Abstract
Administration of 1/5th dose of Inactivated poliovirus vaccine intradermally (fIPV) provides similar immune response as full-dose intramuscular IPV, however, fIPV administration with BCG needle and syringe (BCG NS) is technically difficult. We compared immune response after one fIPV dose administered with BCG NS to administration with intradermal devices, referred to as Device A and B; and assessed feasibility of conducting a door-to-door vaccination campaign with fIPV. In Phase I, 452 children 6–12 months old from Karachi were randomized to receive one fIPV dose either with BCG NS, Device A or Device B in a health facility. Immune response was defined as seroconversion or fourfold rise in polio neutralizing antibody titer 28 days after fIPV among children whose baseline titer ≤362. In Phase II, fIPV was administered during one-day door-to-door campaign to assess programmatic feasibility by evaluating vaccinators’ experience. For all three poliovirus (PV) serotypes, the immune response after BCG NS and Device A was similar, however it was lower with Device B (34/44 (77%), 31/45 (69%), 16/30 (53%) respectively for PV1; 53/78 (68%), 61/83 (74%), 42/80 (53%) for PV2; and; 58/76 (76%), 56/80 (70%), 43/77 (56%) for PV3; p < 0.05 for all three serotypes). Vaccinators reported problems filling Device B in both Phases; no other operational challenges were reported during Phase II. Use of fIPV offers a dose-saving alternative to full-dose IPV.
Keywords: Inactivated polio vaccine, Intradermal injections, fractional dose IPV, Immune response
1. Background
The Global Polio Eradication Initiative (GPEI) is getting ever closer to reaching its goal, with only 34 cases of polio caused by wild poliovirus (WPV) reported from 3 endemic countries (Afghanistan, Pakistan and Nigeria) as of December 20, 2016 [1]. Complete poliovirus eradication, however, requires the disappearance of not only WPVs but of all polioviruses from human populations: including those resulting from use of oral poliovirus vaccine (OPV). The Polio Eradication & Endgame Strategic Plan 2013–2018 provides a framework for interruption of WPV transmission in remaining endemic foci and lays out plans for the new polio endgame, which includes the withdrawal of Sabin strains contained in OPV vaccine, starting with type 2, and the introduction of inactivated poliovirus vaccine (IPV), for risk mitigation purposes [2]. The last case of poliomyelitis caused by type 2 wild poliovirus was reported in 1999 and this serotype is now considered to be eradicated [3].
The switch from trivalent OPV (tOPV) to bivalent OPV (bOPV) without type 2 poliovirus has been conducted in a globally synchronized manner in April 2016. As of December 2016, there were no countries still using type 2 containing OPV, except for outbreak control: in case of outbreaks of type 2 circulating vaccine derived poliovirus (cVDPV2) or wild poliovirus in the post switch era, WHO maintains a stock of monovalent type 2 OPV (mOPV2) reserved for outbreak response [4].
At least one dose of inactivated poliovirus vaccine (IPV) has been planned to be introduced globally in routine immunization of all countries in 2015 and 2016 to provide immunity against type 2 polioviruses. In addition to IPV use in routine immunization, IPV, together with mOPV2, are tools to be used in campaigns as a response to cVDPV 2 outbreaks [5]. However, as of June 2016, there was acute IPV shortage that affected 43 countries and caused either delayed IPV introduction or stock-outs in countries that had already introduced IPV [6], [7]. This global shortage is likely to last at least until end 2018.
Intradermal administration of 1/5th of full IPV dose (0.1 mL instead of 0.5 mL), referred to as fractional IPV (fIPV) has demonstrated good safety and immunogenicity [8], [9], [10], [11], [12], [13], [14], [15]; and can be considered as an alternative to full-dose, intramuscular IPV in routine immunizations, and in outbreak response IPV campaigns [16].
Use of full-dose IPV in campaigns (combined with OPV) has been successfully demonstrated in Kenya, Nigeria and in high risk areas of Pakistan and Afghanistan to accelerate eradication or to control polio outbreaks [17]. The fIPV intradermal administration in campaigns is however, technically difficult with BCG needles and syringes (considered a “classical” intradermal administration performed by insertion of a 26–27 gauge needle nearly parallel to and solely into the skin to raise a visible bleb), requires additional training, and may result in poor intradermal injection. Therefore, new intradermal administration methods are being explored. Needle-free jet injectors, various needle adaptors, or intradermal syringes have been developed to ease intradermal administration and improve injection quality [7].
