Abstract
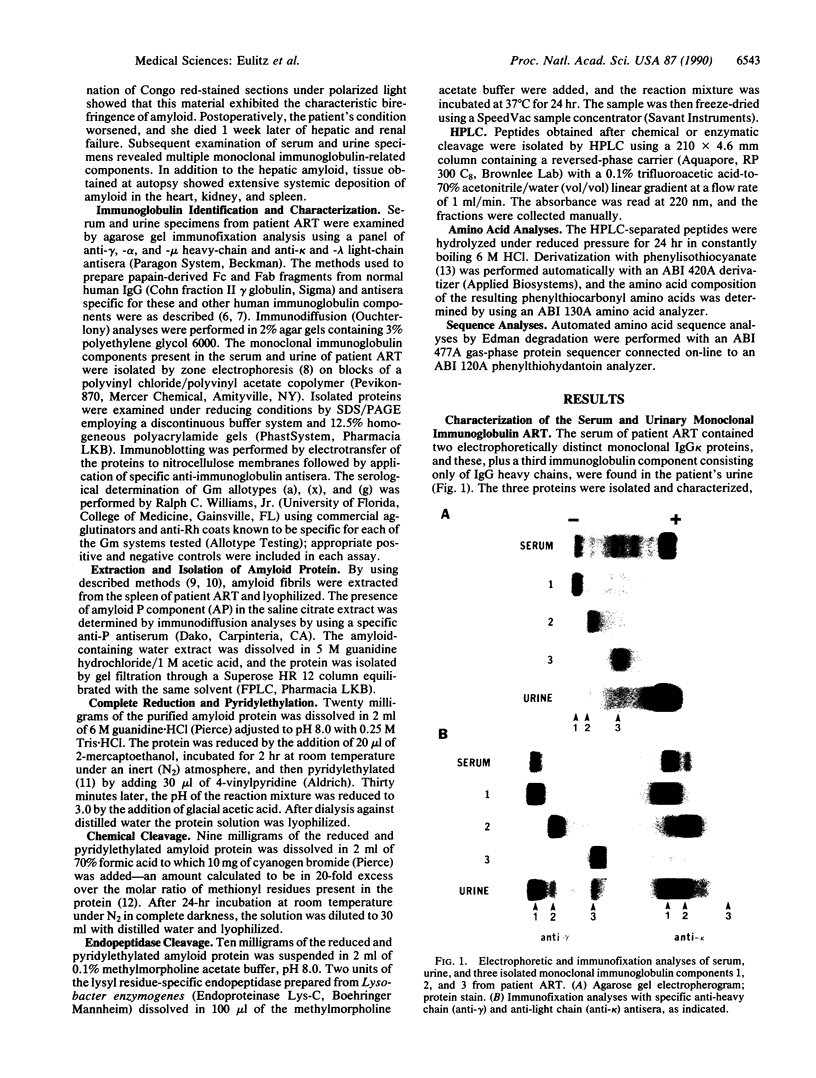
Immunoglobulin- or multiple myeloma-associated amyloidosis has been distinguished by the tissue deposition of Congophilic, fibrillar protein consisting of light chains or light-chain fragments (AL amyloidosis). We now report the isolation and characterization of another form of immunoglobulin-associated amyloid obtained from a patient who had extensive systemic amyloidosis and in whom the amyloid deposits consisted not of light chains but rather of an unusual form of heavy chain. This component, isolated from splenic amyloid extracts, represented an internally deleted IgG1 heavy chain as evidenced by immunochemical, electrophoretic, and amino acid sequence analyses. A comparable immunoglobulin-related monoclonal protein, consisting only of IgG heavy chains, was present in the patient's urine. Based on serologic reactivity with a battery of anti-immunoglobulin antisera, these two immunoglobulin-related components were antigenically identical; however, when compared to normal IgG, both were deficient in Fc-associated gamma-chain determinants. The structural abnormality of the amyloid gamma-chain protein was further evidenced by SDS/PAGE and immuno-blotting analyses: An unusually low molecular mass of approximately 22 kDa was found for this material vs. the expected value of approximately 55 kDa for a normal gamma heavy chain. Despite the lack of certain Fc determinants, the amyloid and urinary heavy-chain proteins expressed the IgG1 subclass allotype marker G1m(a) located on the third constant region (CH3) domain of the internally deleted IgG1 heavy chains. That the amyloid protein contained an intact CH3 domain was established through amino acid sequence analyses of cyanogen bromide fragments and peptides generated by a lysine-specific protease. These studies also revealed that the gamma-chain amyloid protein contained the complete heavy-chain variable (VH) domain [including the diversity (DH) and joining (JH) segments] that was contiguous with the CH3 domain. The low molecular mass of the protein resulted from the total absence of the first (CH1), hinge, and second (CH2) heavy-chain constant regions. Such extensive CH deletions and the presence of a complete VH distinguish this amyloid-associated heavy chain from all other heretofore characterized gamma-heavy-chain disease proteins. This heavy-chain-related form of immunoglobulin-associated amyloidosis is tentatively designated AH amyloidosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlersberg J. B., Grann V., Zucker-Franklin D., Frangione B., Franklin E. C. An unusual case of a plasma cell neoplasm with an IgG3lambda myeloma and a gamma3 heavy chain disease protein. Blood. 1978 Jan;51(1):85–96. [PubMed] [Google Scholar]
- Alexander A., Anicito I., Buxbaum J. Gamma heavy chain disease in man. Genomic sequence reveals two noncontiguous deletions in a single gene. J Clin Invest. 1988 Oct;82(4):1244–1252. doi: 10.1172/JCI113722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Alt F. W. Mechanism and developmental program of immunoglobulin gene rearrangement in mammals. Annu Rev Genet. 1989;23:605–636. doi: 10.1146/annurev.ge.23.120189.003133. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Tucker P. W. Human immunoglobulin heavy chain genes. J Biol Chem. 1989 Aug 5;264(22):12745–12748. [PubMed] [Google Scholar]
