Abstract
Background
Multiple myeloma (MM) risk increases with higher adult body mass index (BMI). Emerging evidence also supports an association of young adult BMI with MM. We undertook a pooled analysis of eight case-control studies to further evaluate anthropometric MM risk factors, including young adult BMI.
Methods
We conducted multivariable logistic regression analysis of usual adult anthropometric measures of 2,318 MM cases and 9,609 controls, and of young adult BMI (age 25 or 30 years) for 1,164 cases and 3,629 controls.
Results
In the pooled sample, MM risk was positively associated with usual adult BMI; risk increased 9% per 5-kg/m2 increase in BMI (odds ratio [OR]=1.09, 95% confidence interval [CI]= 1.04–1.14; p=0.007). We observed significant heterogeneity by study design (p=0.04), noting the BMI-MM association only for population-based studies (p-trend=0.0003). Young adult BMI was also positively associated with MM (per 5-kg/m2; OR=1.2, 95% CI=1.1–1.3; p=0.0002). Further, we observed strong evidence of interaction between younger and usual adult BMI (p-interaction <0.0001); we noted statistically significant associations with MM for persons overweight (25-<30 kg/m2) or obese (30+ kg/m2) in both younger and usual adulthood (v. individuals consistently <25 kg/m2), but not for those overweight or obese at only one time period.
Conclusions
BMI-associated increases in MM risk were highest for individuals who were overweight or obese throughout adulthood.
Impact
These findings provide the strongest evidence to date that earlier and later adult BMI may increase MM risk and suggest that healthy BMI maintenance throughout life may confer an added benefit of MM prevention.
Keywords: multiple myeloma, obesity, body mass index, anthropometric measures, epidemiology
Introduction
Multiple myeloma (MM) is a malignancy of plasma cells that is expected to account for 30,330 new cancer diagnoses and 12,650 cancer deaths in the United States (US) in 2016 (1). In spite of recent therapeutic breakthroughs (2, 3), the relative 5-year survival of MM remains below 50% (4). Prevention strategies informed by knowledge of modifiable risk factors are urgently needed, but the etiology of MM is not known. Established risk factors include older age, male gender, African ancestry and a family history of hematologic malignancy (5, 6). The pre-malignant condition, monoclonal gammopathy of undetermined significance (MGUS) precedes essentially all cases of MM (7, 8) and appears to share demographic risk factors with MM (9), but knowledge of MGUS etiology and risk factors for progression to MM is also limited.
Body mass index (BMI) has emerged as the first and only identified modifiable risk factor for MM (10–12). Most published studies reported an increased risk of MM in relation to adult BMI but lacked sufficient statistical power to examine the association separately by age, sex or race. Comparatively few studies evaluated MM risk in relation to earlier life body size, but the available evidence supports a positive association for younger adult BMI with MM that may be at least as strong as that for later adult BMI (13, 14).
Obesity is associated with dysregulation of several endogenous hormonal pathways (15, 16) that also contribute to MM pathogenesis, including insulin-like growth factor (IGF)-1 (17, 18), insulin (19), interleukin (IL)-6 (20) and adipokines such as adiponectin (21). Further, recent prospective studies have provided evidence for an association of MM risk with pre-diagnosis levels of these hormones (22–24), and a recent in vivo study demonstrated susceptibility to an MM-like condition in mice with diet-induced obesity (25). A clearer understanding of the association of obesity and related anthropometric measures with MM would offer valuable insights for the development of urgently needed prevention strategies.
To further elucidate the association of BMI and related anthropometric measures with MM, we conducted a pooled analysis of eight case-control studies participating in the International Multiple Myeloma Consortium (IMMC)(26). The pooled total of 2,318 confirmed incident cases of MM and 9,609 controls—with young adult BMI data for 1,164 cases and 3,629 controls—makes the present study the largest to date and the best equipped to evaluate the independence of younger and later adult BMI associations with MM and the heterogeneity of BMI-MM associations across demographically defined MM risk strata.
Materials and Methods
Study population
The present study included participants from the eight IMMC case-control studies that collected information on adult height, usual adult weight, and relevant covariables (age, sex, race, education level, tobacco use, alcohol intake). Those included the EpiLymph, Fred Hutchinson Cancer Research Center (FHCRC), National Cancer Institute (NCI) Black-White, NCI-Connecticut, NCI-Surveillance, Epidemiology and End Results (SEER), Nebraska, Roswell Park Cancer Institute (RPCI), and Utah studies (Supplementary Table S1), for which the designs and methods were previously published in detail (27–35). Four of those studies represented multiple study centers, and thus the analysis included a total of 20 study centers. Five study centers utilized a hospital-based design; the remaining centers used a population-based design, based on the source of controls.
MM cases were enrolled either through participating hospitals and physicians (4 studies/9 centers) or through population-based cancer registries (4 studies/11 centers). Case participation ranged from 72% to 96% of eligible cases. Hospital-based controls were recruited among patients admitted to the same hospitals as the cases for a variety of non-malignant conditions. Population-based controls were identified through random-digit telephone dialing, random selection from national health insurance registries or the equivalent, and/or through other population-based registries. Controls were individually or frequency-matched to cases on age, sex, and region or center. One study also matched on age, sex, race, and vital status (Nebraska) (34), and another matched on age, sex, region, and race (NCI-SEER)(33). The NCI-Connecticut study was restricted to women and used frequency-matching of controls to cases on age (31, 32). The controls in the NCI-Connecticut and NCI-SEER studies were obtained from related ongoing studies of non-Hodgkin lymphoma (NHL) and were thus frequency-matched to the NHL rather than to the MM cases (31, 32); controls for the EpiLymph study were frequency-matched by age and sex to cases enrolled in the broader study of all adult hematologic malignancies. The Utah study enrolled both spouse controls and controls identified through registry of motor vehicle records, with the latter frequency-matched to cases by age and sex; we excluded the spouse controls (n=79) from the present analysis. Control participation ranged from 44% to 96% of eligible controls. The protocols for the participating case-control studies were approved by the institutional review board (IRB) or equivalent at the respective host institutions and at each SEER center, and study participants provided written informed consent. The protocol for the present analysis was approved by the IRB of the NCI and each collaborating institution.
