Abstract
Several lines of evidence indicate that microRNAs (miRNAs) modulate tolerance to the analgesic effects of morphine via regulation of pain-related genes, making dysregulation of miRNA levels a clinical target for controlling opioid tolerance. However, the precise mechanisms by which miRNAs regulate opioid tolerance are unclear. In the present study, we noted that the miR-375 level was downregulated but the expression of Janus kinase 2 (JAK2) was upregulated in mouse dorsal root ganglia (DRG) following chronic morphine treatment. The miR-375 levels and JAK2 expression were correlated with the progression of morphine tolerance, and upregulation of miR-375 level could significantly hinder morphine tolerance. This was ameliorated by JAK2 knockdown. Prolonged morphine exposure induced the expression of brain-derived neurotrophic factor (BDNF) in a time-dependent manner in the DRG. This was regulated by the miR-375 and JAK2–signal transducer and activator of transcription 3 (STAT3) pathway, and inhibition of this pathway decreased BDNF production, and thus, attenuated morphine tolerance. More importantly, we found that miR-375 could target JAK2 and increase BDNF expression in a JAK2/STAT3 pathway-dependent manner.
Keywords: morphine tolerance, miR-375, JAK2, BDNF
Introduction
Morphine is commonly prescribed for clinical treatment of neuropathic pain. However, tolerance to its narcotic analgesic effects seriously limits its clinical utility for chronic pain treatment. Although neuropathic pain can be relieved by increasing doses of morphine, the side effects also increase. Changes in dorsal root ganglia (DRG) neuron responses lead to morphine tolerance,1 and it is clinically challenging because of its complex mechanism. Therefore, clarifying the molecular mechanisms is an important goal.
Altered pain-related gene expression or signaling pathways, likely involving epigenetic modification,2 are probable mechanisms involved in the long-term effects of morphine therapy.3 Thus, investigation of epigenetic modifications may lead to better understanding of morphine tolerance. Non-coding RNAs mediate epigenetic modification and chronic neuropathic pain,4,5 and microRNAs (miRNAs) are one class of non-coding RNA that may inhibit messenger RNA (mRNA) translation or promote mRNA degradation via binding to mRNA 3′-untranslated regions (3′-UTRs).6 Previous studies have shown downregulation of miRNA (miR-96, miR-182 and miR-183) levels in rat DRG with pain,7 whereas considerable increases of miR-21 level occur in DRG after various types of peripheral nerve injury.8 These results indicate that miRNAs play vital roles in modulating pain. Recent studies examined the functions of let-7 following high opioid doses to regulate analgesic tolerance via targeting the µ-opioid receptor.9,10 miR-16 could mediate posttranscriptional regulation of µ-opioid receptor in CEM ×174 cells.11 Several other miRNA levels were detected after opioid administration, such as miR-103,12 miR-133b13 and miR-339.14 However, although most of these miRNAs affect tolerance via targeting the opioid receptors, it is unclear whether miR-375 is similarly involved in modulating narcotic actions.
Neuropathic pain involves various signaling pathways, such as the Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) pathways, which can be activated following peripheral nerve injury.15 Additionally, STAT3 expression is widely activated after nerve injury and leads to neuroinflammatory responses.16 Rapid and lasting activation of the JAK2/STAT3 pathway can induce neuropathic pain.17 Moreover, miR-375 can target JAK2 and inhibit the JAK2/STAT3 pathway in Helicobacter pylori-induced gastric tumors to decrease cell migration and proliferation.18 Serum miR-375 is a biomarker of acute pancreatic injury in rats,19 and enhances bone marrow-derived progenitor cell-mediated myocardial repair and function after myocardial infarction by binding with interleukin-10.20 However, whether miR-375 could target JAK2 and regulate morphine tolerance via blocking the JAK2/STAT3 pathway remains unclear.
Brain-derived neurotrophic factor (BDNF) is synthesized in DRG neurons and is a member of the neurotrophin family. It is transported anterograde to the central terminals of the spinal dorsal horn, where transduction of pain inputs is modulated.21 Expressions of BDNF mRNA and protein are dramatically changed in the DRG after peripheral inflammation and nerve injury.22,23 However, the relationship of miR-375 and DRG-derived BDNF in the development of morphine tolerance is poorly understood. In the present study, a mouse model of narcotic tolerance was induced by repeated subcutaneous (s.c.) injections of morphine. In addition, miR-375 levels were negatively correlated with morphine tolerance, whereas JAK2 expression in DRG was positively correlated with tolerance. Gain and loss of tolerance experiments were further performed, showing that ectopic expression of miR-375 or decreasing JAK2 expression could significantly ameliorate the progression of morphine tolerance via injection with miR-375 agomir or small-interfering RNA (siRNA) against JAK2. More importantly, the BDNF production, which is characterized by the mature BDNF expression, was modulated by altered expression of miR-375 or JAK2 in both naïve and morphine-tolerant mice. Our results demonstrate that miR-375 could delay morphine tolerance via blocking the JAK2/STAT3 pathway. Therefore, this study was designed to examine the effects of miR-375 on morphine tolerance in mouse DRG by targeting the JAK2/STAT3 pathway.
