Abstract
Background
Sublingual immunotherapy (SLIT) is a safe/well-tolerated alternative to allergen injection immunotherapy for allergic rhinoconjunctivitis (ARC). Patient adherence is essential and patient-related outcome measures including treatment satisfaction are informative/indicative of adherence.
Objective
The aim was to assess treatment satisfaction with five-grass pollen tablet SLIT under real-life conditions.
Methods
Treatment satisfaction among adults taking SLIT with a five-grass pollen tablet for grass pollen-related ARC was assessed with QUARTIS, a self-report questionnaire dedicated to the management of patients treated with SLIT for ARC. This 1-year prospective, non-interventional, post-authorization study was conducted in Germany between 2008 and 2010.
Results
Of the 327 patients treated with the five-grass pollen tablet, 253 completed the QUARTIS questionnaire before and during (at least one item) treatment and were included in this analysis. Between the baseline and the treatment season, significant improvements were documented in nasal and ocular symptoms, and in the impact of allergy on everyday life. At the end of the first treatment period, patients had an improved opinion of the ease of SLIT intake and a significantly improved perception of SLIT. Compliance, overall satisfaction and motivation to continue SLIT the following year were good. Physicians’ assessments showed reduced symptoms and a reduced need for symptomatic medication throughout the study. SLIT was also well tolerated.
Conclusion
Under real-life conditions, five-grass pollen tablet treatment was associated with a good level of treatment satisfaction, good symptom control, a reduced need for symptomatic medication, and favourable tolerability. These facets impacted favourably on patient functioning, disposition towards this medication, and adherence.
Key Points
| Patients with grass pollen-induced allergic rhinoconjunctivitis show good levels of treatment satisfaction when treated with a five-grass pollen tablet sublingual immunotherapy in routine clinical practice. |
| Patients experienced good symptom control, had less need for symptomatic medication and reported that five-grass pollen tablet was well tolerated. |
Introduction
Allergic rhinoconjunctivitis (ARC) is a major health issue, affecting more than 500 million people worldwide [1]. Commonly referred to as hay fever, ARC is characterized predominately by nasal and ocular symptoms such as rhinorrhoea, nasal congestion, and watery and itchy eyes. These symptoms cause significant impairment to the patients’ quality of life (QOL) [2], impacting negatively on sleep and daily living activities, including the ability to work, with a consequent drop in productivity [3].
Allergen-specific immunotherapy (SIT) is a widely administered treatment for ARC, which can be administered sublingually or subcutaneously. Motivations for using SIT include its potential to alter the course of disease and reduce the requirement for symptomatic medications or reduce dissatisfaction with current pharmacotherapy [4]. Sublingual immunotherapy (SLIT) is accepted as an efficacious, safe, and well-tolerated treatment [1, 5, 6] that can be self-administered, allowing patients more independence and causing less inconvenience in comparison to patients taking subcutaneous immunotherapy (SCIT). Patient evaluation of SLIT has shown this approach is frequently considered easier than SCIT while not inducing side effects [4]. SLIT has also been shown to improve the health-related QOL of patients with ARC and coexisting asthma [7]. For these reasons, it is expected that patients will accept SLIT more readily than SCIT.
Patient adherence to SIT remains a key issue, as with treatment for other chronic diseases. One method of predicting adherence is to measure treatment satisfaction among patients taking the agent by recording the individual’s rating of important attributes of the process and outcomes of their treatment experience [8]. Patient satisfaction has been shown to be positively correlated with patient adherence [9].
The patient–clinician relationship is changing, with patients now seeking more involvement in their own treatment decisions. Assessments of patient-reported outcomes (PROs) are therefore becoming more frequent in clinical trials for allergic conditions [10, 11]. PROs are defined as health-related reports provided by the patient, without involvement or interpretation by physicians [12]. As well as measuring parameters such as safety and efficacy, these outcomes can also assess patients’ preferences and values [13]. This provides information that is unobtainable from other sources such as laboratory measures, caregiver reports or physicians’ judgments [14]. The assessment of outcomes, such as the effect of SLIT on the severity of symptoms, QOL, illness, and treatment perception, as well as adherence, will help identify opportunities and facilitate improvements in patient management.
QUARTIS (Questionnaire sur l’Allergie Respiratoire Traitée par Immunothérapie Sublinguale) is a validated self-report questionnaire that is used to assess PROs in patients with ARC who are treated with SLIT [15] and is therefore a disease-specific tool. It assesses symptoms, effects of allergy on daily living activities, reasons for starting treatment, advantages, disadvantages, side effects and ease of administration of SLIT, compliance, satisfaction and opinions on continuing therapy.
Five-grass pollen tablet is a SLIT for the treatment of ARC that has demonstrated efficacy and safety in both adult and paediatric patients with this condition [16–18]. Following the approval of the tablet in Germany in June 2008, we conducted an observational post-authorization study to assess treatment satisfaction with five-grass pollen tablet in adult patients with ARC.