Case definition
Most study centers considered all individuals with histologically confirmed incident primary diagnoses of MM that occurred during the respective enrollment periods (ICD-O-3 diagnostic codes of 9731.3, 9732.3, 9734.3, ICD-9 of 203, and the equivalent) as eligible for enrollment. The Utah study included both prevalent and incident cases. Sensitivity analyses conducted without the Utah study sample yielded similar findings to those with all eight studies, and thus we focus on the analyses that include the Utah data in this report.
Data collection
Study data were obtained in person by trained interviewers or by self-administered questionnaire. The vast majority of questionnaires or interviews were completed directly by the enrolled cases or controls; three studies obtained data from a proxy respondent when the enrolled case was too ill or deceased. The interviews/questionnaires included items on the participants’ date of birth, sex, education level, race/ethnicity, height, weight during a reference period (typically one year) prior to interview and/or “usual” adult weight, and habits pertaining to use of tobacco or alcohol. Two population-based studies (NCI Black-White, FHCRC) also collected information on weight in younger adulthood, specifically at age 25 or 30, respectively. Participants in the Utah study reported weight at age 40 as a usual adult weight, and therefore cases (n=6) and controls (n=1) from Utah who were younger than 40 years at interview were removed from the analysis.
Classification of anthropometric variables
We determined sex-specific cut-points for quartiles of height (m), usual adult weight (kg, referring to the year pre-interview or [for Utah] at age 40), and younger adult weight (kg, referring to weight at age 25 or 30) among the pooled control subjects with non-missing data on those variables. We also computed the usual adult and younger adult body mass index (BMI, kg/m2) from the height and usual or younger adult weight. We categorized the BMI variables into the World Health Organization (WHO)-defined categories of “underweight” (<18.5 kg/m2), “normal” (18.5 to <25 kg/m2), “overweight” (25 to <30 kg/m2), “obese” (30 to <35 kg/m2), and “severely obese” (≥ 35 kg/m2) (36, 37).
Classification of demographic and other potential confounding variables. We categorized age at interview by decade (<50, 50–59, 60–69, 70+ years) and race/ethnicity into three groups (White, Black, Other/unknown). We harmonized education level into five categories (<12 years of study; 12+ years or high school graduate; some attendance at college, technical, or vocational school after high school; graduation from college without further studies; or, other) and defined participants’ history of tobacco use (ever, never) and alcohol intake (ever, never)(26, 38).
Statistical analysis
To evaluate the association of usual or younger adult BMI, height and younger adult weight with risk of MM, we computed odds ratios (OR) and 95% confidence intervals (CI) using unconditional logistic regression models. We first computed study-specific and study center-specific ORs to evaluate heterogeneity in the associations across the participating studies and study centers. To assess the heterogeneity of the usual adult BMI-MM associations across study populations, we performed a meta-analysis of study center-specific ORs and variances from covariable-adjusted models (see below) using both fixed and random effects models.
We also directly pooled the data across all participating study centers and computed ORs and 95% CIs in the combined population using logistic regression. To evaluate possible sources of heterogeneity we conducted additional analyses that included interaction terms for usual or younger adult BMI with potential effect modifiers, including study design (hospital- v. population-based control ascertainment), study or study center, interview type (participant v. proxy), age group at interview, sex, and race. We assessed the statistical significance of any apparent interaction with the likelihood ratio test. To control for potential confounding we included indicator variables for age group at interview, sex, race/ethnicity, study center, height, education level, and history of tobacco use and alcohol intake; we considered a given covariable to be a confounding variable if its inclusion in a model resulted in a 10% or greater change to the corresponding effect estimate. We examined the joint classification of usual (<25, 25 to <30, ≥30 kg/m2) and younger adult BMI (<25, 25 to <30, ≥30 kg/m2) in pooled data from the NCI Black-White and FHRC studies. To evaluate linear trend across the categories of a given anthropometric variable we assigned exposure category medians to ordinal variables that we modeled as continuous variables in additional multivariable logistic regression models. For comparison we also assessed the increase in MM risk per 5-kg/m2 increase in usual or younger adult BMI.
We utilized SAS (Cary, NC) version 9.2 for all statistical analyses except for the meta-analyses, which we performed using the MiMa package (39) in R. All tests of statistical significance assumed a two-tailed alpha error level of 0.05.
Results
The eight participating case-control studies contributed a total pooled sample of 2,318 cases of incident MM and 9,609 controls to the present analysis (Table 1). Differences between cases and controls in the distribution of study design, demographic and lifestyle variables were statistically significant. We did not observe confounding of usual or younger adult BMI associations with MM by height, education, tobacco use or alcohol intake, and thus we did not retain those covariables in the final logistic regression models for the BMI variables.
Table 1.
Selected characteristics of the pooled study population
| Cases N (%) |
Controls N (%) |
p-value | |
|---|---|---|---|
| Pooled total | 2,318 | 9,609 | |
| Study design* | < 0.0001 | ||
| Hospital-based | 293 (12.64) | 1,817 (18.91) | |
| Population-based | 2,025 (87.36) | 7,792 (81.09) | |
| Interview type | < 0.0001 | ||
| Self | 2,074 (89.47) | 9,005 (93.71) | |
| Proxy | 244 (10.53) | 604 (6.29) | |
| Sex | 0.0001 | ||
| Men | 1,093 (47.15) | 4,947 (51.48) | |
| Women | 1,225 (52.85) | 4,662 (48.52) | |
| Age, years | < 0.0001 | ||
| <50 | 242 (10.44) | 1,758 (18.30) | |
| 50–59 | 515 (22.22) | 2,074 (21.58) | |
| 60–69 | 829 (35.76) | 2,880 (29.83) | |
| 70+ | 730 (31.49) | 2,893 (29.97) | |
| Missing | 2 (0.09) | 4 (0.04) | |
| Education level | 0.001 | ||
| <12 years of education | 815 (35.16) | 3,370 (35.07) | |
| 12+ years or high school graduate | 659 (28.48) | 2,760 (28.73) | |
| College, technical or vocational school | 425 (18.31) | 1,559 (16.22) | |
| College graduate only | 388 (16.72) | 1,676 (17.44) | |
| Other | 24 (1.03) | 219 (2.28) | |
| Missing | 7 (0.30) | 25 (0.26) | |
| Race/ethnicity | < 0.0001 | ||
| White | 1,764 (76.10) | 7,940 (82.63) | |
| Black | 478 (20.62) | 1,485 (15.45) | |
| Other/unknown | 71 (3.06) | 133 (1.38) | |
| Missing | 5 (0.22) | 51 (0.53) | |
| Ever smoke cigarettes | < 0.0001 | ||
| No | 1,034 (44.60) | 3,972 (41.34) | |
| Yes | 1,104 (47.63) | 5,018 (52.22) | |
| Missing | 180 (7.77) | 619 (6.44) | |
| Ever alcohol consumption | < 0.0001 | ||
| No | 531 (22.91) | 1,983 (20.64) | |
| Yes | 709 (30.59) | 3,819 (39.74) | |
| Missing | 1078 (46.50) | 3807 (39.62) |
As determined by source of control subjects.
