Abstract
Tumor necrosis factor-α (TNF)-induced RIP1/RIP3-mediated necroptosis has been proposed to be an alternative strategy for treating apoptosis-resistant leukemia. However, we found that most acute myeloid leukemia (AML) cells, especially M4 and M5 subtypes, produce TNF and show basal level activation of RIP1/RIP3/MLKL signaling, yet do not undergo necroptosis. TNF, through RIP1/RIP3 signaling, prevents degradation of SOCS1, a key negative regulator of interferon-γ (IFN-γ) signaling. Using both pharmacologic and genetic assays, we show here that inactivation of RIP1/RIP3 resulted in reduction of SOCS1 protein levels and partial differentiation of AML cells. AML cells with inactivated RIP1/RIP3 signaling show increased sensitivity to IFN-γ-induced differentiation. RIP1/RIP3 inactivation combined with IFN-γ treatment significantly attenuated the clonogenic capacity of both primary AML cells and AML cell lines. This combination treatment also compromised the leukemogenic ability of murine AML cells in vivo. Our studies suggest that inhibition of RIP1/RIP3-mediated necroptotic signaling might be a novel strategy for the treatment of AML when combined with other differentiation inducers.
INTRODUCTION
Acute myeloid leukemia (AML) is an aggressive hematopoietic malignancy known to have increased incidence with advanced age. Currently, the 5-year overall survival is 40–45% for patients under 60 years of age and <10% for patients 60 years of age and older1–4. Thus, the development of novel treatments for AML with minimal toxicities is urgent. The remarkable success of all-trans retinoic acid (ATRA) in combination with arsenic trioxide in the treatment of acute promyelocytic leukemia (APL)5, 6 encourages us to search for novel differentiation-inducing therapies in the management of non-APL subtypes of AML.
In addition to stimulating NF-κB-mediated survival signaling7–9, tumor necrosis factor-α (TNF) also induces caspase-8-mediated apoptosis and RIP1/RIP3-MLKL-mediated necroptosis, two mutually interconvertible programmed cell death signaling pathways10–14. Inactivation of the apoptotic pathway in the development of leukemia and the progression to drug resistance has been extensively studied15–17. However the role of necroptosis in the pathogenesis of AML has not been significantly evaluated18–20. The normal phenotype of RIP3−/− mice suggested that necroptotic signaling is not necessary for development and tissue regeneration during homeostatic conditions21, suggesting that inhibition of RIP1/RIP3 signaling would be safe.
We found that leukemic cells isolated from many AML patients express TNF, RIP1 and RIP3. Activation of RIP1/RIP3 signaling can be readily detected in AML cells and is required for maintaining the undifferentiated state of such malignant cells. Genetic or pharmacologic inactivation of the RIP1/RIP3 signal induces spontaneous differentiation and represses leukemogenic capacity of AML cells. RIP1/RIP3 signaling-inactivated AML cells are highly sensitive to interferon-γ (IFN-γ)-induced differentiation. Our studies suggest that inhibiting necroptotic signaling may be a new strategy to treat AML when combined with IFN-γ or other differentiation inducers.
METHODS
Ex vivo and in vivo transplantation and leukemogenesis
For ex vivo study, WT MA9-AML cells were treated with IFN-γ and Nec1 individually or in combination, while TNFR−/−, RIP1−/−, and RIP3−/− MA9-AML cells were treated with IFN-γ only (1ng mL−1) for 5 days. The resultant cells were used for transplantation. AML cells with or without pretreatment were transplanted into lethally-irradiated recipient mice (2 month old male) by tail vein injection. Each mouse received 5000 viable AML cells together with 2×105 support BM cells. For in vivo treatment studies, WT, TNFR−/−, RIP1−/−, and RIP3−/− MA9-AML cells were first transplanted into recipient mice and allowed 15 days to engraft. Each recipient mouse received 5000 WT or TNFR−/− AML cells, 20,000 RIP3−/− AML cells, and 50,000 RIP1−/− AML cells. Recipient mice were randomly allocated into two groups and treated with IFN-γ (2.5μg/mouse via i.p. injection) or vehicle daily for two weeks. All mice were monitored for leukemia development by observing for symptoms such as hunched body, significant weight loss, or hind-limb paralysis. The death of mice from leukemia was confirmed by examining WBC and leukemic blasts in PB and infiltration of AML cells into spleens, livers and kidneys. The survival of recipient mice was monitored over time and analyzed by Kaplan-Meier survival graphing.
Competitive transplantation assay
To evaluate the reconstitutive capacity of BM cells following IFN-γ treatment, 2×106 BM MNCs isolated from CD45.1 mice were treated with Nec1 (30μM) for 48 hr. and then mixed with 2×106 freshly-isolated BM MNCs from CD45.2 mice. These cells were equally transplanted into lethally-irradiated CD45.2 mice. PB samples were collected 3 months after transplantation and analyzed for the ratio of CD45.1 to CD45.2 of total MNC counts.
Microarray data analysis
We selectively analyzed the expression of the key components of the TNF, IL1 and TLR signaling pathways in a cohort of 562 AML patients using a microarray dataset GSE3764222 (See Table S1, Supplementary information, for details of the genes). The samples that showed similar patterns of expression of these genes were clustered together.
Statistical analysis
Data are expressed as means ± SD. One-way ANOVA (multiple groups) and Student’s t-test (two groups) were performed to determine the statistical significance of differences among and between experimental groups at p< 0.05. The number of mice used in the experiments was determined by two tail ANOVA analysis to obtain >85% power.
Cytokine profile analysis
WT, RIP1−/− and RIP3−/− AML cells (2×105/ml) were cultured in 4-cytokine medium for 24 hours. Supernatants were collected for cytokine profile analysis by Bio-Plex Pro™ mouse cytokine 23-Plex Assay (Bio-Rad, M60-009RDPD) follow the vender’s instructions.
RESULTS
Basal activation of RIP1/RIP3 signaling in human AML cells
Our analysis of a large-scale AML patient cohort (N = 562, GSE37642 cohort) showed that the expression of many key components of inflammatory signaling pathways (Table S1), such as TNF, TNFRs, TRAIL/TRAIL receptor, IL-1α/β, TLRs and MYD88 were greatly elevated in LCs isolated from M4 and M5 subsets of AML patients (Fig. 1a). We also noticed that the key necroptotic mediator, RIP3 kinase, was greatly elevated in these samples (Fig. 1a). Consistent with previously reports23–26, we found that necroptosis can be readily induced using TNF + Smac-mimetics + a caspase inhibitor in AML cells, which results in extensive cell death within 20 hours in in vitro culture, suggesting Rip3-Mlkl-mediated necroptotic signaling pathway is intact in AML cells (Fig. S1).
