Abstract
Lipoarabinomannan (LAM) lipoglycans have been characterized from a range of mycolic acid-containing actinomycetes and from the amycolate actinomycete Amycolatopsis sulphurea. To further understand the structural diversity of this family, we have characterized the lipoglycan of the otic commensal Turicella otitidis. T. otitidis LAM (TotLAM) has been determined to consist of a mannosyl phosphatidylinositol anchor unit carrying an (α 1→6)-linked mannan core and substituted with terminal-arabinosyl branches. Thus, TotLAM has a novel truncated LAM structure. Using the human monocytic THP-1 cell line, it was found that TotLAM exhibited only minimal ability to induce tumor necrosis factor alpha. These findings contribute further to our understanding of actinomycete LAM diversity and allow further speculation as to the correlation between LAM structure and the immunomodulatory activities of these lipoglycans.
The cell envelopes of gram-positive bacteria contain structurally diverse macroamphiphilic membrane-anchored polymers that can be classified as either lipoteichoic acids or lipoglycans (11, 54, 56). Although considered to play important roles, the functions of these macromolecules remain unknown and it is not known if a common function underlies their structural diversity. Lipoteichoic acids predominate in the Bacillus-Streptococcus-Clostridium (Firmicutes) lineage of gram-positive bacteria, whereas Mollicutes and actinomycete bacteria typically synthesize lipoglycans (54). Actinomycete lipoglycans can be classified into a number of structural archetypes (54), of which the most extensively studied are the mycobacterial lipoarabinomannans (LAM) (6, 7, 39). LAM-like lipoglycans have also been identified in phylogenetically close relatives of the mycobacteria, which share common cell envelope features dominated by the presence of mycolic acids (13, 52, 55), and three lipoglycans from this taxon (the mycolata) have been recently characterized as structurally related members of a LAM family: ReqLAM from the equine pathogen Rhodococcus equi (18), RruLAM from Rhodococcus ruber (22), and TpaLAM from Tsukamurella paurometabola (20). Moreover, an additional representative of the LAM family (AsuLAM) has been characterized from the more distantly related actinomycete Amycolatopsis sulphurea (21), which lacks mycolic acids. In each of the LAM-like lipoglycans, the carbohydrate domain consists of a linear (α 1→6) mannan backbone and an arabinan portion either consisting of few units glycosylating the mannan core or organized as an independent domain, as observed in mycobacteria.
The mycolata lineage also encompasses a few species that appear to have lost the ability to synthesize mycolates, including Turicella otitidis, the type species of the monospecific genus Turicella (16, 17). T. otitidis is part of the normal flora of the ear (15, 29, 51) that may cause opportunistic infections such as acute otitis media (8, 16, 17, 44, 45, 48). However, in contrast to reports that it is an exclusively otic organism, it has also been isolated in a case of bacteremia (33) and from environmental sources (49). T. otitidis apparently shares other cell envelope features with the mycolata. For example, arabinose and galactose as cell wall sugars suggest the presence of an arabinogalactan wall polysaccharide. However, little is known of its precise cell envelope composition. In the present study, we have undertaken the identification and characterization of the lipoglycan present in T. otitidis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The type strain of T. otitidis, DSM 8821, was obtained from the Deutsche Sammlung für Mikroorganismen (Braunschweig, Germany) culture collection. Bacteria were grown in brain heart infusion broth (Oxoid; Unipath Ltd., Basingstoke, United Kingdom) containing 2% (wt/vol) yeast extract at 37°C with shaking (200 rpm). Large-scale cultures were grown overnight and harvested by centrifugation (3,600 × g, 15 min, 4°C). The cell pellets were washed with phosphate-buffered saline and freeze-dried for extraction.
Lipoglycan extraction and purification.
Lyophilized cells were delipidated with chloroform-methanol (1:1 [vol/vol]; 50 mg/ml) at room temperature for 18 h. Cell pellets were recovered by centrifugation (3,600 × g, 10 min), washed twice with phosphate-buffered saline, and permeabilized (1) with mutanolysin (50 U/ml) and lysozyme (25 mg/ml). Lipoglycan was then extracted from the cell suspension by the hot phenol-water method, as described previously (18, 52). Following dialysis to remove phenol traces, the crude aqueous extract containing lipoglycan was lyophilized.
Lipoglycan was purified by hydrophobic interaction chromatography (HIC) as described previously (18, 52). Briefly, the freeze-dried extract was resuspended in 100 mM sodium acetate buffer (pH 4.5) containing 15% (vol/vol) n-propanol and applied to an octyl-Sepharose CL-4B (Amersham Biosciences, United Kingdom) column (1.25 by 17 cm) equilibrated with the same buffer. The column was washed through with 40 ml of this buffer prior to gradient elution with 15 to 65% n-propanol in 100 mM sodium acetate (pH 4.5). Column fractions (ca. 4 ml) were analyzed for carbohydrate and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining. Lipoglycan-containing fractions were pooled, dialyzed to remove propanol, and lyophilized. Contaminants were then removed by gel filtration. The sample (7.7 mg) was dissolved in a mixture of 0.2 M NaCl, 0.25% (wt/vol) sodium deoxycholate (DOC), 1 mM EDTA, and 10 mM Tris, pH 8, to a final concentration of 200 mg/ml, incubated for 2 days at room temperature, and loaded onto a gel permeation Bio-Gel P-100 column (52 by 3 cm) eluted with the same buffer at a flow rate of 5 ml/h.
Analytical methods.
Carbohydrate was assayed by the method of Fox and Robyt (14). Lipoglycan carbohydrate composition was also examined by gas chromatography (GC) as described previously (52), following hydrolysis with 2 M trifluoroacetic acid (TFA; 2 h, 110°C), neutralization over sodium hydroxide pellets, and derivatization to alditol acetates (46). For permethylation analysis, lipoglycan (1.1 mg) was deacylated according to the method of Beachey et al. (5) followed by permethylation according to the method of Dell et al. (9). The permethylated samples were cleaned with a C18 Sep-Pak cartridge (Waters Ltd., Watford, United Kingdom), hydrolyzed, and acetylated as described previously (18).
Lipoglycan fatty acid composition was determined by GC of fatty acid methyl esters (FAME) released by acid-catalyzed methanolysis, as described previously (52). SDS-PAGE and Western blotting were performed as described previously (52). Lipoglycan was detected by silver staining with a periodic acid oxidation step to enhance polysaccharide staining (58).
Acetolysis procedures.
Ten micrograms of lipoglycan (T. otitidis LAM [TotLAM]) was treated with 15 μl of an acetic anhydride-acetic acid-sulfuric acid (10:10:1 [vol/vol/vol]) mixture for 3 h at 40°C. The reaction was quenched by addition of 40 μl of water. Acetolysis products were extracted twice with 40 μl of chloroform and after drying were deacetylated with 20 μl of a methanol-20% aqueous ammonia solution (1:1 [vol/vol]) at 37°C for 18 h. The reagents were removed under a stream of nitrogen. The samples were then submitted to 1-aminopyrene-3,6,8-trisulfonate (APTS) tagging (see below).