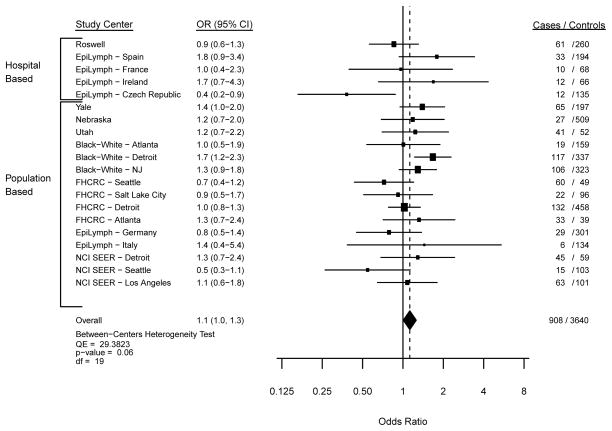
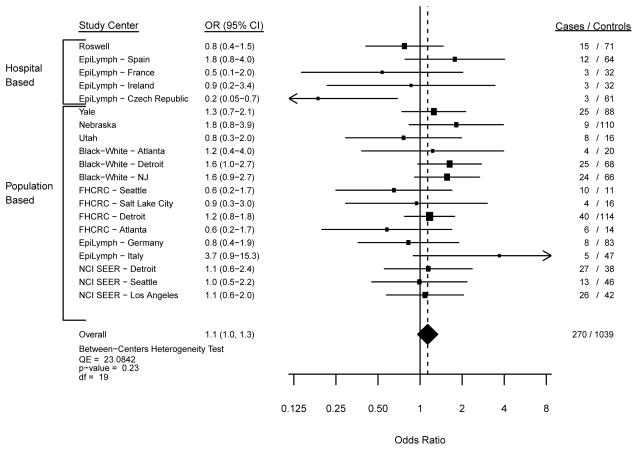
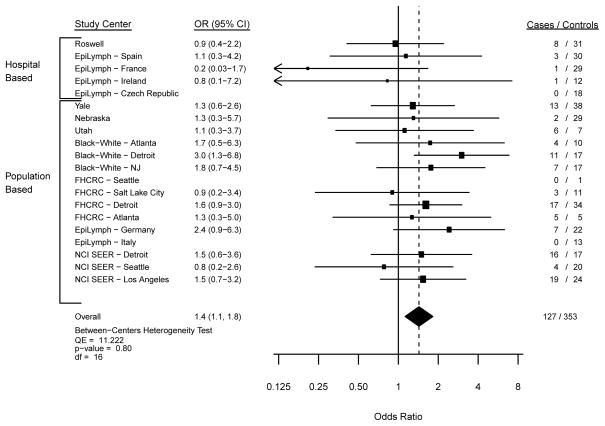
In the meta-analysis of usual adult BMI with MM risk, we observed similar findings from the fixed and random effects models, and thus we focus herein on the fixed effects model data. The Forest plots from models comparing overweight (Figure 1; p-heterogeneity=0.06), obesity (Figure 2; p-heterogeneity=0.23) and severe obesity (Figure 3; p-heterogeneity=0.80) with a normal BMI illustrate statistically non-significant variability in the risk estimates across study centers. Across all participating study centers, the summary ORs indicated that MM risk increased similarly by 10% for overweight (Figure 1) and obese (Figure 2) persons and by 40% for severely obese individuals (Figure 3) compared with those with a usual adult BMI in the WHO-defined normal range.
Figure 1. Risk of Myeloma with a BMI of 25–<30 kg/m2.
Study center-specific and summary odds ratios (OR) and 95% confidence intervals (CI) for the risk of multiple myeloma with a usual adult BMI of 25 to <30 kg/m2 compared to the referent category of 18.5 to <25 kg/m2 in 20 study centers. The study center-specific data are organized by study design (hospital- v. population-based case-control study). The summary measures were obtained by meta-analysis using fixed effects models.
Figure 2. Risk of Myeloma with a BMI of 30–<35 kg/m2 compared with the referent 18.5 to <25 kg/m2.
Study center-specific and summary odds ratios (OR) and 95% confidence intervals (CI) for the risk of multiple myeloma with a usual adult BMI of 30 to <35 kg/m2 compared to the referent category of 18.5 to <25 kg/m2 in 20 study centers. The study center-specific data are organized by study design (hospital- v. population-based case-control study). The summary measures were obtained by meta-analysis using fixed effects models.
Figure 3. Risk of Myeloma with a BMI of 35+ kg/m2 compared with the referent 18.5 to <25 kg/m2.
Study center-specific and summary odds ratios (OR) and 95% confidence intervals (CI) for the risk of multiple myeloma with a BMI of 35+ kg/m2 compared to the referent category of 18.5 to <25 kg/m2 in 20 study centers. The study center-specific data are organized by study design (hospital- v. population-based case-control study). The summary measures were obtained by meta-analysis using fixed effects models.
We observed a similar positive association of usual adult BMI with MM risk in the pooled study sample (p-trend=0.008; Table 2). After controlling for age, sex, race and study center, the ORs for overweight, obesity and severe obesity were virtually identical to those observed in the meta-analysis. When modeled continuously, MM risk increased by 9% per 5-kg/m2 increase in usual adult BMI (OR, 95% CI: 1.09, 1.04–1.14; p=0.007). Likelihood ratio tests indicated significant heterogeneity of the usual adult BMI-MM association by study design (p-heterogeneity=0.04) but not by interview type (p-heterogeneity=0.98; Table 2). We therefore performed subsequent analyses within strata defined by study design.
Table 2.