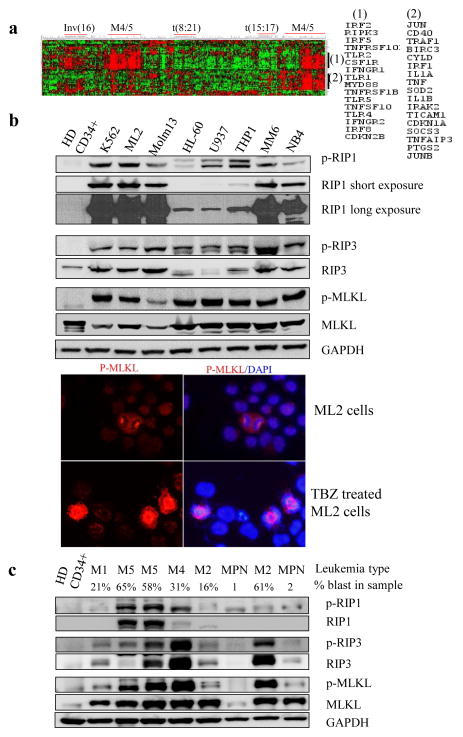
Fig. 1. Human AML cell lines express TNF and show a basal level activation of the RIP1/RIP3 pathway.
a. TNF, TFNR and RIP3 are highly expressed in a subset of M4 and M5 AML samples, as shown by microarray data (n=562). b. Cell lysates prepared from the human AML cells lines ML-2, Molm13, HL60, U937, THP1, MM6, NB4, and K562 were subjected to Western blotting with anti-p-RIP1, anti-RIP1 anti-p-RIP3, anti-RIP3, anti-p-MLKL, and MLKL antibodies. Healthy donor cells (HD CD34+) were used as controls. Immune staining of p-MLKL in ML2 LCs (up-panel) or TBZ (TNF-α plus birinapant and Z-VAD)-induced necroptotic ML2 LCs (lower panel). Similar staining was observed in MM6 and Molm16 cells (data not shown) c. Lysates prepared from mononucleated cells of patient blood samples were subjected to Western blotting for p-RIP1, RIP1, p-RIP3, RIP3, p-MLKL, and MLKL levels (the type of leukemias and percentage of blasts are indicated above the lanes. MPN, myeloproliferative neoplasm).
Necroptotic signaling is primarily stimulated by TNF in most types of cells. We have reported that most AML cells produce TNF, which stimulates the growth of AML cells in an autocrine fashion27. To study whether some degree of activation of necroptotic signaling is present in AML cells, we examined the expression and activity of RIP1/RIP3 signaling by measuring the protein levels of RIP1 and RIP3 as well as the phosphorylation of RIP1, RIP3 and MLKL (key downstream mediator of RIP1/RIP3 activation) in human AML cell lines including ML-2 (M4), Molm13 (M5a), U937 (M5), THP1 (M5a), MM6 (M5), HL-60 (M2), and NB4 (M3), as well as K562, a Bcr-Abl-expressing erythroid leukemia cell line derived from CML. ML-2, Molm13, U937, THP1 and MM6 are MLL-rearranged (MLL-r) AML cells. Phosphorylated forms of RIP1 (p-RIP1-Ser166), RIP3 (p-RIP3-Ser227), and MLKL (p-MLKL Thr357/Ser358) were present in all AML cell lines tested but was barely detectable in healthy CD34+ hematopoietic stem/progenitor cells (HSPCs) (Fig 1b). While most other cell lines express one isoform of RIP1 and RIP3, two splicing isoforms of RIP1 and RIP3 were detected in U937, THP1 and HL60 cells which correlated to reduced expression of both RIP1 and RIP3 (Fig. 1b). Immunofluorescence assays demonstrated that, in contrast to necroptosis induced by TNF-α plus Birinapant and Z-VAD (TBZ) treatment, in which p-MLKL protein is aggregated within the cytoplasm and cell membrane, in AML cells, basal level p-MLKL is localized to the nucleus and stains strongly on the centrosome/spindle of dividing cells (Fig. 2b). In addition, we examined the expression and activation of RIP1 and RIP3 in primary leukemia samples. As shown in Fig 1c, expression of p-RIP1, RIP1, p-RIP3 and RIP3 was detected in all AML patient samples but not in healthy donor samples. Myeloproliferative neoplasm (MPN) samples showed lower levels of RIP3 and p-RIP3 when compared to AML samples. RIP3 and p-RIP3 levels appeared to positively correlate to the percentage of leukemic blasts. These data suggested that a basal level activation of the RIP1/RIP3/MLKL pathway exists in AML cells when grown under standard culture conditions without any stimulation.
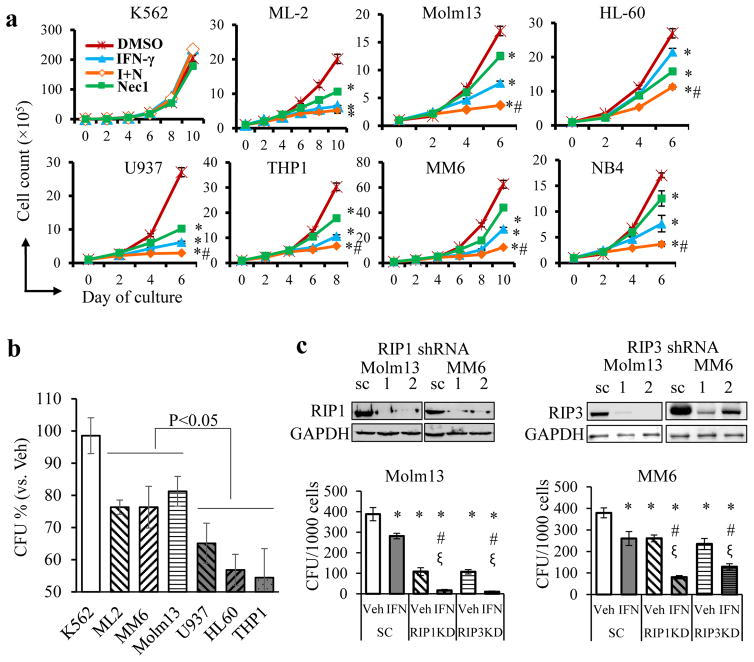
Fig. 2. Blockage of the RIP1/RIP3 pathway enhances the differentiation of human LC lines.
Human CML cell line K562 and AML cell lines ML-2, Molm13, HL60, U937, THP1, MM6, and NB4 were treated with Nec1 (N, 50μM), human IFN-γ (I, 5ng mL−1), IFN-γ +Nec1 (I+N) and DMSO (D, vehicle control), respectively. a. Cell growth was examined by counting viable cells every other day (* p<0.05, compared to DMSO, #, p<0.05, compared to IFN-γ group). b. K562, ML-2, Molm13, MM6, U937, HL60, and THP1 cells were treated with human IFN-γ (5ng mL−1) or vehicle in liquid culture for 4 days. One thousand live cells from each group were used for CFU. Colonies were counted after 10 days and normalized to each cell type’s vehicle-treated group (* p<0.05). c. Molm13 and MM6 cells were infected with scrambled (SC), RIP1 or RIP3 shRNAs with GFP reporter. GFP+ cells were sorted and subjected to Western blotting to determine knockdown efficiency. Cells with the highest efficiency of knockdown for RIP1 (shRNA#1) and RIP3 (shRNA#1) were used for CFU assay with or without IFN-γ (1ng mL−1) treatment. * p<0.05, compared to DMSO of SC group, # p<0.05, compared to IFN-γ-treated SC group; ξ p<0.05, compared to vehicle (Veh) of corresponding shRNA group. Results shown are representative of three independent trials.
