Abstract
Background
Targeted therapies [interferon (IFN), vascular endothelial growth factor (VEGF) inhibitors, and somatostatin analogs (SSA)] have become an integral part of the neuroendocrine tumor (NET) treatment paradigm. We systematically reviewed the available literature to assess the overall beneficial and negative effects of targeted therapy on progression-free survival (PFS), overall survival (OS), response rate (RR), and toxicity.
Methods
Randomized controlled trials (RCT) were identified from MEDLINE, Embase, other major databases, and an electronic search of major conferences. Abstract review, quality assessment, and data abstraction were performed independently by 2 investigators. Meta-analyses were conducted using the generic inverse variance method with a random-effects model, with studies pooled according to drug class and/or control arm for clinical homogeneity.
Results
Fifteen RCT [SSA, n = 2; mammalian target of rapamycin (mTOR)/VEGF inhibitors, n = 4; IFN, n = 3; targeted therapy added to everolimus, n = 2, and other, n = 4] investigating 2,790 patients were included. Overall, targeted agents improved PFS (HR 0.54; 95% CI 0.40–0.73) but not OS (HR 0.86; 95% CI 0.72–1.01). SSA improved PFS (HR 0.41; 95% CI 0.29–0.58) but not OS (HR 1.00; 95% CI 0.58–1.74). mTOR/VEGF inhibitors improved PFS (HR 0.48; 95% CI 0.32–0.72) but not OS (HR 0.82; 95% CI 0.58–1.17). Targeted therapies added to everolimus or IFN did not improve either PFS or OS. The RR overall was improved (OR 2.85; 95% CI 1.77–4.59) but toxicity was increased (meta-analysis not performed).
Conclusions
The addition of targeted therapies improves PFS but not OS in NET. The evidence is strongest for VEGF inhibitors and SSA. There is an ongoing need for well-designed RCT to inform the optimal use of targeted therapies in NET.
Keywords: Neuroendocrine tumor, Targeted therapy, Everolimus, Sunitinib, Interferon, Octreotide, Lanreotide, Systematic review
Introduction
Neuroendocrine tumors (NET) represent a heterogeneous group of tumors that may be difficult both to diagnose and to treat. NET are found in a variety of locations such as the gastroenteropancreatic (GEP) system, along the gastrointestinal tract, and in the respiratory system. The natural history of these tumors is extremely variable, with some being relatively indolent and others aggressive in nature. While some patients present relatively early in the disease trajectory as a result of hormonal symptoms from functioning tumors, delayed presentation and diagnosis with nonfunctional tumors is the norm. Their incidence in recent years has increased globally, likely due to improved detection with gallium-68 and fluorodeoxyglucose positron emission topography (PET) scans as well as increased clinician awareness and vigilance [1, 2]. Their prevalence is also likely to increase with improved management options and consequently an increased survival of patients [3].
From an initial paucity of therapeutic options, the scope has now expanded to include cytotoxic chemotherapy, targeted biological agents, radioactive labeled delivery, and intra-arterial liver-directed therapies. Despite increasing recognition that this tumor group is histologically and biologically different from the more common epithelial cell tumors, a separate staging system for NET has only recently been established by the American Joint Committee on Cancer [4]. There is increasing knowledge that poorly differentiated (G3) tumors behave differently from well-differentiated (G1/G2) ones, and markers of proliferation such as the mitotic count and Ki-67 are increasingly used to guide both prognostication and the selection of optimal treatments [5].
We undertook this systematic review of the current evidence on targeted therapy for NET, with the exclusion of peptide receptor radionuclide therapy (PRRT) as this has been reviewed recently [6]. We analyzed the evidence using the Cochrane method in order to evaluate the overall benefits and harms of treatment to establish a clearer path in the decision-making process for a patient with metastatic well-differentiated NET.
Methods
Criteria for Consideration of Studies for Review
We searched for randomized clinical trials that included adult patients (aged 18 years and over) with a confirmed histological diagnosis of well-differentiated, advanced (locally advanced/unresectable or metastatic) GEP NET with an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2 and radiologically measurable disease.
Eligible studies included patients treated with targeted therapy compared to no targeted therapy or placebo, targeted therapy with another nontargeted therapy, and targeted therapy and other therapy compared to targeted therapy. Targeted therapy was defined to include somatostatin analogs (SSA), monoclonal antibodies, tyrosine kinase inhibitors, mammalian target of rapamycin (mTOR) inhibitors, and immunotherapy. PRRT-related studies were also excluded from this paper due to a recent comprehensive review on this area by Bodei et al. [6].
The primary endpoint was progression-free survival (PFS), and secondary endpoints included overall survival (OS), time to tumor failure or progression, objective response rates (RR), rates of toxicity, and quality of life.
Search Methods for the Identification of Studies
We searched for trials consistent with our inclusion criteria in MEDLINE from 1946 to December 1, 2015, and in Embase from 1996 to December 1, 2015. The searches for patient population, intervention, and systematic or narrative reviews were combined separately, and limits were applied to adults aged 18 years or older.
The database search also included the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, the Cochrane Methodology Register, the ACP Journal Club, and databases of abstracts of reviews of effects and abstracts from major conferences, including the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), the North American Neuroendocrine Tumor Society, and the European Neuroendocrine Tumor Society. In addition, we searched through relevant journals including the Journal of Clinical Oncology, Annals of Oncology, Lancet, Lancet Oncology, the New England Journal of Medicine, the European Journal of Cancer, Neuroendocrinology, and Pancreas.
All members of the author group were asked to identify additional trials. Only trial reports in the English language were included. Two review authors (A.L. and D.L.C.) performed searches independently to identify eligible trials for inclusion.
Data Collection and Analysis
Studies identified from the MEDLINE and Embase searches were screened for randomized controlled trials (RCT) investigating targeted therapy. Uncertainties were resolved via further investigation into full-text articles. All possibly relevant articles were retrieved in full text and assessed with reference to the inclusion criteria.
Study quality and the risk of bias were assessed by at least 2 authors (A.L., D.L.C., and M.H.W.) independently using Cochrane criteria. Where the primary references did not provide sufficient details, we resorted to secondary references, abstracts, presentations, or protocols. Disagreements were resolved after discussion with a third reviewer (N.P.). A meta-analysis was performed on the endpoints listed above, appreciating that some statistical heterogeneity might occur from the pooling of trials investigating different therapies. The statistical analysis was performed using standard meta-analytical techniques, with calculations using a random-effects model given the heterogeneity of the patient characteristics and the treatments investigated. HR and OR were calculated where feasible with 95% CI. Heterogeneity, defined by I2 >30% or p < 0.10, was further explored.