Pooled relative risk of multiple myeloma by category of usual and younger adult BMI, study design and interview type
| Stratification | N* | Usual BMI (kg/m2)† | p-heterogeneity ‡ | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| <18.5 | 18.5–<25 | 25–<30 | 30–<35 | 35+ | |||
|
USUAL ADULT BMI
| |||||||
| All Subjects | N/A | ||||||
| Case N (%) | 2,318 | 36 (1.5) | 977 (42.1) | 908 (39.2) | 270 (11.7) | 127 (5.5) | |
| Control N (%) | 9,609 | 191 (2.0) | 4,354 (45.3) | 3,640 (37.9) | 1,039 (10.8) | 385 (4.0) | |
| OR (CI)§ | 0.8 (0.5–1.1) | 1.0 (ref) | 1.1 (1.0–1.3) | 1.1 (1.0–1.3) | 1.4 (1.1–1.7) | ||
| p-trend=0.008¶ | |||||||
| Study design | 0.042 | ||||||
| Hospital Based | |||||||
| Case N (%) | 293 | 4 (1.4) | 112 (38.2) | 128 (43.7) | 36 (12.3) | 13 (4.4) | |
| Control N (%) | 1,817 | 36 (2.0) | 678 (37.3) | 723 (37.8) | 260 (14.3) | 120 (6.6) | |
| OR (CI)§ | 0.8 (0.3–2.2) | 1.0 (ref) | 1.0 (0.7–1.3) | 0.8 (0.6–1.3) | 0.6 (0.3–1.2) | ||
| p-trend=0.59¶ | |||||||
| Population Based | |||||||
| Case N (%) | 2,025 | 32 (1.6) | 865 (42.7) | 780 (38.5) | 234 (11.6) | 114 (5.6) | |
| Control N (%) | 7,792 | 155 (2.0) | 3,676 (47.2) | 2,917 (37.4) | 779 (10.0) | 266 (3.4) | |
| OR (CI)§ | 0.8 (0.5–1.2) | 1.0 (ref) | 1.1 (1.0–1.3) | 1.2 (1.0–1.4) | 1.6 (1.3–2.1) | ||
| p-trend=0.0003¶ | |||||||
| Interview Type | 0.98 | ||||||
| Self | |||||||
| Case N (%) | 2,074 | 33 (1.6) | 862 (41.2) | 813 (39.2) | 249 (12.0) | 117 (5.6) | |
| Control N (%) | 9,005 | 177 (2.0) | 4,054 (45.0) | 3,417 (38.0) | 990 (11.0) | 367 (4.0) | |
| OR (CI)§ | 0.8 (0.6–1.2) | 1.0 (ref) | 1.1 (1.0–1.3) | 1.2 (1.0–1.4) | 1.4 (1.1–1.8) | ||
| p-trend=0.01¶ | |||||||
| Proxy | |||||||
| Case N (%) | 244 | 3 (1.2) | 115 (45.3) | 95 (37.4) | 21 (8.3) | 10 (3.9) | |
| Control N (%) | 604 | 14 (2.2) | 300 (46.7) | 223 (34.7) | 49 (12.3) | 18 (2.8) | |
| OR (CI)§ | 0.4 (0.04–4.2) | 1.0 (ref) | 0.9 (0.4–1.7) | 1.1 (0.4–3.3) | 1.2 (0.3–5.9) | ||
| p-trend=0.91¶ | |||||||
|
| |||||||
| YOUNGER ADULT BMI (kg/m2)† | |||||||
|
| |||||||
| Case N (%) | 1,164 | 70 (5.6) | 791 (62.9) | 253 (20.1) | 37 (2.1) | 13 (1.0) | N/A |
| Control N (%) | 3,629 | 220 (5.8) | 2,655 (67.8) | 632 (16.6) | 101 (2.7) | 21 (0.6) | |
| OR (CI)§ | 0.9 (0.7–1.3) | 1.0 (ref) | 1.5 (1.2–1.7) | 1.3 (0.9–1.9) | 2.2 (1.1–4.5) | ||
| p-trend=0.0001¶ | |||||||
Abbreviations: BMI, body mass index; OR, odds ratio; CI, confidence interval; N/A = Not Applicable.
Counts across recent BMI categories may not add up to N total because of missing data.
Usual adult BMI was calculated from height at study enrollment and weight as of the study-specific reference period prior to interview. In the two population-based study populations with data on younger adult weight, younger adult BMI was calculated from height at study enrollment and weight reported for younger adulthood.
P-values for heterogeneity were obtained from likelihood ratio tests that compared logistic regression models with only main effects variables to models that also included a term for the interaction of BMI with the specified stratifying variable.
From logistic regression models controlling for age (<50, 50–59, 60–69, >70), sex, race (White, Black, Other/unknown), and study center.
P-values for trend were obtained by modeling category of BMI as an ordinal variable in logistic regression models with the same covariables as in the models that generated the corresponding odds ratios.
From the population-based studies we observed a slightly stronger positive association of usual adult BMI with MM risk than from the full study sample (p-trend=0.0003); for example, in continuous multivariable models, MM risk increased by 11% per 5-kg/m2 increase in usual adult BMI (OR, 95% CI: 1.11, 1.06–1.17; p=0.0001). When modeled by WHO category, overweight and obese individuals had a 10% (OR, 95% CI: 1.1, 1.0–1.3) and 20% (OR, 95% CI: 1.2, 1.0–1.4) greater risk, respectively, and severely obese participants had a 60% greater risk (OR, 95% CI: 1.6, 1.3–2.1) when compared with those with a normal adult BMI. In contrast, we did not observe an association of MM risk with usual adult BMI in the hospital-based studies, whether modeled using WHO categories (p-trend=0.59; Table 2) or 5-kg/m2 incremental increases in BMI (p=0.48). Adult BMI had a significant or suggestive positive association with MM in each age- and race-related stratum of the population-based study participants (Supplementary Table S2), with no clear evidence of heterogeneity by demographic risk factors (p-values all ≥0.30). Usual adult BMI was not associated with MM for all persons or for any stratum of the hospital-based study participants (p-trends all ≥0.26; Supplementary Table S3).