This study was conducted in two phases; in Phase I, we assessed the usability and immune response following fIPV administration with two novel ID adaptors (Device A: Intradermal Adapter by HELM/West Pharmaceutical Services Inc., Exton, USA and Device B: Star Intradermal Syringe by Star Syringe Ltd, East Sussex, UK) and compared this response with the one achieved with traditional BCG syringe which served as a reference. In Phase II we evaluated the feasibility of conducting a door-to-door campaign with intradermal fIPV administered using BCG NS and the two novel devices.
2. Methods
The study was conducted in four low-income areas in and around Karachi (4 peri-urban, contiguous coastal villages: Rehri Goth, Bhains Colony, Ali Akber Shah and Ibrahim Hydri) where the Aga Khan University's Department of Paediatrics and Child Health has well-established Demographic Surveillance System (DSS) which captures population size, pregnancies and births. The population of the study area according to the last census from 2015 is 294,171. Each area has a Primary Health Center (PHC) operated by the Department of Paediatrics and Child Health research program, which also provides Expanded Programme on Immunizations (EPI) services.
Phase I was an un-blinded randomized controlled trial. Children aged 6–12 months living in the target area were enrolled after their guardians provided informed consent. Exclusion criteria were acute illness at the time of enrolment, requiring emergent medical care/hospitalization, refusal of blood testing, contraindication for ID injection or suspicion of immunodeficiency disorder.
The selection of participants was performed using simple random sampling from lists generated by DSS which contained lists of households with age eligible children. Teams of community health workers (CHWs) visited selected households to confirm eligibility and administer informed consent.
Vaccination history with OPV received through routine immunization was assessed from vaccination cards, when cards were not available by parental recall. OPV doses received through SIAs were estimated by the number of SIA rounds that were conducted in the study area during the life of each child. The majority of the SIA rounds in this area were conducted using bivalent OPV vaccine.
All enrolled children received one dose of fIPV (0.1 mL) between November and December 2015; prior to fIPV administration they were randomized into three study arms: in arm A they received fIPV with Device A; in arm B they received fIPV with Device B; and in arm C they received fIPV with regular BCG needle and syringe (BCG NS). The bleb diameter was measured by marking the outer rims of the bleb with a pen and recording the distance between the marks in millimeters with a ruler. Bleb diameter is often interpreted as the extent of intradermal localization of the antigen. Vaccine loss as indicated by liquid on the surface of the skin was measured by applying filter paper to collect liquid on the skin surface immediately after fIPV injection. The wet spot on the filter paper was then circled and the circle diameter compared to a reference template graded 0–5. Vaccine loss was graded using the following: grades 0, 1, and 2 indicated a ≤10% of vaccine loss, or ≤10 µl of a 0.1 ml dose volume. Wetness grades 3, 4, and 5 indicated >10–≤20%, >20–≤40%, and >40% vaccine loss, respectively [7]. Successful intradermal injection was defined as injection resulting in a bleb with diameter ≥5 mm and wetness ≤10%.
Only one attempt for intradermal injection was allowed, even if deemed unsuccessful by the vaccinator (i.e. no bleb or high volume of vaccine spilled).
Device A is a novel injection guide designed for use with 1 mL staked needle disposable syringes. The ID Adapter can help make ID injection easier and more consistent by guiding the angle and limiting the depth of needle insertion [18]. Device B enables simple consistent accurate intradermal (ID) injection without requiring the difficult Mantoux technique, while at the same time facilitating access to all vial sizes and ampoules without any additional devices or manipulation. The device also meets or exceeds WHO global public health standards for auto-disable technology and US standards for needle stick protection [19].
Peripheral blood (2 mL) was collected at the time of enrollment (prior to the study vaccine administration) and 28 days after immunization using venipuncture. Blood specimens collected at the sites were allowed to clot, centrifuged to separate serum, and transported to the Infectious Disease Research Laboratory (IDRL) at the Aga Khan University where they were stored at −20 °C until shipment to the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA, where the anonymized blinded sera were tested for presence of poliovirus neutralizing antibodies using standard neutralization assays [20], [21], [22], [23].