Methods
Study Design and Patients
This was a non-randomized, prospective, open-label, multicentre, non-interventional study of pre- and co-seasonal SLIT using a five-grass pollen tablet under real-life medical conditions to assess patient satisfaction with therapy. The study was conducted between August 2008 and March 2010 at 47 study centres throughout Germany.
Patients aged ≥18 years with immunoglobulin E (IgE)-mediated grass pollen-allergic rhinitis with clinically relevant symptoms, with or without conjunctivitis, whose diagnosis was confirmed by a positive cutaneous test and/or a positive titre of the specific IgE to grass pollen were eligible for enrolment. Patients were excluded if they were receiving treatment with a beta-blocker, had malignancy or systemic diseases affecting the immune system (including autoimmune, immune complex, or immune deficiency), had inflammation of the oral cavity with severe symptoms (e.g. oral lichen planus with ulcerations or severe oral mycosis), or had severe or uncontrolled asthma (forced expiration in 1 s <70% of predicted value).
Study Drug and Administration
Patients were treated with five-grass pollen tablet (Oralair®, Stallergènes SA, Antony, France), containing freeze-dried, standardized allergen extracts of orchard (Dactylis glomerata), meadow (Poa pratensis), perennial rye (Lolium perenne), sweet vernal (Anthoxanthum odoratum) and timothy (Phleum pratense) grasses, in a pre- and co-seasonal regimen. Patients commenced treatment approximately 4 months before, and continued treatment throughout the pollen season. Five-grass tablets were administered once daily, each morning, by mouth and were to be retained under the tongue for at least 1 min. The activity of the five-grass pollen tablet is expressed as index of reactivity (IR), which refers to a specific in-house measurement. Tablets were administered in accordance with the approved dosage regimen. The first dose of 100 IR was administered under medical supervision, which lasted at least 30 min. All subsequent doses were taken at home. On the second day, the patients took two tablets of 100 IR together. From the third day onwards, patients took one tablet of 300 IR daily.
Study Assessments
Details of assessments at each clinic visit are given in Table 1. Patients attended an initial clinic visit (visit 1) to determine baseline characteristics and history, to complete the QUARTIS questionnaire, and to receive the initial dose of five-grass pollen tablet (see below). Patients attended up to four further optional appointments (visits 2–5) during the treatment period if a new prescription was required, during which adverse events were recorded and the QUARTIS questionnaire was completed. After the pollen season had ended, patients attended a post-treatment visit (visit 6). During this final visit, physician assessments were conducted (Table 1) and patients completed the final QUARTIS questionnaire.
Table 1.
Patient and physician assessments
| Study visit | Time (months) | Actions/documentation | Documented by | |
|---|---|---|---|---|
| INV | PAT | |||
| Admission/treatment initiation | 0 | Allergy history–clinical manifestations | x | |
| Allergy history–allergies in need of treatment | x | |||
| Symptoms/symptomatic medication intake (during the last grass pollen season) | x | |||
| Diagnostic tests | x | |||
| Concomitant diseases/medication | x | |||
| Documentation of date of first SLIT dose taken under medical supervision (30 min) | x | |||
| QUARTIS questionnaire (Q1) | x | |||
| Follow-up prescriptions (optional) | 1–4 | Adverse events/side effects | x | |
| Receipt of follow-up prescription | x | |||
| Booking of follow-up/control appointment | x | |||
| QUARTIS questionnaire (Q2) | x | |||
| Post-treatment visit | 5–6 (approximately) | Documentation of date | x | x |
| Date of last intake | x | x | ||
| Treatment conduct (titration/dose) | x | x | ||
| Symptoms/symptomatic medication intake (during the grass pollen season) | x | |||
| Patient well-being | x | x | ||
| Tolerability | x | x | ||
| Adverse events/side effects | x | |||
| Date and reason for premature termination of treatment if applicable | x | |||
| Continuation of treatment | x | |||
| QUARTIS questionnaire (Q2) | x | |||
INV investigators, PAT patients, Q questionnaire, QUARTIS Questionnaire sur l’Allergie Respiratoire Traitée par Immunothérapie Sublinguale, SLIT sublingual immunotherapy
Patient Characteristics
Baseline physician assessments included patient demographics, documentation of grass pollen allergy (diagnosis and prior tests), nature and manifestation of allergic symptoms (time of occurrence, severity during the prior grass pollen season), history of allergy, symptomatic medication intake during the prior grass pollen season, concomitant diseases and medication.
Patient Assessments: Treatment Satisfaction
Treatment satisfaction was measured using QUARTIS, a self-reported questionnaire dedicated to the management of adult patients treated with SLIT for ARC [15]. Two versions of QUARTIS were applied during the course of the study, one for patients about to start SLIT (Q1) and another for patients currently undergoing SLIT (Q2). QUARTIS consists of 27 questions, covering the following domains: reasons for starting SLIT (Q1), advantages of SLIT (Q1), nasal and ocular symptoms (Q1 and Q2), allergy in everyday life (that is, the impairment of daily activities, work-related activities and relationships with other people; Q1 and Q2), disadvantages of SLIT (Q1 and Q2), ease of SLIT intake (Q2), overall satisfaction with SLIT (Q2), adverse reactions of SLIT (Q2), and motivation to continue SLIT (Q2) (see Table 2). All questions are self-rated, using four-, five- or ten-point Likert scales. Compliance was assessed under the domain for “ease of SLIT intake” (Q2) by responses to three categories (1) “Taking the drug is part of my routine”, (2) “I sometimes forget to take the drug when I wake up”, and (3) “I don’t always take my drug with me”. Ease of SLIT intake was also assessed using Q1, but under the heading “Advantages of SLIT”.