Results
Study Selection
The study flow is summarized in a CONSORT diagram (fig. 1). A total of 976 records were identified after de-duplication. Twenty articles were assessed for eligibility. Five articles were removed upon full evaluation (detailed below), leaving 15 studies for qualitative synthesis and 11 for meta-analysis.
Fig. 1.
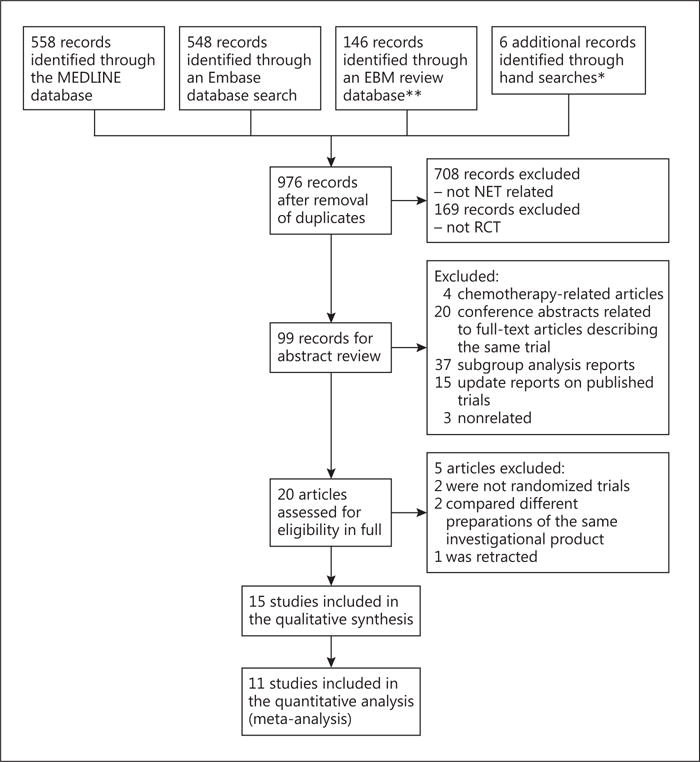
Selection of studies. Abstracts from major oncology and NET conferences; major journals including the Journal of Clinical Oncology, Annals of Oncology, Lancet, Lancet Oncology, and the New England Journal of Medicine.** Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, ACP Journal Club, Database of Abstracts of Reviews of Effects, health technology assessments, and NHS Economic Evaluation Database. EBM = Evidence-based medicine.
Included Studies
Fifteen trials (2,790 patients; table 1) investigated the role of targeted therapy in the treatment of advanced well-differentiated NET. Eleven studies (2,172 patients) were included in the meta-analysis, as 4 studies were not included for reasons detailed below.
Table 1.
Included studies and baseline characteristics
| Trial | Intervention | Comparator | Participants randomized, n | Mean age, years | Primary tumor sites | Liver involvement, % | Included in the quantitative analysis? |
|---|---|---|---|---|---|---|---|
| Rinke et al. [7] | octreotide LAR (30 mg) | placebo | 85 | 62 | midgut | 86 | yes |
| Caplin et al. [8] | lanreotide autogel (120 mg) | placebo | 204 | 54 | GEP | 83 | yes |
| Yao et al. [10] | everolimus | placebo | 410 | 58 | pancreas | 92 | yes |
| Pavel et al. [9] | everolimus + octreotide LAR | placebo + octreotide LAR | 429 | 60 | mixed | 92 | yes |
| Yao [11] | everolimus | placebo | 302 | 63 | lung/gastrointestinal | 84 | yes |
| Raymond et al. [12] | sunitinib | placebo | 171 | 57 | pancreas | 95 | yes |
| Arnold et al. [13] | IFN-α + octreotide | octreotide | 109 | 58 | mixed | NR | yes |
| Kolby et al. [15] | octreotide + IFN-α | octreotide | 68 | 62 | midgut | 100 (all had TACE) | yes |
| Faiss et al. [14] | IFN-α +/− lanreotide | lanreotide | 84 | 58 | mixed | 91 | yes |
| Kulke et al. [16] | everolimus + pasireotide | everolimus | 160 | 58 | pancreas | NR | yes |
| Kulke et al. [17] | everolimus + bevacizumab | everolimus | 150 | 59 | pancreas | NR | yes |
| Wolin et al. [18] | pasireotide | octreotide | 110 | 62 | mixed | NR | no |
| Yao et al. [19] | bevacizumab + octreotide | IFN-α-2b + octreotide | 44 | 55 | mixed | 95 | no |
| Yao et al. [20] | bevacizumab + octreotide | IFN-α-2B + octreotide | 402 | NR | mixed | NR | no |
| Salazar et al. [21] | BEZ235 | everolimus | 62 (part 1) | NR | pancreas | NR | no |
TACE = Transarterial chemoembolization; NR = not reported.
Two studies investigated the impact of SSA compared to placebo. Rinke et al. [7] investigated octreotide long-acting repeatable (LAR) against placebo in midgut NET. Caplin et al. [8] investigated lanreotide versus placebo in GEP NET.
Four studies investigated agents directed against either the mTOR or the vascular endothelial growth factor (VEGF) pathways. Pavel et al. [9] compared everolimus in combination with octreotide LAR against octreotide LAR alone in mixed NET, Yao et al. [10] compared everolimus with placebo in patients with pancreatic NET (pNET), and Yao [11] compared everolimus with placebo in nonpancreatic NET patients (including primary pulmonary NET). Raymond et al. [12] compared sunitinib against placebo in pNET.
Three studies, i.e. those of Arnold et al. [13], Faiss et al. [14], and Kolby et al. [15], investigated the impact of the addition of interferon (IFN)-α in either midgut or mixed NET.
Two studies from 2015 in abstract form investigated the addition of targeted therapy to an everolimus backbone. Kulke et al. [16] investigated the addition of a combination of an SSA (pasireotide) and everolimus compared to everolimus alone in advanced pNET. Kulke et al. [17] also enrolled patients with advanced pNET, but they investigated the impact of adding bevacizumab to everolimus.
The results reported in the above studies are summarized in table 2.
Table 2.