Seropositivity was defined as reciprocal titers of poliovirus neutralizing antibodies ≥8; seroconversion was defined as the change from seronegative to seropositive (from reciprocal titer of <8–≥8); and boosting was defined as ≥4-fold increase in titers. In this study, “immune response” refers to either boosting or seroconversion. The analysis of immune response was restricted to infants with a baseline serological titer of ≤362 to ensure that a 4-fold boosting response could be achieved since the highest tested titer was ≥1:1448.
Subjective assessment of each device was performed by each vaccinator after completion of Phase I; vaccinators were asked to rank and compare the methods of intradermal vaccination and to assess each component of the injection process (process of filling with vaccine, delivery and safety) using a self-administered questionnaire.
Adverse events following vaccination were identified by site investigators and reviewed by the principal investigator. Children were observed for 30 min following the administration of the vaccine for immediate adverse events; parents were instructed to immediately report back to the health centers if adverse events occurred. Serious adverse events were reported for review by the Data and Safety Monitoring Board and by the Ethical Review Committees of the Aga Khan University and the World Health Organization. Druing observations, the study staff were aware of arm allocation of the observed children.
In Phase II, both new ID adaptors and BCG NS were used during regular Government of Pakistan organized supplementary polio immunization activity (SIA) in a door-to-door fashion. The objective of Phase II was to assess feasibility of conducting an intradermal campaign with fIPV. This one-day pilot campaign took place in March 2016 and targeted children younger than five years of age of either gender living in high polio risk area of Karachi. The pilot campaign took place on the same day as regular combined IPV + OPV campaign. Children either received IPV + OPV from regular Pakistan governmental teams or fIPV + OPV from the study teams. Children were not randomized; they were selected based on their residence. After administration of the campaign vaccines, there was no further observation of children participating in Phase II. Each vaccination team was monitored by one person who observed the entire process, measured time with stop-watch that was required to administer intradermal vaccine, and recorded preference of each intradermal method and if programmatic problems occurred. In the end of the session, the monitors collected the used vials and assessed vaccine remaining in the last used vial. Wastage proportion was calculated as number of doses administered subtracted from total number of doses available for administration from used vials and then divided by the number of doses available for administration from used vials.
Community Health Workers (CHWs) and Research Assistants (RA) were trained and certified for administering intradermal injections before the start of the study. One day training on each device was conducted. The government teams were not trained for the intradermal injections and vaccine delivery.
For both phases we used IPV produced by Bilthoven Biologicals B.V., the Netherlands presented in 1-dose vials (0.5 mL), which represented 5 fIPV doses.
Data was analyzed using STATA version 11. The proportion of seroconversion in different study arms was compared by χ2 test for quantitative variables. P-value was calculated to assess difference in immune response between BCG NS and each of the devices. K-sample equality of median test was performed to compare the median titers across the study arms and 95% confidence intervals for median titers were calculated.
The study was approved by the Ethical Review Committee of the Aga Khan University (3519-Ped-ERC-15), the National Bioethics Committee of Pakistan (4-87/15/NB-191/RDC) and the Ethical Review Committee of the World Health Organization, Geneva. All activities followed the guidelines of Good Clinical Practice; the trial protocol was registered at ClinicalTrials.gov with identifier NCT02769923. The World Health Organization assisted in study design, trial monitoring, and contributed to writing of the report. The Aga Khan University conducted the trial. Laboratory testing of de-identified sera were tested at the Centers of Disease Control and Prevention, Atlanta, USA.
3. Results
3.1. Phase I
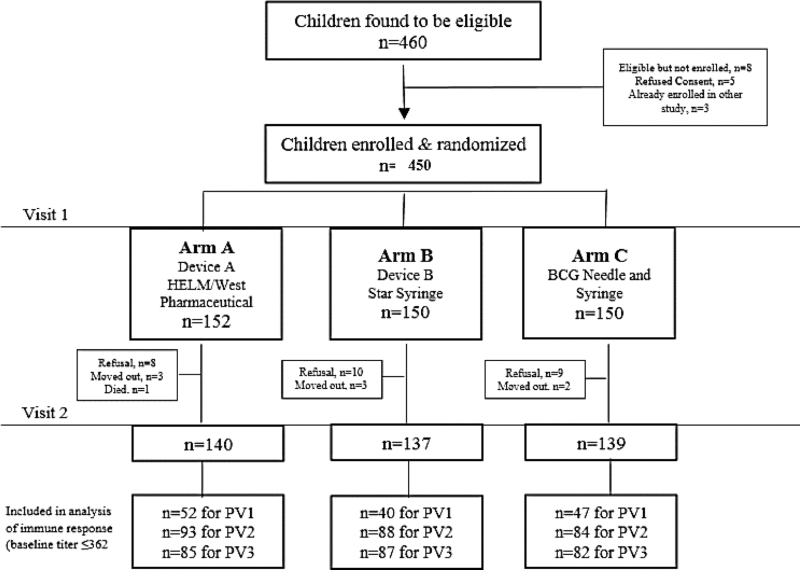
A total of 452 subjects were enrolled; 36/452 (8%) withdrew consent for participation or were lost to follow-up between enrollment and the second blood collection on day 28 (most were lost due to refusal to have blood collection performed) leaving 416 (92%) subjects to be included in the analysis (Table 1).
