defective, weak, and leaky outer membrane open to limited substances with low molecular weights due to a considerable reduction of or the complete loss of Pgm6/7 as an anchor to the peptidoglycan layer. A rich medium such as sTSB did not affect the growth of the mutants, but the synthetic CDM (with 3% BSA), which apparently supports good growth of this organism, dramatically affected growth (Fig. 5), suggesting that an intact outer membrane is essential for growth under certain nutritional conditions. BSA is essential for the growth in CDM, and the mutants as well as the wild type appeared to equally digest BSA (data not shown). When NaCl was added to medium at moderate concentrations (0.15 or 0.2 M), growth was severely decreased in the mutants. In P. aeruginosa, an OmpA homolog, OprF, functions as a major porin (12, 35, 50) and also plays an important role in maintaining the structural integrity of the outer membrane (10, 38, 47). Evidence of this structural role is that P. aeruginosa OprF-deficient mutants were unable to grow or grew very slowly in low-osmolality medium. In contrast, the P. gingivalis mutants were unable to grow in the presence of 0.2 M NaCl. These results may indicate that Pgm6/7 play an important role in the ability of P. gingivalis to survive in inflamed periodontal pockets, an oral environment with high osmotic pressure (11). Preliminary analysis by 2-D gel electrophoresis showed that more protein spots with strong intensity were observed in the rich medium, sTSB, than in the poor medium, CDM, suggesting that there was a global regulation mechanism depending on a shift from rich to poor nutritional circumstances. In CDM, the loss of Pgm6/7 as anchors between the outer membrane and the peptidoglycan layer may be critical in P. gingivalis, and they could not produce essential components working together with Pgm6/7 for growth against a highly osmotic condition.
The three Pgm6/7 mutants had a strong tendency to autoagglutinate and to settle to the bottom of culture tubes, suggesting an alteration of their cell surfaces. Permeability of the outer membrane in the mutants changed, and they became more permeable to p-nitrophenylphosphate (Mr, 217) and thymolphthalein monophosphate (Mr, 509), showing loss of crypticity, but did not allow alkaline phosphatase to leak out, although the double mutant leaked a small amount of enzyme into the supernatant (Fig. 6). However, MICs of various antibiotics that have to pass through the outer membrane did not change even in the double mutant. As an exception, MICs of CHL were 33 to 39 μg/ml for the mutants and 3 μg/ml for the wild-type and W83 strains (Table 3). The increases of CHL resistance were probably due to the higher CAT enzyme activity (data not shown), and not to decreases in CHL penetration of their outer membranes. These observations as to unchanged MICs and loss of crypticity seem paradoxical. However, this is not so unusual because increases or decreases in outer membrane permeability do not necessarily produce large changes in MICs if agents are not efficiently inactivated or pumped out (33). As shown in Table 3, various antibiotics showed very low MICs for P. gingivalis wild-type strains, and these strains have been considered to be intrinsically susceptible to most antibiotics, including β-lactams, partially due to the absence of β-lactamase (16, 37). The antibiotics tested may pass through the outer membrane efficiently enough to reach targets in this rather slow-growing organism, and limited increases in outer membrane permeability seen for alkaline phosphatase activity may not affect MICs, even based on the assumption that no change occurs in the efflux and inactivation systems.
Most of the proteins found in the outer membrane appear to be channel-forming proteins, and they are classified as porins, specific channels, and high-affinity receptors (34). P. gingivalis major outer membrane proteins have been designated Pgm1 through Pgm7 (29), and of these, Pgm1 (RagA), Pgm4 (RagB), and Pgm6/7 are inferred to be channel-forming proteins without specific evidence. RagA and RagB appear to be expressed together and have been assumed to function as a specific channel (3) because RagA is a homolog of the TonB-linked receptors. Since this organism does not seem to have “classical porins,” OmpF and OmpC homologs in the chromosome (13, 31), Pgm6/7 have been strongly inferred to be candidates for the major porin in this organism because of their abundance in the outer membrane and the OmpA homology (29). OmpA-type porins are called “monomeric or slow porins” without strong evidence for a stable oligomeric structure like OprF (29, 32). In swelling experiments with proteoliposomes containing bacterial envelopes, no obvious difference of permeability of the liposomes between the wild type and Pgm6/7 mutants was detected. However, preliminary observations showing that a protein(s) other than Pgm6/7 may play a major role in pore-forming activities were made using a transposon-insertion mutant of P. gingivalis unrelated to the mutants created here.