Table 2.
Distribution of questions in the QUARTIS questionnaire
| QUARTIS domain | Number of questions | |
|---|---|---|
| Q1 | Q2 | |
| Patients about to start SLIT (baseline) | Patient undergoing SLIT | |
| Reasons for starting SLIT | 8 | – |
| Symptoms (nasal/ocular) | 6 | 6 |
| Allergy in everyday life | 3 | 3 |
| Advantages of SLIT | 5 | – |
| Ease of taking SLIT | – | 5 |
| Disadvantages of SLIT | 5 | 5 |
| Patient satisfaction with SLIT | – | 4 |
| Side effects of SLIT | – | 1 |
| Continuation of SLIT | – | 3 |
Q questionnaire, QUARTIS Questionnaire sur l’Allergie Respiratoire Traitée par Immunothérapie Sublinguale, SLIT sublingual immunotherapy
Physician Assessments: Effectiveness, Tolerability and Safety
Effectiveness of therapy was assessed by the evaluation of symptoms, the need for symptomatic medication and patient well-being. Physicians documented the severity of symptoms (nasal, ocular and bronchial) and the requirement of symptomatic medication such as eye drops, antihistamines or corticosteroids in the grass pollen season prior to SLIT (at baseline; visit 1) and in the grass pollen season with SLIT treatment (at post-treatment visit; visit 6). Symptoms were classified as being absent, mild, moderate or severe. Patient well-being was assessed at the final visit (visit 6) in conjunction with the patient by comparing status during the treatment period with that during the prior season, using a subjective scale (“much better”, “better”, “unchanged”, “worse” and “much worse”).
Tolerability was assessed by documentation of adverse events arising over the entire course of treatment and by assessment of tolerability by the investigator and patient. The nature, causality, intensity, time course and outcome of all adverse events were recorded. Adverse events were also classified according to whether they were serious or whether they led to withdrawal of the study medication.
Statistical Analysis
Patients were asked to complete the QUARTIS questionnaires before treatment (Q1) and after the start of treatment (Q2) (see Table 2). Two patient sets were defined for the analyses. Patients who completed the QUARTIS questionnaire before treatment and had at least one evaluable item from the questionnaire during treatment were used to analyse PROs (QUARTIS set), while those who completed the baseline (visit 1) and the final visit (visit 6) were used to analyse physician-reported effectiveness outcomes (effectiveness set). All patients treated with the study drug were included in the demographic and safety analyses.
Descriptive statistics were applied to evaluate treatment satisfaction with the five-grass pollen tablet using the QUARTIS questionnaire and effectiveness of the treatment. The changes in the QUARTIS domains were used to determine the patient’s treatment satisfaction, and the investigator’s observation of changes in symptoms and symptomatic medication intake during the first year of pre- and co-seasonal SLIT were used to determine effectiveness. Exploratory Wilcoxon tests were applied to the mean changes in the QUARTIS domains.
Results
Patient Disposition and Baseline Characteristics
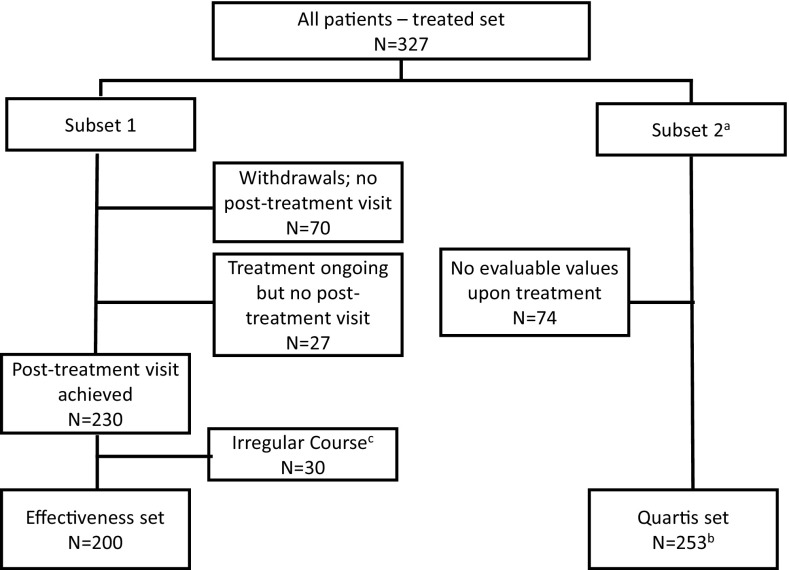
Of the 327 patients enrolled in the study, 253 completed the QUARTIS questionnaire before treatment (Q1) and provided at least one response to the questionnaire during treatment (Q2) and were included in the QUARTIS set, while of the 230 patients that attended the first visit and the final post-treatment visit, 200 were included in the effectiveness set, 30 having taken an “irregular course” of therapy (Fig. 1). Among the 327 patients recruited, 30.9% were lost to follow-up or prematurely withdrew from observation, the main reasons being investigator non-compliance (i.e. no further documentation provided) (6.4%), patient non-compliance (i.e. patient did not reappear or patient refused further treatment) (14.7%), and adverse events (6.4%).