Included studies and outcome measures
| Trial | PFS | OS | Time to tumor failure/progression, months | Objective RR, % |
|---|---|---|---|---|
| Rinke et al. [7] | not reported | HR 0.81, p = 0.77 | 14.3 vs. 6 | 2.4 vs. 2.3b |
| Caplin et al. [8] | HR 0.47, p = 0.0002, not reached vs. 18 months | no difference, p = 0.88 | not reached vs. 18 | not reached vs. 18 months |
| Yao et al. [10] | HR 0.35, p < 0.001, 11 vs. 4.6 months | HR 1.05, p = 0.59 | not reported | 5 vs. 2 |
| Pavel et al. [9] | HR 0.77, p = 0.026, 16.4 vs. 11.3 months | HR 1.22 | not reported | 2.4 vs. 2b |
| Yao [11] | HR 0.48, p < 0.00001, 11.0 vs. 3.9 months | median not reached | not reported | 2 vs. 1 |
| Raymond et al. [12] | HR 0.42, p < 0.001, 11.4 vs. 5.5 months | HR 0.41, p = 0.02 | not reported | 9.3 vs. 0 |
| Arnold et al. [13] | not clearly reportedc | HR 0.82, p = 0.38 | not clearly reported | 50 vs. 45 |
| Kolby et al. [15] | not reported | HR 0.62, p = 0.132 | not reported | not reported |
| Faiss et al. [14] | not reported | not reported | not clearly reportedc, no difference as reported by authors | lanreotide, 4; IFN-α, 3.7; combination, 7.1 |
| Kulke et al. [16] | HR 0.99, p = 0.488, 16.82 vs. 16.59 months | median not reached | not reported | 20.3 vs. 6.2 |
| Kulke et al. [17] | HR 0.80, p = 0.12, 16.7 vs. 14 months | HR 0.75, p = 0.16, 36.7 vs. 35 months | not reported | 31 vs. 12 |
| Wolin et al. [18] | HR 0.46, p = 0.045, 11.8 vs. 6.8 monthsa | not reported | not reported | 2 vs. 3.8 |
| Yao et al. [19] | 16.5 vs. 14 months, p = 0.34 | no difference observed; exact figure not reported | not reported | 18 vs. 0b |
| Yao et al. [20] | HR 0.93, p = 0.55, 16.6 vs. 15.4 months | not reported | 9.9 vs. 5.6, HR 0.72, p = 0.003 | 12 vs. 4 |
| Salazar et al. [21] | HR 1.53 (95% CI 0.72 - 3.25), 8.2 vs. 10.8 months | not reported | not reported | 10 vs. 10 |
Post hoc analysis.
Not reported but calculated from raw data presented in the report.
Actual number not reported. Kaplan-Meier curve presented.
Studies Included in the Qualitative Synthesis but Not in the Meta-Analysis
Wolin et al. [18] investigated pasireotide LAR against octreotide LAR in metastatic functional NET. Their primary outcome was symptom control, i.e. bowel movements and flushing episodes, and no significant differences were observed between the 2 groups. Pasireotide was shown to achieve symptom control in 20.9% of cases and octreotide LAR in 26.7%. The tumor control rate in the 6th month was higher in the pasireotide arm (62.7 vs. 46.2%). PFS, calculated in a post hoc analysis, favored pasireotide LAR (11.8 vs. 6.8 months). Given that that study compared 2 SSA rather than an SSA versus placebo, it was not included with the other studies in the meta-analysis.
Yao et al. [19] compared bevacizumab and IFN-α as part of a phase II study. Given that this study investigated the use of one targeted agent compared to another (as opposed to the addition of a targeted agent in the other 8 studies), it was reported separately instead of being incorporated into the meta-analysis. Enrolled patients had stable octreotide doses before randomization to the addition of either bevacizumab or PEG IFN-α-2b. The primary endpoint was the RR. At progression or after 18 weeks, the patients entered phase II and were given both drugs. The RR was 4/22 in the bevacizumab arm and 0/22 in the IFN arm. In the IFN arm, 2/22 patients achieved a partial response after the addition of bevacizumab in phase II. The median PFS was 66 weeks in the bevacizumab arm and 56 weeks in the IFN arm (p = 0.34). The main differing toxicities between the 2 arms included increased granulocytopenia in the PEG IFN arm (14 vs. 0%, p = 0.02) and hypertension in the bevacizumab arm (18 vs. 0%, p = 0.01).
The phase III RCT that continued to investigate the above combinations, i.e. SWOG S0518 [20], reported preliminary results at the 2015 ASCO Annual Meeting. PFS was not significantly different between the arms, being 16.6 months in the bevacizumab and 15.4 months in the IFN arm. Increased rates of hypertension and proteinuria were noted in the bevacizumab arm, and increased fatigue and neutropenia were seen in the IFN arm. That study was not included in the quantitative synthesis for the same reasons.
The study of Salazar et al. [21] was a phase II randomized trial with a 2-stage design investigating the activity of BEZ235, a dual PI3K/mTOR inhibitor. Patients with advanced pNET were initially randomized to everolimus or BEZ235. Those who were randomized to the everolimus arm were eligible for a second stage in which patients were randomized to either BEZ235 or best supportive care. The results were presented at the 2015 ASCO Annual Meeting. No statistically significant difference was recorded in terms of the median PFS in the first stage (8.2 months in the BEZ235 arm compared to 10.8 months in the everolimus arm). The median PFS has not been presented for stage 2 to date (the PFS at 16 weeks for BEZ235 was 52%). Although stage 2 would be eligible for inclusion at a future date, insufficient results have been reported to date; therefore, we excluded this study from the quantitative analysis until further data are available.
Excluded Studies
There were 5 excluded studies.
The studies of Bajetta et al. [22] and Rubin et al. [23] were excluded as they focused on showing equivalence in response and symptom control of the same drug compound in different preparations. Bajetta et al. [22] tested lanreotide autogel versus lanreotide microparticles, and Rubin et al. [23] used octreotide LAR versus octreotide acetate.
The report of Bajetta et al. [24] (investigating treatment with everolimus and octreotide in GEP and pulmonary NET populations) was not an RCT and was thus excluded.
The report of Yao et al. [25] was a phase II study investigating everolimus versus placebo in patients with pNET. Although the results were fully published and the study was eligible for inclusion, this was subsequently retracted, and thus we have excluded it from the analysis for the time being.
We note a recent study published by Phan et al. [26] investigating the combination of pazopanib long-acting octreotide in 52 patients with advanced well-differentiated NET, with an objective response in 7/32 patients with pancreatic primaries. Given that this was a single-arm trial, it was excluded from the meta-analysis.
Ongoing Studies
The ongoing studies identified by the literature search are summarized in table 3.
Table 3.