Table 1.
Baseline Characteristics of the study population.
| Study Arm A (Device A, West Pharmaceutical) | Study Arm B (Device B, Star Sryine) | Study Arm C (BCG NS) | Total | |
|---|---|---|---|---|
| N = 152 | N = 150 | N = 150 | N = 452 | |
| Median age in months (IQR) | 9 (7–10) | 8 (7–10) | 9 (8–10) | 9 (7–10) |
| Gender: % male | 53% | 50% | 57% | 53% |
| OPV dose history: median # of doses received (IQR) | 5(3–7) | 5 (3–7) | 5 (3–8) | 5 (3–7) |
| Baseline seroprevalence | ||||
| Poliovirus type 1 | ||||
| n, (% positive, CI95%) | 143 (94%, 89–97%) | 139 (93%, 87–96%) | 144 (96%, 92–99%) | 452 (94%, 92–96%) |
| Median Titer | 910 | 1152 | 1152 | 1152 |
| (CI95%) | 576–1152 | 910–1448 | 588–1448 | 910–1152 |
| Poliovirus type 2 | ||||
| n, (% positive, CI95%) | 110 (72%, 65–79%) | 115 (77%, 69–83%) | 117 (78%, 71–84%) | 342 (76%, 71–80%) |
| Median titer | 161 | 202 | 288 | 228 |
| (CI95%) | 91–362 | 102–362 | 120–455 | 144–288 |
| Poliovirus type 3 | ||||
| n, (% positive, CI95%) | 123 (81%, 74–87%) | 131 (87%, 81–92%) | 124 (83%, 76–88%) | 378 (84%, 80–87%) |
| Median Titer | 228 | 242 | 288 | 288 |
| (CI95%) | 144–455 | 181–402 | 181–455 | 181–362 |
| Proportion of children with baseline titer <362 | ||||
| Polio virus type 1 n/N (%) | 52/152 (34%) | 40/150 (27%) | 47/150 (31%) | 139/452 (31%) |
| Poliovirus type 2 n/N (%) | 93/152 (61%) | 88/150 (59%) | 84/150 (56%) | 265/452 (59%) |
| Poliovirus type 3 n/N (%) | 85/152 (46%) | 87/150 (58%) | 82/150 (55%) | 254/452 (56%) |
In Phase I, there were nine vaccinators who administered ID injections; each of the vaccinators used all three types of ID delivery. Total of 152 injections were administered using Device A and 150 injections each were administered using Device B and BCG NS.
Baseline characteristics of enrolled children did not significantly differ between study arms. There were 53% female participants; median age was 9 months; the average number of OPV doses received prior to enrollment (from routine immunizations or SIAs) was 5 (Table 2). Baseline seroprevalence to all 3 poliovirus serotypes was high (94%, 76% and 84% respectively for serotypes 1, 2 and 3) and did not differ significantly between arms (Table 2).
Table 2.
Study consort.
 |
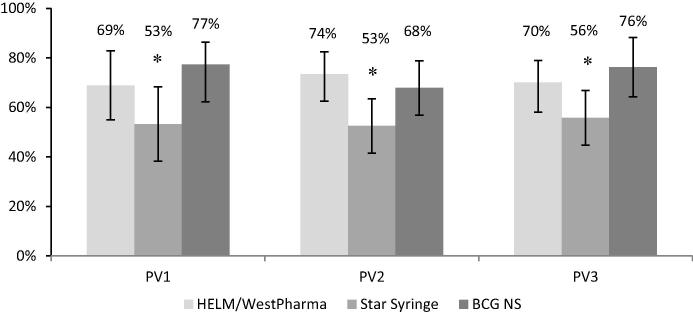
Immune response (seroconversion or boosting) following a single dose of fIPV was assessed among children with baseline titer of ≤362 (Table 2, Fig. 1). There was significantly lower immune response induced with Device B compared with BCG NS (p = 0.03, p = 0.04, p = 0.01 for serotypes 1, 2 and 3 respectively). There was no statistical difference in immune response induced by Device A compared to BCG NS.