Fig. 1.
Disposition of patients. QUARTIS Questionnaire sur l’Allergie Respiratoire Traitée par Immunothérapie Sublinguale. a Completion of the QUARTIS questionnaire (number of patients): baseline/Visit 1=253; Visit 2=225; Visit 3=140; Visit 4=75; Visit 5=44; Visit 6=164. b Analysis based on the last individual questionnaire per patient. c Irregular course: patients with a treatment start during the season and/or a post-treatment visit before the end of the pollen season
Baseline patient demographics are detailed in Table 3. Of the 327 patients who received at least one dose of five-grass pollen, the mean age was 31.5 years, 52.3% were male, 94.5% had allergic rhinitis and 75.2% had conjunctivitis. A smaller proportion of patients also had asthma or neurodermatitis. All patients had a documented history of symptomatic ARC due to grass pollen at study entry, and 73.1% of patients had required medication for symptomatic relief in the grass pollen season prior to SLIT. Five-grass pollen tablet was the first immunotherapy treatment in 88.6% of patients.
Table 3.
Patient demographics and baseline characteristics (n = 327)
| Demographic or baseline characteristics | Results |
|---|---|
| Age distribution | 31.5 ± 13.3 years (mean ± SD) 30 years (median) |
| Gender | 52.3% (171) male 47.7% (156) female |
| Immunotherapy | 88.6% (288) first time immunotherapy 9.2% (30) new immunotherapy after a previous complete immunotherapy 2.2% (7) change over from a different preparation |
| Allergological history (multiple entries) | 94.5% (309) rhinitis 75.2% (246) conjunctivitis 28.4% (93) asthma 9.2% (30) neurodermatitis 2.4% (8) unspecified |
| Severity of ARC symptoms (in the grass pollen season preceding SLIT) | |
| Rhinitis | 87.8% (287) moderate-to-severe |
| Conjunctivitis | 60.6% (198) moderate-to-severe |
| Requirement of symptomatic medication (in the grass pollen season preceding SLIT) | 73.1% (239) yes 26.9% (88) no |
| Allergies in need of treatment (multiple entries) | 100% (327) grasses 47.4% (155) cereals, rye 28.1% (92) birch 22.3% (73) alder 21.7% (71) hazel 14.4% (47) house dust mites 6.7% (22) others |
ARC allergic rhinoconjunctivitis, SD standard deviation, SLIT sublingual immunotherapy
Patient Assessments: Treatment Satisfaction
Changes in the QUARTIS domains were measured using a Likert scale, with a decreased score indicating a favourable result. The results demonstrated significant improvements between the baseline assessment before treatment start and upon treatment in mean scores (±standard deviation) for nasal symptoms (−3.8 ± 4.3, p < 0.0001), ocular symptoms (−2.0 ± 2.8, p < 0.0001) and allergy in everyday life (−2.5 ± 3.6, p < 0.0001) (Table 4). A significant improvement in mean scores for perceived ease of SLIT intake (−0.2 ± 1.0, p < 0.0067) and disadvantages of SLIT (−0.8 ± 2.9, p < 0.0001) was also recorded (Table 4). At the end of the first year of pre- and co-seasonal SLIT with the five-grass pollen tablet, 99.2% of patients agreed that SLIT was easy to take and that “The sublingual method is quick and only takes a couple of minutes in the morning”. At the end of the first year of pre- and co-seasonal SLIT, QUARTIS domain scores suggested that compliance with SLIT was good, as was satisfaction with SLIT and motivation to continue SLIT (Table 5). Patients were motivated to continue SLIT throughout the pre- and co-seasonal treatment period and to restart SLIT during the following year. A large proportion (74.6%) of patients strongly agreed with the statement “I want to complete the course of my sublingual immunotherapy”. When asked to name potential/actual reasons that may decrease or had decreased motivation to continue SLIT, patients provided the following responses (calculated for each reason separately vs. those who did not consider the category as a reason for discontinuation): symptoms improved sufficiently (26.5 vs. 73.5%); treatment is difficult to follow (9.5 vs. 90.5%); treatment causes local reactions (13.0 vs. 87.0%); treatment is not effective enough (24.1 vs. 75.9%); and treatment is expensive (14.2 vs. 85.8%). A better tolerability than expected was reported by 70.4% of patients, and the overall tolerability was graded “good” or “very good” in approximately 90% of the evaluable cases.