APTS derivatization.
Lipoglycan (1 μg) was hydrolyzed with strong acid hydrolysis (20 μl of 2 M TFA at 110°C for 2 h), mild hydrolysis (20 μl of 0.1 M HCl at 110°C for 30 min), very mild hydrolysis (20 μl of 0.01 M HCl at 110°C for 30 min), or acetolysis (described above). The samples were then dried and mixed with 0.4 μl of 0.2 M APTS (Interchim, Montluçon, France) in 15% (vol/vol) acetic acid and 0.4 μl of a 1 M sodium cyanoborohydride solution dissolved in tetrahydrofuran (28). The reaction was performed for 90 min at 55°C and was quenched by addition of 20 μl of water. Dilutions of 1 to 5 μl of the APTS derivatives were prepared in a total of 20 μl of water before analysis by capillary electrophoresis (CE).
CE.
The electropherograms were acquired and stored on a Dell XPS P60 computer, using the System Gold software package (Beckman Instruments, Inc.). APTS derivatives were loaded by applying a 0.5-lb/in2 (3.45 kPa) vacuum for 5 s (6.5 nl injected). Separations were performed with an uncoated fused-silica capillary column (Sigma, Division Supelco, Saint-Quentin-Fallavier, France) with a 50-μm internal diameter and 40-cm effective length (47-cm total length). Analyses were usually performed on a P/ACE capillary electrophoresis system (Beckman Instruments, Inc.) with the cathode on the injection side and the anode on the detection side (reverse polarity). They were carried out at a temperature of 25°C with an applied voltage of −20 kV and using acetic acid 1% (wt/vol)-30 mM triethylamine in water, pH 3.5, as the running electrolyte. For Fig. 3B, the electropherogram was recorded in normal mode, at a temperature of 25°C with an applied voltage of +25 kV and borate buffer (20 mM, pH 9.2) as a running electrolyte. The detection system consisted of a Beckman laser-induced fluorescence (LIF) device equipped with a 4-mW argon-ion laser with an excitation wavelength of 488 nm and emission wavelength filter of 520 nm.
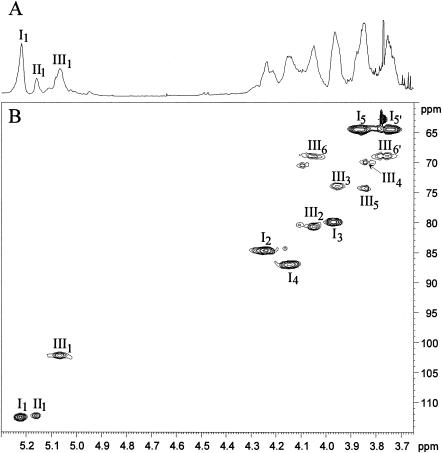
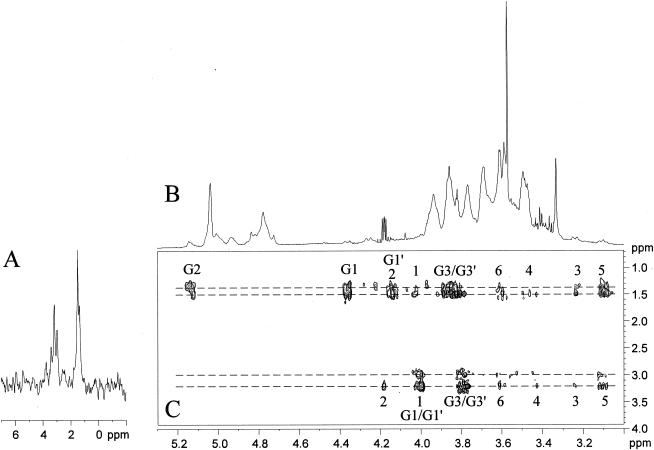
FIG. 3.
Expanded regions of the 1D 1H spectrum (δ 1H, 3.65 to 5.30) (A) and of 2D 1H-13C HMQC spectrum (δ 1H, 3.65 to 5.30; and δ 13C, 60 to 115) (B) in D2O at 313 K of the TotLAM. I, t-α-Araf; II, t-α-Araf; III, 2,6-α-Manp.
NMR spectroscopy.
Prior to nuclear magnetic resonance (NMR) spectroscopic analysis, fractions were exchanged in D2O (99.9% purity; Eurisotop, Saint Aubin, France) at room temperature with intermediate freeze-drying and then dissolved in 400 μl of Me2SO-d6 (99.8% purity; Eurisotop, Saint Aubin, France). TotLAM (7 mg) was analyzed in 535-PP NMR tubes (200 by 5 mm) at 313 K on a Bruker DMX-500 500-MHz NMR spectrometer equipped with a double-resonance (1H/X)-BBi z-gradient probe head. Data were processed on a Bruker-X32 workstation using the xwinnmr program. Proton and carbon chemical shifts are expressed in ppm downfield from dimethyl sulfoxide (δH/tetramethylsilane [TMS] 2.52 and δC/TMS 40.98). The one-dimensional (1D) phosphorus (31P) spectra were measured at 202.46 MHz, and phosphoric acid (85%) was used as the external standard (δP 0.0). All 2D NMR data sets were recorded without sample spinning, and data were acquired in the phase-sensitive mode by the time-proportional phase increment method (34). Four 2D homonuclear Hartmann-Hahn (HOHAHA) spectra were recorded with MLEV-17 mixing sequences of 113 ms (2). The 1H-13C and 1H-31P single-bond correlation spectra (heteronuclear multiple quantum coherence [HMQC]) were obtained with Bax's pulse sequence (3). The GARP sequence (47) at the carbon or phosphorus frequency was used as a composite pulse decoupling during acquisition. The pulse sequence used for 1H-detected heteronuclear relayed spectra (HMQC-HOHAHA) was that of Lerner and Bax (32) and the pulse sequence used for homonuclear multiple bond coherence (HMBC) was that of Bax and Summers (4).
TNF-α production by macrophages.
The THP-1 macrophage human cell line was maintained as nonadherent cells in continuous culture with RPMI 1640 medium (Life Technologies) in 10% fetal calf serum (Life Technologies) in an atmosphere of 5% CO2 at 37°C. The various stimuli were added at 10 or 20 μg/ml in duplicate to macrophage cells (5 × 105 cells/well) in 24-well culture plates and then incubated for 20 h at 37°C. Three independent assays were conducted; one representative experiment is shown. Supernatants were assayed for TNF-α by sandwich enzyme-linked immunosorbent assay using commercially available kits and according to the manufacturer's instructions (R&D Systems). Lipopolysaccharide (LPS) was from Escherichia coli O55:B5 (Sigma), ManLAM and lipomannan (LM) were from Mycobacterium bovis BCG, and ReqLAM was from Rhodococcus equi (18).