Ongoing (unreported) phase II/III randomized controlled studies involving targeted therapies (non-PRRT, no chemotherapy) for well-differentiated GEP NET
| National clinical trial ID No. (study name) | Phase | Patients, n | Intervention | Comparator | Population | Study start | Primary completion | Status |
|---|---|---|---|---|---|---|---|---|
| NCT02031536 | II | 150 | everolimus | placebo | pancreatic NET with liver metastases previously treated with surgery | January 2014 | October 2016 | not yet recruiting |
| NCT01841736 | II | 165 | pazopanib | placebo | progressive carcinoid tumors | June 2013 | December 2016 | recruiting |
| NCT01744249 | II | 80 | sandostatin + LAR axitinib | placebo | advanced well-differentiated nonpancreatic NET | November 2011 | December 2013 | recruiting |
| NCT01731925 (SUNLAND) | II | 104 | lanreotide + sunitinib | lanreotide + placebo | advanced midgut carcinoid tumors | December 2012 | March 2016 | recruiting |
| NCT01755182 (LOTUS) | II | 140 | TAE | Octreotide LAR | metastatic NET to the liver: with or without upfront TAE | July 2013 | December 2017 | recruiting |
| NCT01678664 (EVACEL) | II | 72 | everolimus | TACE (doxorubicin) | digestive endocrine tumor | October 2012 | April 2017 | recruiting |
| NCT00781911 | II | 43 | IMC-A12 | Octreotide depot | islet cell cancer | February 2009 | July 2011 | active, not recruiting |
| NCT02288377 | II/III | 222 | lanreotide | placebo | duodenopancreatic NET | September 2014 | June 2017 | recruiting |
| NCT02231762 | II | 40 | lanreotide + temozolomide | placebo | GEP NET | February 2015 | October 2018 | recruiting |
| NCT02031536 | II | 150 | everolimus | placebo | pancreatic NET: after liver metastasis resection | January 2014 | October 2016 | recruiting |
| NCT02246127 | III | 180 | everolimus | STZ-5FU | pancreatic NET | October 2014 | September 2018 | recruiting |
TAE = Transarterial embolization; TACE = transarterial chemoembolization.
Quality of the Trials
On the whole, the quality of the trials was good. Of the 15 trials included (table 4), 8 were of good quality. While minor issues were noted with regard to blinding, these did not affect the assessment of the results. For instance, the report of Arnold et al. [13] was an open-label study with the primary endpoint of time to treatment failure, but radiological assessment was blinded. In the study of Wolin et al. [18], complete double blinding was not achievable due to different appearances of LAR formulations, but only the independent study nurse/coordinator was privy to the assignment, with the participant, investigator, and assessors remaining blinded throughout the study. Yao [11] has yet to report on all of his prespecified outcome measures (such as quality of life and biomarkers), but he has stated that he will do so in future publications.
Table 4.
Risk-of-bias assessment of the included studies
| Trial | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of assessors | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Rinke et al. [7] | + | + | + | + | + | + | + |
| Caplin et al. [8] | + | + | + | + | + | + | + |
| Yao et al. [10] | + | + | + | + | + | + | + |
| Pavel et al. [9] | + | + | + | + | + | + | + |
| Yao [11] | + | + | + | + | ? | + | + |
| Raymond et al. [12] | + | + | + | + | + | + | + |
| Arnold et al. [13] | + | + | − | + | + | − | + |
| Kolby et al. [15] | + | − | − | − | + | + | + |
| Faiss et al. [14] | + | − | − | − | − | − | + |
| Kulke et al. [16] | ? | ? | ? | ? | ? | ? | ? |
| Kulke et al. [17] | ? | ? | ? | ? | ? | ? | ? |
| Wolin et al. [18] | + | + | − | + | + | ? | + |
| Yao et al. [19] | + | − | − | − | + | + | + |
| Yao et al. [20] | ? | ? | ? | ? | ? | ? | ? |
| Salazar et al. [21] | ? | ? | ? | ? | ? | ? | ? |
+ = Low risk of bias; ? = unclear risk of bias; − = high risk of bias.
Four studies, i.e. those of Kulke et al. [16, 17], Yao et al. [20], and Salazar et al. [21], have only been reported in abstract form thus far, with insufficient information to fully assess the risk of bias.
Three studies were considered to have a moderate to high risk of bias, i.e. those of Yao et al. [19], Kolby et al. [15], and Faiss et al. [14]. Yao et al. [19] randomized patients but did not specify blinding of patients/investigators to the treatment arms or to the tumor response. The study of Faiss et al. [14] was also an open-label RCT with assessment of cases by unblinded local investigators. Of the 80 cases, 13 were considered critical and were reviewed independently by a blinded radiologist, but the rest were not, resulting in assessment of a high risk of bias. The report of Kolby et al. [15] similarly was an open-label RCT without an independent blinded review of radiology and PFS as one of the stated endpoints.
Publication Bias
A funnel plot for PFS (fig. 2) revealed a possible mild publication bias toward trials which favored a benefit from targeted therapy.
Fig. 2.
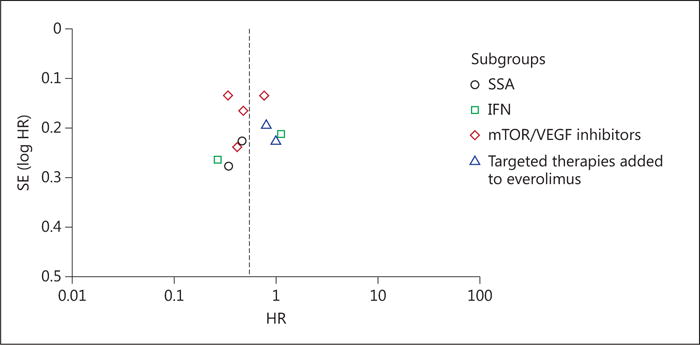
Funnel plot for the included trials – PFS.
Progression-Free Survival
Overall, 10 RCT (investigating 2,088 patients) investigated the effect of targeted therapy for NET on PFS. The addition of targeted therapy improved PFS with an HR of 0.54 (95% CI 0.40–0.73, p < 0.0001; fig. 3). Significant statistical heterogeneity was present (p < 0.00001, I2 = 83%), likely attributable to pooling of studies investigating different treatment modalities (IFN, VEGF inhibitors, and SSA). Use of the leave-one-out strategy only resulted in a decrease in heterogeneity to I2 = 79%, with the exclusion of Yao et al. [10]. Therefore, these results should be interpreted with caution.
Fig. 3.