Table 4.
Mean change in QUARTIS scores during the first year of SLIT with five-grass pollen tablet (n = 253)
| QUARTIS domain | Range of scorea (min–max) | Q1 score (mean ± SD) before SLIT | Q2 score (mean ± SD) during SLIT | Change in score (mean ± SD) until end of SLIT | p valueb |
|---|---|---|---|---|---|
| Nasal symptoms | 4–20 | 14.0 ± 3.7 | 10.2 ± 4.1 | −3.8 ± 4.3 | <0.0001 |
| Ocular symptoms | 2–10 | 6.4 ± 2.5 | 4.4 ± 2.2 | −2.0 ± 2.8 | <0.0001 |
| Allergy in everyday life | 3–15 | 9.8 ± 3.1 | 7.3 ± 3.2 | −2.5 ± 3.6 | <0.0001 |
| Ease of SLIT intake | 2–8 | 2.5 ± 1.0 | 2.3 ± 0.7 | −0.2 ± 1.0 | 0.0067 |
| Disadvantages of SLIT | 5–25 | 8.7 ± 3.5 | 7.9 ± 2.8 | −0.8 ± 2.9 | <0.0001 |
A decrease in nasal symptoms, ocular symptoms, allergy in everyday life, ease of SLIT intake, or disadvantages of SLIT represents a favourable result
Q questionnaire, QUARTIS Questionnaire sur l’Allergie Respiratoire Traitée par Immunothérapie Sublinguale, SD standard deviation, SLIT sublingual immunotherapy
aSum of the ratings of all items in the domain
bWilcoxon test within the subgroup of patients who filled in the QUARTIS questionnaire before the start of SLIT and at least once thereafter
Table 5.
QUARTIS scores at the end of the first year of SLIT with five-grass pollen tablet (n = 253)
| QUARTIS domaina | Range of scoreb (min–max) | Score at study end (mean ± SD) |
|---|---|---|
| Compliance with SLIT | 3–12 | 6.5 ± 2.1 |
| Satisfaction with SLIT | 4–20 | 8.8 ± 3.0 |
| Motivation to continue SLITc | 0–10 | 8.0 ± 2.4 |
QUARTIS Questionnaire sur l’Allergie Respiratoire Traitée par Immunothérapie Sublinguale, SD standard deviation, SLIT sublingual immunotherapy
aLow values for compliance with SLIT and satisfaction with SLIT and high values for motivation to continue SLIT at study end represent favourable results
bSum of the ratings of all items in the domain
cMotivation to continue SLIT is an item in the domain “Continuation of SLIT” in questionnaire 2
Physician Assessments: Effectiveness, Safety and Tolerability
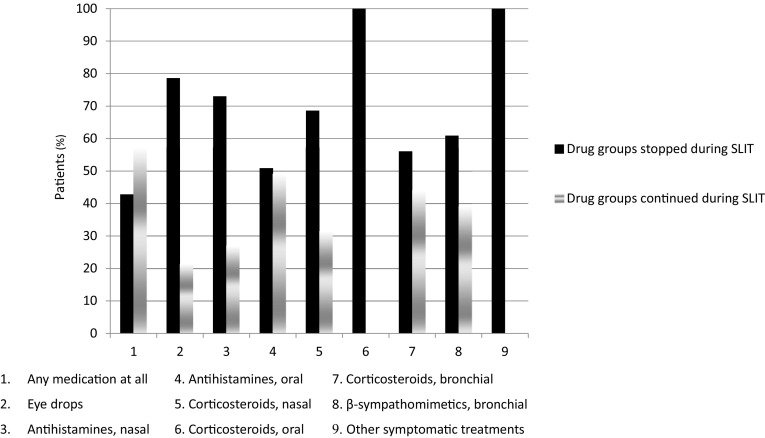
Treatment with five-grass pollen tablet was clearly effective as determined by assessment of symptoms, symptomatic medication use and patient well-being. When compared with the season prior to SLIT, an improvement in symptoms or an absence of symptoms was recorded for 83.1% of patients for nasal symptoms, 79.4% for bronchial symptoms and 74.3% for eye symptoms. The proportion of patients stopping or continuing medication after commencing SLIT is shown in Fig. 2. Overall the proportion of patients using symptomatic medication decreased from 72.5% in the season preceding SLIT with the five-grass pollen tablet to 42.5% in the first season of SLIT use. Only 1.0% of patients who had not used symptomatic medication in the season preceding SLIT started using symptomatic medication during treatment with SLIT. Patient well-being during the SLIT season was assessed at the post-treatment visit as “better” or “much better” by investigators (83.0%) and patient self-assessment (83.6%). The remaining investigators and patients assessed patient well-being as “unchanged” or “worse” (no-one recorded patient well-being as “much worse”).
Fig. 2.