In pooled analyses across the two population-based studies (comprising 7 study centers) with data on younger adult weight we observed a significant positive association of younger adult BMI with risk of MM (p-trend=0.0001; Table 2). In the continuous models, MM risk increased by 20% per 5-kg/m2 increase in younger adult BMI (OR, 95% CI: 1.2, 1.1–1.3; p=0.0002). Participants who reported severe obesity in younger adulthood had a more than two-fold increase in MM risk compared with those with a normal younger adult BMI (multivariable OR, 95% CI: 2.2, 1.1–4.5), and those who were overweight (OR, 95% CI: 1.5, 1.2–1.7) or obese (OR, 95% CI: 1.3, 0.9–1.9) in younger adulthood had suggestive modest increases in risk (Table 2). Younger adult BMI did not demonstrate significant interaction with study center, age, sex or race (all p-values for interaction ≥0.46). In this participant subgroup, the association of usual adult BMI with MM was similar to that for younger adult BMI (usual adult BMI, OR per 5-kg/m2: 1.16, 1.07–1.26). Further, usual and young adult weight (Spearman r=0.73, p=0.0001) and BMI (Spearman r=0.56, p=0.0001) were significantly correlated.
We observed a highly significant interaction between younger adult and usual adult BMI (p-heterogeneity < 0.0001; Table 3). In models that examined a joint classification of younger and usual adult BMI, we observed significantly elevated MM risk only for persons classified as overweight or obese at both early and later adulthood compared with individuals with BMI <25 kg/m2 on both measures. Individuals who were overweight (OR 1.2, 95% CI: 0.8–1.9) or obese (OR 1.0, 95% CI: 0.3–3.1) in early adulthood but not in the reference period prior to diagnosis did not have a clearly increased risk of MM, nor did those with both a normal younger adult BMI and a usual adult BMI in the overweight (OR 1.1, 95% CI: 0.9–1.3) or obese categories (OR 1.0, 95% CI: 0.7–1.4).
Table 3.
Joint classification of younger adult BMI and usual BMI and pooled relative risk of multiple myeloma in two population-based case-control studies
| Stratification | N* | Younger adult BMI (kg/m2)† | |||
|---|---|---|---|---|---|
| <25 | 25–<30 | 30+ | |||
| Usual BMI (kg/ m2)‡ | P§= <0.0001 | ||||
| <25 | |||||
| Case N (%) | 560 | 523 (93.4) | 33 (5.9) | 4 (0.7) | |
| Control N (%) | 1,861 | 1,772 (95.2) | 77 (4.1) | 12 (0.6) | |
| OR (CI)¶ | 1.0 (ref) | 1.2 (0.8–1.9) | 1.0 (0.3–3.1) | ||
| 25–<30 | |||||
| Case N (%) | 454 | 283 (19.5) | 156 (79.5) | 15 (1.0) | |
| Control N (%) | 1,384 | 936 (67.6) | 412 (29.8) | 36 (2.6) | |
| OR (CI)¶ | 1.1 (0.9–1.3) | 1.4 (1.1–1.8) | 1.3 (0.7–2.5) | ||
| 30+ | |||||
| Case N (%) | 150 | 55 (36.4) | 64 (43.0) | 31 (20.5) | |
| Control N (%) | 384 | 167 (43.4) | 143 (37.2) | 74 (19.3) | |
| OR (CI)¶ | 1.0 (0.7–1.4) | 1.7 (1.2–2.3) | 1.7 (1.1–2.6) | ||
Abbreviations: BMI, body mass index; OR, odds ratio; CI, confidence interval.
Percentages across rows may not add up to 100% due to rounding. 389 subjects were excluded from analysis due to missing data.
Calculated from height at study enrollment and weight reported for young adulthood.
Calculated from height at study enrollment and weight as of the study-specific reference period prior to interview.
P-value for heterogeneity from a likelihood ratio test that compared logistic regression models with only main effects variables to a model that also included a term for the interaction of younger adult BMI with usual BMI.
From logistic regression models controlling for age (<50, 50–59, 60–69, >70), sex, race (White, Black, Other/unknown), and study center.
In the analysis of other anthropometric measures (Supplementary Table S4), persons in the highest quartile (Q4) of usual adult weight had a modest increase in MM (v. Q1, multivariable OR, 95% CI: 1.3, 1.1–1.4; p-trend=0.15) that was statistically significant in population-based studies (v. Q1; OR, 95% CI: 1.3, 1.2–1.6; p-trend=0.04) but not evident in hospital-based studies (v. Q1; OR, 95% CI: 0.8, 0.6–1.2; p-trend=0.08; p-heterogeneity by study design=0.01). Quartile of younger adult weight also demonstrated a modest positive association with MM risk (in two population-based studies, Q4 v. Q1, multivariable OR, 95% CI: 1.5, 1.2–1.8; p-trend=0.001). We did not observe an association of adult height with MM risk for all subjects or for any separately evaluated demographically defined risk stratum (i.e., age, sex, race; p-trends all ≥0.35).
Discussion
In this large pooled IMMC analysis we observed a positive association of both usual and younger adult BMI with risk of MM, as well as with usual and younger adult weight. Further, we noted that usual and younger adult BMI interact with one another, such that increased MM risk was strongest for individuals who were overweight or obese in both time periods, and weaker or absent for those obese or overweight at only one of the time periods.
The positive association that we observed for usual adult BMI and MM is consistent with most (but not all) previous studies, as summarized in recent meta-analyses (10–12). Of interest, in our pooled study sample the association of usual adult BMI with MM was restricted to the population-based studies, suggesting that one or more attributes of the hospital-based study design—possibly an association of BMI with control diagnoses or factors that influenced participation—may have introduced bias. The increases in MM risk that we observed in severely obese, obese and overweight population-based study participants are roughly consistent in magnitude with the meta-analysis results for obese and overweight persons (10–12); the meta-analyses did not separately report findings for more severe obesity. Our findings are also similar to those from a prospective study of MM mortality in the Cancer Prevention Study II population of nearly 1.2 million US residents (36). In that analysis, women and men with a baseline BMI of 35 to 39.9 kg/m2 (i.e., severely obese) had a 44% and 75% increase in MM mortality, respectively, and those with a baseline BMI classified as overweight (25 to 29.9 kg/m2) or obese (30 to 34.9 kg/m2) had 12% to 47% higher MM mortality, compared with those with a normal baseline BMI (36). The meta-analyses did not separately evaluate the MM-BMI association by age, sex, or race, and individual studies had limited statistical power to assess heterogeneity by these MM risk factors. Our findings provide strong evidence that the association of usual adult BMI with MM is similarly detectable across demographic MM risk groups including race. The recent in vivo demonstration by Lwin et al. (25) of a link between the development of MM-like conditions and diet-induced obesity further strengthens the evidence that obesity is causally associated with MM.
