Abstract
Genetic variation in CACNA1C, which codes for the L-type calcium channel (LTCC) Cav1.2, is associated with clinical diagnoses of bipolar disorder, depression, and schizophrenia. Dysregulation of the mesolimbic dopamine (DA) system is linked to these syndromes and LTCCs are required for normal DAergic neurotransmission between the ventral tegmental area (VTA) and nucleus accumbens (NAc). It is unclear, however, how variations in CACNA1C genotype, and potential subsequent changes in expression levels in these regions, modify risk. Using constitutive and conditional knockout mice, and treatment with the LTCC antagonist nimodipine, we examined the role of Cacna1c in DA-mediated behaviors elicited by psychomotor stimulants. Using fast-scan cyclic voltammetry (FSCV), DA release and reuptake in the NAc were measured. We find that subsecond DA release in Cacna1c haploinsufficient mice lacks normal sensitivity to inhibition of the DA transporter (DAT). Constitutive haploinsufficiency of Cacna1c led to attenuation of hyperlocomotion following acute administration of stimulants specific to DAT, and locomotor sensitization of these mice to the DAT antagonist GBR12909 did not reach the same level as wild type mice. The maintenance of sensitization to GBR12909 was attenuated by administration of nimodipine. Sensitization to GBR12909 was attenuated in mice with reduced Cacna1c selectively in the VTA but not in the NAc. Our findings reveal that Cacna1c is crucial for normal behavioral responses to DA stimulants and that its activity in the VTA is required for behavioral sensitization. Cacna1c likely exerts these effects through modifications to presynaptic mesolimbic DA system function.
INTRODUCTION
A genetic component substantially influences risk for developing mood disorders. Genome wide association studies (GWAS) have identified genetic variants in CACNA1C as a risk factor for the development of bipolar disorder and depression, as well as schizophrenia (Ripke et al., 2011, Sklar et al., 2011, Smoller et al., 2013, Ripke et al., 2014, Bhat et al., 2012, Ferreira et al., 2008, Green et al., 2010, Green et al., 2012, Hamshere et al., 2013, Liu et al., 2011, Moskvina et al., 2009, Nyegaard et al., 2010, Ripke et al., 2013, Sklar et al., 2008). Despite the significance of this human genetic finding, the mechanism by which genetic variants in CACNA1C modify the risk of developing a psychiatric illness remains largely unknown. CACNA1C codes for the α1C subunit of the Cav1.2 channel, which contains the voltage sensor, the conduction pore, and is a primary target for both drugs and second messengers acting on L-type calcium channels (LTCCs) (Catterall et al., 2005). As the identified single nucleotide polymorphisms are found in an intronic (non protein-coding) region of CACNA1C, mechanisms whereby genetic changes modify risk are likely via altered levels of CACNA1C in specific regions of the brain. Studies have supported this, showing that the CACNA1C risk allele is associated with increased mRNA expression of CACNA1C in the human postmortem dorsolateral prefrontal cortex and in induced human neurons (Bigos et al., 2010, Yoshimizu et al., 2014). Decreased expression of CACNA1C was also identified in the human postmortem cerebellum and parietal cortex samples (Gershon et al., 2014) and in an analysis of human brain available from multiple sources (Roussos et al., 2014).
There is evidence that Cacna1c is involved in regulation of mesolimbic-dopamine ML-DA system mediated behaviors in rodents, including reinstatement of cocaine seeking after LTCC activation in the NAc (Anderson et al., 2008), and LTCC mediated changes in calcium currents in the NAc following repeated cocaine administration (Zhang et al., 2002). In rats, sensitization to amphetamine is associated with an increase in Cacna1c mRNA and protein in the VTA (Rajadhyaksha et al., 2004). Although there is mounting evidence that normal Cacna1c function is important in ML-DA system mediated behaviors, the specific neural substrates through which it acts are largely unknown. ML-DA system function modulation by Cav1.2 may be related to the pathophysiology of psychiatric disorders linked to genetic changes in CACNA1C. Dysregulation of the ML-DA system is implicated in the expression of endophenotypes of bipolar mania and schizophrenia, as well as depression (Basar et al., 2010, Grace, 2016, Nestler & Carlezon, 2006, Ryding et al., 2008, Salamone et al., 2016, Whitton et al., 2015). Drugs that acutely increase release, or reduce reuptake of DA result in mania and psychosis phenotypes in humans (Anand et al., 2000, Drevets et al., 2001, Leyton et al., 2002, Lieberman et al., 1987, Murphy et al., 1971). Antipsychotics, as well as other treatments that impede DAergic neurotransmission, diminish mania, as well as psychotic symptoms, in humans (Creese et al., 1976, Mctavish et al., 2001, Perlis et al., 2006).
We previously found that d-amphetamine-induced hyperlocomotion was attenuated in mice lacking one copy of Cacna1c (Dao et al., 2010). Here, we further characterized the role of that decreased Cacna1c function on ML-DA system-mediated behaviors and hypothesized that stimulant-induced DA release and/or reuptake would be modulated by Cacna1c levels. Using rodent models of reduced Cacna1c function both globally and in specific brain regions, we show that Cacna1c modulates DA dependent stimulant-induced locomotor activity and sensitization. Additionally, we used fast-scan cyclic voltammetry (FSCV) to directly examine the role of Cacna1c in subsecond DA release and reuptake, finding that reduced Cacna1c leads to an attenuated response to DA reuptake blockers following stimulant administration. Therefore we show that Cacna1c modulates DA reuptake and that its function in the VTA is necessary for the development of stimulant-induced sensitization.