Materials and methods
Animals
All animal experiments were performed with the approval of the Ethics Committee for Animal Experimentation of the People’s Hospital of Henan Province. Eight to 10 weeks of male CD-1 mice were purchased from the medical center of Yangzhou University. These mice were housed in a pathogen-free facility and were acclimated to the new environment for a week before surgery. All experiments were conducted in accordance with the National Institutes of Health guidelines, and great efforts were made to minimize the amount of animals used and their suffering.
Morphine analgesic tolerance models
Morphine was purchased from Hospira, which was freshly prepared in saline. The procedure was referred to the previous study.24 Briefly, for testing acute tolerance, single morphine (100 mg/kg, s.c.) was injected into mice and after 24 h, again a single morphine (20 mg/kg, s.c.) was injected to elicit acute morphine tolerance for 2 h. For testing chronic tolerance, mice were treated with morphine (20 mg/kg, s.c.) twice a day at 12 h intervals.
Behavioral tests
The hot-plate test was performed to evaluate the thermal antinociceptive behavior, and thus, to examine the morphine tolerance. The detailed procedure was referred to the aforementioned study.4 The percentage of maximum potential efficiency (MPE%) was calculated by this following formulation: MPE% = (drug response latency − basal latency)/(30 s − basal latency) × 100%. In addition, Hargreaves’ test was also carried out to assess paw withdrawal latency (PWL) according to the classical protocol.25
miRNA agomir, antagomir and siRNA
miR-375 agomir, scrambled negative control (NC), miR-375 antagomir and JAK2 siRNA were purchased from GenePharma (Shanghai, People’s Republic of China).
Drug administration
miR-375 agomir and NC (20 μM, 5 μL), JAK2 siRNA (5 μM, 5 μL), scrambled siRNA (5 μM, 5 μL) or phosphate-buffered saline (PBS, 5 μL) were delivered via intrathecal (i.t.) injection. miR-375 antagomir (20 μM, 5 μL) or NC (20 μM, 5 μL) was delivered via intraganglionic (i.g.) injection into the right L4–L5 DRGs. For i.t. injection, intraspinal puncture was performed with a 30-gauge syringe needle between the L5 and L6 interspace to deliver the drug. The correct subarachnoid positioning of the needle tip was verified by a tail- or paw-flick response. For i.g. injection, the paraspinal muscles and tissues were removed in order to identify the intervertebral foramen. The right L4 and L5 DRGs were exposed, and 5 μL of miR-375 antagomir or NC was injected into each DRG following wound closure. Motor function was also evaluated at 2 min before behavioral test. Mice with movement disorders were ruled out.
qRT-PCR
Total RNA was extracted from DRG or spinal cord using Trizol reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. Then total RNA was reverse transcribed into complementary DNA using GoScript™ Reverse Transcription System (Promega Corporation, Fitchburg, WI, USA) and miRNA Reverse Transcription System (Haigene, Harbin, People’s Republic of China) according to the standard procedures. The mRNA expression and miRNAs levels were examined following the protocols of SYBR Premix Ex Taq™kit (Takara Bio, Inc., Otsu, Shiga, Japan) on an ABI Prism 7500 Detection System (Applied Biosystems, Inc., Waltham, MA, USA). Each reaction was performed in triplicate. mRNA expression was normalized to GAPDH expression, and U6 was used for internal reference of miR-375 level. The relative gene expression levels were analyzed via a method.
Western blot
The detailed procedure was referred to the previous study.26 The anti-JAK2 (sc-34479) and anti-STAT3 (sc-293151) antibodies were purchased from Santa Cruz. p-STAT3 (Cat #9145) was purchased from Cell Signaling Technology, and anti-BDNF (ab205067) and anti-β-actin (ab8227) were purchased from Abcam. Blots were washed and incubated with a peroxidase-conjugated antibody, and chemiluminescence was detected using an enhanced chemiluminescence kit (Tanon, Shanghai, People’s Republic of China) following by visualization using the Detection System (Tanon). Protein expression levels were quantified by density analysis using Quantity One Software and normalized to β-actin.
Statistical analysis
All data were presented as mean ± standard error of the mean, and all statistical analysis were carried out by GraphPad Prism 5. The miRNA level and protein expression were examined by one-way analysis of variance (ANOVA). Changes in behavioral response were evaluated using two-way ANOVA. Pearson’s correlation was used for the linear correlation analysis. *P<0.05 or **P<0.01 was considered to be significant.