Our analysis of younger adult BMI and MM risk, including 1,164 cases and 3,629 controls, is the largest to date. We observed an association with MM for younger adult BMI of comparable size to that for usual adult BMI, and a significant correlation between those two measures. Of the previous studies that have reported on both earlier and later life body size and MM risk, three prospective investigations observed significant associations with BMI in early and later adulthood (13, 14, 40, 41). Three other studies—including two prospective analyses restricted to women with 92 and 111 MM cases (42, 43) and a pooled case-control analysis (44)—did not find an association of MM risk with BMI at any age. The latter null studies may reflect limited statistical power from smaller case counts, and in particular from limited numbers of cases included in higher categories of younger adult BMI.
A key finding of our investigation is that a significantly increased MM risk was apparent only for participants who were overweight or obese during both early and later adulthood when participants were jointly classified for both time periods. Similar patterns of association with BMI across early and later adulthood were also observed in joint analyses from two cohort investigations of MM, although tests of interaction did not reach statistical significance (13, 14). Although it is not possible in our data to conclusively distinguish a long-lasting influence of younger adult BMI from an influence of persistent obesity throughout adulthood, our findings are consistent with a conceptual model in which pathogenic effects of BMI influence myelomagenesis both at earlier and later stages, a model also supported by recent evidence relating BMI to MGUS prevalence (45) and to progression from MGUS to MM (46). These findings also have important public health implications; they suggest that maintaining a healthy body size throughout adulthood is optimal for reducing MM risk and further, that adults with a history of carrying excess weight may reduce their MM risk by achieving a normal BMI later in life.
The present findings for usual and younger adult weight and MM risk were weaker than our observations for usual and younger adult BMI but were generally consistent with a positive association of weight with MM risk at both time periods. Likewise, in other studies that reported findings for both weight and BMI at a given age, the results for weight tended to be similar to or weaker than those for BMI (41, 43, 44, 47, 48). Some studies (43, 44, 47, 49) also reported a suggestive, usually modest positive association of MM risk with height, primarily for women (43, 44, 47), whereas other large prospective studies did not note any associations for height (13, 40, 41, 48). Our observation of no clear association for height with MM risk supports the collective evidence that adiposity is the more important component of body size underlying the association of BMI with MM. We could not assess measures of central adiposity in the present study to further clarify whether specific types of adiposity have a stronger association with MM (13, 47, 48).
A rapidly expanding body of literature addresses biologic processes that are active or dysregulated in both obesity and oncogenesis and may underlie observed associations of obesity with cancer [recently reviewed by De Pergola and Silvestris (16)]. Several of the implicated pathways are known contributors to MM pathogenesis (15). For example, IGF-1 (17, 18), insulin (19), and IL-6 (20) are growth factors for MM. Further, some byproducts of lipid metabolism can activate nuclear factor (NF)-κB, a transcription factor that mediates aspects of normal B-cell hematopoiesis but is up-regulated in MM and cooperates with other pathways to promote cell proliferation and survival (50). More recently it has also been recognized that adiponectin, an anti-inflammatory adipocyte-derived cytokine that is inversely correlated with BMI, has anti-proliferative effects on MM in vivo (21). In support of a role for these pathways in MM etiology, pre-diagnosis peripheral blood concentrations of IGF-1 binding protein (IGFBP)-1, the soluble IL-6 receptor (sIL6R) and adiponectin demonstrated significant associations with the development of MM in recent prospective studies (22–24).
The major strength of the present study is its large sample size, which enabled the separate examination of severe obesity and the joint analysis of BMI in early and later adulthood. However, we recognize that small numbers in some of the joint analysis categories of BMI in early and later adulthood—in particular, the small number of participants who were heavy in younger but not later adulthood—limit our ability to assess the relative importance of excess weight in one age period versus the other in affecting MM risk. Another limitation is the lack of information on other potentially relevant anthropometric measures such as waist and hip circumference, other measures of adiposity and on MGUS status as a second outcome. The IMMC member studies reported varying levels of participation among eligible cases and controls, and we cannot rule out an influence of selection or other biases on our results. However, the overall consistency of our findings with those from prospective studies is reassuring, as is the noted biologic plausibility of our observations. Lastly, notwithstanding our large pooled sample size, we had limited statistical power for analyses of non-White strata and could not evaluate anthropometric measure associations with MM separately for Asian and Hispanic participants for whom MM risk factor data are generally more limited. Further evaluation of these associations using larger and more diverse study samples would provide important insights to clarify whether individuals from those understudied racial/ethnic groups could also expect an MM risk reduction from maintaining a healthy BMI throughout adulthood.
In conclusion, we have reported some of the strongest evidence to date in support of a positive association of BMI with MM. In particular, our study demonstrates that these effects begin in young adulthood, and are most apparent for individuals who carry excess weight throughout adulthood. Whether those findings reflect a persistent influence of younger adult obesity or a cumulative influence of lifelong BMI on myelomagenesis warrants further investigation. Given that obesity remains the only known modifiable risk factor for this as yet incurable malignancy, public health efforts to reduce the prevalence of obesity among both younger and older adults currently represents the best available strategy for reducing even a modest proportion of the incidence and burden of MM.
Supplementary Material
Acknowledgments
Financial support: Financial support for this study was provided by the NCI Intramural Program Division of Cancer Epidemiology and Genetics, and by NCI grants K07 CA115687 (BMB), R01 CA149445, R01 CA127435, P30 CA014089, R21CA198239 and R13 CA159842. BMB was also supported in part by the American Cancer Society (RSG- 11-020-01-CNE), and WC was supported in part by Federal funds from the National Cancer Institute (SEER): N01-CP-67010 SOW 16. Additional support was provided by Rio Hortega (CM13/00232), MV15/00025 and public grants from Health Ministry of Spain (PI11/01810 and PI14/01219) and the Catalan Government (2014SGR756). The Utah studies were supported by the Research Informatics Shared Resource which in part is funded by NCI P30CA042014 and Leukemia and Lymphoma Society award 6067-09 (NJC). EpiLymph was supported by European Commission 5th Framework Programme (QLK4-CT-2000-00422); 6th Framework Programme (FOOD-CT-2006-023103); Carlos III Institute of Health (FIS PI081555, RCESP C03/09, RTICESP C03/10, RTICRD06/0020/0095, CIBERESP and European Regional Development Fund-ERDF); Marató TV3 Foundation (051210); International Agency for Research on Cancer (IARC-5111); MH CZ - DRO (MMCI, 00209805), RECAMO CZ.1.05/2.1.00/03.0101; Fondation de France (1999 008471; EpiLymph-France); Italian Association for Cancer Research (AIRC, Investigator Grant 11855); Italian Ministry of Education, University and Research, PRIN programme (2007WEJLZB, 20092ZELR2); the German Federal Office for Radiation Protection (StSch4261 and StSch4420; EpiLymph Germany); and the Health Research Board, Ireland. The collection of patients used in this publication was supported in part by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, the American Cancer Society or other funding agencies noted above. The authors declare no competing financial interests.