MATERIALS AND METHODS
Animals
Male and female Cacna1c haploinsufficient (Cacna1c+/−) founder mice were obtained from Jackson Laboratories (Bar Harbor, ME) as previously described (Dao et al., 2010). Cacna1c+/+ and Cacna1c+/− mice were the product of in-house breeding of Cacna1c+/− males generated in our own colony and WT C57BL/6 females obtained from Jackson Laboratories and were backcrossed for at least 10 generations (Dao et al., 2010). We previously showed that Cacna1c+/− mice have ~50% decreased Cav1.2 protein levels and ~30% decrease in mRNA levels in the hippocampus, as well as decreased L-VGCC current density in CA1 compared to their wild-type littermates (Dao et al., 2010, Zanos et al., 2015). We previously found that there were baseline differences in locomotor activity in female, but not male, haploinsufficient mice (Dao et al., 2010), consistent with an overall decrease in locomotor activity observed in Cacna1c haploinsufficient mice across 30 inbred mouse strains (Sittig et al., 2016). We therefore only used male animals for experiments, as it would have been difficult to interpret any results obtained using female haploinsufficient mice, due to the baseline difference in locomotor activity. In the conditional Cacna1c knockout mouse line, exons 14 and 15 of Cacna1c are excised in the presence of Cre, leading to a premature stop codon and removing all known functional significance of the resulting protein (Jeon et al., 2010). A line of conditional Cacna1c knockout mice (Jeon et al., 2010) were also bred on a C57BL/6 background and maintained as homozygous for the floxed allele. All mice used were group housed males between 8–20 weeks of age at the time of behavioral testing and between 10–14 weeks at the time of testing in FSCV and tissue collection for immunoblotting. Cacna1c+/+ and Cacna1c+/− mice used in experiments were littermates, and likewise AAV-Cre-GFP and AAV-Cre injected mice from the conditional Cacna1c knockout mouse line were littermates. Mice were tested during the light phase. All experimental procedures were approved by the University of Maryland Animal Care and Use Committee and were conducted in full accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Fast-Scan Cyclic Voltammetry
Carbon fiber microelectrodes were prepared as previously described (Cheer et al., 2004). Stimulation electrodes were made by twisting electrodes (Plastics One) and cutting ends evenly. Ag/AgCl reference electrodes were made from 0.5 mm Ag wire (Sigma-Aldrich, St. Louis, MO, USA) through electrolysis in HCl (Sigma-Aldrich).
Voltammetry experiments were performed based on a protocol previously described (Loewinger et al., 2012). Animals were anesthetized with urethane (1.5 g/kg, i.p.) and placed in a stereotaxic apparatus. Holes were drilled for the carbon fiber microelectrode to access the NAc (+1.2 AP, +1.1 ML from bregma), for a bipolar stimulating electrode to access the VTA (−3.1 AP; +0.7 ML from bregma), and for an Ag/AgCl reference electrode placed in the contralateral hemisphere. Once positioned, the carbon fiber electrode was held at −0.4 V in reference to the Ag/AgCl electrode. Cyclic voltammograms were collected at 10 Hz by ramping up to +1.3 V and back in a triangular fashion at 400 V/s. Stimulation, voltage, and data collection were controlled through the Tarheel Echem suite (University of North Carolina, Chapel Hill, NC, USA). After experimentation, changes in electrical current were converted to DA concentration changes via post-calibration of the carbon fiber electrodes to a known concentration of DA.
To determine DA release and reuptake following dopamine transporter (DAT) blockade, peak amplitude and decay rates following a 1-second, 60Hz, 300 μA stimulation were recorded following a 5 ml/kg i.p. saline injection and at 21 minutes following an i.p. injection of GBR12909 (16mg/kg) (Sigma-Aldrich). To determine decay rate, % of peak DA concentration vs. time plots were exported to Prism version 5 (Graph Pad, La Jolla, CA, USA). One-phase decay regression lines were fit to the data and software generated values were determined for the decay constant tau. Tau indicates the time at which DA levels have dropped to 37% of the maximum, and was used as a measure of DA reuptake (Yorgason et al., 2011).
Western Blots
Western blots were performed using a previously published protocol (Gould et al., 2004). Immediately following decapitation the brain was sectioned into 1.0mm slices using a matrix (ASI Instruments, Warren, MI, USA) and the NAc was obtained using a 1.5mm punch (Miltex, Inc., York, PA, USA). Nucleus accumbens tissue samples from homozygous dopamine transporter knock-out (DAT−/−) and wild-type mice (DAT+/+) (Giros et al., 1996) were obtained from Dr. Sara Jones. Samples were homogenized in RIPA buffer containing protease and phosphatase inhibitors (Sigma-Aldrich). The homogenates were centrifuged at 12000g for 20 minutes at 4°C. Protein concentrations were determined using a BCA assay (Peirce Biotechnology, Inc., Rockford, IL, USA). For immunoblotting, 1ug of each sample was loaded on to a 4–12% Bis-Tris gel (Life Technologies, Grand Island, NY, USA) and transferred onto PVDF membranes (Life Technologies). Membranes were incubated overnight at 4°C with rat anti-DAT at a 1:4 000 dilution (Millipore MAB369) and rabbit anti-GAPDH at a 1:40 000 dilution (Millipore #5174) (EMD Millipore, Billerica, MA, USA). Membranes were washed and incubated with HRP-tagged anti-rat (Cell Signaling Technology Inc, Danvers, MA) and anti-rabbit (KPL, Inc., Gaithersburg, MD, USA) secondary antibodies, visualized using a chemiluminescence reaction (ClarityTM Western ECL Substrate, Bio-Rad Laboratories, Inc., Hercules, CA, USA), and quantified using densitometry (Image J (Schneider et al., 2012)). Results are expressed as relative optical density with DAT values normalized to GAPDH.
Virus Injections
Mice were anesthetized with isoflurane and stereotaxically received an injection of 0.7μl AAV-CMV-Cre-GFP or AAV-CMV-GFP (UNC Vector Core, Chapel Hill, NC, USA) bilaterally into the NAc (+1.6 anterior/posterior, +1.5 lateral, and −4.4 dorsal/ventral, 10° angle) or the VTA (−3.2 anterior/posterior, +1.0 lateral, and −4.6 dorsal/ventral, 7° angle). Injections were performed at a rate of 0.1ul/minute and the needle was left in place for 10 minutes prior to being removed. Following injections, a two-week recovery period was given prior to experiments. Following experiments, mice were euthanized. Immediately following decapitation the brain was sectioned into 1.0 mm slices using a matrix (ASI Instruments), and placed in cold PBS. Brain slices were then visualized through a fluorescent microscope (Leica Microsystems GmbH, Wetzlar, Germany). Mice that did not show fluorescence bilaterally in the targeted structures were excluded from the results. In the NAc injected group, this included 9 mice, and in the VTA injected group this included 6 mice.
qPCR and Immunohistochemistry
mRNA was extracted from 1.5mm tissue punches from AAV-injected mice. Tissue samples were homogenized in RNAzol RT (Sigma-Aldrich) using BashingBead lysis tubes (Zymo Research Corporation, Irvine, CA, USA) in a disrupter genie (Scientific Industries, Bohemia, NY, USA) for 10 minutes at 3000RPM. mRNA was isolated and DNAse treated using the Directzol RNA mini prep kit, according to manufacturer directions (Zymo Research). Using an iScript cDNA Synthesis Kit (Bio-Rad), total RNA was reverse transcribed into cDNA. Real-time RT-PCR was conducted using a SensiFast SYBER Lo-ROX Kit (Bioline, Taunton, MA, USA) in a 15μl reaction. A primer pair for the target gene, Cacna1c (5′-GTGCTGAGATGTGTGCGGTTG-3′, 5′-GCACTGAGTTCAGCAAGGATGC-3′), as well as the control genes Tfrc (5′-TGCTAATCCAATTGCTGTCTCT-3′, 5′-TGGATAAAGTTGTCCTTGGTACT-3′), and Rplp0 (5′-GCACAGTGACCTCACACG-3′, 5′-AGAAACTGCTGCCTCACATC-3′) were used (Integrated DNA Technologies, Coralville, IA, USA). The PCR reactions were run on a ViiA 7 Real-Time PCR System (Life Technologies) with a reaction volume of 15μl and an annealing temperature of 60°C. ViiA 7 software (Life Technologies) was used to determine Ct values. Cacna1c levels were normalized to the mean of Tfrc and Rplp0, and fold difference was determined using the 2−ΔΔCt method (Livak & Schmittgen, 2001). Three mice that had Cacna1c expression levels that were two standard deviations from the mean in the NAc were excluded from the results.