RIP1/RIP3 inhibition sensitizes human AML cells to IFN-γ-induced differentiation
To study the roles of basal level activation of RIP1/RIP3 signaling in AML cells, we examined cell growth, morphology and clonogenic ability in a variety of AML cell lines after blocking RIP1/RIP3 interaction using Necrostatin 1 (Nec1), the best-characterized necroptotic inhibitor28, 29. We and others determined that 20–30μM of Nec1 can largely prevent TNF-induced necroptosis in most types of cells30, 31. We also determined that Nec1 has negligible influence on healthy HSPCs when its concentration is less than 150 μM30. Therefore, we used 30–50μM Nec1 to treat AML cells in the current study. We found that the reduced growth, proliferation and survival of AML cells could only be observed 4 days after Nec1 treatment (Fig. 2a and S2). Consistently Nec1 treatment inhibits the clonogenic ability of AML cells in almost all examined AML lines to some extent (Fig. S3). We believed that the growth-inhibitory effect of Nec1 on AML cells was due to differentiation, as demonstrated by mature monocyte/macrophage morphology (Fig. S4a) and increased expression of the differentiation markers CD11B and CD14 (Fig. S4b).
Consistent with previous studies, IFN-γ inhibits growth by inducing the differentiation of AML cells32–34 (Fig. S4). The partially differentiated morphology of AML cells after Nec1 treatment encouraged us to test whether Nec1 treatment combined with IFN-γ could induce a more complete differentiation in AML cells. As expected, combination treatment of Nec1 with IFN-γ further promoted differentiation and repressed growth/clonogenic ability in most tested AML cell lines (Fig. 2a, S3, S4), indicating the synergistic functions of these two molecules. In addition, it was noted that the sensitivity of these human AML cells to IFN-γ was correlated to the expression levels of RIP1 and RIP3. As shown in Fig. 2b, following 4 days of treatment with IFN-γ, cells with low levels of RIP1 and RIP3 (U937, HL-60 and THP1, Fig. 1b) showed a greater reduction in CFU capacity compared to cells with high levels of RIP1 and RIP3 (ML2, Molm13 and MM6, Fig. 1b). To validate this observation, we used shRNA to knock down RIP1 and RIP3 in Molm13 and MM6 cells (Fig. 2c). We found that RIP1 and RIP3 knockdown repressed proliferation but did not induce cell death in AML cells (Fig. S5a, b). Knockdown of RIP1 and RIP3 also increased the sensitivity of these cells to IFN-γ-induced differentiation, as shown by their morphology (Fig. S5d), CD11b expression (Fig. S5e) and a reduction in the number of colonies (Fig. 2c). These data suggested that basal activation of RIP1/RIP3 necroptotic signaling is required for maintaining the undifferentiated state of AML cells. Inhibition of the RIP1/RIP3 necroptotic pathway induces partial differentiation of AML cells and sensitizes AML cells to IFN-γ-induced differentiation.
RIP1/RIP3 knockout attenuates leukemogenic capacity
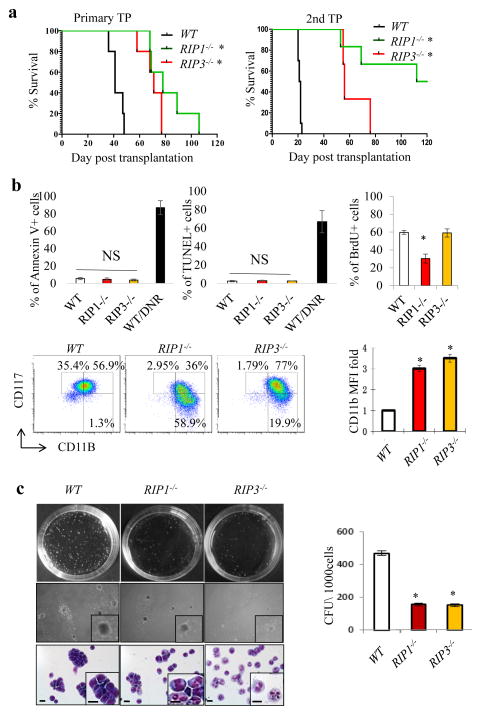
To more specifically understand the role of necroptotic signaling in the pathogenesis of AML, we examined the effects of genetic inactivation of RIP1 and RIP3 on AML development and progression using a murine Mll-AF9 (MA9)-AML model (M5 subtype). We found that mice receiving MA9-transduced RIP1−/− (RIP1 knockout) or RIP3−/− (RIP3 knockout) HSPCs showed a significantly longer latency to AML development than mice receiving MA9-transduced WT (wild-type) HSPCs. Significantly delayed leukemia development and reduced leukemia-related mortality were also observed in mice receiving RIP1−/− and RIP3−/− AML cells upon secondary transplantation (Fig. 3a). RIP1 and RIP3 knockout in AML cells was verified by Western blotting (Fig. S6a). To study the mechanism by which inactivation of RIP1/RIP3 signaling influences AML development, we compared the cell cycle, proliferation, survival and differentiation of WT, RIP1−/− and RIP3−/− AML cells. We found that proliferation was reduced in RIP1−/− AML cells but was not altered in RIP3−/− AML cells compared to WT AML cells, as shown by the percentage of cells in S-G2/M phase (Fig. S7a) and BrdU pulse-labeling (Fig. 3b). Survival of RIP1−/− and RIP3−/− AML cells was comparable to that of WT AML cells as demonstrated by Annexin-V staining and TUNEL assays (Fig. 3b). The undifferentiated CD117+CD11Blow population35 was significantly reduced in both RIP1−/− and RIP3−/− AML cells compared to WT AML cells. Both RIP1−/− and RIP3−/− AML cells express increased levels of the differentiation marker CD11b compared to WT AML cells (Fig. 3b). The partially differentiated feature of RIP1−/− and RIP3−/− AML cells was further verified by a reduction in colony-forming ability. Compared to WT AML cells, RIP1−/− and RIP3−/− AML cells generated fewer and smaller-sized colonies. Moreover, the AML cells in RIP1−/− and RIP3−/− colonies showed a differentiated morphology (Fig. 3c). The gene-mutant AML cells also showed a differentiated morphology in peripheral blood smears (Fig. S7b) and there was a delay in the development of leukemia in recipient mice (Fig. 3a, S7c). These data support the notion that RIP1/RIP3 signaling plays a role in maintaining the undifferentiated state of AML cells.
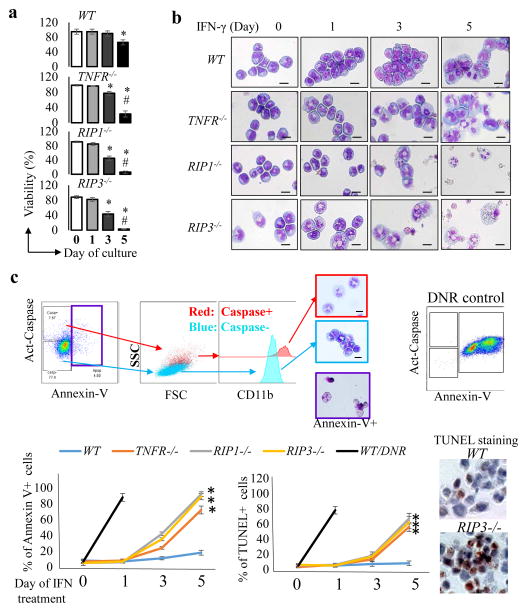
Fig. 3. Genetic inactivation of RIP1/RIP3 signaling leads to partial differentiation and compromised leukemogenic ability of murine MA9-AML cells.
a. Survival curves of mice receiving WT, RIP1−/−, and RIP3−/− MA9-AML cells in primary and secondary transplantations (n=5, * p<0.05, compared to WT). b. WT, RIP1−/− and RIP3−/− murine MA9-AML cells were analyzed for cell death using Annexin-V and TUNEL assays, for cell proliferation using BrdU pulse-labeling assay, and for expression of CD117 and CD11b. Percentages of CD117highCD11blow and CD117low/−CD11Bhigh populations are denoted. Mean fluorescence intensity (MFI) of CD11b was calculated and normalized to WT value. c. CFU assay was performed at a seeding density of 1000 cells/dish. Colonies were photographed and counted on day 7 of culturing. Cells were collected from colonies for morphologic analysis after Giemsa staining (bottom panel, scale bar=15μm). Results shown in b and c are representative of three independent trials.