RESULTS
Purification and initial characterization of the T. otitidis lipoglycan.
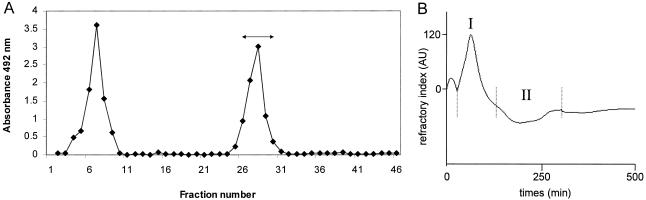
Lipoglycan was extracted from lyophilized and delipidated cells of T. otitidis by a conventional phenol-water extraction method that has been used for the extraction of macroamphiphiles such as LPS, lipoteichoic acids, and lipoglycans, including mycobacterial LAM (13, 18, 31, 52, 61). Purified lipoglycan was recovered from the crude extract by HIC, as shown in Fig. 1A. Initial elution of the HIC column removed hydrophilic contaminant material, and a single carbohydrate-containing peak was retained by the column until gradient eluted with n-propanol. Fractions 26 to 30 were pooled after verification by SDS-PAGE that these fractions contained a diffuse band centered around ca. 20 kDa, which is comparable to the electrophoretic mobility of the truncated LAM of R. equi (18).
FIG. 1.
Purification of the lipoglycan fraction from T. otitidis. (A) Crude phenol extract was loaded onto the HIC column and washed through until fraction 11 to remove hydrophilic contaminant material. Gradient elution with increasing concentrations of propanol (15 to 65% [vol/vol]; fractions 11 to 40) was used to recover one peak of interest. Fractions from 41 onwards were eluted with 65% (vol/vol) propanol. Fractions (4 ml) were monitored by assay for carbohydrate (♦). (B) Gel filtration analysis in DOC buffer of the HIC-purified T. otitidis lipoglycan. Fractions of interest (I) were identified by SDS-PAGE analysis and pooled. Other fractions analyzed (II) did not contain lipoglycan. AU, arbitrary units.
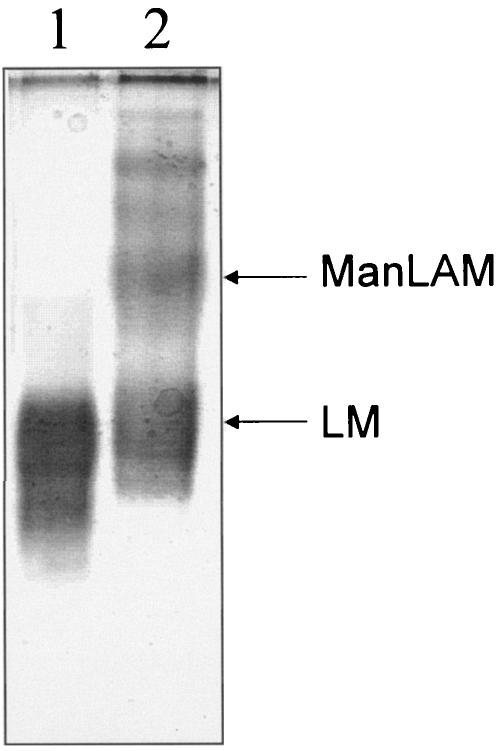
Subsequently, NMR studies revealed that this fraction was still contaminated by small mannose-containing molecules. This fraction was further purified by gel filtration in presence of DOC buffer. The gel filtration chromatographic profile contained two peaks, I and II (Fig. 1B). Peak I was assigned to the putative LAM, based on its electrophoretic mobility on SDS-PAGE (Fig. 2), which is distinct from that of Mycobacterium tuberculosis strains and M. bovis BCG ManLAM. This mobility is in agreement with a matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) spectrum showing a broad peak centered at m/z 9,000 (data not shown) indicating a molecular mass for the most abundant molecular species of 9 kDa. A molecular mass around 17 kDa was established for the BCG ManLAM (41, 59), whereas MALDI-TOF MS has established similar molecular masses for other LAM lipoglycans, notably ReqLAM (ca. 8 kDa) (18) and AsuLAM (ca. 10 kDa) (21).
FIG. 2.
SDS-PAGE analysis of T. otitidis and related M. bovis BCG lipoglycans. The gel was stained with a silver stain containing periodic acid. Lane 1, lipoglycan-containing fraction from T. otitidis; lane 2, LM and ManLAM from M. bovis BCG.
The carbohydrate composition of the lipoglycan was investigated by GC and CE following acid hydrolysis (TFA, 2 M, 2 h at 110°C) and appropriate derivatizations. In both cases, only two peaks were observed, which identified arabinose and mannose. The relative composition of the polysaccharidic backbone was determined by peak integration of CE signals as 44% arabinose and 56% mannose (data not shown). Minor amounts of glycerol and inositol were also detected by GC and GC-MS. The fatty acid composition of the HIC-purified lipoglycan was analyzed by acid hydrolysis followed by GC of the FAME (data not shown). The predominant FAME recovered were methyl palmitate (C16:0; 41%), methyl stearate (C18:0; 21%), and methyl octadecenoate (C18:1; 32%). The lipoglycan FAME profile was similar to that of the whole bacterial cells (data not shown), except that minor FAME, including 10-methyloctadecanoate (tuberculostearate; C19:0), were not detected. The FAME profile was consistent with the previously reported fatty acid composition of T. otitidis (16, 17).
In summary, the presence of arabinose and mannose, as well as glycerol, inositol and fatty acids, the basic components of a phosphatidyl-myo-inositol anchor, provided the first indications of a lipoglycan with a structure related to mycobacterial LAM. The lipoglycan was subsequently termed TotLAM. However, Western blot analysis revealed that the lipoglycan did not cross-react significantly with a polyclonal anti-LAM antibody raised against ManLAM from M. tuberculosis strain H37Rv (data not shown).
Structural characterization of the TotLAM polysaccharidic backbone.
The glycosidic linkages present in TotLAM were analyzed by per-O-methylation analysis. The partially per-O-methylated, per-O-acetylated alditols identified by GC-MS were terminal-arabinofuranose (t-Araf) and 2,6-mannopyranose (Manp).
Purified TotLAM was then analyzed by NMR in order to precisely determine the saccharidic domain. The 1D 1H-NMR anomeric zone, recorded with TotLAM dissolved in D2O (Fig. 3A), was composed of several signals, assignment of which required 2D NMR experiments, as 1H-13C HMQC (Fig. 3B) and 1H-1H HOHAHA with different mixing times (not shown). Then the different spin systems which compose the TotLAM were characterized (Table 1), and the sequences of these monosaccharidic units were established through 1H-1H nuclear Overhauser effect spectroscopy (NOESY) experiments. This strategy was derived from previous NMR analysis in D2O of the ReqLAM of R. equi (18) and from the ManLAM (unpublished data) and ManAM (36) of the mycobacterial strain M. bovis BCG.