Results
miR-375 level was downregulated and negatively correlated with JAK2 expression in DRG after chronic morphine treatment
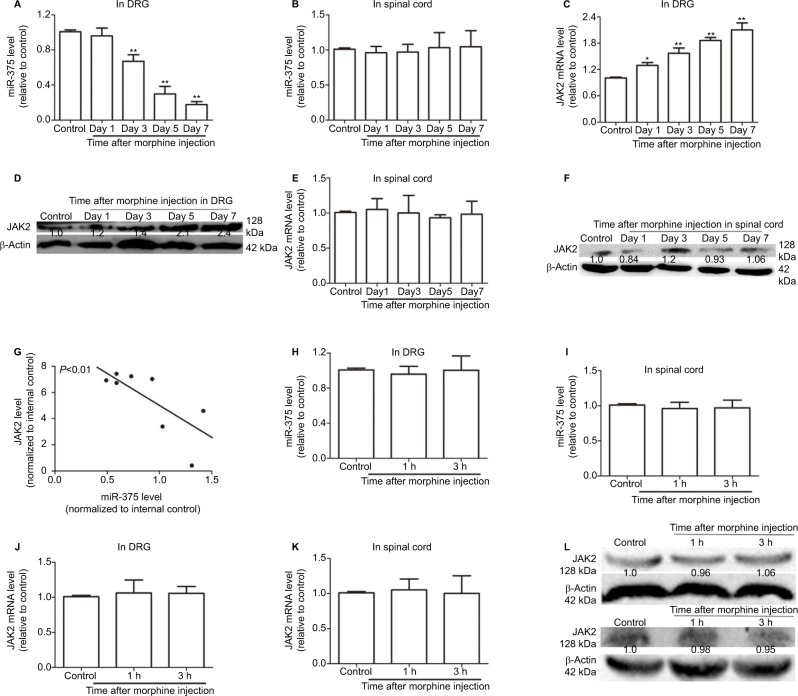
To investigate whether miR-375 was involved in the regulation of morphine tolerance, quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the miR-375 level in DRG after repeated morphine treatment (20 mg/kg, s.c.). Although miR-375 was downregulated in a time-dependent manner in DRG (Figure 1A), the miR-375 level in the dorsal horn of the spinal cord was unaffected (Figure 1B). Based on the previous report that miR-375 could target JAK2 in H. pylori-induced gastric carcinogenesis,18 we assumed that the interaction between miR-375 and JAK2 existed in DRG after repeated morphine treatment. As shown in Figure 1C and D, JAK2 expression levels were also upregulated in a time-dependent manner in DRG after repeated injection of morphine but not changed in the dorsal horn of the spinal cord (Figure 1E and F). Meanwhile, JAK2 expression was negatively correlated with the miR-375 level (Figure 1G). In addition, miR-375 and JAK2 expression levels were detected after a single 100 mg/kg (s.c.) morphine injection following a single 20 mg/kg (s.c.) morphine injection at 24 h. The results of Figure 1H–L showed that neither miR-375 nor JAK2 expression levels were altered in the DRG or the dorsal spinal cord. Therefore, our results suggested that miR-375 modulated morphine tolerance and was negatively correlated with JAK2 expression in DRG.
Figure 1.
miR-375 level is downregulated and negatively correlated with JAK2 expression in DRG after chronic morphine treatment. (A and B) qRT-PCR is used to detect the levels of miR-375 in DRG (A) and spinal cord (B). (C and D) JAK2 mRNA and protein levels are examined by qRT-PCR and Western blot assays in DRG after chronic morphine treatment. (E and F) qRT-PCR and Western blot analysis are used to test the mRNA (E) and protein (F) levels of JAK2 in spinal cord after chronic morphine treatment. (G) qRT-PCR results show that miR-375 level is negatively correlated with JAK2 mRNA level in DRG following morphine treatment. (H and I) miR-375 level is detected in DRG (H) and spinal cord (I) after acute morphine treatment. (J–L) JAK2 mRNA and protein expression levels are examined in DRG (J and L upper) and spinal cord (K and L lower) following acute morphine treatment. Data were presented as mean ± SD; **P<0.01 vs. control.
Abbreviations: DRG, dorsal root ganglia; mRNA, messenger RNA; JAK2, Janus kinase 2; qRT-PCR, quantitative real-time polymerase chain reaction; SD, standard deviation.