RIP1/RIP3 knockout sensitizes murine MA9-AML cells to IFN-γ-induced differentiation
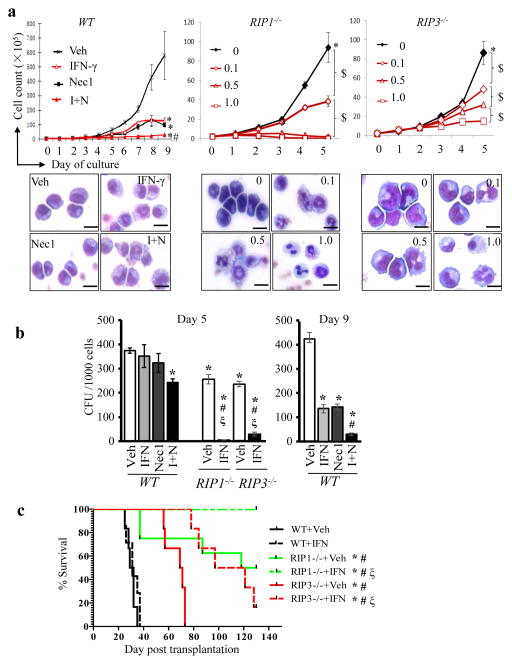
Consistent with data from human AML cell lines, Nec1 also induced inhibition of cell growth and partial differentiation as well as enhanced IFN-γ-induced differentiation in murine AML cells (Fig. 4a). Furthermore, genetic deletion of RIP1 or RIP3 led to a remarkably increased sensitivity of AML cells to IFN-γ-induced differentiation. Significant growth arrest and differentiation of WT AML cells were only observed at a concentration of >1 ng mL−1 of IFN-γ (Fig. S8), while significant growth arrest and differentiation of RIP1−/− or RIP3−/− AML cells was observed at a concentration of 0.1–0.5 ng mL−1 and complete growth arrest and full differentiation were obtained at a concentration of 1 ng mL−1 of IFN-γ (Fig. 4a). Nec1 treatment sensitized WT AML cells to IFN-γ-induced differentiation but did not further inhibit the growth nor enhance IFN-γ-induced differentiation in RIP3−/− AML cells (Fig. S9), suggesting that Nec1 had a reasonable specificity in terms of blocking RIP1/RIP3 signaling. To evaluate whether inactivation of the RIP1/RIP3 signal synergizes with IFN-γ to repress the colony-forming ability of AML cells, WT AML cells were treated with Nec1 and IFN-γ individually or in combination, while RIP1−/− or RIP3−/− AML cells treated with 1ng mL−1 IFN-γ were seeded into methylcellulose for CFU assay. As shown in Fig. 4b, after inactivation of the RIP1/RIP3 signal by Nec1 treatment or genetic deletion, IFN-γ treatment results in a remarkably reduced number of colonies.
Fig. 4. Inactivation of the RIP1/RIP3 pathway sensitizes IFN-γ-induced differentiation and represses leukemia development in vivo.
a. Murine WT MA9-AML cells were treated with DMSO (Veh), Nec1 (30μM), mouse IFN-γ (1ng mL−1), and Nec1 + IFN-γ (I+N) respectively. RIP1−/− and RIP3−/− MA9-AML cells were treated with mIFN-γ at concentrations of 0.1, 0.5 and 1ng mL−1, respectively. Cells were counted daily. Cell morphology was examined on day 5 of treatment (scale bar=15μm). b. Cells in each treatment group of WT AML cells and 1ng mL−1 the IFN-γ treatment group of RIP1−/− and RIP3−/−AML cells from (a) were collected at indicated times of treatment and seeded onto methylcellulose for CFU assay. Colony counts were obtained and compared. c. WT, RIP1−/−, and RIP3−/− MA9-AML cells were transplanted into lethally-irradiated mice together with supporting cells. Each recipient mouse received 5000 WT AML cells (n=5/group), 20,000 RIP3−/− AML cells (n=6/group), or 50,000 RIP1−/− AML cells (n=10/group). Beginning 15 days post-transplantation, half of the recipient mice from each group were treated with IFN-γ daily for two weeks. Animal survival curves were plotted and statistically analyzed based on daily monitoring for mouse mortality. $ indicated p<0.05 for the indicated groups; * p<0.05, compared to WT+Veh; # p<0.05, compared to WT+IFN; ξ p<0.05, compared to Veh group of the same cell type. Results shown in a and b are representative of three independent trials.
To evaluate whether the differentiation induced by combining IFN-γ with inactivation of RIP1/RIP3 signaling was capable of reducing the leukemogenic capability of MA9-AML cells, an index of reduction of leukemogenic capability, we treated WT AML cells with Nec1 (30 μM) and IFN-γ (1 ng mL−1) individually or in combination and RIP1−/− and RIP3−/− MA9 AML cells with IFN-γ (1 ng mL−1) alone for five days. We subsequently collected live cells for transplantation and leukemogenic analysis. As indicated in Fig. S10, while all recipient mice receiving Nec1-treated WT MA9-AML cells and most recipients receiving IFN-γ treated WT MA9-AML cells died 25–50 days post-transplantation, recipient mice that received WT AML cells treated with a combination of IFN-γ and Nec1 showed increased latency to leukemia (around 50 days) and reduced mortality, with 40% mice remaining alive and leukemia-free for up to 120 days. In contrast, mice receiving RIP1−/−, and RIP3−/− MA9-AML cells treated with IFN-γ did not develop leukemia for up to 120 days. These data suggested that RIP1/RIP3 inactivation plus IFN-γ-induced differentiation results in a reduction of leukemogenic capability.
To evaluate the efficacy of IFN-γ treatment in vivo, we transplanted WT, RIP1−/−, and RIP3−/− MA9- AML cells into lethally-irradiated recipient mice along with supporting BM cells. Starting 15 days post-transplantation, recipient mice were given IFN-γ (2.5 μg/mouse) daily for two weeks. While IFN-γ showed very limited effects in inhibiting leukemogenesis in WT AML cells, it significantly delayed leukemia development and reduced mortality in RIP1−/− and RIP3−/− MA9 AML cells (Fig. 4c). These data suggest that the RIP1/RIP3 pathway is a promising target for anti-AML therapy.