In conclusion, Pgm6/7 seem to form heterotrimers as a stable structure to function mainly as stabilizers of the outer membrane, presumably to anchor it to the peptidoglycan layer through the C-terminal domain, and are unlikely to function as a major porin.
Acknowledgments
We thank H. Nikaido (Department of Molecular and Cell Biology, University of California) for valuable suggestions and T. Hirose (Center for Instrumental Analysis, Hokkaido University) for analyzing amino acid sequences. We also thank W. C. Wimley (Department of Biochemistry, Tulane University Health Sciences Center) and M. Homma (Graduate School of Science, Nagoya University) for β-barrel prediction of proteins and for allowing us to use MS, respectively.
This study was supported by Grants-in-Aid for Scientific Research (16791149 to K.N., 15591957 to F.Y., and 15591958 to Y.M.) from the Japan Society for the Promotion of Science (JSPS) and the AGU High-Tech Research Center Project from The Ministry of Education, Culture, Sports, Science and Technology, Japan. F.Y. was also supported partly by the Waksman Foundation in Japan. E.K.R. was supported by a JSPS postdoctoral fellowship for U.S. researchers (short-term, ID no. PU02209).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigelow, H. R., D. S. Petrey, J. Liu, D. Przybylski, and B. Rost. 2004. Predicting transmembrane beta-barrels in proteomes. Nucleic Acids Res. 32:2566-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonass, W. A., P. D. Marsh, R. S. Percival, J. Aduse-Opoku, S. A. Hanley, D. A. Devine, and M. A. Curtis. 2000. Identification of ragAB as a temperature-regulated operon of Porphyromonas gingivalis W50 using differential display of randomly primed RNA. Infect. Immun. 68:4012-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Cascales, E., A. Bernadac, M. Gavioli, J. C. Lazzaroni, and R. Lloubes. 2002. Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity. J. Bacteriol. 184:754-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Mot, R., and J. Vanderleyden. 1994. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both gram-positive and gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol. Microbiol. 12:333-334. [DOI] [PubMed] [Google Scholar]
- 7.Diaz, P. I., P. S. Zilm, V. Wasinger, G. L. Corthals, and A. H. Rogers. 2004. Studies on NADH oxidase and alkyl hydroperoxide reductase produced by Porphyromonas gingivalis. Oral Microbiol. Immunol. 19:137-143. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher, H. M., H. A. Schenkein, R. M. Morgan, K. A. Bailey, C. R. Berry, and F. L. Macrina. 1995. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect. Immun. 63:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fountoulakis, M., and H. Langen. 1997. Identification of proteins by matrix-assisted laser desorption ionization-mass spectrometry following in-gel digestion in low-salt, nonvolatile buffer and simplified peptide recovery. Anal. Biochem. 250:153-156. [DOI] [PubMed] [Google Scholar]
- 10.Gotoh, N., H. Wakebe, E. Yoshihara, T. Nakae, and T. Nishino. 1989. Role of protein F in maintaining structural integrity of the Pseudomonas aeruginosa outer membrane. J. Bacteriol. 171:983-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths, G. S. 2003. Formation, collection and significance of gingival crevice fluid. Periodontol. 2000 31:32-42. [DOI] [PubMed] [Google Scholar]
- 12.Hancock, R. E., and F. S. Brinkman. 2002. Function of Pseudomonas porins in uptake and efflux. Annu. Rev. Microbiol. 56:17-38. [DOI] [PubMed] [Google Scholar]
- 13.Hongo, H., E. Osano, M. Ozeki, T. Onoe, K. Watanabe, O. Honda, H. Tani, H. Nakamura, and F. Yoshimura. 1999. Characterization of an outer membrane protein gene, pgmA, and its gene product from Porphyromonas gingivalis. Microbiol. Immunol. 43:937-946. [DOI] [PubMed] [Google Scholar]
- 14.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217:270-279. [DOI] [PubMed] [Google Scholar]