Ectopic expression of miR-375 or JAK2 is involved in chronic morphine tolerance
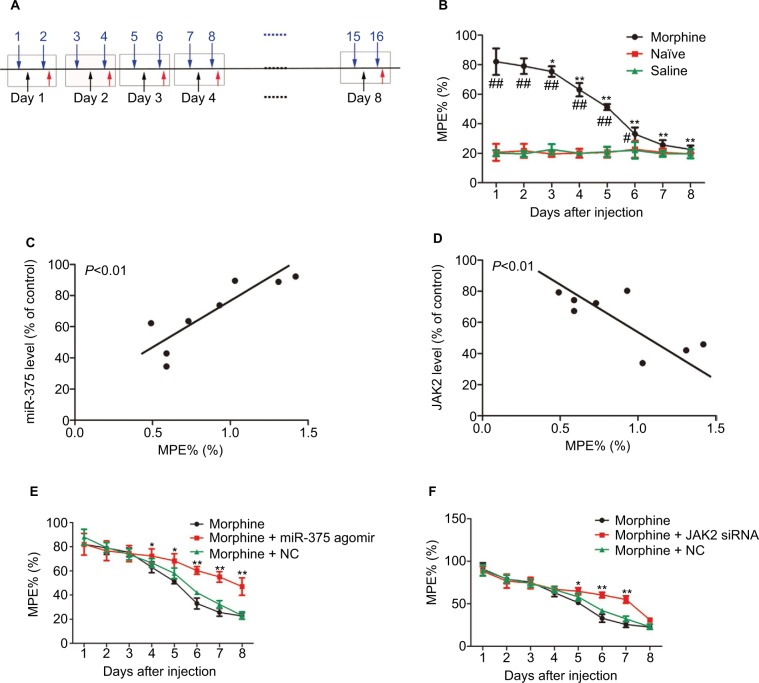
From day 1, robust analgesia was produced by the repeated morphine injection. Morphine exerted an antinociceptive effect until day 8, which is evaluated by MPE% compared with the control groups (Figure 2A). However, from days 4 to 8, the increased MPE% of morphine declined gradually, indicating that tolerance to morphine was developing (Figure 2B). The MPE% did not change in the control groups. Moreover, the miR-375 level was positively correlated with the MPE% of morphine (Figure 2C). On the contrary, JAK2 expression was negatively correlated with the MPE% (Figure 2D). In addition, we investigated the antinociceptive effects of upregulation of miR-375 or downregulation of JAK2 on morphine-tolerant behaviors. miR-375 agomir or JAK2 siRNA was delivered i.t. once daily along with the second, fourth and sixth morphine injection. The behavioral results demonstrated that repetitive i.t. injection of miR-375 agomir remarkably impaired MPE%, leading to progressive reduction from days 4 to 8 after morphine treatment compared with the NC-treated groups, which did not alter the declined MPE% of morphine-injected mice (Figure 2E). Additionally, after sustained morphine injection, the morphine tolerance was also delayed by the i.t. injection of JAK2 siRNA characterized by reduction from days 5 to 7 (Figure 2F). These results indicate that upregulation of miR-375 level or downregulation of JAK2 expression could ameliorate the morphine tolerance.
Figure 2.
Ectopic expression of miR-375 and JAK2 is related to the chronic morphine tolerance. (A) Schematic illustration of morphine injection, drug application and behavioral test for (B), (E) and (F). Blue arrows indicate morphine injection; black arrows indicate drug application and red arrowheads indicate behavioral test. (B) Repetitive morphine injection (20 mg/kg, s.c., twice a day) elicits a progressive reduction in MPE% during 8 days. (C) The miR-375 level in the DRG is positively correlated with the MPE% of chronic morphine-injected mice. (D) The JAK2 expression in the DRG is negatively correlated with the MPE% of chronic morphine-injected mice. (E and F) Morphine-induced induction of analgesic tolerance is delayed by miR-375 agomir treatment (E) or JAK2 siRNA (F). Data were presented as mean ± SD; *P<0.05, **P<0.01 vs. morphine treatment alone, #P<0.05 vs. the saline group, ##P<0.01 vs. the saline group.
Abbreviations: DRG, dorsal root ganglia; JAK2, Janus kinase 2; MPE%, maximum potential efficiency; NC, negative control; s.c., subcutaneous; siRNA, small-interfering RNA; SD, standard deviation.