Incidences of medications that were stopped upon treatment with SLIT and those that were continued (effectiveness set; n = 200). SLIT sublingual immunotherapy
Five-grass tablet was generally well tolerated. Twenty-two patients (6.7%) experienced 34 adverse events after the first dose of SLIT (4.3% gastrointestinal disorders; 1.5% respiratory, thoracic and mediastinal disorders). Over the entire course of treatment, a total of 52 patients (15.9%) experienced 93 adverse events (9.2% gastrointestinal disorders such as swelling of lips or tongue, or mouth oedema; 4.6% respiratory, thoracic and mediastinal disorders; 1.8% skin and subcutaneous tissue disorders; 1.2% infections and infestations). In 48 patients (14.7%) the adverse events were causally related to five-grass pollen tablet intake. Serious adverse events were reported in two patients, in one of which (dyspnoea and burning tongue) causality with the five-grass pollen tablet was assessed certain. The other serious adverse event (pneumonia) was assessed as not being related to therapy. Treatment was discontinued due to adverse events in 6.4% of patients, and emergent adverse events prompted the use of medication in 3.7% of patients.
Discussion
Patient satisfaction with therapy for allergic rhinitis is generally low, with only 33.5% of 260 patients stating they were satisfied with their treatment in one Italian survey [19]. Treatment dissatisfaction in particular was associated with female gender, comorbidity, rhinitis severity and antihistamine use. Similarly, a survey of 499 Italian patients with symptomatic house dust mite allergy also reported a high level of treatment dissatisfaction (56.2% of patients) and a high frequency of moderate to severe rhinitis [20]. These results are in contrast to those obtained with SLIT. In a study of 1289 adult or paediatric patients with house dust mite allergy, more than half of those included reported that they were satisfied with their therapy [21]. Treatment satisfaction with five-grass pollen therapy has also been reported to be high in a multicentre observational study of 226 patients with ARC in Spain [22–24], while treatment satisfaction improved significantly with this approach in a small observational study of 47 patients with ARC in Italy [25].
Against this background, the results of this study demonstrate that consistently good levels of treatment satisfaction can be achieved by treating ARC with pre- and co-seasonal SLIT using a five-grass pollen tablet. Throughout the first year of treatment, patients’ ocular, nasal, and bronchial symptoms improved, the tolerability of the treatment was reportedly good, and patients therefore remained motivated to adhere to SLIT. Although, 30.9% of patients were lost to follow-up, non-compliance was only recorded for 14.7% of patients in the study population. At the end of this first year, patients were also highly motivated to restart SLIT in the following season.
Previous studies have demonstrated the safety and clinical efficacy of SLIT for treating and altering the course of allergic disease [11, 12]. However, the success of this treatment in clinical practice also relies heavily on patient adherence, as it is self-administered over a period of 3–5 years. The findings of this study are therefore important, as they indicate that patients will remain largely adherent throughout the course of treatment.
The results of our investigation are in accord with those of a large cross-sectional observational survey conducted in 2010 to assess patient satisfaction, among other factors, on SIT. Surveys from 434 patients treated with either SCIT or SLIT were analysed and given a score on a visual analogue scale (VAS) [4]. The mean global satisfaction score for treatment with SIT was 74 (VAS score range 0–100). Compared with SCIT, a higher percentage of participants taking SLIT reported that treatment was easy to take (p = 0.016), did not induce side effects (p = 0.023), prevented the development of new allergies (p < 0.001), and had benefits that exceeded its costs (p = 0.034). Furthermore, a higher percentage of patients treated with SCIT said that they would prefer to change their mode of SIT administration (p < 0.0001) [4]. As previously stated, our results are also in accord with good levels of patient satisfaction noted for five-grass pollen tablet in patients with ARC [22–25].
Other previous studies of SLIT have focused on outcomes such as changes to QOL as indicators of the treatment’s success, rather than treatment satisfaction. In a previous non-interventional study, the impact on QOL of SLIT with standardized grass and/or cereal pollen allergen extract in sublingual solution was assessed using the German adapted version of a specific QOL questionnaire for patients with allergic rhinitis with coexisting asthma (RHINASTHMA GAV) [26]. The improvement of QOL during seasonal SLIT was clinically relevant and reached scores close to normal in the first pollen season. Similar results were reported from a prospective study in 167 polysensitized patients using a rhinoconjunctivitis quality of life questionnaire (RQLQ) at baseline and after the first year of SLIT [27]. The three most commonly used allergen extracts administered as sublingual solution were house dust mite, grass pollen, and Parietaria pollen. The mean RQLQ scores decreased significantly from 3.96 at baseline to 2.89 after 1 year of SLIT. In a randomized, double-blind, placebo-controlled study, the impact on health-related QOL of 1-year SLIT with a sublingual tablet containing P. pratense grass pollen extract was assessed with RQLQ [7]. In the highest dose group, SLIT improved QOL compared with placebo, in both subgroups of patients, i.e. those receiving loratadine as rescue medication and those receiving placebo rescue medication. Lastly, administration of five-grass tablet over three pollen seasons was associated with a marked improvement in QOL when compared with placebo when administered in a pre- and co-seasonal regimen [18]. Overall, in these studies of SLIT with standardized grass pollen allergen extracts or standardized allergen extracts containing other allergens, similar improvements in QOL were demonstrated regardless of study design and type of allergen extract.