TABLE 1.
Proton and carbon chemical shifts of TotLAM
| Residue | Chemical shift (ppm)a
|
|||||
|---|---|---|---|---|---|---|
| H-1 C-1 | H-2 C-2 | H-3 C-3 | H-4 C-4 | H-5(/H-5′) C-5 | H-6/H-6′ C-6 | |
| t-α-Araf (I) | 5.22 | 4.24 | 3.97 | 4.15 | 3.87/3.74 | ND |
| 112.5 | 84.6 | 79.9 | 87.0 | 64.5 | ||
| t-α-Araf (II) | 5.17 | 4.22 | 3.96 | 4.12 | 3.85/3.74 | ND |
| 112.2 | 84.8 | |||||
| 2,6-O-α-Manp (III) | 5.06 | 4.05 | 3.96 | 3.83 | 3.84 | 4.06/3.77 |
| 102.2 | 80.6 | 73.9 | 70.0 | 74.3 | 68.9 | |
Chemical shifts were measured at 313 K in D2O. ND, not determined.
The anomeric proton resonance region of TotLAM is dominated by three intense signals at δ 5.22 (I), δ 5.17 (II), and δ 5.06 (III) (Fig. 3A). As revealed by the 1H-13C HMQC experiment (Fig. 3B), their corresponding anomeric carbons resonate at δ 112.5 (I), δ 112.2 (II), and δ 102.2 (III), respectively. The spin system I was unambiguously assigned to Araf as the spin system could be defined up to H-5/C-5 (Table 1). The chemical shifts of the protons and carbons proved that this unit is terminal. The furanose ring could be deduced from the C-4 chemical shift and from the intense cross peak observed on the 1H-13C HMBC spectrum between H-1 (δ 5.22) and C-4 (δ 87.0) and between C-1 (δ 112.5) and H-4 (δ 4.15) (not shown). The α-anomeric configuration was deduced from the C-1 chemical shift at δ 112.5 compared to α-Araf units observed around 110 ppm and α-Araf units (α-Araf δC-1 103.4) in mycobacterial LAM (24). Therefore, unit I was unambiguously characterized as t-α-Araf. Unit II could only be defined up to H-5 and C-2 (Table 1). All of the defined protons and carbons were very close to the corresponding one of system I. Therefore, this unit was also characterized as t-α-Araf. The spin system III was assigned to 2,6-α-Manp according to the following evidence. The different chemical shifts (Table 1) were deduced from the 1H-13C HMQC-HOHAHA spectra (data not shown), as the HOHAHA spectrum did not allow the characterization of all the protons. Glycosylations at positions 2 and 6 were evidenced by the deshielding of the corresponding carbon resonances at δ 80.6 for C-2 and at δ 68.9 for C-6 (Table 1) compared to unsubstituted t-α-Manp in mycobacterial arabinomannan (36) (Δδ 7.5 ppm for C-2 and 4.7 ppm for C-6). The 1H and 13C chemical shifts of this system were found to be very similar to the ones described for the 2,6-α-Manp of mycobacterial arabinomannan (36). The α-anomeric configuration was deduced from the values of the one bound coupling constant (1JC1, H1) around 170 Hz, measured on a nondecoupled 1H-13C HMQC spectrum (data not shown).
Subsequently, the sequence of the different units was investigated by 1H-1H NOESY (data not shown). Interestingly, H-1 of the t-α-Araf unit (I) at δ 5.22 showed an intense interresidue nOe contact with H-2 of the 2,6-α-Manp unit (III) at δ 4.05, indicating that t-α-Araf units glycosylated 2,6-α-Manp units at their O-2 position.
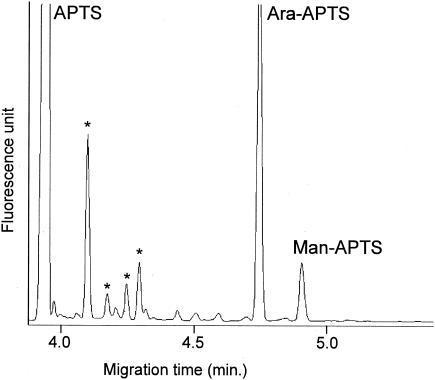
NMR data indicated that the main structural feature of the TotLAM carbohydrate backbone is a completely branched polymer composed of an (α 1→6) Manp chain replaced at O-2 by single t-α-Araf units. Nevertheless, from these data, we could not exclude the presence in very small abundance of other structural sequences found in LAM-like molecules, such as [(t-Manp)n→Araf], (Araf→Araf), or (t-Manp→Manp). The absence of [(t-Manp)n→Araf] sequence was verified by TotLAM mild acidic hydrolysis (0.1 M HCl for 30 min at 110°C) followed by APTS derivatization and CE analysis (37). Mild hydrolysis leads to selective cleavage of Araf links and release of oligomannosides with one Ara unit at the reducing end (23, 35, 41). The electropherogram (Fig. 4) was dominated by the peak assigned to Ara-APTS. Man-APTS was found in a small amount, but no peak corresponding to oligosaccharide APTS was detected, supporting the absence of t-Manp→Araf sequence. Subsequently, the absence of Araf→Araf sequence was confirmed by very mild acidic hydrolysis (0.01 M HCl for 30 min at 110°C) of TotLAM followed by APTS derivatization and CE analysis. The resulting electropherogram (data not shown) was similar to that in Fig. 4 and showed only two peaks corresponding to Ara-APTS and Man-APTS, supporting the absence of oligoarabinoside sequence. Then attempts were made to detect (Manp→2Manp) sequence, which would correspond to Manp units directly substituting for the (α 1→6) Manp chain. TotLAM was submitted to acetolysis, followed by deacetylation, APTS derivatization, and CE analysis. Indeed, under particular conditions, acetolysis allows preferential cleavage of 6-O-linked hexopyranose and Araf linkages but keeps intact (α 1→2) Manp linkages (41). Only two peaks with similar intensity were observed, which were assigned to Ara-APTS and Man-APTS. No peak which would have allowed confirmation of (Manp→2Manp) sequence could be detected under the different conditions of acetolysis. Cumulatively, these data allowed us to propose the structural model depicted in Fig. 5.
FIG. 4.
CE-LIF electropherograms of monosaccharide and oligosaccharide APTS derivatives resulting from partial acid hydrolysis of TotLAM. Peaks labeled with asterisks arise from the reagent.
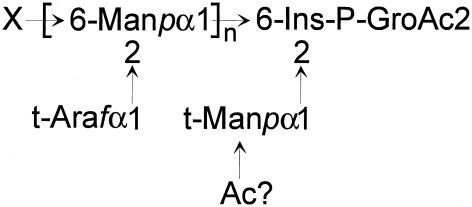
FIG. 5.
Structural model of TotLAM. From the molecular mass given by MALDI-MS, n could be estimated to 28.