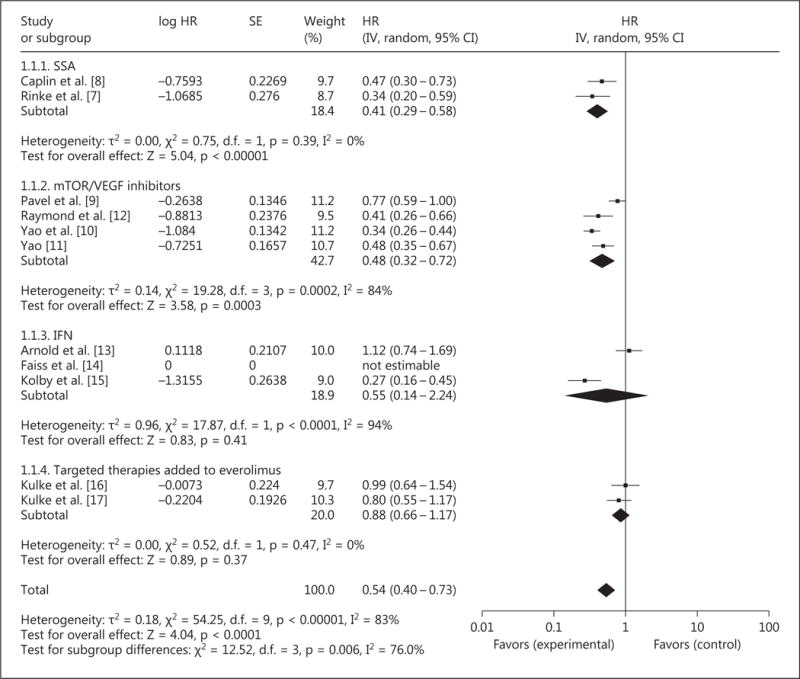
Forest plot – PFS.
Considering the different classes of targeted therapy separately, the addition of SSA (2 studies and 289 patients), compared to placebo, improved PFS with an HR of 0.41 (95% CI 0.29–0.58, p < 0.0001). No significant statistical heterogeneity was present.
The addition of mTOR/VEGF inhibitors (4 studies and 1,312 patients) improved PFS with an HR of 0.48 (95% CI 0.32–0.72, p = 0.0003). Significant statistical heterogeneity was present (p < 0.0001, I2 = 84%), likely due to the pooling of studies investigating these agents as monotherapy and studies investigating them in combination with other active agents (e.g. Pavel et al. [9]). Restriction to those trials investigating monotherapy versus best supportive care resulted in no further heterogeneity being present (p = 0.24, I2 = 31%) and persistence of a PFS benefit (HR 0.40, 95% CI 0.32–0.50, p < 0.00001).
The addition of IFN (2 studies and 177 patients) did not significantly improve PFS with an HR of 0.55 (95% CI 0.14–2.24, p = 0.41). Significant statistical heterogeneity was also present (p < 0.0001, I2 = 94%), possibly due to the selection of NET of mixed primaries in the study of Arnold et al. [13] compared to restriction to midgut NET in the study of Kolby et al. [15]. Because there were only 2 studies, exclusion of either study resulted in no further heterogeneity. This result should be interpreted with caution given the significant heterogeneity and the relatively low number of patients investigated.
The addition of targeted therapies to an everolimus backbone (2 trials and 310 patients) did not significantly improve PFS with an HR of 0.88 (95% CI 0.66–1.17, p = 0.37). No significant statistical heterogeneity was present.
Sensitivity analysis excluding studies considered to have a moderate-to-high risk of bias demonstrated persistence of improvements in PFS with an HR of 0.52 (95% CI 0.37–0.72, p < 0.0001).
Overall Survival
Overall, 10 RCT (investigating 2,088 patients) investigated the effect of targeted therapy for NET on OS. The addition of targeted therapy did not improve OS with an HR of 0.86 (95% CI 0.72–1.01, p = 0.07; fig. 4). No significant statistical heterogeneity was present.
Fig. 4.
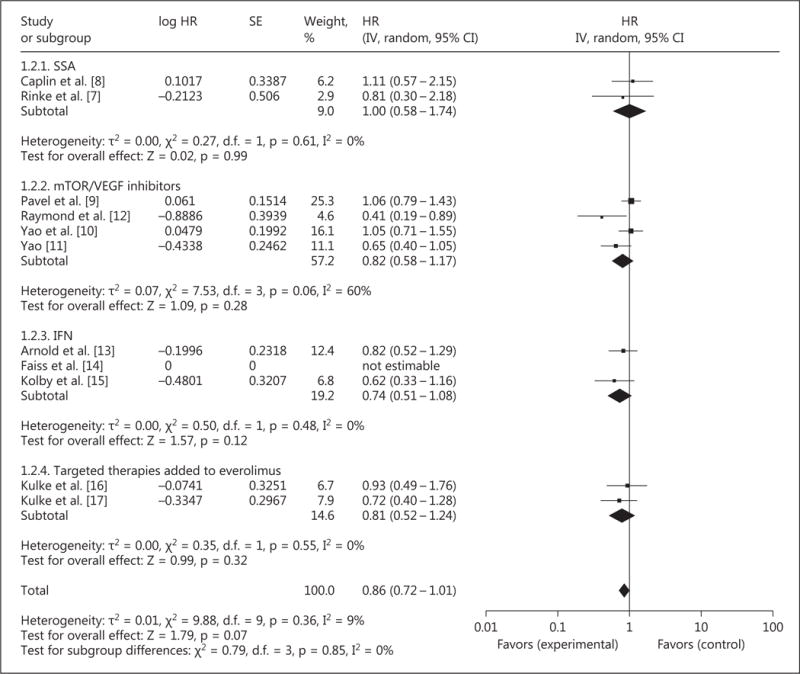
Forest plot – OS.
Considering the different classes of targeted therapy separately, the addition of SSA (2 studies and 289 patients), compared to placebo, did not improve OS with an HR of 1.00 (95% CI 0.58–1.74, p = 0.99). No significant statistical heterogeneity was present.
The addition of VEGF inhibitors (5 studies and 1,462 patients) did not improve OS with an HR of 0.81 (95% CI 0.60–1.09, p = 0.17). Mild statistical heterogeneity (I2 = 51%, p = 0.09) was present, likely due to the combination of studies investigating agents with different targets of action.
The addition of IFN (2 studies and 177 patients) did not improve OS with an HR of 0.74 (95% CI 0.51–1.08, p = 0.12). No significant statistical heterogeneity was present.
The addition of targeted therapies to an everolimus backbone (2 trials and 310 patients) did not significantly improve OS with an HR of 0.81 (95% CI 0.52–1.24, p = 0.32). No significant statistical heterogeneity was present.
Sensitivity analysis excluding studies considered to have a moderate-to-high risk of bias demonstrated no improvement in OS with an HR of 0.87 (95% CI 0.70–1.09, p = 0.23).