- 15.Hruby, D. E., and E. M. Wilson. 1992. Use of fluorescent chloramphenicol derivative as a substrate for chloramphenicol acetyltransferase assays. Methods Enzymol. 216:369-376. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda, T., and F. Yoshimura. 2002. A resistance-nodulation-cell division family xenobiotic efflux pump in an obligate anaerobe, Porphyromonas gingivalis. Antimicrob. Agents Chemother. 46:3257-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeanteur, D., J. H. Lakey, and F. Pattus. 1991. The bacterial porin superfamily: sequence alignment and structure prediction. Mol. Microbiol. 5:2153-2164. [DOI] [PubMed] [Google Scholar]
- 18.Kelley, L. A., R. M. MacCallum, and M. J. Sternberg. 2000. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J. Mol. Biol. 299:499-520. [DOI] [PubMed] [Google Scholar]
- 19.Kennell, W., and S. C. Holt. 1990. Comparative studies of the outer membranes of Bacteroides gingivalis, strains ATCC 33277, W50, W83, 381. Oral Microbiol. Immunol. 5:121-130. [DOI] [PubMed] [Google Scholar]
- 20.Koebnik, R., K. P. Locher, and P. Van Gelder. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37:239-253. [DOI] [PubMed] [Google Scholar]
- 21.Laine, M. L., and A. J. van Winkelhoff. 1998. Virulence of six capsular serotypes of Porphyromonas gingivalis in a mouse model. Oral Microbiol. Immunol. 13:322-325. [DOI] [PubMed] [Google Scholar]
- 22.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamont, R. J., and H. F. Jenkinson. 2000. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol. Immunol. 15:341-349. [DOI] [PubMed] [Google Scholar]
- 24.Luckey, M., R. Ling, A. Dose, and B. Malloy. 1991. Role of a disulfide bond in the thermal stability of the LamB protein trimer in Escherichia coli outer membrane. J. Biol. Chem. 266:1866-1871. [PubMed] [Google Scholar]
- 25.Lugtenberg, B., J. Meijers, R. Peters, P. van der Hoek, and L. van Alphen. 1975. Electrophoretic resolution of the “major outer membrane protein” of Escherichia coli K12 into four bands. FEBS Lett. 58:254-258. [DOI] [PubMed] [Google Scholar]
- 26.MacLellan, S. R., and C. W. Forsberg. 2001. Properties of the major non-specific endonuclease from the strict anaerobe Fibrobacter succinogenes and evidence for disulfide bond formation in vivo. Microbiology 147:315-323. [DOI] [PubMed] [Google Scholar]
- 27.Milner, P., J. E. Batten, and M. A. Curtis. 1996. Development of a simple chemically defined medium for Porphyromonas gingivalis: requirement for α-ketoglutarate. FEMS Microbiol. Lett. 140:125-130. [DOI] [PubMed] [Google Scholar]
- 28.Murakami, Y., M. Imai, Y. Mukai, S. Ichihara, H. Nakamura, and F. Yoshimura. 2004. Effects of various culture environments on expression of major outer membrane proteins from Porphyromonas gingivalis. FEMS Microbiol. Lett. 230:159-165. [DOI] [PubMed] [Google Scholar]
- 29.Murakami, Y., M. Imai, H. Nakamura, and F. Yoshimura. 2002. Separation of the outer membrane and identification of major outer membrane proteins from Porphyromonas gingivalis. Eur. J. Oral Sci. 110:157-162. [DOI] [PubMed] [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. 1997. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 5th ed., vol. 21. Approved standard M11-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 31.Nelson, K. E., R. D. Fleischmann, R. T. DeBoy, I. T. Paulsen, D. E. Fouts, J. A. Eisen, S. C. Daugherty, R. J. Dodson, A. S. Durkin, M. Gwinn, D. H. Haft, J. F. Kolonay, W. C. Nelson, T. Mason, L. Tallon, J. Gray, D. Granger, H. Tettelin, H. Dong, J. L. Galvin, M. J. Duncan, F. E. Dewhirst, and C. M. Fraser. 2003. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J. Bacteriol. 185:5591-5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikaido, H. 1989. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob. Agents Chemother. 33:1831-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikaido, H. 1994. Porins and specific diffusion channels in bacterial outer membranes. J. Biol. Chem. 269:3905-3908. [PubMed] [Google Scholar]