Microscopy
30μm coronal sections of paraformaldehyde perfused brains were cut in a cryostat and placed in 1x PBS. Sections were then blocked in 20% Triton X-100 (Sigma-Aldrich) for 30 minutes and incubated overnight with primary antibody (Chicken anti-GFP, 1:4 000, Aves Labs, Inc., Tigard, OR, USA) at room temperature. Sections were then washed and incubated in secondary antibody for two hours at room temperature (Donkey anti-Chicken Alexa-488 Green, 1:1 000, Life Technologies), mounted and cover slipped. After drying, sections were visualized under a confocal microscope (Olympus Fluoview) and images were obtained.
Acute locomotor response to stimulants
Mice were habituated to an open field (50x50cm; illuminated at 30 lux) for 30 minutes, after which they received an injection of d-amphetamine (2 mg/kg i.p.), cocaine (10 mg/kg s.c.), GBR12909 (16 mg/kg i.p.), or MK-801 (0.3 mg/kg i.p.) (Sigma-Aldrich, St. Louis, MO) and were returned to the open field for an additional 45 (d-amphetamine and cocaine) or 90 (GBR12909 and MK-801) minutes. Doses and route of administration were based on those established previously in the literature as resulting in a moderate hyperlocomotor response, in order to allow a further increase to be possible without inducing stereotypic behaviors. (Hirabayashi et al., 1991, Liljequist et al., 1991, Mcnamara et al., 2006, Young et al., 2010). All compounds were dissolved in 0.9% saline on the day of testing. Distance travelled was assessed using TopScan tracking software (CleverSys, Inc., Reston, VA, USA).
Sensitization to GBR12909
GBR12909, unlike amphetamine and cocaine, works specifically through blockade of the DAT (Andersen, 1989, Heikkila & Manzino, 1984). GBR12909 was therefore used to specifically evaluate activity at the DAT, eliminating confounding effects of drugs such as amphetamine or cocaine that affect other monoamine transporters. Mice were tested for GBR12909-induced locomotor sensitization in an open field (50 cm x 50 cm) illuminated at 30 lux. During the first three days of testing, mice were habituated to the open field following saline injections. Mice were then administered 16 mg/kg GBR 12909 (Sigma-Aldrich) over six consecutive sensitization days. In experiments where nimodipine was used, mice were habituated to the open field following saline injections for three (4 mg/kg) or four (6 mg/kg) days and on the fourth (or fifth) day were given a nimodipine (Alexis Biochemicals, San Diego, CA, USA) injection (4mg/kg or 6mg/kg, suspended in 20% DMSO (Sigma-Aldrich), 1.5% Tween-80 (Sigma-Aldrich) and saline) or vehicle. Over six consecutive sensitization days mice received 4mg/kg or 6 mg/kg nimodipine or vehicle once per day, were returned to the home cage, and after 30 minutes received 16 mg/kg GBR12909 and were placed in the open field. In a protocol based on that used by Giordano et al. 2010, the mice in the experiment using 4mg/kg nimodipine were tested again at 1 week, 3 weeks, and 4 weeks after the last sensitization day During the 4-week test, half of the mice previously treated with nimodipine received a vehicle injection and half of the mice previously treated with vehicle received 4 mg/kg nimodipine prior to administration of GBR12909. The low volume of DMSO included in the nimodipine vehicle did not lead to any locomotor changes in control experiments. All sessions were one hour, and distance travelled was analyzed using CleverSys tracking software (CleverSys, Inc.).
Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 5 (GraphPad Software). The statistics used were two-tailed t test or repeated measure two-way ANOVA, either paired or unpaired depending on the experimental design, and post hoc comparisons utilized the Bonferroni method. The results of final (4 week) challenge using 4 mg/kg nimodipine were analyzed with a planned comparison t test. Data are reported as mean ± SEM and p < 0.05 was considered significant.
RESULTS
DA release and reuptake in Cacna1c+/+ and Cacna1c+/− mice
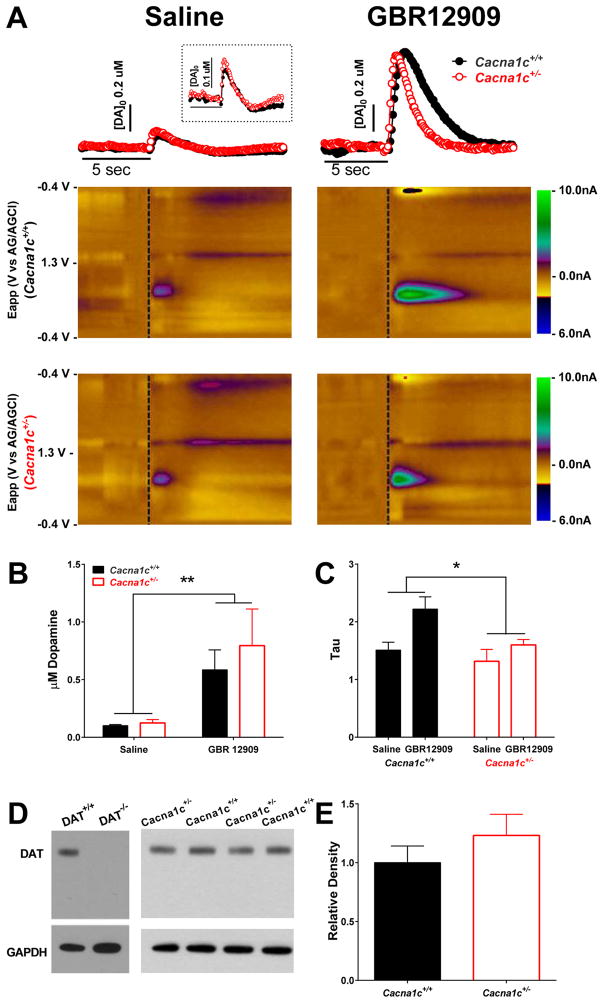
Using FSCV, we assessed DA release and reuptake in the NAc following electrical stimulation in the VTA of Cacna1c+/+ and Cacna1c+/− mice following saline and GBR12909 administration (Figure 1A). Administration of GBR12909 led to increased extracellular DA concentrations (Figure 1A, B) and slowed DA reuptake compared to saline administration (Figure 1A, C). This is indicated by an overall significant increase in DA following a 300 μA stimulation (Figure 1B; F(1,10) = 11.82, p < 0.01) and increased decay rate (tau; Figure 1C; F(1,10) = 8.71, p < 0.05). Additionally, there was a significant overall effect of genotype on tau following GBR12909 administration (Figure 1C; F(1, 10) = 5.65, p < 0.05). There was no effect of genotype on DA levels (F(1, 10) = 0.36, p = 0.56), and no significant genotype by drug interaction for increase in DA (Figure 1B; F(1, 10) = 0.31, p = 0.59) or increase in tau (Figure 1C; F(1, 10) = 1.60, p = 0.23). While Cacna1c+/+ and Cacna1c+/− mice had similar levels of DA reuptake following saline administration, an exploratory Bonferroni analysis indicated that Cacna1c+/+ mice had a significantly higher tau value compared to Cacna1c+/− mice only following GBR12909 administration (p < 0.05), indicating that Cacna1c haploinsufficiency is associated with attenuated effects of GBR12909 on DA reuptake (Figure 1C).