TNFR knockout sensitizes AML cells to differentiation in vitro but not in vivo
Because: 1) AML cells produce TNF (Table S2 and Fig. S6b); 2) necroptosis is primarily induced by TNF in AML cells; and 3) TNF inhibitors have been used clinically, we examined whether TNF is the key stimulator of RIP1/RIP3 signaling in AML and evaluated the potential application of this for anti-TNF therapy. We found that although the morphology, CD117+CD11blow population percentage and clonogenic ability (Fig. S11a–c) of freshly-isolated TNFR−/− AML cells were comparable to those of WT AML cells, TNFR−/− AML cells showed remarkably increased sensitivity to IFN-γ treatment in vitro when compared to WT AML cells (Fig. S8). The growth of WT AML cells was inhibited by IFN-γ in a dose-dependent manner (Fig. S8); even 10 ng mL−1 of IFN-γ failed to completely repress the growth of WT AML cells. In contrast, the growth inhibition of TNFR−/− AML cells was observed at as low as 0.1 ng mL−1 of IFN-γ, and complete inhibition was achieved at 0.5 ng mL−1 (Fig. S11). By day 5 of treatment, TNFR−/− AML cells showed massive death and the remaining viable cells showed terminally differentiated morphology (Fig. S8, 11). IFN-γ treatment also reduced the clonogenic capability of TNFR−/− AML cells in vitro (Fig. S11d–f). However, ex vivo, but not in vivo, IFN-γ treatment delayed AML development in both WT and TNFR−/− AML cells (Fig. S11g–h). We speculate that RIP1/RIP3 signaling is primarily activated by TNF in in vitro culture, but can also be activated by other inflammatory factors in vivo, including TLR, TRAIL and IL-1β.
Induced cell differentiation was followed by apoptosis
In normal tissue cells, RIP1 prevents IFN-γ-induced RIP3-medicated necroptosis in a kinase activity-independent manner13, 36, 37. Interestingly, we found that RIP3 levels were dramatically decreased in RIP1−/− AML cells (Fig. S6), which might explain why RIP1−/− AML cells did not die of necroptosis upon IFN-γ treatment (Fig. 5). To exclude the potential that the increased sensitivity of murine TNFR−/−, RIP1−/− and RIP3−/− AML cells to IFN-γ treatment is due to a direct effect of IFN-γ-induced apoptosis or necroptosis, we conducted a time-course study for cell viability and differentiation early during the period of treatment. In the first 48 hours of IFN-γ treatment, increased cell counts were observed in AML cells without showing decreased viability despite TNFR-RIP1-RIP3 activity. Significant cell death was observed after 3 days of treatment in TNFR−/− or RIP1−/− or RIP3−/− AML cells but only after 4 days in WT AML cells, as demonstrated by Trypan Blue exclusion analysis, Annexin V and TUNEL assays (Fig. 5a, c and S12a–b). These observations were consistent with the time at which cells show an obviously differentiated morphology (Fig. 5b and S12e). Detailed analysis demonstrated that Annexin-V-negative cells with activated caspase 3/8 showed increased expression of CD11b and a differentiated morphology compared to caspase 3/8-negative AML cells (Fig. 5c). We speculated that caspase activation and apoptosis are secondary events in differentiation, which explains why AML cells become short-lived following the induction of differentiation. This was supported by our data showing that the inhibition of caspase activity later on in treatment slightly increased cell counts (Fig. S12c) and delayed cell death (Fig. S12d), but failed to block differentiation (Fig. S12e). Such a phenomenon was also detected in several human AML cell lines. In these cell lines, we found that Nec1 and IFN-γ-induced AML cell differentiation was followed by cell cycle arrest and activation of caspases (Fig. S13), indicating an induced normal maturation process in these malignant cells.
Fig. 5. Apoptosis of MA9 AML cells after IFN-γ treatment is secondary to differentiation.
WT, TNFR−/−, RIP1−/− and RIP3−/− MA9 AML cells were treated with mIFN-γ (1ng/ml) for 5 days. Cell viability was measured by Trypan Blue exclusion (a) and cell morphology (b) was examined by Giemsa staining every other day (* p<0.05, compared to day 0; # p<0.05, compared to day 3; scale bar=15 μm). c. A representative procedure (up-panel) for the analysis of Caspase activity, CD11b expression, Annexin V+ cells and cell morphology. Apoptotic death of Annexin-V+ cells were further verified by TUNEL staining. DNR (daunorubicin) treated WT AML cells were used as positive controls in all experiments for cell death analysis. Percentages of Annexin-V+ and TUNEL+ cells were quantitated and compared among WT, TNFR−/−, RIP1−/− and RIP3−/− AML cells on days 0, 1, 3, and 5 of IFN-γ treatment. Representative TUNEL-staining images of WT and RIP3−/− AML cells at day 5 of IFN-γ treatment were shown. (* p<0.05, compared to WT AML cells). Results shown in a are representative of three independent trials.
IFN-γ and Nec1 show minimal inhibition of normal HSPCs
One concern for the use of IFN-γ and RIP1 inhibitors to treat AML is their potentially repressive effects on normal HSPCs. Therefore, we examined the influence of Nec1 and IFN-γ treatment on the survival and colony-forming ability of BM HSPCs. We found that Nec1 was toxic and had inhibitory effects on colony formation of WT HSPCs when its concentration was higher than 200 μM (Fig. S14a–b), in contrast to 10 μM for MA9-AML cells (Fig. S14c). IFN-γ appears to have minimal effects on the clonogenic ability of BM HSPCs even at a concentration of 10 ng mL−1, regardless of the intact state (WT) or inactivated state (TNFR−/− or RIP3−/−) of TNFR-RIP3 signaling (Fig. S14d–e). In contrast, the effective dose of IFN-γ was 0.1 ng mL−1 for TNFR−/− and RIP3−/− AML cells (Fig. 4 and S11). In addition, we found that doses of 100μM Nec1 and 1ng mL−1 IFN-γ have no significant effects on the reconstitutive capacity of normal HSPCs (Fig. S15f–g). Such a dose-response difference between AML cells and HSPCs to IFN-γ and Nec1 provides us with a valuable window for these agents to be used to specifically treat leukemia with a negligible deleterious influence on normal hematopoiesis.
RIP1/RIP3 inhibition sensitizes primary AML cells to differentiation
Consistent with what was observed in murine HSPCs, neither Nec1 nor IFN-γ individually nor in combination, had a negative impact on the differentiation and colony formation of normal human CD34+ HSPCs (Fig. 6a and b). To study the efficacy of Nec1 and IFN-γ treatment on human primary AML samples, we examined the responses of 10 human primary LC samples (5 newly-diagnosed cases: 1 M0, 2 M1, and 2 M5; and 4 cases secondary to MDS, 1 case secondary to therapy, see Table S2 in Supplementary Information) to Nec1 and IFN-γ treatment. Colony growth was achieved in 7 samples, while 3 samples from patients with AML secondary to MDS failed to grow colonies. Among these 7 colony-growing samples, Nec1 and IFN-γ treatment had at least an additive effect on all of them in terms of repression of clonogenic capacity regardless of their disease subtype (Fig. 6, Table S2). Following treatment, AML cells isolated from all 7 responsive samples underwent differentiation, as demonstrated by morphologic analysis (Fig. 6a) and increased expression of the differentiation markers CD11b (Fig. 6c) and CD14 (Fig. S15a), as well as by reduced size and number of colonies (Fig. 6a–b). Significant cell cycle arrest following Nec1 and IFN-γ treatment was observed after 5 days of treatment, which is in accordance with the time when the cells showed a pronounced differentiated morphology (Fig. S15b). Among these 7 AML samples, two have MLL-fusion genes, 4 samples have NPM mutations while 1 has DNMT3a mutation. These results suggested that RIP1/RIP3 inhibition combined with IFN-γ might be a good treatment option for human the treatment of AML cases showing these genomic abnormalities (Table S2).