Upregulation of miR-375 level could inhibit BDNF expression by downregulating JAK2 in the DRG of morphine-tolerant mice
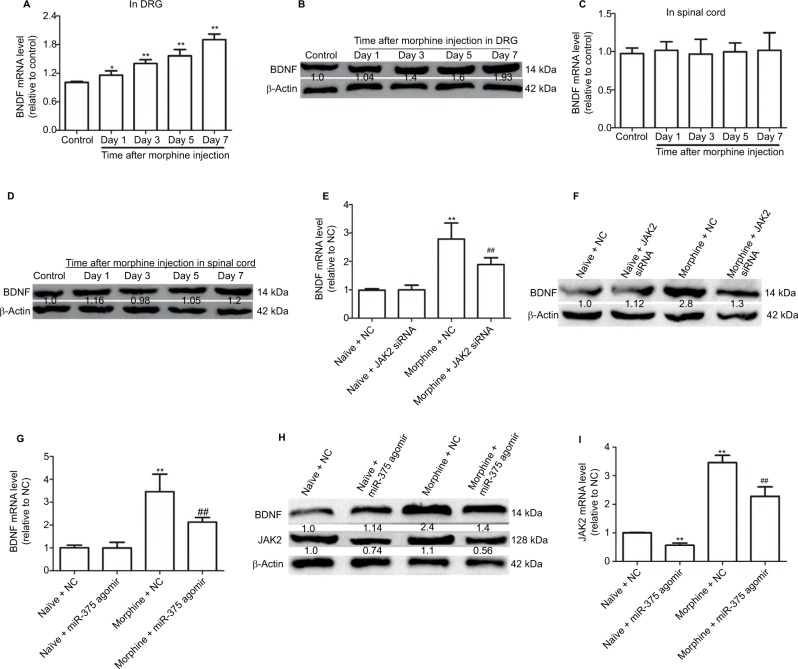
Here, we further investigated whether miR-375 could regulate BDNF expression in the DRG of morphine-tolerant mice. The expression levels of BDNF were significantly increased in a time-dependent manner in DRG but not in spinal cord (Figure 3A–E). Moreover, we found that the increased BDNF expression was also inhibited by JAK2 siRNA treatment (Figure 3F), and that injection of miR-375 agomir could further inhibit the expression of BDNF (Figure 3G and H). Also, JAK2 expression was inhibited whether or not morphine treatment was provided (Figure 3H and I), which confirmed the interaction between miR-375 and JAK2. Collectively, our results demonstrate that upregulation of miR-375 level or downregulation of JAK2 expression inhibits morphine-induced BDNF expression in the DRG of morphine-tolerant mice.
Figure 3.
Upregulation of miR-375 level inhibits BDNF expression by downregulating JAK2 in the DRG of morphine-tolerant mice. (A and B) qRT-PCR and Western blot assays are used to detect BDNF mRNA and protein expression levels in DRG after morphine treatment. (C and D) mRNA and protein expression levels of BDNF are examined in spinal cord via qRT-PCR and Western blot analysis after morphine treatment. (E and F) Administration of JAK2 siRNA suppresses the increased protein and mRNA expression of BDNF at day 6 following morphine injection. (G and H) Injection with miR-375 agomir suppresses the increased protein and mRNA expression of BDNF at day 6 following morphine injection. (H and I) Injection with miR-375 agomir suppresses the increased protein and mRNA expression of JAK2 at day 6 following morphine injection. Data were presented as mean ± SD; *P<0.05, **P<0.01 vs. control; ##P<0.01 vs. morphine + NC.
Abbreviations: BDNF, brain-derived neurotrophic factor; DRG, dorsal root ganglia; mRNA, messenger RNA; NC, negative control; JAK2, Janus kinase 2; qRT-PCR, quantitative real-time polymerase chain reaction; SD, standard deviation; siRNA, small-interfering RNA.
Downregulation of miR-375 level could induce BDNF expression and elicit pain-like behavior and spinal neuronal sensitization partly depending on JAK2 expression
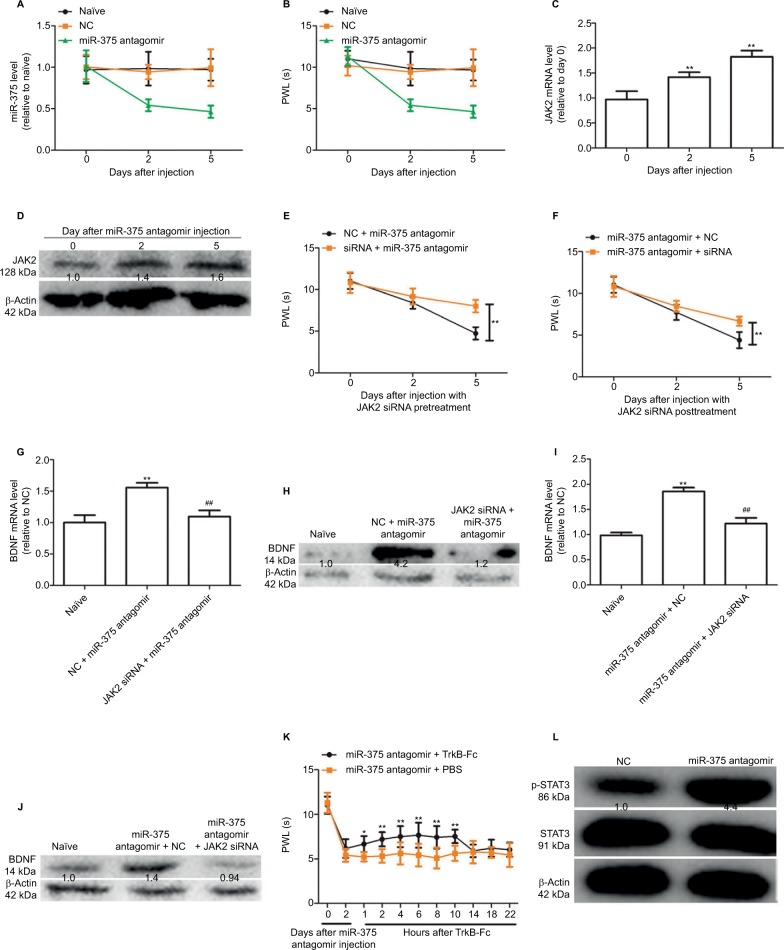
We further investigated whether the downregulation of miR-375 level could trigger hyperalgesia via i.g. injection of miR-375 antagomir into the naïve mice. This behavior was then tested. As shown in Figure 4A and B, injection with miR-375 antagomir significantly decreased miR-375 levels in DRGs and thus attenuated the PWL lasting for 5 days. Meanwhile, JAK2 expression was increased with miR-375 antagomir treatment at days 2 and 5 (Figure 4C and D). However, coadministration of JAK2 siRNA either before (Figure 4E) or after (Figure 4F) miR-375 antagomir injection could attenuate the inhibitory effects by miR-375 antagomir on PWL. In addition, BDNF expressions were significantly increased in DRGs after co-administration of JAK2 siRNA either before (Figure 4G and H) or after (Figure 4I and J) miR-375 antagomir injection, which was significantly inhibited by JAK2 siRNA