Figure 1. Dopamine release and reuptake following saline and GBR12909 administration.
(A) Representative concentration trace over time (top) and color plots (middle, bottom) showing voltammetric current (z-axis) against applied scan potential (y-axis) and time (x-axis) of dopamine (DA) release in Cacna1c+/+ (center) and Cacna1c+/− (bottom) mice following saline (top left) and GBR12909 (top right) administration. (B) Administration of GBR12909 (n=6/group) led to an overall significant increase in DA release in both Cacna1c+/+ and Cacna1c+/− mice (p<0.01). (C) Administration of GBR12909 led to an overall significant increase in tau (p<0.05), and an overall significant effect of genotype on DA reuptake (p<0.05). (D, E) There was no significant effect of genotype on dopamine transporter (DAT) protein levels in the nucleus accumbens (p=.33) as measured by immunoblot. n=8/group.
DAT protein levels in Cacna1c+/+ and Cacna1c+/− mice
Both Cacna1c+/+ and Cacna1c+/− mice had similar levels of DAT protein in the NAc as assessed by western blot (Figure 1D, E; t(14) = 1.01, p = .33).
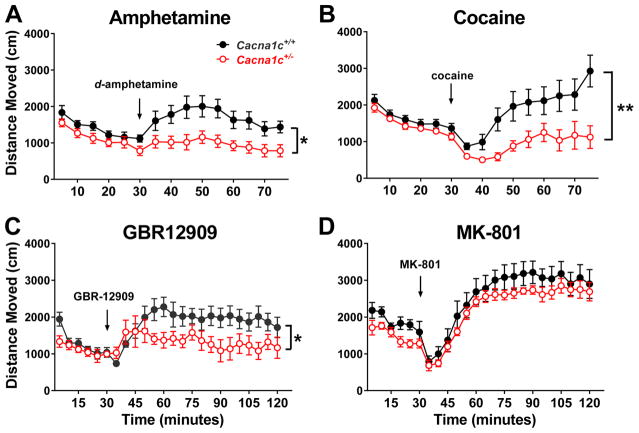
Response to acute psychostimulant administration in Cacna1c+/+ and Cacna1c+/− mice
Reduction of sensitivity to DAT blockade in Cacna1c+/− mice predicts an effect of Cacna1c haploinsufficiency on stimulant-induced behaviors. Acute psychostimulant-induced hyperlocomotion was assessed in Cacna1c+/+ and Cacna1c+/− mice. There were no significant baseline differences between genotypes during a 30-minute habituation in the open field (Figure 2 A–D). Compared with Cacna1c+/+ mice, Cacna1c+/− mice displayed a significant decrease in hyperlocomotor response following administration of d-amphetamine (Figure 2A; F(1,14) = 8.12, p < 0.05) and cocaine (Figure 2B; F(1,29) = 8.31, p < 0.01). As both of these stimulants have non-specific actions on multiple monoamine neurotransmitters, we also assessed hyperlocomotion following administration of the specific DAT inhibitor GBR12909. There was a significant difference in hyperlocomotor response to GBR12909 in Cacna1c+/− mice compared to that observed in Cacna1c+/+ mice (Figure 2C; F(1,14) = 4.60, p < 0.05). In contrast, there was no significant difference in hyperlocomotor activity between genotypes following administration of the NMDA receptor antagonist MK-801 (Figure 2D; F(1,13) = 0.92, p = 0.3546). Following administration of all psychostimulants, two-way ANOVAs further revealed there was a significant overall effect of day (d-amphetamine (Figure 2A) F(8, 112) = 4.99, p < 0.05; cocaine (Figure 2B) F(8, 232) = 2.96, p < 0.01; GBR12909 (Figure 2C) F(17, 238) = 2.54, p < 0.001; MK-801 (Figure 2D) F(17, 221) = 68.52, p < 0.0001) and a significant interaction between time and genotype following cocaine (Figure 2B; F(8, 232) = 12.68, p < 0.0001) and GBR12909 (Figure 2C; F(17, 238) = 2.01, p < 0.05) administration. There was no significant interaction following d-amphetamine (Figure 2A; F(8, 112) = 0.72, p = 0.67) or MK-801 (Figure 2D; F(17, 221) = 0.49, p = 0.96) administration.
Figure 2. Altered hyperlocomotor response to dopamine-acting stimulants in Cacna1c+/− mice.
Cacna1c+/+ and Cacna1c+/− mice were habituated to the open field for 30 minutes and then received either (A) d-amphetamine (2 mg/kg i.p., n=8/group), (B) cocaine (10 mg/kg s.c., n=15–16/group), (C) GBR12909 (16 mg/kg i.p., n=8/group), or (D) MK-801 (0.3 mg/kg i.p., n=7–8/group). * indicates an overall significant effect of genotype during the time following stimulant administration. *p<0.05, **p<0.01.