Fig. 1.
Immune response (seroconversion or boosting of antibody titer) after one fIPV dose among children who completed all study visits and had baseline titer ≤362 [error bars: 95%CI]. * – Significant difference between Device B and BCG NS study arms (p < 0.05). n/N. PV1: HELM-31/45; Star-16/30; BCG-34/44; Total-81/119. PV2: HELM-61/83; Star-42/80; BCG-53/78; Total-156/241. PV3: HELM-56/80; Star-43/77; BCG-58/76; Total-157/233.
We did not analyze seroconversion for serotype 1 because only very few children were seronegative at baseline (6%). Among the seronegative children at baseline, for serotype 2, there were 33/41 (80%, 95% Confidence Interval or CI95%: 63.5–90.7%), 18/35 (51%, CI95%: 33.1–69.8%), and 25/33 (76%, CI95%: 57.7–90.1%) children in arms A, B, and C respectively who seroconverted after one fIPV dose; and 14/28 (50%, CI95%: 30.6–69.4%), 7/18 (39%, CI95%: 17.3–64.3%), and 11/25 (44%, CI95%: 23.2–65.5%) for serotype 3. Seroconversion after fIPV administered with Device B for serotype 2 was significantly lower than with BCG NS (p = 0.03). There was no statistical difference for serotype 3.
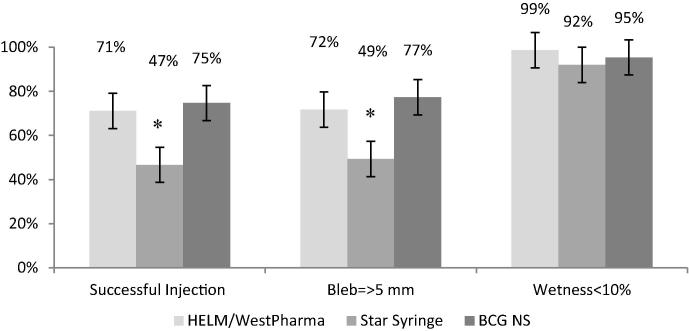
Ability to induce bleb with diameter ≥5 mm was significantly lower with Device B when compared to BCG NS (p < 0.01); and “successful injection” was achieved in lower proportion of children with Device B when compared to BCG NS. All ID methods achieved >90% of injections with less than 10% of wetness (Fig. 2).
Fig. 2.
Indicators of successful injection (from Phase I) [error bars: 95%CI]. * – Significant difference between Device B and BCG NS study arms (p < 0.05).
Two thirds (6/9, 67%) of the vaccinators reported Device A as the most preferred one followed by BCG NS (22%). Device B was the least preferred device among the vaccinators, mainly because of the filling process which was found to be cumbersome, and resulted in vaccine loss (5/9 vaccinators though the filling was difficult and 3/9 were unable to properly fill the syringe with IPV).
3.2. Phase II
Six vaccinators, all of whom participated also in Phase I, divided into three teams participated in Phase II of this study. During the one-day door-to-door campaign the three teams delivered 104 intradermal injections with Device A, 101 with Device B, and 103 with BCG NS. The average time from opening of the syringe blister to discarding the syringe in the biosafety box was shortest for the Device B (1 min 42 s, range 1–7 min) followed by BCG NS (1 min 52 s, range 1–3 min) and Device A device (2 min 32 s, range 1–5 min). Some vaccinators reported problems to properly fill Device B.
Wastage of IPV vaccine was highest for Device B (37% of IPV was wasted), compared with 10% for Device A and 6% for BCG NS. After Phase II, the vaccinators reported that crying among children was less common with Device B when compared to either Device A or BCG NS. No operational obstacles to conducting an intradermal campaign were found.
No severe adverse events related to study procedures were reported in Phase I; one child died between fIPV administration and blood collection on day 28, however, the PI together with DSMB concluded that the death was not study related, the cause of death was determined to be severe diarrhea with dehydration. There was no monitoring of children in Phase II.