Response Rate
An RR was reported in 9 studies (1,837 patients). The odds of a radiological tumor response were increased with an OR of 2.85 (95% CI 1.77–4.59, p < 0.0001; fig. 5) and pooled rates of 75/976 (7.7%) and 26/861 (3.0%), respectively, resulting in a net RR increase of 4.7%. No significant statistical heterogeneity was present.
Fig. 5.
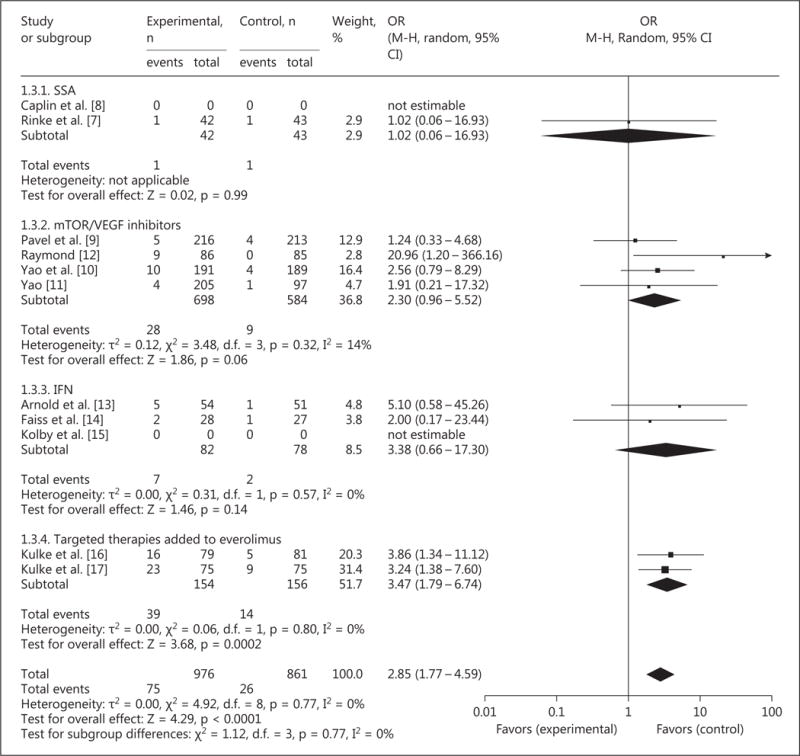
Forest plot – RR.
Sensitivity analysis excluding studies considered to have a moderate-to-high risk of bias confirmed persistent RR improvement with an OR of 2.33 (95% CI 1.14–4.76, p = 0.02).
Biochemical Response
The chromogranin A (CgA) response criteria and rates, where reported, are summarized in table 5. Given the different definitions of CgA response, a meta-analysis was not performed. Decreases in 5-hydroxyindoleacetic acid (5-HIAA) were seldom reported, but in studies where they were reported they mirrored CgA RR (data not shown).
Table 5.
CgA response rates of the included studies
| Trial | Definition of CgA response | RR (experimental arm) | RR (control arm) | p value |
|---|---|---|---|---|
| Arnold et al. [13] | decrease to >50% from baseline | 8/16 | 4/12 | 0.46 |
| Faiss et al. [14] | not reported | NS | ||
| Pavel et al. [9] | decrease to normal or >50% from baseline | 75/164 | 53/146 | 0.0041 |
| Caplin et al. [8] | decrease to >50% from baseline | 27/64 | 3/64 | <0.0001 |
| Rinke et al. [7] | normalization of CgA | 9/26 | 4/30 | 0.11 |
NS = Not significant.
Toxicity
Given the variable reporting criteria and measures of toxicity, the decision was made to report toxicity outcomes in descriptive terms rather than quantitatively in a meta-analysis.
Caplin et al. [8] recorded similar rates of diarrhea, which was the most common adverse event, in both arms. Both groups had 3 patients each who dropped out of the trials due to adverse events. Rinke et al. [7] reported higher discontinuation rates in the octreotide LAR arm compared to placebo. Serious adverse events (most commonly gastrointestinal symptoms) were otherwise balanced in both arms.
Pavel et al. [9] reported higher rates of severe adverse events (57%) and therapy discontinuation (19%) in the experimental arm. The most common severe adverse events included stomatitis, fatigue, diarrhea, hyperglycemia, and thrombocytopenia. Yao et al. [10] reported a 13% discontinuation rate in the everolimus arm, with the most common grade 3 or 4 toxicities in the everolimus arm being stomatitis (7%), anemia (6%), and hyperglycemia (5%). Yao [11] demonstrated toxicity profiles similar to those found in previous everolimus trials. Patients in the everolimus arm reported more toxicity events (i.e. stomatitis, diarrhea, fatigue, and infection) compared to those in the placebo group. All individual grade 3 or 4 events had a frequency of less than 10%, and the discontinuation rate in the everolimus arm was also comparable to previous data at 12%.
Raymond et al. [12] reported an increase in grade 3 or 4 events in the sunitinib arm compared to placebo, with the most common events being neutropenia (12%), hypertension (10%), and palmar-plantar erythrodysesthesia (6%).
Arnold et al. [13] reported discontinuation of treatment due to side effects in 20% of the patients in the experimental arm. Faiss et al. [14] reported side effects leading to interruption of therapy in 25% of the patients in the IFN + lanreotide arm, 15% of the patients in the IFN arm, and 12% of the patients in the lanreotide arm. Kolby et al. [15] reported 1 patient in the experimental arm with a severe side effect without further clarification.
In the study of Kulke et al. [16], both groups had a significant incidence of overall grade 3–4 toxicity, i.e. 77% in the combination arm compared to 69% in everolimus alone group. In another study of Kulke et al. [17] (CALGB80701), the combination of everolimus and bevacizumab resulted in a higher frequency of grade 3 or 4 adverse events, including diarrhea, hyponatremia, hypophosphatemia, proteinuria, and hypertension.
In the other bevacizumab trials, i.e. those by Yao et al. [19, 20], adverse events were as expected, with the bevacizumab group recording more hypertension, proteinuria, and headaches and the IFN group having more neutropenia, leukopenia, and nausea. Fatigue occurred in both groups with more events recorded against IFN. As expected, fatigue increased in frequency in the study of Yao et al. [20] when the 2 cohorts merged (octreotide + bevacizumab + IFN).
In the study of Salazar et al. [21], more adverse events were noted with BEZ235 than with everolimus, including diarrhea, stomatitis, nausea, and vomiting. These adverse events are usually strongly associated with everolimus, and its increased frequency in BEZ235 further limited its potential for future developments. Wolin et al. [18] recorded more grade 3 or 4 severe adverse events in the pasireotide group compared to the octreotide group, with the most common events being hyperglycemia, diabetes mellitus, diarrhea, liver function derangements, and abdominal pain.