Sensitization to GBR12909 in Cacna1c+/+ and Cacna1c+/− mice
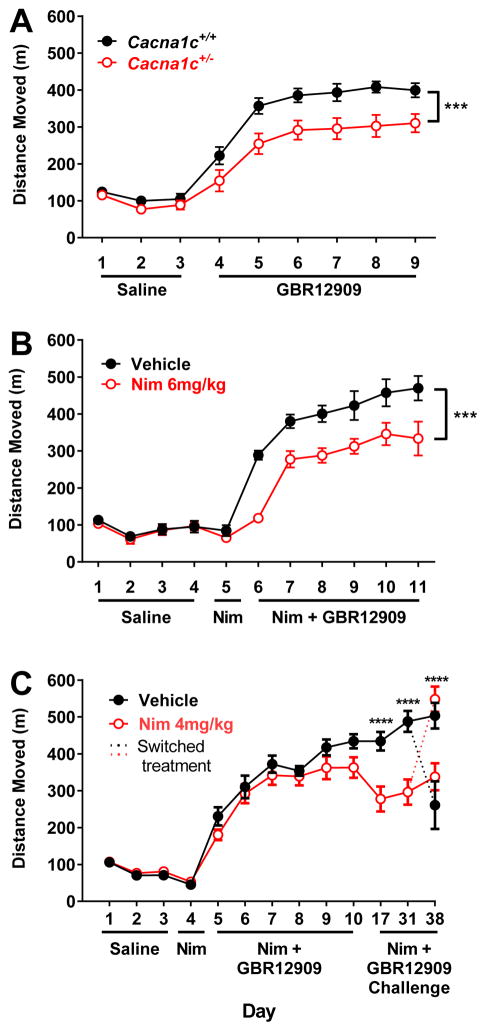
Compared to Cacna1c+/+ mice, Cacna1c+/− mice displayed a different hyperlocomotor activity in response to the specific DAT inhibitor GBR12909, which was maintained after repeated administration. There was no significant difference in baseline locomotor activity during the habituation period (Figure 3A; Day 1–3). However, Cacna1c+/− mice displayed a reduced hyperlocomotor response to GBR12909 during the sensitization period. A repeated measures two-way ANOVA revealed a significant overall effect of day (Figure 3A; F(5, 105) = 26.11, p < 0.0001) and genotype (Figure 3A; F(8,21) = 3.32, p < 0.01), with no significant interaction (F(5, 105) = .29, p = 0.92) . The reduced response to GBR12909 in Cacna1c+/− mice is evident at the beginning of treatment, and is most apparent on the second through sixth day of sensitization (Figure 3A; Day 5–9).
Figure 3. Altered locomotor sensitization to GBR12909 in Cacna1c+/− mice and following L-type calcium channel blockade.
Locomotor activity was measured during habituation to saline injections and sensitization to GBR12909 injections. (A) In Cacna1c+/+ compared to Cacna1c+/− mice there is a significant genotype and day interaction on locomotor sensitization to GBR12909 (p<0.001). Post-hoc tests revealed a significant difference in locomotor activity on the second through sixth days of GBR12909 administration. (B) There is a significant overall effect of 6 mg/kg nimodipine (Nim) on locomotor sensitization to GBR12909 (p<0.0001). Post-hoc tests revealed a significant difference in locomotor activity on the first through sixth days of GBR12909 administration. (C) There is a significant overall effect of 4 mg/kg nimodipine on locomotor sensitization to GBR12909 (p<0.0001). Bonferroni post-hoc tests revealed a significant difference in locomotor activity during 1 week and 3 week challenges. On week 4, planned comparisons t-tests revealed that mice whose treatment was switched to nimodipine from vehicle or vehicle from nimodipine displayed an attenuation of sensitization or normal sensitization, respectively. * indicates a significant effect of genotype or nimodipine. *p<0.05, **p<0.01, ***p<0.001,****p<0.0001 n= 11–12/group.
Locomotor responses to GBR12909 in mice with a pharmacological blockade of LTCCs
The LTCC antagonist nimodipine (6 or 4 mg/kg) was administered 30 minutes prior to administration of GBR12909 during the sensitization period. There were no baseline differences during habituation to the open field between groups (Figure 3B; Day 1–4; Figure 3C; Day 1–3) or following 6 mg/kg or 4 mg/kg nimodipine alone (Figure 3B, Day 5; Figure 3C; Day 4). Two-way repeated measures ANOVA revealed that there was a significant overall effect of GBR12909 treatment over days (F(5, 100) = 23.2, p < 0.0001), an overall effect of nimodipine treatment (F(1, 20) = 19.41, p < 0.001), and an no significant interaction (F(5, 100) = 0.69, p = 0.63) in mice treated with vehicle or 6 mg/kg nimodipine prior to GBR12909. In mice treated with vehicle or 4 mg/kg nimodipine prior to GBR12909 a two-way repeated measures ANOVA revealed a significant overall effect of GBR12909 treatment over days (F(7, 154) = 28.59, p < 0.0001), a significant overall effect of nimodipine treatment (F(1, 22) = 6.40, p < 0.05) and a significant interaction (F(7, 154) = 7.48, p < 0.0001). One week following the last sensitization day, mice were challenged with GBR12909 following treatment with vehicle or 4 mg/kg nimodipine. Bonferroni post-hoc tests revealed that mice treated with 4 mg/kg nimodipine manifested an attenuated sensitization to GBR12909 compared to vehicle treated mice (Figure 3C, p < 0.0001). At the three-week challenge, mice treated with 4 mg/kg nimodipine again manifested attenuated sensitization (Figure 3C, p < 0.0001).
At four weeks following the initial sensitization procedure half of the mice previously treated with nimodipine received a vehicle injection and half of the mice previously treated with vehicle received 4 mg/kg nimodipine prior to administration of GBR12909. The other half received the previously assigned treatment. As outlined in Table 1, planned comparison t-tests revealed that mice that were sensitized to GBR12909 with treatment of 4 mg/kg nimodipine, but were given vehicle prior to the four week GBR12909 challenge dose displayed no significant difference in locomotor activity from mice that received only vehicle during sensitization and challenge and displayed a significant difference from mice that received nimodipine both during sensitization and prior to the 4-week challenge. Mice that had been treated previously with vehicle, but that received 4 mg/kg nimodipine prior to GBR12909 on the challenge day showed no significant difference in locomotor activity compared to mice that received only nimodipine injections throughout the study, but manifested attenuated sensitization to GBR12909 compared to mice that received only vehicle during sensitization and challenge, Mice that were treated with nimodipine both during sensitization and prior to the 4-week challenge manifested significantly attenuated locomotor activity compared to mice given only vehicle injections. Furthermore, mice that were sensitized to GBR12909 with treatment of 4 mg/kg nimodipine, but were given vehicle prior to the four week GBR12909 challenge dose manifested attenuated locomoter activity compared to mice that were treated with nimodipine during sensitization but were given a vehicle injection prior to the 4-week GBR-12909 challenge. These findings suggest that pharmacological blockade of LTCCs at a higher dose leads to an attenuation of locomotor response to GBR12909 similar to that seen in Cacna1c+/− mice (Figure 3B); however a lower dose does not result in the acute reduction of GBR12909 induced locoomotor activity. Instead, similar to findings of Giordano et al. 2010, this dose attenuates the maintenance of sensitization, indicating an effect of Cav1.2 blockade on sensitization to psychostimulants even in the absence of an acute effect (Figure 3C).