4. Discussion
This was the first study to pilot the use of fIPV in a campaign setting and it demonstrated that it is feasible to implement an intradermal campaign in a resource-poor setting.
The immune response achieved with one dose of fIPV was similar to previously reported data [13], [15]; the rate of seroconversion for poliovirus serotype 2 was high and provided further evidence that fIPV can be successfully used for the primary immunization series as an alternative to full dose IPV in the era when type 2 containing OPV is no longer in use.
Device A needle adaptor was preferred among the vaccinators; and the immune response achieved with Device A was similar to the one achieved with BCG NS. It is unclear why the immune response achieved with Device B was significantly lower than with BCG NS. We consider two possible hypotheses: (1) the intradermal needle on the Device B is too long for young children (1.5 mm) and delivers the vaccine too deep into the skin – this would be supported by significantly smaller proportion of children in whom the bleb size was ≥ 5 mm when compared with BCG NS; (2) the difficulty in filling the Device B may have resulted in <0.1 mL of the vaccine delivered to the skin.
In the campaign setting, the fastest time was achieved with the Device B which was also preferred by parents because their children cried less. All vaccinators received one day of training in use of each administration method; this proved to be sufficient time to properly master the intradermal administration either in health centers or in the campaign. The advantage of the devices compared to BCG NS was in easier intradermal administration; however, we demonstrated that it is also possible to conduct a successful intradermal campaign if only BCG NS is available.
The study had some limitations; we found that the majority of children received large number of doses of poliovirus vaccines and had high poliovirus antibody titers at baseline. In fact, we lost >50% of children for analysis of immune response due to the fact that their baseline titer was >362. This could have affected the proportion of those who responded to the fIPV [10], and limited our ability to measure seroconversion as well as immune response. In addition, this was a trial designed to answer basic programmatic question: is there a programmatic advantage or disadvantage to use intradermal devices when compared to BCG NS? As such the power of the trial was unable to demonstrate non-inferiority or equivalence but rather to demonstrate usability of intradermal devices in difficult field conditions and whether or not these devices are safe and induce comparable immunogenicity when compared with BCG NS.fIPV has repeatedly demonstrated good safety, and immunogenicity [13]. Our study provided evidence that fIPV can be successfully used in a campaign. GPEI urgently needs to respond to the current IPV shortage. Use of fIPV for outbreak response as well as for routine immunizations offers a dose-saving alternative to full dose intramuscular IPV and should be considered as a tool to overcome current IPV shortage, and in longer term, as a strategy to save resources for immunization programs in resource poor settings.
Funding
The World Health Organization.
Conflict of interest
All authors – no conflict of interest declared.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of CDC and other contributing agencies.
Acknowledgements
Dr Ali F. Saleem received research training support from the National Institute of Health’s Fogarty International Center (1 D43 TW007585-01). The Centers for Disease Control and Prevention supported the project in-kind by provision of laboratory testing and expertise in interpretation. We thank Dr Mark A Pallansch, Director Division of Viral Diseases at the CDC and the team conducting the neutralization assays at the CDC Laboratory including Deborah Moore, Yiting Zhang, Sharla McDonald, Larin McDuffie, Will Hendley, Patricia Mitchell, and Mario Nicolas. We also thank Ms Shahida Qureshi, Aneeta Hotwani from the Infectious Disease Research Laboratory, Aga Khan University for the storage and shipment of the samples. Mr Najeeb Ahmed (Data management Unit Aga Khan University), Dr Usman Chachar (Coordinator of EOC Sindh), Dr Temesgen Demeke (Team Leader WHO PEI Sindh), Government of Sindh in Bin Qasim and Landhi Town for constant support during the project.
References
- 1.Cases of wild poliovirus by country and year. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpolioviruslist.aspx [accessed 12/20/2016].
- 2.Sutter R.W., Platt L., Mach O., Jafari H., Aylward R.B. The new polio eradication end game: rationale and supporting evidence. J Infect Dis. 2014;210(Suppl 1):S434–S438. doi: 10.1093/infdis/jiu222. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease C, Prevention Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mortal Wkly Rep. 2001;50:222–224. [PubMed] [Google Scholar]
- 4.GPEI. Global switch in oral polio vaccines. Available at: http://maps.who.int/OPV_switch/ [accessed 07/06/2016].
- 5.WHO. Outbreak response: a package of guidelines and materials. Available at: http://www.polioeradication.org/Resourcelibrary/Resourcesforpolioeradicators/Technicalguidelines.aspx.