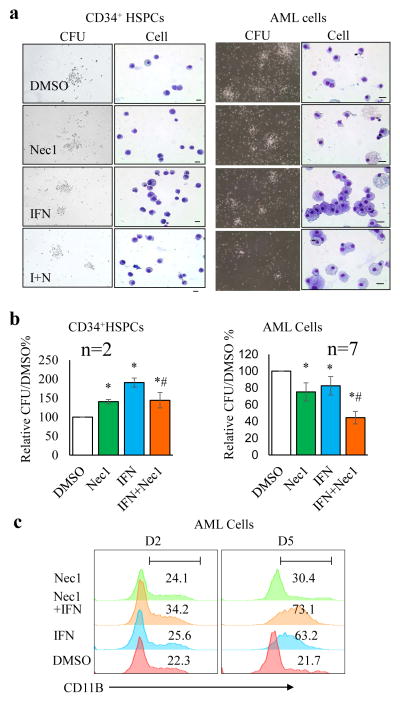
Fig. 6. Blockage of the RIP/RIP pathway enhanced human primary AML cell differentiation.
CD34+ cells purified from two healthy donors’ peripheral blood and primary AML cells isolated from 7 AML patients were seeded for CFU assay (40,000 cells/dish, in triplicate) in the presence of DMSO, Nec1 (50 μM), hIFN-γ (5 ng mL−1), or IFN-γ + Nec1 (I+N), respectively. After culturing for 9 days, colonies and cell morphologies were examined and photographed (a) and total numbers of CFUs were counted and compared (b). * p<0.05, compared to vehicle control, # p<0.05, compared to IFN-γ group; scale bar=15 μm). Relative CFU was normalized to DMSO group. Absolute CFU numbers are shown in Table S2. c. AML cells were cultured in liquid medium and treated with Nec1 and IFN-γ individually or in combination. Differentiation was evaluated by FACS for CD11b on day 2 (D2) and day 5 (D5). Data in a and c are representative of one of two healthy donors and one of the 7 colony-forming AML samples.
TNF-RIP1/RIP3 signaling maintains SOCS1 levels
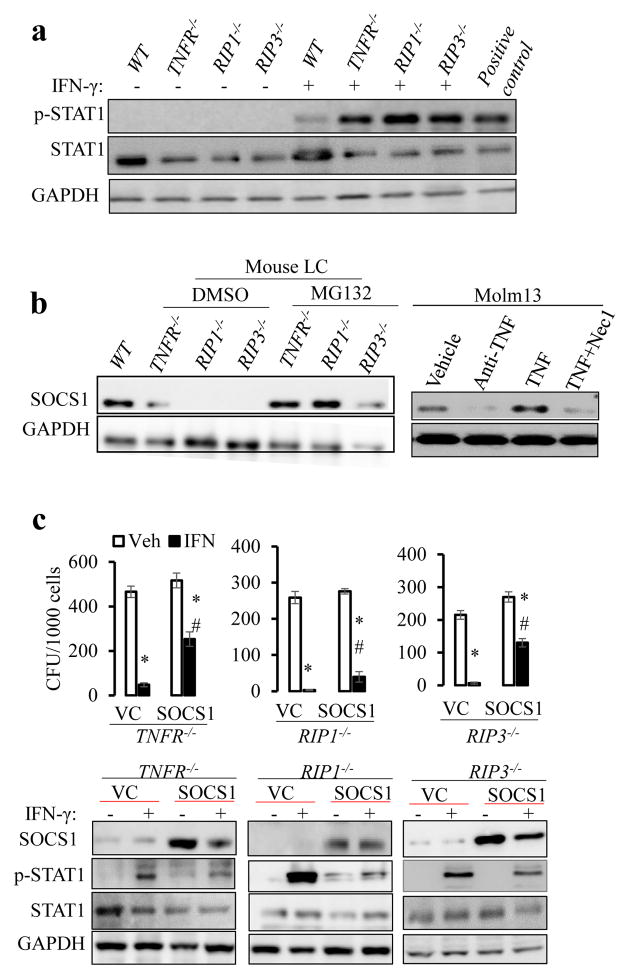
To understand the underlying mechanism by which inactivation of the TNFR-RIP1/RIP3 pathway facilitates IFN-γ-induced LC differentiation, we first examined the influence of TNFR-RIP1/RIP3 signaling inactivation on IFN-γ signaling transduction using murine MA9-AML cells. Despite lower levels of total STAT1 expression in TNFR−/−, RIP1−/−, and RIP3−/− AML cells compared to WT AML cells, IFN stimulation induced significantly higher phosphorylated-STAT1 (p-STAT1) in these gene-mutant AML cells than in WT AML cells (Fig. 7a). Reduction of negative regulators, increased expression of INFGR, or alteration of cytokine profiling are potential mechanisms to explain the enhanced response of RIP1−/− and RIP3−/− AML cells to IFN-γ treatment. We excluded the latter two mechanisms by showing that the levels of INFGR (Fig. S16) and cytokine profiling were comparable among WT, RIP1−/− and RIP3−/− AML cells (only a slight reduction of IL1β in the gene-mutant AML cells) (Table S3). SOCS1 is a well-known negative regulator of IFN-γ, so we determined SOCS1 levels in WT, TNFR−/−, RIP1−/−, and RIP3−/− AML cells. As shown in Fig. 7b, while SOCS1 expression was readily detected in WT AML cells, it was diminished in TNFR−/−, RIP1−/−, and RIP3−/− AML cells. In addition, we found that SOCS1 levels correlated with RIP1/RIP3 levels in human AML cells (Fig. S17). TNF stimulation resulted in increased SOCS1 expression in Molm13 cells, which can be inhibited by Nec1, suggesting that RIP1/RIP3 signaling also regulates SOCS1 levels in human AML cells. However, SOCS1 mRNA levels in these gene-mutant AML cells were only slightly reduced when compared to WT AML cells (data not shown), suggesting that a post-transcriptional mechanism might be involved in regulating SOCS1 protein levels. The restoration of SOCS1 protein in TNFR−/−, RIP1−/−, and RIP3−/− AML cells by treatment with the proteasome inhibitor MG132 suggested that TNF-RIP1-RIP3 signaling prevents SOCS1 degradation (Fig. 7b). To confirm that loss of SOCS1 is responsible for the increased sensitivity of the gene-mutant AML cells to IFN-γ-induced differentiation, we over-expressed SOCS1 in these AML cells and tested their responses to IFN-γ. We found that over-expression of SOCS1 partially repressed IFN-γ-stimulated STAT1 activation and restored resistance to IFN-γ-induced differentiation in TNFR−/−, RIP1−/−, and RIP3−/− AML cells in in vitro culture (Fig. 7c and S18). In addition, overexpression of SOCS1 also partially restored the refractoriness of RIP1−/− and RIP3−/− AML cells to IFN-γ treatment in vivo (Fig. S19). Altogether, these data establish the functional role of TNFR-RIP1/RIP3-signaling in the maintenance of the lack of differentiation in AML cells. This effect is partially mediated by the repression of IFN-γ-STAT1 signaling, which maintains SOCS1 levels.