The authors thank Emily Steplowski and Joe Krzystan for data management and statistical programming. We also gratefully acknowledge the guidance and support of Rao (Mahesh) Divi and the participants and staff of each participating study, without whose dedication and contributions the present study would not be possible.
Footnotes
Conflicts of interest:
Marc Maynadié (Author #19)
Consultant/Advisory Board
-
Entity: RocheRelationship: MyselfCompensation: CompensatedType: Minor ($10,000 or less)
-
Entity: JANSSENRelationship: MyselfCompensation: CompensatedType: Minor ($10,000 or less)
The remaining authors declare no potential conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Pozzi S, Marcheselli L, Bari A, Liardo EV, Marcheselli R, Luminari S, et al. Survival of multiple myeloma patients in the era of novel therapies confirms the improvement in patients younger than 75 years: a population-based analysis. Br J Haematol. 2013;163:40–6. doi: 10.1111/bjh.12465. [DOI] [PubMed] [Google Scholar]
- 3.Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist. 2011;16:1600–3. doi: 10.1634/theoncologist.2011-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: Apr, 2016. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- 5.Birmann BM, Chiu BCH, Muench K, Suppan CA, Cozen W. Epidemiology and etiology of multiple myeloma. In: Podar K, Anderson KC, editors. Multiple myeloma -- a new era of treatment strategies. Bentham Science Publishers; 2011. pp. 15–57. [Google Scholar]
- 6.Landgren O, Kristinsson SY, Goldin LR, Caporaso NE, Blimark C, Mellqvist UH, et al. Risk of plasma cell and lymphoproliferative disorders among 14621 first-degree relatives of 4458 patients with monoclonal gammopathy of undetermined significance in Sweden. Blood. 2009;114:791–5. doi: 10.1182/blood-2008-12-191676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–22. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23:1691–7. doi: 10.1038/leu.2009.134. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Wolk A. Body mass index and risk of multiple myeloma: a meta-analysis. Int J Cancer. 2007;121:2512–6. doi: 10.1002/ijc.22968. [DOI] [PubMed] [Google Scholar]
- 11.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 12.Wallin A, Larsson SC. Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47:1606–15. doi: 10.1016/j.ejca.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Teras LR, Kitahara CM, Birmann BM, Hartge PA, Wang SS, Robien K, et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. Br J Haematol. 2014 doi: 10.1111/bjh.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann JN, Moore SC, Lim U, Park Y, Baris D, Hollenbeck AR, et al. Body mass index and physical activity at different ages and risk of multiple myeloma in the NIH-AARP diet and health study. American journal of epidemiology. 2013;177:776–86. doi: 10.1093/aje/kws295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtman MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist. 2010;15:1083–101. doi: 10.1634/theoncologist.2010-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge NL, Rudikoff S. Insulin-like growth factor I is a dual effector of multiple myeloma cell growth. Blood. 2000;96:2856–61. [PubMed] [Google Scholar]
- 18.Qiang YW, Kopantzev E, Rudikoff S. Insulinlike growth factor-I signaling in multiple myeloma: downstream elements, functional correlates, and pathway cross-talk. Blood. 2002;99:4138–46. doi: 10.1182/blood.v99.11.4138. [DOI] [PubMed] [Google Scholar]
- 19.Sprynski AC, Hose D, Kassambara A, Vincent L, Jourdan M, Rossi JF, et al. Insulin is a potent myeloma cell growth factor through insulin/IGF-1 hybrid receptor activation. Leukemia. 2010;24:1940–50. doi: 10.1038/leu.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano T. Interleukin 6 (IL-6) and its receptor: their role in plasma cell neoplasias. Int J Cell Cloning. 1991;9:166–84. doi: 10.1002/stem.5530090303. [DOI] [PubMed] [Google Scholar]
- 21.Fowler JA, Lwin ST, Drake MT, Edwards JR, Kyle RA, Mundy GR, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–82. doi: 10.1182/blood-2011-01-330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birmann BM, Neuhouser ML, Rosner B, Albanes D, Buring JE, Giles GG, et al. Prediagnosis biomarkers of insulin-like growth factor-1, insulin, and interleukin-6 dysregulation and multiple myeloma risk in the Multiple Myeloma Cohort Consortium. Blood. 2012;120:4929–37. doi: 10.1182/blood-2012-03-417253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann JN, Liao LM, Pollak MN, Wang Y, Pfeiffer RM, Baris D, et al. A prospective study of circulating adipokine levels and risk of multiple myeloma. Blood. 2012;120:4418–20. doi: 10.1182/blood-2012-06-438606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmann JN, Birmann BM, Teras LR, Pfeiffer RM, Wang Y, Albanes D, et al. Low Levels of Circulating Adiponectin Are Associated with Multiple Myeloma Risk in Overweight and Obese Individuals. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lwin ST, Olechnowicz SW, Fowler JA, Edwards CM. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia. 2015;29:507–10. doi: 10.1038/leu.2014.295. [DOI] [PubMed] [Google Scholar]
- 26.Andreotti G, Birmann B, De Roos AJ, Spinelli J, Cozen W, Camp NJ, et al. A pooled analysis of alcohol consumption and risk of multiple myeloma in the international multiple myeloma consortium. Cancer Epidemiol Biomarkers Prev. 2013;22:1620–7. doi: 10.1158/1055-9965.EPI-13-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortuny J, de Sanjose S, Becker N, Maynadie M, Cocco PL, Staines A, et al. Statin use and risk of lymphoid neoplasms: results from the European Case-Control Study EPILYMPH. Cancer Epidemiol Biomarkers Prev. 2006;15:921–5. doi: 10.1158/1055-9965.EPI-05-0866. [DOI] [PubMed] [Google Scholar]