Quality of Life
The reported results regarding quality of life are listed in table 6.
Table 6.
Quality-of-life results of the included studies
| Trial | Scale used | Global score change | Other subscales |
|---|---|---|---|
| Arnold et al. [13] | EORTC QLQ-C30 | octreotide, +11.4; octreotide + IFN, −6.4 (p = 0.003) | |
| Raymond et al. [12] | EORTC QLQ-C30 | no overall difference in global score | worsening of diarrhea (21.4 points, p < 0.001) and clinically insignificant worsening of insomnia (7.8 points, p = 0.04) in the sunitinib arm |
| Caplin et al. [8] | EORTC QLQ-C30 | −0.31 (95% CI -5.73 to 5.10), p = NS in the SSA group | |
| Rinke et al. [7] | EORTC QLQ-C30 | −2.1 (95% CI −7.8 to 12.0), p = 0.67 in the SSA group |
NS = Not significant.
Discussion
The treatment of well-differentiated (grade 1/2) NET has changed significantly in recent years, with newer trials supporting the use of targeted therapeutic agents that have demonstrated clear improvements in PFS based on robust randomized clinical trials. While multiple chemotherapy regimens were initially used and formed the basis of the treatment paradigm for a number of years, these were found to have limited benefits, and the trials were susceptible to bias. No single chemotherapy combination has been demonstrated to be superior to another. In addition, no chemotherapy protocol has been tested against placebo or best supportive care. The strength of this review lies in its broad investigation of the role of targeted therapies for NET and rigorous evaluation of the trial evidence according to study quality.
Considered overall, the addition of targeted agents improved PFS with a clinically significant HR of 0.54 (p < 0.00001). This supports the ongoing investigation of such agents in these trials, and the adoption of agents such as SSA and everolimus/sunitinib in routine clinical practice. Significant heterogeneity was found, which was somewhat expected given the pooling of results from studies of differing agents with quite distinct modes of action.
Benefits were observed in the SSA and mTOR/VEGF inhibitor subgroups but not in the IFN subgroup. The subgroup of trials investigating the addition of targeted agents to an everolimus backbone also did not show a PFS benefit, which could be attributable to either the efficacy of everolimus as a single agent (thus diluting any clinical benefit from the addition of a second agent) or the alternatively decreased efficacy of targeted agents such as pasireotide and bevacizumab when coadministered with everolimus. Sensitivity analyses excluding trials with a moderate-to-high risk of bias did not alter the significance of the above results.
In contrast, no OS benefit was observed, either overall or in any of the subgroups. This is somewhat surprising given the magnitude of the PFS benefit. The significant number of trials which allowed crossover to the experimental arm after progression may explain this disparity between PFS and OS efficacy, as may the relatively long survival of some NET patients, resulting in increased access to later-line treatments. The toxicity was manageable, but the quality of life was mostly unchanged and worsened in one trial (i.e. Arnold et al. [13]). Significant heterogeneity was noted in the mTOR/VEGF inhibitor subgroup, with a significant OS benefit reported by Raymond et al. [12] (HR 0.41) which was not replicated in the everolimus trials.
Interestingly, Pavel et al. [9], Yao et al. [10], Yao [11], and Kulke et al. [16] (CALGB 80701) demonstrated significant PFS benefits with everolimus but no OS benefit. Early trial termination may have resulted in overestimation of the true benefit of sunitinib in the study of Raymond et al. [12], and significant crossover in the RADIANT-2 and RADIANT-3 trials (58% of the placebo arm in the study of Pavel et al. [9] and 73% in the study of Yao et al. [10]) may have diluted any OS benefit. This is supported by the trend towards improved OS in RADIANT-4, which did not allow crossover after progression. The role of everolimus in the treatment of NET has been confirmed by the above trials, and even more so recently, with confirmation of its efficacy in bronchial and gastrointestinal carcinoids in addition to pNET.
The differential OS outcomes in the interventional arms of RADIANT-2 (16.4 months on everolimus and octreotide) and RADIANT-3 (11 months on everolimus alone) are intriguing, especially given the significant benefit of mTOR inhibition as monotherapy. However, it would be difficult to attribute the differential OS to the addition of octreotide given the investigation of different primary sites (midgut NET in RADIANT-2 and pNET in RADIANT-3) and the known prognostic implications of these sites.
The role of bevacizumab also deserves further investigation. The 2 trials of Yao et al. [19] would indicate some activity against NET, although the comparator employed, i.e. IFN-α, is not widely used currently because of its significant toxicity profile. This was further supported by the data from Kulke et al. [16] where the combination of everolimus and bevacizumab led to superior PFS compared to everolimus alone. Bevacizumab is certainly associated with increased adverse events, particularly hypertension and proteinuria, but these can be managed easily with either temporary cessation of therapy or introduction of antihypertensives. Further trials, particularly investigating the combination of bevacizumab with other targeted therapies, are warranted.
To our knowledge, this is the first review to summarize all currently published RCT investigating targeted agents in NET. A rigorous literature search was performed with an evaluation of trial quality according to Cochrane criteria. The key strength of this review is its accurate assessment of the risk of bias and the quality of evidence. In addition, the demonstration of a PFS benefit overall strongly argues for ongoing research into the best way to sequence these agents in the treatment paradigm for NET.
The main limitation of this study is the significant statistical heterogeneity observed in the meta-analysis when pooling studies of differing agents in different clinical settings. Individual high-quality trials (such as PROMID and CLARINET) should be considered as stand-alone pieces of evidence. This is mainly due to the inclusion of mixed GEP NET in multiple trials and the use of different molecular targeted therapies. The different biology and prognosis of pNET versus other GEP NET have already been established. Inclusion of all GEP NET can certainly confound the results in a systematic review of trial level data. The use of individual patient data meta-analyses would decrease the risk of bias and provide greater statistical certainty regarding the benefit of specific targeted agents, and it would allow further subgroup analyses.
There are a number of unanswered questions regarding the use of targeted therapies in metastatic NET. Firstly, the best targeted therapy has not been demonstrated, since there are no head-to-head trials comparing different SSA, sunitinib, and everolimus. Secondly, whether targeted therapy can replace chemotherapy, particularly in high-grade NET, is not known, again due to the lack of randomized data comparing 2 groups of therapy. Thirdly, the optimal timing and sequence for starting molecular targeted therapy are unknown. PROMID and CLARINET suggest a very long PFS with the use of SSA (14.3 months and not reached) compared to placebo used as the initial therapy. RADIANT-2 indicates a 23% improvement in PFS with the combination of everolimus and octreotide, but the PFS in the control arm is still fairly long at 11 months. Of the patients on placebo and octreotide, 58% crossed over on progression. After adjusting for prognostic factors, there were no difference between the experimental and placebo groups. This suggests that early versus delayed use of everolimus may not alter long-term outcomes in the setting of early use of SSA. However, this will need to be assessed in an RCT. Fourthly, the sequence of surgery of the primary and debulking in the setting of molecular targeted therapy is unknown. Removal of the primary and debulking of the tumor burden have been well described in international guidelines largely based on expert consensus and prospective non-randomized studies. In an era in which somatatostatin analogs have been proven to be largely effective in symptom control and progression delay with tolerable side effects, the role of surgery, particularly in widespread metastatic disease, has now become less certain.