Fig. 7. TNF antagonizes IFN-γ-induced differentiation by maintaining SOCS1 level.
a. WT and TNFR−/−, RIP1−/−, and RIP3−/− murine MA9-AML cells were treated with or without IFN-γ (1ng mL−1) for 4 hours. Cell lysates were prepared and subjected to Western blotting for total STAT1 and phosphorylated STAT1 (p-STAT1). Cell lysate from IFN-γ treated Hela cells provided by vender was used as positive control. b. Western blotting was used to determine SOCS1 levels in WT, TNFR−/−, RIP1−/−, and RIP3−/− MA9-AML cells with or without of 12 hours of MG132 treatment. Molm13 cells were cultured for 14 hours in the presence of anti-TNF monoclonal antibody (5μg mL−1/ml), TNF (20 ng mL−1), or combined TNF+Nec1 (20ng mL−1 and 50μM, respectively). Cell lysates were prepared for Western blotting for SOCS1. c. TNFR−/−, RIP1−/− and RIP3−/− MA9-AML cells transduced with vector-only (VC) or SOCS 1 were treated with mIFN-γ (1ng mL−1). On day 5, 1000 viable cells from each treatment group were collected and seeded for CFU assay. After one week in culture, colonies were counted and compared. SOCS1 levels and Stat1 activity (determined by p-Stat1 levels) were examined at 4 hours after IFN-γ treatment. * p<0.05, compared to VC + Veh; # p<0.05, compared to VC + IFN-γ. Results shown in c are representative of three independent trials.
DISCUSSION
Chemical-induced necroptosis has been investigated as a means of treating drug-resistant cancers25, 38–40. Its value is still controversial because of several concerns. First, although short-term reductions of tumor size have been observed in certain types of cancer, any long-term benefits associated with such treatment appear to be limited41–43. Second, spontaneous necroptosis has been observed in some solid tumors but is associated with poor prognosis44–46. Third, necroptosis is involved in many clinical disorders such as myocardial infarction and stroke, atherosclerosis, ischemia–reperfusion injury, pancreatitis, and inflammatory bowel diseases13, 14, 19. Lastly, necroptotic cells release molecules that induce systemic inflammatory reactions13, 14, 19 and may stimulate tumor cell growth47, 48. Therefore, induced necroptosis as a treatment for cancer might not appear to be an ideal strategy. To that end, our current studies indicate that the inhibition of necroptotic signaling may benefit certain AML patients and should be safe, without the induction of deleterious side-effects. In addition, although our array data showed that the components of inflammatory signaling and RIP3 are more highly expressed in M4 and M5 subtypes of AML, the synergistic repressive effects of Nec1 plus IFN-γ were observed in all of the primary AML samples we studied regardless of their subtype, suggesting that such treatment might not limited to monocytic leukemia. While this manuscript was under in revision, a report by Hockendorf et al, demonstrated that TNF-RIP3 signaling restricts FLT3-IDT and RUNX-ETO-induced AML development by repressing IL1-induced differentiation and MLKL-mediated cell death49. We previous reported that TNF stimulates the growth of MLL-AF9 AML cells but represses the growth of RUNX-ETO AML cells. Thus, we speculate that the role of RIP3 signaling in AML pathogenesis might be cell context-dependent, determined by the leukemic driver genes. The AML patient samples in our study contain MLL-fusion genes, NPM1 or DNMT3a mutations. We did not find FLT3-IDT and RUNX-ETO in our patient samples. However, a large number of patient samples needs to be tested in order to determine the correlation of patient genetic abnormalities to the responses to Nec1 and IFN-γ treatment.
The inhibition of RIP1−/− and RIP3−/− AML by IFN injection in vivo suggested that the combination of a necroptosis inhibitor plus IFN might be an effective treatment for AML patients. Inactivation of TNF signaling in AML cells can reproduce the RIP1−/− and RIP3−/− AML phenotype in in vitro culture. However, the reduced sensitivity of TNFR−/− AML cells to IFN-γ treatment in vivo suggests that anti-TNF may be insufficient to fully block RIP1/RIP3 signaling in vivo due to other stimulating agents50 and that directly targeting RIP1/RIP3 is necessary. Unfortunately, due to the short life of Nec1 in vivo, we were unable to show the effects of pharmacologic inhibition of necroptosis on AML development in vivo. We intend to test optimized, stable necroptotic inhibitors such as Nec1-stable (Nec1-s)51 to further evaluate the translational potential of our study results. Although we currently do not know whether RIP1−/− and RIP3−/− AML cells are more sensitive to the toxicity of the immune response, we found that RIP1−/− and RIP3−/− AML cells are highly sensitive to IFN-γ-induced differentiation in vitro. We believe that a RIP1/RIP3 inhibitor will be an effective treatment for AML when combined with specific chimeric antigen receptor T cells (which express high levels of IFN-γ) by inducing AML cell differentiation.
Because the leukemogenic capacity of RIP1−/− AML cells was much more compromised than it was in RIP3−/− AML cells, we speculate that other signaling pathway(s) downstream of RIP1 and parallel to the RIP3 pathway might also be involved in AML development. Several recent studies suggest that the scaffolding activity of RIP1 plays an essential role in maintaining tissue homeostasis by preventing TNF-induced apoptosis and IFN-γ or TLR signaling-stimulated necroptosis in skin, intestine and hematopoietic tissues10, 11, 52–54. Further studies are needed to elucidate the role of TNF and the scaffolding activity of RIP1 in the pathogenesis of AML. In addition, the scaffolding activity of RIP3 is required for caspase 8-mediated apoptosis55, 56. It will be interesting to determine whether RIP3 kinase inhibitors are more effective than RIP1 inhibitors in eliminating AML cells when combined with IFN-γ.
Supplementary Material
Acknowledgments
The authors thank the staff of the Department of Comparative Medicine of Loyola University Medical Center for animal care services, as well as Drs. Manuel Diaz, Nancy Zeleznik-Le, Andrew Dingwall and Wei Qiu for their ongoing professional collaboration and scientific suggestions and discussions, which have improved the present studies. We also thank the members of Dr. Wei Qiu’s and Dr. Stephanie Watkins’s laboratories for imaging assistance. We appreciate laboratory support from Patience Oladeinde, Danielle Howard and Emma Yao, and FACS sorting and analysis assistance from Patricia Simms, Ashley Hess, Shwetha Ravichandran, and Veronica Volgina.
This work was supported by NIH (grants R01-HL095896 and R21-CA181970 to J. Z. through Loyola University Chicago) and the Leukemia Research Foundation New Investigator Award (8th Annual George Richard Memorial Grant to J.X.). J.X. was also supported in part by a grant from the Muscular Dystrophy Association (MDA202906). A. V was supported by NRSA F31 Fellowship (F31CA17417). JL and Jun Z were supported by National Basic Research Program of China (project 2013CB966800), the Program for Basic Research of Shanghai Municipal Science and Technology Commission (Grant No.13JC1406403).
Footnotes
Contributions:
JX designed the experiments, analyzed the data, and drafted the manuscript; JWZ supervised the overall research, designed the experiments, analyzed the data, and edited the manuscript; JX, JL, Jun Z, RS, DY, AV, AN, GN, RG, JC, JP, WW, ZX, YC, PB, HC, J Zhu and JKZ collectively contributed to data collection and interpretation of the results; JC provided microarray data; MMP, PCK, SN, and AK provided study samples, clinical data, helped to write and revised the manuscript. PB helped to write, and also edited and refined the manuscript.
Conflict of interest statement
The authors declare no conflict interests.