- 28.Becker N, Deeg E, Nieters A. Population-based study of lymphoma in Germany: rationale, study design and first results. Leuk Res. 2004;28:713–24. doi: 10.1016/j.leukres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Koepsell TD, Daling JR, Weiss NS, Taylor JW, Olshan AF, Lyon JL, et al. Antigenic stimulation and the occurrence of multiple myeloma. American journal of epidemiology. 1987;126:1051–62. doi: 10.1093/oxfordjournals.aje.a114744. [DOI] [PubMed] [Google Scholar]
- 30.Brown LM, Gridley G, Pottern LM, Baris D, Swanso CA, Silverman DT, et al. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control. 2001;12:117–25. doi: 10.1023/a:1008937901586. [DOI] [PubMed] [Google Scholar]
- 31.Landgren O, Zhang Y, Zahm SH, Inskip P, Zheng T, Baris D. Risk of multiple myeloma following medication use and medical conditions: a case-control study in Connecticut women. Cancer Epidemiol Biomarkers Prev. 2006;15:2342–7. doi: 10.1158/1055-9965.EPI-06-0097. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Holford TR, Leaderer B, Zahm SH, Boyle P, Morton LM, et al. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15:419–28. doi: 10.1023/B:CACO.0000027506.55846.5d. [DOI] [PubMed] [Google Scholar]
- 33.Chatterjee N, Hartge P, Cerhan JR, Cozen W, Davis S, Ishibe N, et al. Risk of non-Hodgkin’s lymphoma and family history of lymphatic, hematologic, and other cancers. Cancer Epidemiol Biomarkers Prev. 2004;13:1415–21. [PubMed] [Google Scholar]
- 34.Zahm SH, Weisenburger DD, Babbitt PA, Saal RC, Vaught JB, Blair A. Use of hair coloring products and the risk of lymphoma, multiple myeloma, and chronic lymphocytic leukemia. American journal of public health. 1992;82:990–7. doi: 10.2105/ajph.82.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moysich KB, Bonner MR, Beehler GP, Marshall JR, Menezes RJ, Baker JA, et al. Regular analgesic use and risk of multiple myeloma. Leuk Res. 2007;31:547–51. doi: 10.1016/j.leukres.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Report of a WHO Expert Committee. Geneva: World Health Organization; 1995. Physical Status: The use and interpretation of anthropometry. Report No.: WHO Technical Report Series No. 854. [PubMed] [Google Scholar]
- 38.Andreotti G, Birmann BM, Cozen W, De Roos AJ, Chiu BC, Costas L, et al. A pooled analysis of cigarette smoking and risk of multiple myeloma from the international multiple myeloma consortium. Cancer Epidemiol Biomarkers Prev. 2015;24:631–4. doi: 10.1158/1055-9965.EPI-14-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viechtbauer W. MiMa: An S-Plus/R function to fit meta-analytic mixed-, random-, and fixed-effects models [Computer software and manual] 2006 Retrieved from http://www.wvbauer.com/
- 40.Pylypchuk RD, Schouten LJ, Goldbohm RA, Schouten HC, van den Brandt PA. Body mass index, height, and risk of lymphatic malignancies: a prospective cohort study. American journal of epidemiology. 2009;170:297–307. doi: 10.1093/aje/kwp123. [DOI] [PubMed] [Google Scholar]
- 41.Troy JD, Hartge P, Weissfeld JL, Oken MM, Colditz GA, Mechanic LE, et al. Associations between anthropometry, cigarette smoking, alcohol consumption, and non-Hodgkin lymphoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. American journal of epidemiology. 2010;171:1270–81. doi: 10.1093/aje/kwq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Roos AJ, Ulrich CM, Ray RM, Mossavar-Rahmani Y, Rosenberg CA, Caan BJ, et al. Intentional weight loss and risk of lymphohematopoietic cancers. Cancer Causes Control. 2010;21:223–36. doi: 10.1007/s10552-009-9453-5. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Sullivan-Halley J, Henderson KD, Ma H, Horn-Ross PL, Reynolds P, et al. Anthropometric characteristics and multiple myeloma risk. Epidemiology. 2010;21:272–3. doi: 10.1097/EDE.0b013e3181cc9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang SS, Voutsinas J, Chang ET, Clarke CA, Lu Y, Ma H, et al. Anthropometric, behavioral, and female reproductive factors and risk of multiple myeloma: a pooled analysis. Cancer Causes Control. 2013;24:1279–89. doi: 10.1007/s10552-013-0206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landgren O, Rajkumar SV, Pfeiffer RM, Kyle RA, Katzmann JA, Dispenzieri A, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood. 2010;116:1056–9. doi: 10.1182/blood-2010-01-262394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas T, Chang S-H, Luo S, O’Brian K, Colditz GA, Carson KR. Is body mass index related to the progression of monoclonal gammopathy of undetermined significance to multiple myeloma? Blood. 2014;124:2016. [Google Scholar]
- 47.Britton JA, Khan AE, Rohrmann S, Becker N, Linseisen J, Nieters A, et al. Anthropometric characteristics and non-Hodgkin’s lymphoma and multiple myeloma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Haematologica. 2008;93:1666–77. doi: 10.3324/haematol.13078. [DOI] [PubMed] [Google Scholar]
- 48.Blair CK, Cerhan JR, Folsom AR, Ross JA. Anthropometric characteristics and risk of multiple myeloma. Epidemiology. 2005;16:691–4. doi: 10.1097/01.ede.0000172135.61188.2d. [DOI] [PubMed] [Google Scholar]
- 49.Patel AV, Diver WR, Teras LR, Birmann BM, Gapstur SM. Body mass index, height and risk of lymphoid neoplasms in a large United States cohort. Leuk Lymphoma. 2013;54:1221–7. doi: 10.3109/10428194.2012.742523. [DOI] [PubMed] [Google Scholar]
- 50.Demchenko YN, Glebov OK, Zingone A, Keats JJ, Bergsagel PL, Kuehl WM. Classical and/or alternative NF-kappaB pathway activation in multiple myeloma. Blood. 2010;115:3541–52. doi: 10.1182/blood-2009-09-243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.