Although these studies were investigating the effect of treatment on the patients’ QOL, rather than treatment satisfaction, the results from our study using the QUARTIS questionnaire support the findings that treatment with SLIT has a positive effect on patient reported outcomes.
The main limitation of the present investigation is that it was a non-interventional study conducted in a real-life setting. Lack of a control (placebo) group and variability in grass pollen loads between the season preceding treatment (2008) and the treatment season (2009) are also confounding factors. Indeed, the 2009 grass pollen season was documented as being less severe than that for the previous year [28]. Nevertheless, although effectiveness was assessed in the present study, the focus was treatment satisfaction. Study outcomes were also based on patients’ subjective and retrospective assessments, the latter being open to recall bias.
Conclusions
In conclusion, SLIT has become established as a standard therapy for ARC, and is accepted and adhered to by patients in a highly motivated manner. It is important to document good levels of treatment satisfaction, tolerability and effectiveness of SLIT reported in this non-interventional study under routine medical conditions, as these are important factors that may help to improve treatment adherence and success.
Acknowledgements
The authors wish to thank the participating investigators not included as authors for contributing to the acquisition of the data, former Stallergenes GmbH medical staff members for their work on the design, analysis and interpretation of the study, and Newmed Publishing Services for medical writing assistance.
Compliance with Ethical Standards
Funding sources
This study was sponsored by Stallergenes GmbH, Kamp-Lintfort, Germany. They were involved in conception and planning of the study, analysis and interpretation of the data. Medical writing and editorial assistance was provided by Newmed Publishing Services, and funded by the sponsor. Open access fees were funded by the sponsor.
Conflict of interest
Udo Schäfer and Andrea Kienle-Gogolok were participating investigators in this study and received remuneration from Stallergenes for the documentation of patient data from their practices. They declare no further conflicts of interest. Efstrathios Karagiannis and Meike Hadler are full-time employees of Stallergenes. Sylvia Schnitzer was medical consultant and participating investigator for this study, and received remuneration from Stallergenes for the documentation of patient data from her practice. She declares no further conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of the Faculty of Medicine, University of Rostock.
Informed consent
All patients provided informed consent to participate.
Footnotes
Parts of the results of this non-interventional study were presented as a poster at the European Academy of Allergy and Clinical Immunology (EAACI) Congress in Barcelona, Spain, 6–10 June 2015.
References
- 1.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen. Allergy. 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 2.Leynaert B, Neukirch C, Liard R, Bousquet J, Neukirch F. Quality of life in allergic rhinitis and asthma: a population-based study of young adults. Am J Respir Care Med. 2000;162:1391–1396. doi: 10.1164/ajrccm.162.4.9912033. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer EO, Gross GN, Katial R, Storms WW. Allergic rhinitis substantially impacts patients quality of life: findings from the Nasal Allergy Survey Assessing Limitations. J Fam Pract. 2012;61:S5–S10. [PubMed] [Google Scholar]
- 4.Baiardini I, Puggioni F, Menoni S, Boot JD, Diamant Z, Brado F, et al. Patient knowledge, perceptions, expectations and satisfaction on allergen-specific immunotherapy: a survey. Respir Med. 2013;107:361–367. doi: 10.1016/j.rmed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Windom HH, Lockey RF. An update on the safety of specific immunotherapy. Curr Opin Allergy Clin Immunol. 2008;8:571–576. doi: 10.1097/ACI.0b013e32831845fb. [DOI] [PubMed] [Google Scholar]
- 6.Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rak S, Yang WH, Pedersen MR, Durham SR. Once-daily sublingual allergen-specific immunotherapy improves quality of life in patients with grass pollen-induced allergic rhinoconjunctivitis: a double-blind, randomised study. Qual Life Res. 2007;16:191–201. doi: 10.1007/s11136-006-9110-3. [DOI] [PubMed] [Google Scholar]
- 8.Weaver M, Patrick DL, Markson LE, Martin D, Frederic I, Berger M. Issues in the measurement of satisfaction with treatment. Am J Manag Care. 1997;3:579–594. [PubMed] [Google Scholar]
- 9.Kumar RN, Kirking DM, Hass SL, Vinolur AD, Taylor SD, Atkinson MJ, et al. The association of consumer expectations, experiences and satisfaction with newly prescribed medications. Qual Life Res. 2007;16:1127–1136. doi: 10.1007/s11136-007-9222-4. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. doi: 10.2147/PPA.S24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancuso CA, Rincon M, McCulloch CE, Charlson ME. Self-efficacy, depressive symptoms, and patients’ expectations predict outcomes in asthma. Med Care. 2001;39:1326–1338. doi: 10.1097/00005650-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Fox P, Porter PG, Lob SH, Boer JH, Rioch DA, Adelson JW. Improving asthma-related health outcomes among low-income, multiethnic, school-aged children: results of a demonstration project that combined continuous quality improvement and community health worker strategies. Pediatrics. 2007;120:902–911. doi: 10.1542/peds.2006-1805. [DOI] [PubMed] [Google Scholar]
- 13.Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. Patient-reported outcomes to support medical product labelling claims: FDA perspective. Value Health. 2007;10:S125–S137. doi: 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 14.Baiardini I, Bousquet PJ, Brzoza Z, Canonica GW, Compalati E, Fiocchi E, et al. Recommendations for assessing patient-reported outcomes and health-related quality of life in clinical trials on allergy: a GA2LEN taskforce position paper. Allergy. 2010;65:290–295. doi: 10.1111/j.1398-9995.2009.02263.x. [DOI] [PubMed] [Google Scholar]
- 15.Didier A, Vervloet D, Fontaine J, Haddad T, Mathelier-Fusade P, Rufin P, et al. Development and validation of a questionnaire dedicated to the management of adult patients treated with sublingual immunotherapy for allergic rhinitis. 25th Congress of the European Academy of Allergology and Clinical Immunology. Vienna, 2006: Abstract 1398.