- 35.Nikaido, H., K. Nikaido, and S. Harayama. 1991. Identification and characterization of porins in Pseudomonas aeruginosa. J. Biol. Chem. 266:770-779. [PubMed] [Google Scholar]
- 36.Nishikawa, K., and F. Yoshimura. 2001. The response regulator FimR is essential for fimbrial production of the oral anaerobe Porphyromonas gingivalis. Anaerobe 7:255-262. [Google Scholar]
- 37.Pajukanta, R., S. Asikainen, B. Forsblom, M. Saarela, and H. Jousimies-Somer. 1993. β-Lactamase production and in vitro antimicrobial susceptibility of Porphyromonas gingivalis. FEMS Immunol. Med. Microbiol. 6:241-244. [DOI] [PubMed] [Google Scholar]
- 38.Rawling, E. G., F. S. Brinkman, and R. E. Hancock. 1998. Roles of the carboxy-terminal half of Pseudomonas aeruginosa major outer membrane protein OprF in cell shape, growth in low-osmolarity medium, and peptidoglycan association. J. Bacteriol. 180:3556-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross, B. C., L. Czajkowski, D. Hocking, M. Margetts, E. Webb, L. Rothel, M. Patterson, C. Agius, S. Camuglia, E. Reynolds, T. Littlejohn, B. Gaeta, A. Ng, E. S. Kuczek, J. S. Mattick, D. Gearing, and I. G. Barr. 2001. Identification of vaccine candidate antigens from a genomic analysis of Porphyromonas gingivalis. Vaccine 19:4135-4142. [DOI] [PubMed] [Google Scholar]
- 40.Ross, B. C., L. Czajkowski, K. L. Vandenberg, S. Camuglia, J. Woods, C. Agius, R. Paolini, E. Reynolds, and I. G. Barr. 2004. Characterization of two outer membrane protein antigens of Porphyromonas gingivalis that are protective in a murine lesion model. Oral Microbiol. Immunol. 19:6-15. [DOI] [PubMed] [Google Scholar]
- 41.Shi, Y., D. B. Ratnayake, K. Okamoto, N. Abe, K. Yamamoto, and K. Nakayama. 1999. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J. Biol. Chem. 274:17955-17960. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara, E., and H. Nikaido. 1992. Pore-forming activity of OmpA protein of Escherichia coli. J. Biol. Chem. 267:2507-2511. [PubMed] [Google Scholar]
- 43.Ueda, O., and F. Yoshimura. 2003. Transposon-induced norfloxacin-sensitive mutants of Bacteroides thetaiotaomicron. Microbiol. Immunol. 47:17-25. [DOI] [PubMed] [Google Scholar]
- 44.van Winkelhoff, A. J., B. J. Appelmelk, N. Kippuw, and J. de Graaff. 1993. K-antigens in Porphyromonas gingivalis are associated with virulence. Oral Microbiol. Immunol. 8:259-265. [DOI] [PubMed] [Google Scholar]
- 45.Veith, P. D., G. H. Talbo, N. Slakeski, and E. C. Reynolds. 2001. Identification of a novel heterodimeric outer membrane protein of Porphyromonas gingivalis by two-dimensional gel electrophoresis and peptide mass fingerprinting. Eur. J. Biochem. 268:4748-4757. [DOI] [PubMed] [Google Scholar]
- 46.Wimley, W. C. 2002. Toward genomic identification of β-barrel membrane proteins: composition and architecture of known structures. Protein Sci. 11:301-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodruff, W. A., and R. E. Hancock. 1989. Pseudomonas aeruginosa outer membrane protein F: structural role and relationship to the Escherichia coli OmpA protein. J. Bacteriol. 171:3304-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamashita, Y., K. Toyoshima, M. Yamazaki, N. Hanada, and T. Takehara. 1990. Purification and characterization of alkaline phosphatase of Bacteroides gingivalis 381. Infect. Immun. 58:2882-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimura, F., and H. Nikaido. 1982. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J. Bacteriol. 152:636-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshimura, F., L. S. Zalman, and H. Nikaido. 1983. Purification and properties of Pseudomonas aeruginosa porin. J. Biol. Chem. 258:2308-2314. [PubMed] [Google Scholar]