treatment. More notably, the hyperalgesia induced by miR-375 antagomir was also reversed by tyrosine receptor kinase B-Fc treatment, which is a scavenger of BDNF (Figure 4K).12 We further detected the expression of STAT3 and phosphorylated STAT3 (p-STAT3) after injection with miR-375 antagomir. Western blot analysis showed that miR-375 antagomir treatment increased the expression of p-STAT3 but had no effect on STAT3 expression in the DRG after 3 or 5 days (Figure 4L). Therefore, our results indicate that miR-375 could ameliorate morphine tolerance partly depending on the JAK2/STAT3 pathway.
Figure 4.
Downregulation of miR-375 level induces BDNF expression and elicits pain-like behavior and spinal neuronal sensitization partly dependent on JAK2 expression. (A) qRT-PCR results show that miR-375 level is downregulated after injection with miR-375 antagomir at days 2 and 5. (B) Administration of miR-375 antagomir markedly decreases paw withdrawal latency (PWL) at days 2 and 5 after injection. (C and D) JAK2 mRNA and protein levels were examined by qRT-PCR and Western blot analysis after miR-375 antagomir injection. (E and F) Pre- (E) or posttreatment (F) of JAK2 siRNA 2 days before or after miR-375 antagomir administration alleviates miR-375-antagomir-induced decline of PWL. (G–J) Pre- (G and H) or posttreatment (I and J) of JAK2 siRNA 2 days before or after miR-375 antagomir administration alleviates miR-375-antagomir-induced increase of BDNF. (K) Delivery of TrkB-Fc, 2 days after miR-375-antagomir injection, alleviates miR-375-induced PWL reduction in a time-dependent manner. (L) p-STAT3 and STAT3 levels were detected by Western blot assays after miR-375 antagomir injection. Data were presented as mean ± SD; *P<0.05, **P<0.01 vs. control; ##P<0.01 vs. NC + miR-375 antagomir.
Abbreviations: BDNF, brain-derived neurotrophic factor; JAK2, Janus kinase 2; mRNA, messenger RNA; NC, negative control; PBS, phosphate buffered saline; qRT-PCR, quantitative real-time polymerase chain reaction; siRNA, small-interfering RNA; SD, standard deviation; STAT3, signal transducer and activator of transcription 3; TrkB-Fc, tyrosine receptor kinase B-Fc.
Discussion
Morphine has been utilized for relieving pain in humans for past many centuries. Nevertheless, long-time use of morphine may lead to adverse effects like analgesic tolerance.27 Endogenous morphine has been proved to be involved in regulating metabolic homeostasis and energy production.28 However, the concrete cellular mechanisms of morphine tolerance remain unclear. This study focused on this unknown mechanism.
miRNAs have been proved to be posttranscriptional repressors that inhibit target gene expression via translation inhibition or mRNA degradation. As crucial modulators, these could control neurophysiological processes, including neural development,29 synaptic plasticity30 and morphine-induced analgesic tolerance.3 Increasing studies indicate that dysregulation of miRNAs contributes to pain-related gene or pathway alterations, and thus, influences the development and maintenance of pain processes.14 This evidence suggests that miRNAs represent potential therapeutic or diagnostic targets for pain prevention and relief.