- 6.SAGE. SAGE discussion and statement in relation with the IPV supply situation. Available at: http://www.who.int/immunization/sage/meetings/2016/april/SAGE_statement_IPV_situation.pdf [accessed 23/06/2016].
- 7.Resik S., Tejeda A., Mach O. Needle-free jet injector intradermal delivery of fractional dose inactivated poliovirus vaccine: association between injection quality and immunogenicity. Vaccine. 2015;33:5873–5877. doi: 10.1016/j.vaccine.2015.06.071. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed A.J., AlAwaidy S., Bawikar S. Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med. 2010;362:2351–2359. doi: 10.1056/NEJMoa0909383. [DOI] [PubMed] [Google Scholar]
- 9.Nirmal S., Cherian T., Samuel B.U., Rajasingh J., Raghupathy P., John T.J. Immune response of infants to fractional doses of intradermally administered inactivated poliovirus vaccine. Vaccine. 1998;16:928–931. doi: 10.1016/s0264-410x(97)00293-4. [DOI] [PubMed] [Google Scholar]
- 10.Resik S., Tejeda A., Mach O. Immune responses after fractional doses of inactivated poliovirus vaccine using newly developed intradermal jet injectors: a randomized controlled trial in Cuba. Vaccine. 2015;33:307–313. doi: 10.1016/j.vaccine.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Estivariz C.F., Jafari H., Sutter R.W. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6–9 months in Moradabad, India: a community-based, randomised controlled trial. Lancet Infect Dis. 2012;12:128–135. doi: 10.1016/S1473-3099(11)70190-6. [DOI] [PubMed] [Google Scholar]
- 12.Soonawala D., Verdijk P., Wijmenga-Monsuur A.J. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine. 2013;31:3688–3694. doi: 10.1016/j.vaccine.2013.05.104. [DOI] [PubMed] [Google Scholar]
- 13.Nelson K.S., Janssen J.M., Troy S.B., Maldonado Y. Intradermal fractional dose inactivated polio vaccine: a review of the literature. Vaccine. 2012;30:121–125. doi: 10.1016/j.vaccine.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Resik S., Tejeda A., Lago P.M. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis. 2010;201:1344–1352. doi: 10.1086/651611. [DOI] [PubMed] [Google Scholar]
- 15.Resik S., Tejeda A., Sutter R.W. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med. 2013;368:416–424. doi: 10.1056/NEJMoa1202541. [DOI] [PubMed] [Google Scholar]
- 16.Polio vaccines: WHO position paper. Wkly Epidemiol Rec. 2016;91(March):145–168. [PubMed] [Google Scholar]
- 17.Sheikh M.A., Makokha F., Hussein A.M. Combined use of inactivated and oral poliovirus vaccines in refugee camps and surrounding communities – Kenya, December 2013. MMWR Morb Mortal Wkly Rep. 2014;63:237–241. [PMC free article] [PubMed] [Google Scholar]
- 18.WestPharma. Introducing the west intradermal adapter. Available at: http://www.westpharma.com/en/events/Pages/7832-RN-ID-Adapter.aspx [accessed 08/06/2016].
- 19.Syringe S. Star ID: intradermal delivery made easy. Available at: http://www.starsyringe.com/product-starid.htm.
- 20.Sutter R.W., Pallansch M.A., Sawyer L.A., Cochi S.L., Hadler S.C. Defining surrogate serologic tests with respect to predicting protective vaccine efficacy: poliovirus vaccination. Ann N Y Acad Sci. 1995;754:289–299. doi: 10.1111/j.1749-6632.1995.tb44462.x. [DOI] [PubMed] [Google Scholar]
- 21.WHO Expanded Programme on Immunization. World Health Organization. Division of communicable diseases. Manual for the virological investigation of poliomyelitis. Geneva: World Health Organization; 1990.
- 22.Fiore L., Plebani A., Buttinelli G. Search for poliovirus long-term excretors among patients affected by agammaglobulinemia. Clin Immunol. 2004;111:98–102. doi: 10.1016/j.clim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Weldon W.C., Oberste M.S., Pallansch M.A. Standardized methods for detection of poliovirus antibodies. Methods Mol Biol. 2016;1387:145–176. doi: 10.1007/978-1-4939-3292-4_8. [DOI] [PubMed] [Google Scholar]