References
- 1.Burnett AK, Hills RK, Milligan DW, Goldstone AH, Prentice AG, McMullin MF, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2010;28:586–595. doi: 10.1200/JCO.2009.22.9088. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 4.Pulte D, Gondos A, Brenner H. Expected long-term survival of patients diagnosed with acute myeloblastic leukemia during 2006–2010. Ann Oncol. 2010;21:335–341. doi: 10.1093/annonc/mdp309. [DOI] [PubMed] [Google Scholar]
- 5.Chen SJ, Zhou GB, Zhang XW, Mao JH, de The H, Chen Z. From an old remedy to a magic bullet: molecular mechanisms underlying the therapeutic effects of arsenic in fighting leukemia. Blood. 2011;117:6425–6437. doi: 10.1182/blood-2010-11-283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lallemand-Breitenbach V, Zhu J, Chen Z, de The H. Curing APL through PML/RARA degradation by As2O3. Trends Mol Med. 2012;18:36–42. doi: 10.1016/j.molmed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZJ. Ubiquitination in signaling to and activation of IKK. Immunol Rev. 2012;246:95–106. doi: 10.1111/j.1600-065X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 10.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Roderick JE, Hermance N, Zelic M, Simmons MJ, Polykratis A, Pasparakis M, et al. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. Proc Natl Acad Sci U S A. 2014;111:14436–14441. doi: 10.1073/pnas.1409389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16:689–697. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- 13.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 14.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schimmer AD. Apoptosis in leukemia: from molecular pathways to targeted therapies. Best Pract Res Clin Haematol. 2008;21:5–11. doi: 10.1016/j.beha.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 Inhibition Targets Oxidative Phosphorylation and Selectively Eradicates Quiescent Human Leukemia Stem Cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goff DJ, Court Recart A, Sadarangani A, Chun HJ, Barrett CL, Krajewska M, et al. A Pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell. 2013;12:316–328. doi: 10.1016/j.stem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su Z, Yang Z, Xie L, DeWitt JP, Chen Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016 doi: 10.1038/cdd.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014 Nov;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Fulda S. The mechanism of necroptosis in normal and cancer cells. Cancer Biol Ther. 2013;14:999–1004. doi: 10.4161/cbt.26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Bugno J, Hu C, Yang Y, Herold T, Qi J, et al. Eradication of Acute Myeloid Leukemia with FLT3 Ligand-Targeted miR-150 Nanoparticles. Cancer Res. 2016;76:4470–4480. doi: 10.1158/0008-5472.CAN-15-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisberg E, Ray A, Barrett R, Nelson E, Christie AL, Porter D, et al. Smac mimetics: implications for enhancement of targeted therapies in leukemia. Leukemia. 2010;24:2100–2109. doi: 10.1038/leu.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Servida F, Lecis D, Scavullo C, Drago C, Seneci P, Carlo-Stella C, et al. Novel second mitochondria-derived activator of caspases (Smac) mimetic compounds sensitize human leukemic cell lines to conventional chemotherapeutic drug-induced and death receptor-mediated apoptosis. Investigational new drugs. 2011;29:1264–1275. doi: 10.1007/s10637-010-9475-6. [DOI] [PubMed] [Google Scholar]
- 25.Steinhart L, Belz K, Fulda S. Smac mimetic and demethylating agents synergistically trigger cell death in acute myeloid leukemia cells and overcome apoptosis resistance by inducing necroptosis. Cell Death Dis. 2013;4:e802. doi: 10.1038/cddis.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nature reviews Drug discovery. 2012;11:109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 27.Volk A, Li J, Xin J, You D, Zhang J, Liu X, et al. Co-inhibition of NF-kappaB and JNK is synergistic in TNF-expressing human AML. J Exp Med. 2014 doi: 10.1084/jem.20130990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 29.Degterev A, Maki JL, Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20:366. doi: 10.1038/cdd.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Y, Li H, Zhang J, Volk A, Zhang S, Wei W, et al. TNF-alpha/Fas-RIP-1-induced cell death signaling separates murine hematopoietic stem cells/progenitors into 2 distinct populations. Blood. 2011;118:6057–6067. doi: 10.1182/blood-2011-06-359448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ball ED, Guyre PM, Shen L, Glynn JM, Maliszewski CR, Baker PE, et al. Gamma interferon induces monocytoid differentiation in the HL-60 cell line. J Clin Invest. 1984;73:1072–1077. doi: 10.1172/JCI111292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao S, Li D, Zhu HQ, Song MG, Pan XR, Jia PM, et al. RIG-G as a key mediator of the antiproliferative activity of interferon-related pathways through enhancing p21 and p27 proteins. Proc Natl Acad Sci U S A. 2006;103:16448–16453. doi: 10.1073/pnas.0607830103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mi JQ, Li JM, Shen ZX, Chen SJ, Chen Z. How to manage acute promyelocytic leukemia. Leukemia. 2012;26:1743–1751. doi: 10.1038/leu.2012.57. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Seet CS, Sun C, Li J, You D, Volk A, et al. p27 maintains a subset of leukemia stem cells in the quiescent state in murine MLL-leukemia. Mol Oncol. 2013 doi: 10.1016/j.molonc.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, et al. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci U S A. 2014;111:E3206–3213. doi: 10.1073/pnas.1407068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen DJ, Huerta S. Smac mimetics as new cancer therapeutics. Anticancer Drugs. 2009;20:646–658. doi: 10.1097/CAD.0b013e32832ced78. [DOI] [PubMed] [Google Scholar]
- 39.Bai L, Smith DC, Wang S. Small-molecule SMAC mimetics as new cancer therapeutics. Pharmacology & therapeutics. 2014;144:82–95. doi: 10.1016/j.pharmthera.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulda S. Inhibitor of Apoptosis (IAP) proteins in hematological malignancies: molecular mechanisms and therapeutic opportunities. Leukemia. 2014;28:1414–1422. doi: 10.1038/leu.2014.56. [DOI] [PubMed] [Google Scholar]
- 41.Dean E, Jodrell D, Connolly K, Danson S, Jolivet J, Durkin J, et al. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J Clin Oncol. 2009;27:1660–1666. doi: 10.1200/JCO.2008.19.5677. [DOI] [PubMed] [Google Scholar]
- 42.Schimmer AD, Estey EH, Borthakur G, Carter BZ, Schiller GJ, Tallman MS, et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J Clin Oncol. 2009;27:4741–4746. doi: 10.1200/JCO.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter BZ, Mak DH, Morris SJ, Borthakur G, Estey E, Byrd AL, et al. XIAP antisense oligonucleotide (AEG35156) achieves target knockdown and induces apoptosis preferentially in CD34+38− cells in a phase 1/2 study of patients with relapsed/refractory AML. Apoptosis. 2011;16:67–74. doi: 10.1007/s10495-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Rehak P, et al. Histologic tumor necrosis is an independent prognostic indicator for clear cell and papillary renal cell carcinoma. Am J Clin Pathol. 2012;137:283–289. doi: 10.1309/AJCPLBK9L9KDYQZP. [DOI] [PubMed] [Google Scholar]
- 45.You D, Xin J, Volk A, Wei W, Schmidt R, Scurti G, et al. FAK mediates a compensatory survival signal parallel to PI3K-AKT in PTEN-null T-ALL cells. Cell reports. 2015;10:2055–2068. doi: 10.1016/j.celrep.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallagher KK, Spector ME, Pepper JP, McKean EL, Marentette LJ, McHugh JB. Esthesioneuroblastoma: updating histologic grading as it relates to prognosis. The Annals of otology, rhinology and laryngology. 2014;123:353–358. doi: 10.1177/0003489414526368. [DOI] [PubMed] [Google Scholar]
- 47.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 48.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 49.Hockendorf U, Yabal M, Herold T, Munkhbaatar E, Rott S, Jilg S, et al. RIPK3 Restricts Myeloid Leukemogenesis by Promoting Cell Death and Differentiation of Leukemia Initiating Cells. Cancer Cell. 2016;30:75–91. doi: 10.1016/j.ccell.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 53.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014 doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 56.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.