Structural characterization of the TotLAM anchor unit.
The structure of the mannosyl phosphatidylinositol (MPI) anchor was investigated by 1D 31P and 2D 1H-31P NMR experiments in Me2SO-d6. Indeed, it was shown recently that Me2SO-d6 is a suitable solvent for recording high-resolution 1D 31P NMR spectra of multiacylated mycobacterial ManLAM (23). The 1D 31P spectrum (Fig. 6A) of TotLAM dissolved in Me2SO-d6 exhibited four main resonances at 1.37, 1.50, 2.97, and 3.18 ppm. The 31P resonance assignments were performed with the help of 2D 1H-31P NMR spectroscopy. The 1H-31P HMQC-HOHAHA spectrum of TotLAM (Fig. 6b) exhibited four lines of correlations. The two phosphates at 1.37 and 1.50 ppm were derived from esterified diacylated glycerol, while the phosphates at 2.97 and 3.18 ppm were derived from esterified monoacyl glycerol. Indeed, the diacylated glycerol units are typified by the presence of the deshielded glycerol H-2 proton at 5.14 ppm and the deshielded glycerol H-1 protons at 4.36/4.15 ppm (25) (Table 2). By analogy to mycobacterial ManLAM (38), these phosphates were labeled P3 (di-acyl) and P5 (mono-acyl). In all of these cases, the myo-inositol unit was never acylated, as proven by the H-3 resonance of the myo-inositol at 3.27 ppm (compared to an expected resonance around 4.60 ppm when acylated). However, the presence of two lines of correlation for both the mono- and diacylated glycerol anchors is consistent with the additional acylation of the putative Manp at the 0-2 position of the myo-inositol unit, although this is not definitively proven.
FIG. 6.
Expanded regions of the 1D 31P spectrum (δ 31P, −2 to 7) (A) and 1D 1H spectrum (δ 1H, 5.3 to 3.0) and 2D 1H-31P HMQC-HOHAHA spectrum (δ 31P, 0.7 to 4.0; δ 1H, 5.3 to 3.0) (B) of the TotLAM in Me2SO-d6 at 343 K. Numerals alone correspond to the proton number of the myo-inositol units, and numerals following the letter G correspond to the proton number of the glycerol units.
TABLE 2.
P3/P5 Gro and myo-insositol 1H chemical shifts of TotLAM
| Phosphatea | Chemical shift (ppm)b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
myo-Inositol
|
Groc
|
||||||||
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | H-1/H-1′ | H-2 | H-3/H-3′ | |
| P-3 | 4.02 | 4.15 | 3.23 | 3.46 | 3.10 | 3.60 | 4.36/4.15 | 5.14 | 3.85 |
| P-5 | 4.01 | 4.18 | 3.24 | 3.43 | 3.10 | 3.61 | 4.01 | 3.80 | |
Chemical shifts were measured at 343 K in Me2SO-d6.
By analogy to mycobacterial ManLAM, the phosphates associated with a diacyl anchor were labeled P3, whereas those associated with a mono-acyl anchor were labeled P5.
Gro, glycerol.
TNF-α production by macrophages.
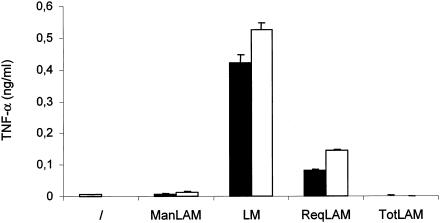
The potency of TotLAM, in comparison to M. bovis BCG ManLAM and LM and R. equi ReqLAM, to stimulate the production of TNF-α was investigated with human THP-1 macrophage cell lines. As expected, LM (43) and ReqLAM (18) induced the production of TNF-α by THP-1 cells in a dose-dependent fashion (Fig. 7). In contrast, TotLAM, tested at concentrations of 10 or 20 μg/ml, showed no TNF-α-inducing activity. Indeed, the amount of TNF-α elicited by TotLAM was even smaller than that induced by M. bovis BCG ManLAM, which is already known to be a poor inducer of proinflammatory cytokines (24, 40, 59).
FIG. 7.
TNF-α production by THP-1 cells in response to various stimuli. ManLAM, LM, ReqLAM, and TotLAM were tested at 10 (black bars) and 20 (white bars) μg/ml. ManLAM and LM were from M. bovis BCG, and ReqLAM was from R. equi. /, medium alone.
DISCUSSION
The present study has allowed the characterization of a novel member of the LAM family of lipoglycans (Fig. 5). TotLAM is comparable to ReqLAM and RruLAM in that its arabinan domain is confined to the presence of t-Araf residues, but its structure is distinguished by the apparent absence of t-Manp associated with the mannan core.
Several themes are now emerging from our detailed understanding of the structures of the LAM lipoglycan family. Consistently these macromolecules have been shown to consist of an MPI anchor unit and an (α1→6) Manp core (18, 20, 22, 39), although in some cases this can be quite short (21). However, there is evidently considerable variation in the nature of the substituting arabinan domains, varying from single t-Ara substituents in ReqLAM (18), RruLAM (22), and TotLAM (Fig. 5) to the more elaborate branched arabinan domain of AsuLAM (21) and the linear arabinan of TpaLAM (20). Thus, the LAM family can be subdivided into truncated LAM types (ReqLAM, RruLAM, and TotLAM) or variant LAM (AsuLAM, TpaLAM, and LAM from Gordonia bronchialis; N. Garton and I. C. Sutcliffe, unpublished data) with structures more typical of (albeit distinct from) the mycobacterial prototypes (Table 3).
TABLE 3.
Summary of LAM family lipoglycan characteristics
| Organism and LAM | Size (kDa) from MALDI-TOF MS | TNF-α induction | Structural comments | Source or reference |
|---|---|---|---|---|
| A. sulfurea AsuLAM | ∼10 | Minimalb | Short branched mannan core with t-Man, branched arabinan, ∼1 cap per LAM | 21 |
| R. equi ReqLAM | ∼8 | Moderate | Truncated LAM, t-Ara substituents on branched mannan core, some t-Man, no caps | 18; this study |
| R. ruber RruLAM | ∼7a | Minimal | Truncated LAM, t-Ara and t-Man substituents on branched Man core; no caps | 22 |
| T. paurometabola TpaLAM | ∼12.5 | Moderate; increased by removing arabinan | Short unbranched mannan core; linear arabinan; oligomannose substitutents | 20 |
| T. otitidis TotLAM | ∼9 | Minimal | Truncated LAM, t-Ara substituents on branched mannan core; t-Man not detected; no caps | This study |
Value calculated from the deduced structure.
Extent of TNF-α induction judged in relation to that induced by mycobacterial ManLAM and LPS as reported in the original studies cited and as shown in Fig 7 in the present study.