- Dickson J. R., Harth M., Bell D. A., Komar R., Chodirker W. B. Gamma heavy chain disease and rheumatoid arthritis. Semin Arthritis Rheum. 1989 May;18(4):247–251. doi: 10.1016/0049-0172(89)90045-0. [DOI] [PubMed] [Google Scholar]
- Eulitz M., Breuer M., Linke R. P. Is the formation of AL-type amyloid promoted by structural peculiarities of immunoglobulin L-chains? Primary structure of an amyloidogenic lambda-L-chain (BJP-ZIM). Biol Chem Hoppe Seyler. 1987 Jul;368(7):863–870. doi: 10.1515/bchm3.1987.368.2.863. [DOI] [PubMed] [Google Scholar]
- Eulitz M., Linke R. Amyloid fibrils derived from V-region together with C-region fragments from a lambda II-immunoglobulin light chain (HAR). Biol Chem Hoppe Seyler. 1985 Sep;366(9):907–915. doi: 10.1515/bchm3.1985.366.2.907. [DOI] [PubMed] [Google Scholar]
- Frangione B., Franklin E. C. Heavy chain diseases: clinical features and molecular significance of the disordered immunoglobulin structure. Semin Hematol. 1973 Jan;10(1):53–64. [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Prelli F. C., Frangione B. Immunoglobulin G (IgG). Methods Enzymol. 1985;116:3–25. doi: 10.1016/s0076-6879(85)16003-9. [DOI] [PubMed] [Google Scholar]
- Guglielmi P., Bakhshi A., Cogne M., Seligmann M., Korsmeyer S. J. Multiple genomic defects result in an alternative RNA splice creating a human gamma H chain disease protein. J Immunol. 1988 Sep 1;141(5):1762–1768. [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Ichihara Y., Abe M., Yasui H., Matsuoka H., Kurosawa Y. At least five DH genes of human immunoglobulin heavy chains are encoded in 9-kilobase DNA fragments. Eur J Immunol. 1988 Apr;18(4):649–652. doi: 10.1002/eji.1830180426. [DOI] [PubMed] [Google Scholar]
- Isobe T., Osserman E. F. Patterns of amyloidosis and their association with plasma-cell dyscrasia, monoclonal immunoglobulins and Bence-Jones proteins. N Engl J Med. 1974 Feb 28;290(9):473–477. doi: 10.1056/NEJM197402282900902. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J. A new supporting medium for preparative electrophoresis. Scand J Clin Lab Invest. 1960;12:33–37. [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzanski W., Katz A., Nyburg S. C., Freedman M. H. In vitro production of an amyloid-like substance from gamma 3 heavy chain disease protein. Immunol Commun. 1974;3(5):469–476. doi: 10.3109/08820137409061126. [DOI] [PubMed] [Google Scholar]
- Skinner M., Shirahama T., Cohen A. S., Deal C. L. The association of amyloid P-component (AP) with the amyloid fibril: an updated method for amyloid fibril protein isolation. Prep Biochem. 1982;12(5):461–476. doi: 10.1080/10826068208070597. [DOI] [PubMed] [Google Scholar]
- Solomon A. Light chains of human immunoglobulins. Methods Enzymol. 1985;116:101–121. doi: 10.1016/s0076-6879(85)16008-8. [DOI] [PubMed] [Google Scholar]
- Stone M. J. Amyloidosis: a final common pathway for protein deposition in tissues. Blood. 1990 Feb 1;75(3):531–545. [PubMed] [Google Scholar]