Table 1. Effects of nimodipine on the maintenance of sensitization to GBR12909.
Mice were sensitized to GBR12909 following administration of either vehicle (Veh) or 4 mg/kg nimodipine (Nim). At four weeks following the initial sensitization procedure half of the mice previously treated with nimodipine received a vehicle injection and half of the mice previously treated with vehicle received 4 mg/kg nimodipine prior to administration of GBR12909. Planned comparisons t-tests revealed that mice whose treatment was switched to nimodipine from vehicle, or vehicle from nimodipine displayed an attenuation of sensitization or normal sensitization, respectively. For all comparisons, df = 10 and n = 6/group.
| Veh-Veh | Nim-Nim | Veh-Nim | Nim-Veh | |
|---|---|---|---|---|
| Veh-Veh | t=3.29, p=0.01 | t=2.99, p=0.01 | t=0.92, p=0.38 | |
| Nim-Nim | t=3.29, p=0.01 | t=0.50, p=0.63 | t=3.29, p=0.01 | |
| Veh-Nim | t=2.99, p=0.01 | t=0.50, p=0.63 | t=3.67, p=0.004 | |
| Nim-Veh | t=0.92, p=0.38 | t=3.29, p=0.01 | t=3.67, p=0.004 |
Locomotor responses to GBR12909 in mice with a knock-down of Cacna1c in the NAc
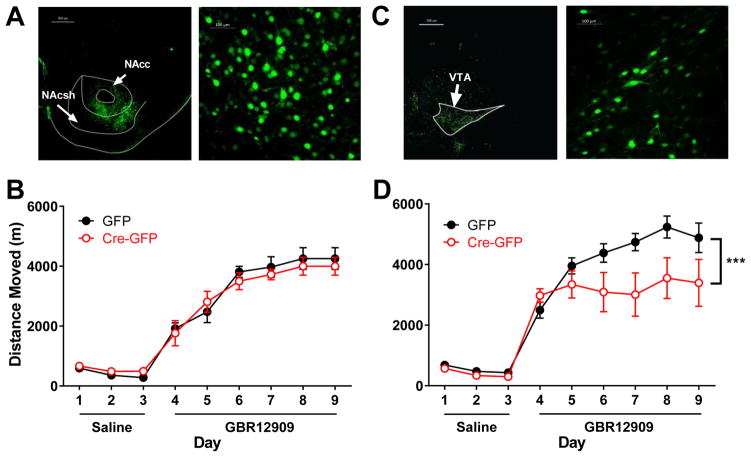
To determine if the attenuated response to GBR12909 was mediated by Cacna1c in the NAc, sensitization to GBR12909 in mice with virally mediated knock-down of Cacna1c in the NAc was measured. AAV-Cre-GFP was expressed in the NAc of mice containing a floxed Cacna1c allele. To control for any non-specific effects of the viral injection, control mice received a GFP-only expressing virus. Expression of GFP-tagged Cre in the NAc was confirmed by the use of fluorescence microscopy to visualize GFP expression in neurons in the NAc (Figure 4A). Injection of Cre-GFP significantly reduced the level of Cacna1c expression, as measured by qPCR, in the NAc by ~ 40% (t(17) = 4.43, p < 0.001).
Figure 4. Attenuated sensitization to GBR12909 following knock-out of Cacna1c in the ventral tegmental area.
Conditional Cacna1c knockout mice received AAV-CMV-Cre-GFP or AAV-CMV-GFP bilaterally in the nucleus accumbens (NAc) or ventral tegmental area (VTA). (A) Representative image of GFP fluorescent tag indicates injection region (left) and cell specificity (right) in the NAc. (B) In NAc injected mice, there was no effect of Cre injection on sensitization. (C) Representative image of GFP fluorescent tag indicates injection region (left) and cell specificity (right) in the VTA. (D) In VTA Cre-injected mice, there was a significant interaction of day and injection during sensitization (p<0.001). *** indicates the significant interaction, n=7/GFP group, 8/Cre-GFP group.
In NAc-injected mice, Cre-GFP injection did not lead to baseline differences in locomotor activity during the habituation period compared to GFP-only injected mice (Figure 4B).
During the sensitization period, there was a significant effect of repeated GBR12909 administration (F(8, 96) = 78.44, p < 0.0001), however there was no overall effect of Cacna1c knockdown in the NAc (F(1,12) = 0.054, p = 0.82) and no significant interaction (F(8, 96) = 0.43, p = 0.90 (Figure 4B).
Locomotor responses to GBR12909 in mice with a knock down of Cacna1c in the VTA
Expression of GFP- tagged Cre in the VTA was indicated by the use of fluorescence microscopy to visualize GFP expression in neuronal cells in the VTA (Figure 4C). While each injection was visually evaluated, we note that an unavailability of VTA RNA from these samples prevented qPCR analysis of the level of Cacna1c knockdown as had been performed with the NAc samples. Compared to GFP-only injected mice, mice with a VTA knockdown of Cacna1c displayed a significant difference in sensitization to GBR12909, as revealed by a significant interaction between day and injection type (Figure 4D; F(8,13) = 3.97, p < 0.001). There was an overall significant effect of day (F(5, 65) = 8.54, p < 0.0001, but no overall effect of Cacna1c knockdown in the VTA (F(1, 13) = 3.41, p = 0.09).
DISCUSSION
We have shown that Cacna1c haploinsufficient mice manifest an attenuated response to the specific DAT blocker GBR12909, indicating that Cacna1c critically regulates DA terminal function. We also demonstrate that mice with one functional allele of the Cacna1c gene manifest a diminished locomotor response to acute and chronic administration of DA elevating psychostimulants, an effect that is replicated with pharmacological blockade of LTCCs. A lower dose of pharmacological LTCC blockade results in attenuation of a maintained locomotor sensitization response, without modifying acute responses to GBR12909. Moreover, reduced levels of Cacna1c in the VTA, but not the NAc, led to attenuation of sensitization to GBR12909. Overall, the data in this study indicate that Cacna1c modulates ML-DA dependent behavior and DA terminal dynamics, potentially through altered DA reuptake by VTA neurons.