The genetic basis for the evident diversity of LAM types can be proposed to primarily reflect a discontinuous distribution of arabinosyltransferases which has no clear parallel with the current taxonomic structure of the actinomycetes. Further structural diversity is imparted to the LAM family by the variable distribution of different capping motifs. Thus, mycobacterial LAM may be classified as either AraLAM (27), ManLAM, or PILAM (24), depending on the absence of caps or the presence of either mannose oligosaccharide or phosphoinositol caps (39). Moreover, a variety of other substituents, such as succinate and 5-methylthiopentose, have also been identified (10, 26, 57). Mannose oligosaccharide capping motifs are also evident in the variant LAM of A. sulphurea (21) and T. paurometabola (20) but have not yet been reported in the representatives of the truncated LAM subfamily.
Less-well-characterized LAM family lipoglycans have been identified in a number of other representatives of the mycolata (12, 13, 19, 30, 42, 52, 53; Sutcliffe and Garton, unpublished data). It thus seems likely that the structural diversity of the LAM family has yet to be fully defined. Typically, cross-reactions with polyclonal anti-ManLAM sera are evident with variant LAM types (e.g., see reference 13), whereas these are not generally apparent with truncated LAM types (e.g., see reference 18 and this study). It is also apparent from these studies that there can be considerable intrageneric diversity in the LAM-like molecules present. Thus, rhodococci synthesize both truncated LAM (ReqLAM and RruLAM) and variant LAM (12), and there are differences apparent in the lipoglycan compositions of corynebacteria (19, 42). An additional issue with respect to lipoglycan diversity and function is that in several actinomycetes producing variant LAM there is preliminary evidence (12, 13, 21, 42) for the production of a distinct LM fraction, as in mycobacteria. However, distinct LM lipoglycans have yet to be identified in those members of the mycolata synthesizing truncated LAM (18, 52; this study).
The lipoglycans of pathogens, notably mycobacteria, are of considerable interest as potential immunomodulators (6, 7, 39, 50). The ability of several well-characterized members of the LAM family to stimulate TNF-α production has been investigated, and recently it has been hypothesized that the proinflammatory activities of these lipoglycans derive from the LM core structure and that this activity is reduced when the LM core is sterically masked by a significant arabinan domain (6). Perhaps most strikingly in this respect, Vignal et al. (60) have demonstrated that progressive chemical hydrolysis of Ara residues from the LAM of Mycobacterium kansasii led to increased capacity to induce TNF-α induction. Likewise, the intact TpaLAM variant gave a moderate TNF-α induction, yet removal of the arabinan domain by mild acid hydrolysis (yielding the LM core) significantly increased its immunostimulatory capability (20). However, the precise structural basis for immunostimulation remains obscure, as there is minimal TNF-α induction by some truncated LAM (RruLAM and TotLAM) and variant LAM (AsuLAM), whereas other truncated LAM (ReqLAM) give moderate induction (Fig. 7) (18, 21, 22). As the immunostimulatory activities of these molecules have typically been studied with monocytic cell lines such as THP-1 (20-22; this study), further studies with primary human cells are now desirable.
The widespread distribution and structural diversity of the LAM family of lipoglycans also have implications for the function(s) of these macromolecules. Mycobacterial LAM structural types have yet to be clearly correlated with the virulence of the parent mycobacteria, and these lipoglycans are clearly synthesized by bacteria from a wide range of ecological niches, including marine and soil environments (Rhodococcus and Tsukamurella); human, insect, and fish commensals (Corynebacterium matruchotii, Dietzia maris, Rhodococcus rhodnii, T. paurometabola, and T. otitidis); opportunist pathogens such as gordoniae and tsukamurellae; as well as human and animal pathogens. Although their precise role(s) remains elusive, this widespread distribution suggests a fundamental contribution to actinomycete cell envelope biology independent of pathogenic potential. However, it is notable that a lipoglycan-less mutant of Corynebacterium glutamicum has apparently been constructed in vitro (19).
The present study has extended our knowledge of the structural diversity of the LAM family of lipoglycans and of their immunostimulatory capabilities (Table 3). It is also significant that, as in A. sulphurea, TotLAM is localized within a cell envelope lacking mycolic acids. Thus the distribution of the LAM family of lipoglycans does not correlate exclusively with the presence of a mycolate cell envelope, even though these lipoglycans are clearly widely distributed throughout the mycolata. Determination of the function of these enigmatic molecules remains a significant goal for future studies.
Acknowledgments
We are grateful to John Belisle (Department of Microbiology, Colorado State University) for supplying polyclonal anti-LAM antibody (under NIH sponsorship N01-AI-25147).
This project was supported by The Wellcome Trust (grant 04762/Z/96).
REFERENCES
- 1.Assaf, N. A., and W. A. Dick. 1993. Spheroplast formation and plasmid isolation from Rhodococcus spp. BioTechniques 15:1010-1012, 1014-1015. [PubMed] [Google Scholar]
- 2.Bax, A., and D. G. Davis. 1985. MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J. Magn. Reson. 65:355-360. [Google Scholar]
- 3.Bax, A., and S. Subramanian. 1986. Sensitivity-enhanced two-dimensional heteronuclear shift correlation NMR spectroscopy. J. Magn. Reson. 67:565-569. [Google Scholar]
- 4.Bax, A., and M. F. Summers. 1986. H-1 and C-13 assignments from sensitivity enhanced detection of heteronuclear multiple bound connectivity by 2D multiple quantum NMR. J. Am. Chem. Soc. 108:2093-2094. [Google Scholar]
- 5.Beachey, E. H., J. B. Dale, W. A. Simpson, J. D. Evans, K. W. Knox, I. Ofek, and A. J. Wicken. 1979. Erythrocyte binding properties of streptococcal lipoteichoic acids. Infect. Immun. 23:618-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briken, V., S. A. Porcelli, G. S. Besra, and L. Kremer. 2004. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol. Microbiol. 53:391-403. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee, D., and K. H. Khoo. 1998. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 8:113-120. [DOI] [PubMed] [Google Scholar]
- 8.Dana, A., R. Fader, and D. Sterken. 2001. Turicella otitidis mastoiditis in a healthy child. Pediatr. Infect. Dis. J. 20:84-85. [DOI] [PubMed] [Google Scholar]
- 9.Dell, A., A. J. Reason, K. H. Khoo, M. Panico, R. A. McDowell, and H. R. Morris. 1994. Mass spectrometry of carbohydrate-containing biopolymers. Methods Enzymol. 230:108-132. [DOI] [PubMed] [Google Scholar]
- 10.Delmas, C., M. Gilleron, T. Brando, A. Vercellone, M. Gheorghui, M. Riviere, and G. Puzo. 1997. Comparative structural study of the mannosylated-lipoarabinomannans from Mycobacterium bovis BCG vaccine strains: characterization and localization of succinates. Glycobiology 7:811-817. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, W. 1994. Lipoteichoic acids and lipoglycans, p. 199-215. In J. M. Ghuysen and R. Hakenbeck (ed.), New comprehensive biochemistry: bacterial cell wall, vol. 27. Elsevier Science, Amsterdam, The Netherlands.