Here, we focused on the roles of miR-375 in the development of morphine tolerance in the DRG for the following reasons: 1) Morphine tolerance is a type of hyperalgesia that has similar but distinct mechanisms to inflammatory or neuropathic pain, especially in the peripheral nervous system.31,32 2) BDNF is mainly synthesized within DRG neurons and acts as a neuromodulator between DRG neurons during inflammatory and neuropathic pain.22 3) As nociceptive responses are modulated largely in the DRG, investigating the roles of miRNAs in the DRG could further elucidate the mechanisms of pain modulation. Our study found that miR-375 was downregulated in DRG after chronic morphine treatment, and ectopic expression of miR-375 could modulate morphine tolerance characterized by induced spinal neuronal sensitization and pain behavior, suggesting that downregulation of miR-375 might be a potential biomarker for morphine tolerance. Our results also revealed that miR-375 could inhibit JAK2 expression and decrease BDNF expression dependent on JAK2 expression, indicating that JAK2 served as a miR-375 target, which is consistent with the previous study.18 These parallel findings on the interaction of miR-375 and JAK2 could be viewed as the common mechanism between morphine tolerance and inflammation. Moreover, inhibiting JAK2 expression could exert similar effects as upregulating miR-375 level, which demonstrates that the JAK2/STAT3 pathway could be a critical regulator of morphine tolerance. Further study in this work proved that miR-375 could modulate the JAK2/STAT3 pathway in the spinal cord after morphine treatment, and that blocking the JAK2/STAT3 pathway could attenuate the inhibitory effects of miR-375 antagomir on BDNF expression, proving that miR-375 exerts its effects partly via the JAK2/STAT3 pathway. Interestingly, we did not find any expression change of miR-375 in the DRG following establishment of acute morphine tolerance, which suggests that different mechanisms might exist for acute and chronic morphine tolerance. Notably, although downregulation of miR-219 has been shown to enhance the BDNF expression in mouse DRG that mediates morphine tolerance,14 further studies are needed to investigate whether upregulation of miR-375 and miR-219 levels exerts additive or synergistic effects in mouse DRG.
BDNF is largely expressed in pain-related neural regions, including the DRG and spinal dorsal horn, and is correlated with the development of pathological pain.30 BDNF may modulate synaptic transmission in the spinal cord,23 and inhibition of BDNF expression may decrease hyperalgesia caused by nerve injury.33 Here, BDNF expression was found to be increased in the DRG after chronic morphine treatment.
Conclusion
In summary, our results are consistent with the hypothesis that DRG-derived BDNF is a functional downstream marker of miR-375/JAK2-mediated morphine tolerance.
Overall, our results document, for the first time, the role of miR-375/JAK2 signaling in the development of chronic morphine tolerance. This study indicates that miRNA-375 could negatively target JAK2 to suppress BDNF expression, thus blocking the development of tolerance, which could make it a potential target mechanism or strategy for long-term maintenance of the therapeutic response to stable doses of narcotic analgesics.
Acknowledgments
This work was supported by the Natural Science Foundation of Beijing (7123219). The authors also thank Professor Wang for critically reviewing this work.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Li Q, Zhao X, Zhong LJ, Yang HY, Wang Q, Pu XP. Effects of chronic morphine treatment on protein expression in rat dorsal root ganglia. Eur J Pharmacol. 2009;612(1–3):21–28. doi: 10.1016/j.ejphar.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 2.Hori N, Narita M, Yamashita A, et al. Changes in the expression of IL-6-Mediated MicroRNAs in the dorsal root ganglion under neuropathic pain in mice. Synapse. 2016;70(8):317–324. doi: 10.1002/syn.21902. [DOI] [PubMed] [Google Scholar]
- 3.Tapocik JD, Ceniccola K, Mayo CL, et al. MicroRNAs are involved in the development of morphine-induced analgesic tolerance and regulate functionally relevant changes in Serpini1. Front Mol Neurosci. 2016;9:20. doi: 10.3389/fnmol.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu XM, Cao SB, Zhang HL, et al. Downregulation of miR-219 enhances brain-derived neurotrophic factor production in mouse dorsal root ganglia to mediate morphine analgesic tolerance by upregulating CaMKIIgamma. Mol Pain. 2016;12(47):14670–14683. doi: 10.1177/1744806916666283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ligon CO, Moloney RD, Greenwood-Van Meerveld B. Targeting epigenetic mechanisms for chronic pain: a valid approach for the development of novel therapeutics. J Pharmacol Exp Ther. 2016;357(1):84–93. doi: 10.1124/jpet.115.231670. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Zheng L, Zhang F, et al. STARD13-correlated ceRNA network inhibits EMT and metastasis of breast cancer. Oncotarget. 