Stimulant-induced blockade of DAT in VTA DA synaptic terminals within the NAc leads to elevated DA levels, slowed DA reuptake, and hyperlocomotion in rodents. The hyperlocomotor response induced by acute psychostimulants and sensitization, as well as changes in DA release and reuptake, are widely used to model aspects of mania and psychosis in rodents (Einat & Manji, 2006, Nestler & Hyman, 2010, O’donnell & Gould, 2007). In this study, we show that Cacna1c has a role in subsecond DA reuptake kinetics, in particular DA reuptake in VTA neurons projecting to the NAc. While Cacna1c is not expressed exclusively in neurons (Cacna1c expression has also been identified in astrocytic cells (D’ascenzo et al., 2004, Latour et al., 2003)) DA reuptake through the DAT in response to stimulant administration occurs primarily through neuronal, and not astrocyte expressed DAT (Takeda et al., 2002). GBR12909, unlike amphetamine and cocaine, works specifically through blockade of the DAT (Andersen, 1989, Heikkila & Manzino, 1984), giving increased specificity for interpreting the results of DAT inhibition on DA terminal dynamics. Therefore, GBR12909 was used to evaluate specific activity at the DAT, eliminating confounding effects of drugs such as amphetamine or cocaine that affect other monoamine transporters. GBR12909 administration produced the expected decrease in reuptake in wild-type mice; however, in mice with genetically reduced levels of Cacna1c this effect was attenuated.
One mechanism by which the behavioral effects of DAT blockade may be attenuated is through altered levels of the DAT protein. Animal models in which DAT expression level is changed have an altered response to psychostimulants. Amphetamine and cocaine-induced hyperlocomotion is absent in mice with a mutation in a DAT gene that leads to deletion of DAT (Giros et al., 1996) and cocaine does not slow DA reuptake in DAT knockout mice (Budygin et al., 2002). Additionally, overexpression of DAT leads to an increase in amphetamine-induced response in mice (Salahpour et al., 2008). Our western blot results indicate that the overall level of DAT is not significantly altered in Cacna1c+/− mice, however the finding that GBR12909 does not block reuptake of DA in Cacna1c+/− mice to the same degree as it does in Cacna1c+/+ mice indicates that DAT function is likely altered by Cacna1c haploinsufficiency in some way other than regulation of total DAT protein levels. Further studies are needed to determine how Cacna1c, which is localized mainly somatodendritically (Hell et al., 1993, Leitch et al., 2009), may alter terminal DAT function.
Reduced stimulant-induced slowed DA reuptake in Cacna1c haploinsufficient mice indicates that there may be an effect of reduced Cacna1c levels on stimulant-induced locomotor activity. While stimulant-induced locomotion is a rudimentary model of mania in rodents, it has been commonly used to assess mania related behaviors due to a lack of alternative measures (Einat & Manji, 2006, Nestler & Hyman, 2010, O’donnell & Gould, 2007). We have previously shown that male Cacna1c haploinsufficient mice manifest a reduced hyperlocomotor response to acute and chronic amphetamine administration. While female Cacna1c haploinsufficient mice also manifest reduced stimulant induced hyperlocomotion, baseline locomotor differences would confound our capacity to interpret results (Dao et al., 2010). In the present study we replicate our previous finding in male mice, as well as show that Cacna1c haploinsufficiency leads to an attenuated hyperlocomotor response to both acute and repeated administration of several psychostimulants. This suggests that reduced levels of Cacna1c likely represent a protective phenotype against mania related behavior. While the dose of psychostimulants used in this study were selected to produce a moderate effect based on what has been published previously in the literature (Hirabayashi et al., 1991, Liljequist et al., 1991, Mcnamara et al., 2006, Young et al., 2010), the use of only one dose of psychostimulant is a limitation. Previous studies have indicated that calcium influx through brain LTCCs is necessary for mediating psychostimulant-induced behavior and Cacna1c is particularly important in sensitization to psychostimulants. In rats, the calcium channel blocker flunarizine attenuated the increased locomotor response induced by chronic cocaine administration (Mills et al., 2007) and nifedipine blocks expression of amphetamine or cocaine induced sensitization (Giordano et al., 2010). Following sensitization to psychostimulants, LTCC dependent calcium uptake increases (Mills et al., 2007) and signaling pathways downstream of Cacna1c are activated in the NAc (Giordano et al., 2010).
The locomotor response to psychostimulants is largely mediated through the ML-DA pathway, although in the NAc, both DAergic and glutamatergic inputs are important for the psychostimulant induced response (Vanderschuren & Kalivas, 2000, Wolf & Khansa, 1991). Our finding that there was no attenuation of the hyperlocomotor response induced by the NMDA receptor antagonist MK-801 in Cacna1c+/− mice indicates that DA, rather than glutamate, system function is likely altered as a result of reduced Cacan1c. Previous studies support this conclusion, showing that LTCCs mediate cocaine-induced elevations of monoamine levels in terminal regions (Mills et al., 2007, Okita et al., 2000).
In the present study, we further identified a role for Cacna1c in regions of the ML-DA circuit that underlie sensitization to GBR12909. While reduction of Cacna1c in the NAc was not sufficient to attenuate sensitization to GBR12909, we found that when Cacna1c was reduced in the VTA, sensitization above the initial acute response to GBR12909 was completely absent. This result suggests that Cacna1c in the VTA is essential for normal psychostimulant induced sensitization. This finding is consistent with previous research, which has found that pharmacological blockade of LTCCs directly in the VTA blocks sensitization (Licata & Pierce, 2003), while activation of LTCCs augments sensitization (Licata et al., 2000). Furthermore, sensitization leads to increased expression of Cacna1c mRNA and Cav1.2 protein in the VTA (Rajadhyaksha et al., 2004). While Cacna1c does influence the downstream effects of psychostimulant exposure in the NAc, such as through increases in calcium uptake increases and activation of signaling pathways (Mills, Ansah et al. 2007, Giordano, Tropea et al. 2010), blocking LTCCs exclusively in the NAc does not lead to attenuation of the psychostimulant induced hyperlocomotion (Pierce, Quick et al. 1998). Our findings, combined with those of previous studies and our own FSCV results, indicate that Cacna1c in the VTA may have a significant role in presynaptic regulation of the response to psychostimulants.