- 12.Flaherty, C., D. E. Minnikin, and I. C. Sutcliffe. 1996. A chemotaxonomic study of the lipoglycans of Rhodococcus rhodnii N445 (NCIMB 11279). Zentbl. Bakteriol. 285:11-19. [DOI] [PubMed] [Google Scholar]
- 13.Flaherty, C., and I. C. Sutcliffe. 1999. Identification of a lipoarabinomannan-like lipoglycan in Gordonia rubropertincta. Syst. Appl. Microbiol. 22:530-533. [DOI] [PubMed] [Google Scholar]
- 14.Fox, J. D., and J. F. Robyt. 1991. Miniaturization of three carbohydrate analyses using a microsample plate reader. Anal. Biochem. 195:93-95. [DOI] [PubMed] [Google Scholar]
- 15.Frank, D. N., G. B. Spiegelman, W. Davis, E. Wagner, E. Lyons, and N. R. Pace. 2003. Culture-independent molecular analysis of microbial constituents of the healthy human outer ear. J. Clin. Microbiol. 41:295-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funke, G., G. E. Pfyffer, and A. Von Graevenitz. 1993. A hitherto undescribed coryneform bacterium isolated from patients with otitis media. Med. Microbiol. Lett. 2:183-190. [Google Scholar]
- 17.Funke, G., S. Stubbs, M. Altwegg, A. Carlotti, and M. D. Collins. 1994. Turicella otitidis gen. nov., sp. nov., a coryneform bacterium isolated from patients with otitis media. Int. J. Syst. Bacteriol. 44:270-273. [DOI] [PubMed] [Google Scholar]
- 18.Garton, N. J., M. Gilleron, T. Brando, H. H. Dan, S. Giguere, G. Puzo, J. F. Prescott, and I. C. Sutcliffe. 2002. A novel lipoarabinomannan from the equine pathogen Rhodococcus equi. Structure and effect on macrophage cytokine production. J. Biol. Chem. 277:31722-31733. [DOI] [PubMed] [Google Scholar]
- 19.Gibson, K. J., L. Eggeling, W. N. Maughan, K. Krumbach, S. S. Gurcha, J. Nigou, G. Puzo, H. Sahm, and G. S. Besra. 2003. Disruption of Cg-Ppm1, a polyprenyl monophosphomannose synthase, and the generation of lipoglycan-less mutants in Corynebacterium glutamicum. J. Biol. Chem. 278:40842-40850. [DOI] [PubMed] [Google Scholar]
- 20.Gibson, K. J., M. Gilleron, P. Constant, T. Brando, G. Puzo, G. S. Besra, and J. Nigou. 2004. Tsukamurella paurometabola lipoglycan, a new lipoarabinomannan variant with pro-inflammatory activity. J. Biol. Chem. 279:22973-22982. [DOI] [PubMed] [Google Scholar]
- 21.Gibson, K. J., M. Gilleron, P. Constant, G. Puzo, J. Nigou, and G. S. Besra. 2003. Identification of a novel mannose-capped lipoarabinomannan from Amycolatopsis sulphurea. Biochem. J. 372:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson, K. J., M. Gilleron, P. Constant, G. Puzo, J. Nigou, and G. S. Besra. 2003. Structural and functional features of Rhodococcus ruber lipoarabinomannan. Microbiology 149:1437-1445. [DOI] [PubMed] [Google Scholar]
- 23.Gilleron, M., L. Bala, T. Brando, A. Vercellone, and G. Puzo. 2000. Mycobacterium tuberculosis H37Rv parietal and cellular lipoarabinomannans. Characterization of the acyl- and glyco-forms. J. Biol. Chem. 275:677-684. [DOI] [PubMed] [Google Scholar]
- 24.Gilleron, M., N. Himoudi, O. Adam, P. Constant, A. Venisse, M. Riviere, and G. Puzo. 1997. Mycobacterium smegmatis phosphoinositols-glyceroarabinomannans. Structure and localization of alkali-labile and alkali-stable phosphoinositides. J. Biol. Chem. 272:117-124. [DOI] [PubMed] [Google Scholar]
- 25.Gilleron, M., J. Nigou, B. Cahuzac, and G. Puzo. 1999. Structural study of the lipomannans from Mycobacterium bovis BCG: characterisation of multiacylated forms of the phosphatidyl-myo-inositol anchor. J. Mol. Biol. 285:2147-2160. [DOI] [PubMed] [Google Scholar]
- 26.Guerardel, Y., E. Maes, V. Briken, F. Chirat, Y. Leroy, C. Locht, G. Strecker, and L. Kremer. 2003. Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: novel structural features and apoptosis-inducing properties. J. Biol. Chem. 278:36637-36651. [DOI] [PubMed] [Google Scholar]
- 27.Guerardel, Y., E. Maes, E. Elass, Y. Leroy, P. Timmerman, G. S. Besra, C. Locht, G. Strecker, and L. Kremer. 2002. Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with alpha 1,3-mannopyranose side chains. J. Biol. Chem. 277:30635-30648. [DOI] [PubMed] [Google Scholar]
- 28.Guttman, A., F. T. Chen, R. A. Evangelista, and N. Cooke. 1996. High-resolution capillary gel electrophoresis of reducing oligosaccharides labeled with 1-aminopyrene-3,6,8-trisulfonate. Anal. Biochem. 233:234-242. [DOI] [PubMed] [Google Scholar]
- 29.Holzmann, D., G. Funke, T. Linder, and D. Nadal. 2002. Turicella otitidis and Corynebacterium auris do not cause otitis media with effusion in children. Pediatr. Infect. Dis. J. 21:1124-1126. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda-Fujita, T., S. Kotani, M. Tsujimoto, T. Ogawa, I. Takahashi, H. Takada, H. Shimauchi, S. Nagao, S. Kokeguchi, K. Kato, et al. 1987. Possible existence of a novel amphipathic immunostimulator in the phenol-water extracts of Mycobacteriaceae. Microbiol. Immunol. 31:289-311. [DOI] [PubMed] [Google Scholar]
- 31.Leopold, K., and W. Fischer. 1993. Molecular analysis of the lipoglycans of Mycobacterium tuberculosis. Anal. Biochem. 208:57-64. [DOI] [PubMed] [Google Scholar]
- 32.Lerner, L., and A. Bax. 1986. Sensitivity-enhanced two-dimensional heteronuclear relayed coherence transfer NMR spectroscopy. J. Magn. Reson. 69:375-380. [Google Scholar]
- 33.Loiez, C., F. Wallet, A. Fruchart, M. O. Husson, and R. J. Courcol. 2002. Turicella otitidis in a bacteremic child with acute lymphoblastic leukemia. Clin. Microbiol. Infect. 8:758-759. [DOI] [PubMed] [Google Scholar]
- 34.Marion, D., and K. Wuthrich. 1983. Application of phase sensitive two dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem. Biophys. Res. Commun. 113:967-974. [DOI] [PubMed] [Google Scholar]