- 16.Didier A, Malling HJ, Worm M, Horak F, Jager S, Montagut A, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120:1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Wahn U, Tabar A, Kuna P, Halken S, Montagut A, de Beaumont O, et al. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2009;123:160–166. doi: 10.1016/j.jaci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Didier A, Worm M, Horak F, Sussman G, de Beaumont O, Le Gall M, et al. Sustained 3-year efficacy of pre- and coseasonal 5-grass-pollen sublingual immunotherapy tablets in patients with grass pollen–induced rhinoconjunctivitis. J Allergy Clin Immunol. 2011;128:559–566. doi: 10.1016/j.jaci.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Ciprandi G, Incorvaia C, Scurati S, Puccinelli P, Soffia S, Frati F, Rossi O. Patient-related factors in rhinitis and asthma: the satisfaction with allergy survey. Curr Med Res Opin. 2011;27:1005–1011. doi: 10.1185/03007995.2011.559580. [DOI] [PubMed] [Google Scholar]
- 20.Frati F, Scurati S, Dell’Albani I, Puccinelli P, Incorvaia C, Passalacqua G. Evaluation of house dust mite allergy in real life: patients’ characteristics and satisfaction with treatment. Eur Ann Allergy Clin Immunol. 2014;46:17–21. [PubMed] [Google Scholar]
- 21.Trebuchon F, David M, Demoly P. Medical management and sublingual immunotherapy practices in patients with house dust mite-induced respiratory allergy; a retrospective observational study. Int J Immunopathol. 2012;25:193–206. doi: 10.1177/039463201202500122. [DOI] [PubMed] [Google Scholar]
- 22.Antolin D, Valbuena T, Valls A, Garrido S, Blanco C, Garcia MA. One season of treatment with 5 grass pollen tablets in adults demonstrated a reduction in disease symptoms and impacts. Findings of the SMILE study. Presented at: European Academy of Allergy and Clinical Immunology (EAACI) Annual Meeting 2013, Milan.
- 23.Fernandez-Nieto M, Alvarez JA, Alvarado MI, Reaño MM, Blanco C, Garcia MA. What is the opinion of the patients under 5 grass pollen tablets immunotherapy? First season assessment in adults. Findings of the SMILE study. Presented at: European Academy of Allergy and Clinical Immunology (EAACI) Annual Meeting 2013, Milan.
- 24.Lyseng-Williamson KA. Sublingual five-grass pollen tablets (Oralair®): a guide to their use as allergen immunotherapy for grass pollen-induced allergic rhinoconjunctivitis. Drugs Ther Perspect. 2014 [Google Scholar]
- 25.Pastorello EA, Losappio L, Milani S, Manzotti G, Fanelli V, Pravettoni V, Agostinis F, D’Arcais AF, Dell’albani I, Puccinelli P, Incorvaia C, Frati F. 5-grass pollen tablets achieve disease control in patients with seasonal allergic rhinitis unresponsive to drugs: a real-life study. J Asthma Allergy. 2013;6:127–133. doi: 10.2147/JAA.S53801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieber J, Gross A, Shah-Hosseini K, Mösges R. The RHINASTHMA GAV scores without SLIT, at the beginning and at the end of seasonal SLIT. Asian Pac J Allergy Immunol. 2010;28:232–236. [PubMed] [Google Scholar]
- 27.Ciprandi G, Cadario G, Valle C, Ridolo E, Verini M, Di Gioacchino M, et al. Sublingual immunotherapy in polysensitized patients: effect on quality of life. J Investig Allergol Clin Immunol. 2010;20:274–279. [PubMed] [Google Scholar]
- 28.Pfaar O, Richter HG, Klimek L, Sieber J, Hadler M, Karagiannis E. Sublingual immunotherapy with a five-grass pollen tablet in adult patients with allergic rhinitis: an open, prospective, noninterventional, multicenter study. Biomed Res Int. 2015;2015:584291. doi: 10.1155/2015/584291. [DOI] [PMC free article] [PubMed] [Google Scholar]