We anticipate that future trials will look at determining the best strategy for sequencing an SSA agent, everolimus, sunitinib, and possibly even bevacizumab in the context of PRRT and liver-directed therapy. The use of PRRT instead of/together with targeted therapies will be the subject of ongoing investigations given the impressive results of NETTER-1 reported recently [27]. Alternating the administration of everolimus and sunitinib, as has been done in some metastatic renal cell carcinoma trials, may be a possible strategy to derive benefits from both agents while minimizing the impact of their adverse events. Traditional chemotherapy does not appear to have the same benefit and is generally less tolerable than newer biologics, and hence their role in the treatment paradigm remains uncertain.
Acknowledgments
We thank Drs. S. Faiss, B. Wiedenmann, and J. Yao for provision of information regarding the conduction of the studies of Faiss et al. [14] and Yao et al. [19], respectively.
References
- 1.Mocellin S, Nitti D. Gastrointestinal carcinoid: epidemiological and survival evidence from a large population-based study (n = 25,531) Ann Oncol. 2013;24:3040–3044. doi: 10.1093/annonc/mdt377. [DOI] [PubMed] [Google Scholar]
- 2.Lepage C, Bouvier AM, Faivre J. Endocrine tumours: epidemiology of malignant digestive neuroendocrine tumours. Eur J Endocrinol. 2013;168:R77–R83. doi: 10.1530/EJE-12-0418. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, et al. One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB, Compton CC, et al., editors. AJCC Cancer Staging Manual. New York: Springer; 2010. Exocrine and endocrine pancreas; pp. 241–249. [Google Scholar]
- 5.Jamali M, Chetty R. Predicting prognosis in gastroentero-pancreatic neuroendocrine tumors: an overview and the value of Ki-67 immunostaining. Endocr Pathol. 2008;19:282–288. doi: 10.1007/s12022-008-9044-0. [DOI] [PubMed] [Google Scholar]
- 6.Bodei L, et al. Yttrium-labelled peptides for therapy of NET. Eur J Nucl Med Mol Imaging. 2012;39(suppl 1):S93–S102. doi: 10.1007/s00259-011-2002-y. [DOI] [PubMed] [Google Scholar]
- 7.Rinke A, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 8.Caplin ME, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 9.Pavel ME, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 10.Yao JC, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao J. Everolimus plus best supportive care vs placebo plus best supportive care in the treatment of patients with advanced neuroendocrine tumors (GI or lung origin) (RADIANT-4). Eur Soc Med Oncol/Eur Cancer Congress; Vienna. 2015. [Google Scholar]
- 12.Raymond E, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. erratum in N Engl J Med 2011;364:1082. [DOI] [PubMed] [Google Scholar]
- 13.Arnold R, et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol. 2005;3:761–771. doi: 10.1016/s1542-3565(05)00481-7. [DOI] [PubMed] [Google Scholar]
- 14.Faiss S, et al. Prospective, randomized, multi-center trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors – the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 15.Kolby L, et al. Randomized clinical trial of the effect of interferon alpha on survival in patients with disseminated midgut carcinoid tumours. Br J Surg. 2003;90:687–693. doi: 10.1002/bjs.4149. [DOI] [PubMed] [Google Scholar]
- 16.Kulke M, et al. A randomized open-label phase II study of everolimus alone or in combination with pasireotide LAR in advanced, progressive pancreatic neuroendocrine tumors (pNET): COOPERATE-2 trial. 12th Ann Eur Neuroendocr Tumor Soc Conf Diagn Treat Neuroendocr Tumor Dis; Barcelona. 2015. [Google Scholar]
- 17.Kulke M, et al. Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E+B) in patients (Pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (Alliance). Annu Am Soc Clin Oncol Meet; Chicago. 2015. [Google Scholar]
- 18.Wolin EM, et al. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Dev Ther. 2015;9:5075–5086. doi: 10.2147/DDDT.S84177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao JC, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316–1323. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]
- 20.Yao JC, Guthrie K, Moran C, Strosberg JR, Kulke MH, Chan JA. SWOG S0518: phase III prospective randomized comparison of depot octreotide plus interferon alpha-2b versus depot octreotide plus bevacizumab (NSC #704865) in advanced, poor prognosis carcinoid patients ( NCT00569127) J Clin Oncol. 2015;33:4004. doi: 10.1200/JCO.2016.70.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salazar R, et al. Phase II studies of BEZ235 in patients with advanced pancreatic neuroendocrine tumors (pNET) J Clin Oncol. 2015;33:4102. [Google Scholar]
- 22.Bajetta E, et al. Lanreotide autogel every 6 weeks compared with lanreotide microparticles every 3 weeks in patients with well differentiated neuroendocrine tumors: a phase III study. Cancer. 2006;107:2474–2481. doi: 10.1002/cncr.22272. [DOI] [PubMed] [Google Scholar]
- 23.Rubin J, et al. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600–606. doi: 10.1200/JCO.1999.17.2.600. [DOI] [PubMed] [Google Scholar]
- 24.Bajetta E, et al. Everolimus in combination with octreotide LAR as the first-line treatment for advanced neuroendocrine tumors: a phase II trial of the I.T.M.O. (Italian Trials in Medical Oncology) group. J Clin Oncol. 2013;31:4136. [Google Scholar]
- 25.Yao J, et al. A randomized phase II study of everolimus for advanced pancreatic neuroendocrine tumors in Chinese patients. Med On-col. 2014;31:251. doi: 10.1007/s12032-014-0251-x. [DOI] [PubMed] [Google Scholar]
- 26.Phan AT, et al. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: a multicentre, single-group, phase 2 study. Lancet Oncol. 2015;16:695–703. doi: 10.1016/S1470-2045(15)70136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruszniewski P, et al. 177 Lu-DOTATATE significantly improves progression-free survival in patients with mid-gut neuroendocrine tumours: results of the phase III NETTER-1 trial. Eur Cancer Congress; Vienna. 2015. [Google Scholar]