- 35.Monsarrat, B., T. Brando, P. Condouret, J. Nigou, and G. Puzo. 1999. Characterization of mannooligosaccharide caps in mycobacterial lipoarabinomannan by capillary electrophoresis/electrospray mass spectrometry. Glycobiology 9:335-342. [DOI] [PubMed] [Google Scholar]
- 36.Nigou, J., M. Gilleron, T. Brando, A. Vercellone, and G. Puzo. 1999. Structural definition of arabinomannans from Mycobacterium bovis BCG. Glycoconj. J. 16:257-264. [DOI] [PubMed] [Google Scholar]
- 37.Nigou, J., M. Gilleron, B. Cahuzac, J. D. Bounery, M. Herold, M. Thurnher, and G. Puzo. 1997. The phosphatidyl-myo-inositol anchor of the lipoarabinomannans from Mycobacterium bovis bacillus Calmette Guerin. Heterogeneity, structure, and role in the regulation of cytokine secretion. J. Biol. Chem. 272:23094-23103. [DOI] [PubMed] [Google Scholar]
- 38.Nigou, J., M. Gilleron, and G. Puzo. 1999. Lipoarabinomannans: characterization of the multiacylated forms of the phosphatidyl-myo-inositol anchor by NMR spectroscopy. Biochem. J. 337:453-460. [PMC free article] [PubMed] [Google Scholar]
- 39.Nigou, J., M. Gilleron, and G. Puzo. 2003. Lipoarabinomannans: from structure to biosynthesis. Biochimie 85:153-166. [DOI] [PubMed] [Google Scholar]
- 40.Nigou, J., M. Gilleron, M. Rojas, L. F. Garcia, M. Thurnher, and G. Puzo. 2002. Mycobacterial lipoarabinomannans: modulators of dendritic cell function and the apoptotic response. Microbes Infect. 4:945-953. [DOI] [PubMed] [Google Scholar]
- 41.Nigou, J., A. Vercellone, and G. Puzo. 2000. New structural insights into the molecular deciphering of mycobacterial lipoglycan binding to C-type lectins: lipoarabinomannan glycoform characterization and quantification by capillary electrophoresis at the subnanomole level. J. Mol. Biol. 299:1353-1362. [DOI] [PubMed] [Google Scholar]
- 42.Puech, V., M. Chami, A. Lemassu, M. A. Laneelle, B. Schiffler, P. Gounon, N. Bayan, R. Benz, and M. Daffe. 2001. Structure of the cell envelope of corynebacteria: importance of the non-covalently bound lipids in the formation of the cell wall permeability barrier and fracture plane. Microbiology 147:1365-1382. [DOI] [PubMed] [Google Scholar]
- 43.Quesniaux, V. J., D. M. Nicolle, D. Torres, L. Kremer, Y. Guerardel, J. Nigou, G. Puzo, F. Erard, and B. Ryffel. 2004. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. 172:4425-4434. [DOI] [PubMed] [Google Scholar]
- 44.Renaud, F. N. R., A. Grégory, C. Barreau, D. Aubel, and J. Freney. 1996. Identification of Turicella otitidis isolated from a patient with otorrhea associated with surgery: differentiation from Corynebacterium afermentans and Corynebacterium auris. J. Clin. Microbiol. 34:2625-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds, S. J., M. Behr, and J. McDonald. 2001. Turicella otitidis as an unusual agent causing a posterior auricular abscess. J. Clin. Microbiol. 39:1672-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saddler, G. S., P. Tavecchia, S. Lociuro, M. Zanol, L. Colombo, and E. Selva. 1991. Analysis of madurose and other actinomycete whole cell sugars by gas chromatography. J. Microbiol. Methods 14:185-191. [Google Scholar]
- 47.Shaka, A. J., P. B. Barker, and R. Freeman. 1985. Computer-optimized decoupling scheme for wideband applications and low level operation. J. Magn. Res. 64:547-552. [Google Scholar]
- 48.Simonet, M., D. De Briel, I. Boucot, R. Minck, and M. Veron. 1993. Coryneform bacteria isolated from middle ear fluid. J. Clin. Microbiol. 31:1667-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strauss, J. M., C. A. du Plessis, and K. H. J. Riedel. 2000. Empirical model for biofiltration of toluene. J. Environ. Eng. ASCE 126:644-648. [Google Scholar]
- 50.Strohmeier, G. R., and M. J. Fenton. 1999. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect. 1:709-717. [DOI] [PubMed] [Google Scholar]
- 51.Stroman, D. W., P. S. Roland, J. Dohar, and W. Burt. 2001. Microbiology of normal external auditory canal. Laryngoscope 111:2054-2059. [DOI] [PubMed] [Google Scholar]
- 52.Sutcliffe, I. C. 2000. Characterisation of a lipomannan lipoglycan from the mycolic acid containing actinomycete Dietzia maris. Antonie Leeuwenhoek 78:195-201. [DOI] [PubMed] [Google Scholar]
- 53.Sutcliffe, I. C. 1995. Identification of a lipoarabinomannan-like lipoglycan in Corynebacterium matruchotii. Arch. Oral Biol. 40:1119-1124. [DOI] [PubMed] [Google Scholar]
- 54.Sutcliffe, I. C. 1994. The lipoteichoic acids and lipoglycans of gram-positive bacteria: a chemotaxonomic perspective. Syst. Appl. Microbiol. 17:467-480. [Google Scholar]
- 55.Sutcliffe, I. C. 1997. Macroamphiphilic cell envelope components of Rhodococcus equi and closely related bacteria. Vet. Microbiol. 56:287-299. [DOI] [PubMed] [Google Scholar]
- 56.Sutcliffe, I. C., and N. Shaw. 1991. Atypical lipoteichoic acids of gram-positive bacteria. J. Bacteriol. 173:7065-7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treumann, A., F. Xidong, L. McDonnell, P. J. Derrick, A. E. Ashcroft, D. Chatterjee, and S. W. Homans. 2002. 5-Methylthiopentose: a new substituent on lipoarabinomannan in Mycobacterium tuberculosis. J. Mol. Biol. 316:89-100. [DOI] [PubMed] [Google Scholar]
- 58.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 59.Venisse, A., J. M. Berjeaud, P. Chaurand, M. Gilleron, and G. Puzo. 1993. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J. Biol. Chem. 268:12401-12411. [PubMed] [Google Scholar]
- 60.Vignal, C., Y. Guerardel, L. Kremer, M. Masson, D. Legrand, J. Mazurier, and E. Elass. 2003. Lipomannans, but not lipoarabinomannans, purified from Mycobacterium chelonae and Mycobacterium kansasii induce TNF-alpha and IL-8 secretion by a CD14-toll-like receptor 2-dependent mechanism. J. Immunol. 171:2014-2023. [DOI] [PubMed] [Google Scholar]
- 61.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]