2016;7(17):23197–23211. doi: 10.18632/oncotarget.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience. 2009;164(2):711–723. doi: 10.1016/j.neuroscience.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai A, Suzuki H. Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochem Biophys Res Commun. 2013;435(2):176–181. doi: 10.1016/j.bbrc.2013.04.089. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Wang ZJ. Let-7 microRNAs and Opioid Tolerance. Front Genet. 2012;3:110. doi: 10.3389/fgene.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci. 2010;30(30):10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou W, Li H, Jiang W, Zhang C, McNutt MA, Li G. Simian immunodeficiency virus impacts microRNA-16 mediated post-transcriptional regulation of mu opioid receptor in CEM ×174 Cells. J Cell Biochem. 2016;117:84–93. doi: 10.1002/jcb.25251. [DOI] [PubMed] [Google Scholar]
- 12.Williams JT, Ingram SL, Henderson G, et al. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65(1):223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Simon FM, Zhang XX, Loh HH, Law PY, Rodriguez RE. Morphine regulates dopaminergic neuron differentiation via miR-133b. Mol Pharmacol. 2010;78(5):935–942. doi: 10.1124/mol.110.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Q, Hwang CK, Zheng H, et al. MicroRNA 339 down-regulates mu-opioid receptor at the post-transcriptional level in response to opioid treatment. FASEB J. 2013;27(2):522–535. doi: 10.1096/fj.12-213439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem. 2008;107(1):50–60. doi: 10.1111/j.1471-4159.2008.05566.x. [DOI] [PubMed] [Google Scholar]
- 16.Peppin JF, Webster L. Letter to the editor in response to “The evidence for pharmacological treatment of neuropathic pain,” by Finnerup et al. Pain. 2011;152(6):1440. doi: 10.1016/j.pain.2011.03.031. Author reply 1440. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M. SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci. 2010;30(16):5754–5766. doi: 10.1523/JNEUROSCI.5007-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao L, Liu K, Xie M, Xing Y, Xi T. miR-375 inhibits Helicobacter pylori-induced gastric carcinogenesis by blocking JAK2-STAT3 signaling. Cancer Immunol Immunother. 2014;63(7):699–711. doi: 10.1007/s00262-014-1550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvano J, Edwards G, Hixson C, Burr H, Mangipudy R, Tirmenstein M. Serum microRNAs-217 and -375 as biomarkers of acute pancreatic injury in rats. Toxicology. 2016;368–369:1–9. doi: 10.1016/j.tox.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Garikipati VN, Krishnamurthy P, Verma SK, et al. Negative regulation of miR-375 by Interleukin-10 enhances bone marrow-derived progenitor cell-mediated myocardial repair and function after myocardial infarction. Stem Cells. 2015;33(12):3519–3529. doi: 10.1002/stem.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lou W, Zhang X, Hu XY, Hu AR. MicroRNA-219-5p inhibits morphine-induced apoptosis by targeting key cell cycle regulator WEE1. Med Sci Monit. 2016;22:1872–1879. doi: 10.12659/MSM.895439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merighi A, Salio C, Ghirri A, et al. BDNF as a pain modulator. Prog Neurobiol. 2008;85(3):297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55(1):1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Liu WT, Han Y, Liu YP, Song AA, Barnes B, Song XJ. Spinal matrix metalloproteinase-9 contributes to physical dependence on morphine in mice. J Neurosci. 2010;30(22):7613–7623. doi: 10.1523/JNEUROSCI.1358-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 26.Zheng L, Li X, Meng X, et al. Competing endogenous RNA networks of CYP4Z1 and pseudogene CYP4Z2P confer tamoxifen resistance in breast cancer. Mol Cell Endocrinol. 2016;427:133–142. doi: 10.1016/j.mce.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Sun X, Zhang Y, et al. Morphine promotes apoptosis via TLR2, and this is negatively regulated by beta-arrestin 2. Biochem Biophys Res Commun. 2009;378(4):857–861. doi: 10.1016/j.bbrc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Kream RM, Stefano GB. Interactive effects of endogenous morphine, nitric oxide, and ethanol on mitochondrial processes. Arch Med Sci. 2010;6(5):658–662. doi: 10.5114/aoms.2010.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olde Loohuis NF, Kos A, Martens GJ, Van Bokhoven H, Nadif Kasri N, Aschrafi A. MicroRNA networks direct neuronal development and plasticity. Cell Mol Life Sci. 2012;69(1):89–102. doi: 10.1007/s00018-011-0788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62(3):259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 32.Mayer DJ, Mao J, Price DD. The development of morphine tolerance and dependence is associated with translocation of protein kinase C. Pain. 1995;61(3):365–374. doi: 10.1016/0304-3959(95)00023-L. [DOI] [PubMed] [Google Scholar]
- 33.Yajima Y, Narita M, Usui A, et al. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem. 2005;93(3):584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]