Our finding that selective reduction of Cacna1c levels in only the VTA or NAc did not modify acute locomotor responses to GBR12909 is consistent with previous studies that have found no acute locomotor response to cocaine following administration of an LTCC antagonist to the NAc (Karler et al., 1991, Pierce et al., 1998). It is of interest to note the difference in the response to chronic psychostimulant administration in haploinsufficient mice compared to mice with conditional knockdown of Cacna1c. In Cacna1c+/− mice, sensitization was reduced, but not completely blocked, as opposed to the complete lack of sensitized response when Cacna1c was selectively knocked down in the VTA. In Cacna1c+/− mice, the different outcome could be due to the involvement of reduced Cacna1c in additional circuits contributing to psychostimulant induced behavior, or the constitutive nature of the knock out in Cacna1c+/− mice leading to compensatory mechanisms that modulate aspects of this behavior. In support of this, a low dose of nimodipine is sufficient to selectively block the maintenance of sensitization to GBR12909; however when a higher level of LTCC blockade is present, the locomotor response is attenuated both acutely and during sensitization.
The results from this study demonstrate that when levels of Cacna1c are reduced, a potential protective phenotype against mania- or psychosis-related behavior emerges. In addition, these studies indicate that the attenuation of mania- or psychosis-related behavior following reduction of Cacna1c levels is due at least in part to altered terminal DA reuptake. As dysregulation of the DAergic system contributes to the etiology of mood disorders, the knowledge that Cacna1c is important for normal function of the ML-DA system has considerable implications for our understanding of how Cacna1c may confer risk. Additional studies are needed to further understand how DA reuptake is altered following sensitization to GBR12909, as well as the specific mechanism through which Cacna1c modifies DA reuptake. As the risk associated SNPs identified in CACNA1C likely influence risk through altered levels of expression of CACNA1C (Bigos et al., 2010, Eckart et al., 2016, Gershon et al., 2014, Roussos et al., 2014, Yoshimizu et al., 2014), these findings are important steps toward understanding the ramifications of altered expression in brain regions particularly relevant to psychiatric disorders.
Acknowledgments
This study was supported by US NIH grant MH103847 and National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award 20230 to TDG, and R01DA025890 to JFC. Dr. Gould reports receiving consulting fees from Sunovion Pharmaceuticals and Janssen Pharmaceuticals, and research funding from Janssen Pharmaceuticals and Roche Pharmaceuticals during the preceding three years. The other authors report no biomedical financial interests or potential conflicts of interest. Cacna1c conditional KO mice were provided by Jean-Pierre Kinet, Harvard University. DAT knock-out (DAT−/−) and wild-type mice (DAT+/+) tissue was provided by Sara R. Jones, Wake Forest University.
References
- Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, Innis RB. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- Andersen PH. The dopamine inhibitor GBR 12909: selectivity and molecular mechanism of action. Eur J Pharmacol. 1989;166:493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JHJ, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nature Neuroscience. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Basar K, Sesia T, Groenewegen H, Steinbusch HWM, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Progress in Neurobiology. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Bhat S, Dao DT, Terrillion CE, Arad M, Smith RJ, Soldatov NM, Gould TD. CACNA1C (Cav1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol. 2012;99:1–14. doi: 10.1016/j.pneurobio.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, Hyde TM, Lipska BK, Kleinman JE, Weinberger DR. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Jones SR. Lack of cocaine effect on dopamine clearance in the core and shell of the nucleus accumbens of dopamine transporter knock-out mice. J Neurosci. 2002;22:RC222. doi: 10.1523/JNEUROSCI.22-10-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacological Reviews. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo M, Vairano M, Andreassi C, Navarra P, Azzena GB, Grassi C. Electrophysiological and molecular evidence of L-(Cav1), N- (Cav2.2), and R- (Cav2.3) type Ca2+ channels in rat cortical astrocytes. Glia. 2004;45:354–363. doi: 10.1002/glia.10336. [DOI] [PubMed] [Google Scholar]
- Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O’Donnell P, Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, Levinson DF, Thompson SM, Potash JB, Gould TD. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 2010;68:801–810. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Eckart N, Song Q, Yang R, Wang R, Zhu H, McCallion AS, Avramopoulos D. Functional Characterization of Schizophrenia-Associated Variation in CACNA1C. PLoS One. 2016;11:e0157086. doi: 10.1371/journal.pone.0157086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59:1160–1171. doi: 10.1016/j.biopsych.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St CD, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon ES, Grennan K, Busnello J, Badner JA, Ovsiew F, Memon S, Alliey-Rodriguez N, Cooper J, Romanos B, Liu C. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol Psychiatry. 2014;19:890–894. doi: 10.1038/mp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Tropea TF, Satpute SS, Sinnegger-Brauns MJ, Striessnig J, Kosofsky BE, Rajadhyaksha AM. Molecular switch from L-type Ca v 1.3 to Ca v 1.2 Ca2+ channel signaling underlies long-term psychostimulant-induced behavioral and molecular plasticity. J Neurosci. 2010;30:17051–17062. doi: 10.1523/JNEUROSCI.2255-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, Gordon-Smith K, Fraser C, Forty L, Russell E, Hamshere ML, Moskvina V, Nikolov I, Farmer A, McGuffin P, Holmans PA, Owen MJ, O’Donovan MC, Craddock N. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry. 2010;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Hamshere M, Forty L, Gordon-Smith K, Fraser C, Russell E, Grozeva D, Kirov G, Holmans P, Moran JL, Purcell S, Sklar P, Owen MJ, O’Donovan MC, Jones L, Jones IR, Craddock N. Replication of bipolar disorder susceptibility alleles and identification of two novel genome-wide significant associations in a new bipolar disorder case-control sample. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamshere ML, Walters JT, Smith R, Richards AL, Green E, Grozeva D, Jones I, Forty L, Jones L, Gordon-Smith K, Riley B, O’Neill T, Kendler KS, Sklar P, Purcell S, Kranz J, Morris D, Gill M, Holmans P, Craddock N, Corvin A, Owen MJ, O’Donovan MC. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry. 2013 doi: 10.1038/mp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L. Behavioral properties of GBR 12909, GBR 13069 and GBR 13098: specific inhibitors of dopamine uptake. Eur J Pharmacol. 1984;103:241–248. doi: 10.1016/0014-2999(84)90483-7. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi M, Okada S, Tadokoro S. Comparison of sensitization to ambulation-increasing effects of cocaine and methamphetamine after repeated administration in mice. J Pharm Pharmacol. 1991;43:827–830. doi: 10.1111/j.2042-7158.1991.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karler R, Turkanis SA, Partlow LM, Calder LD. Calcium channel blockers and behavioral sensitization. Life Sci. 1991;49:165–170. doi: 10.1016/0024-3205(91)90029-b. [DOI] [PubMed] [Google Scholar]
- Latour I, Hamid J, Beedle AM, Zamponi GW, Macvicar BA. Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia. 2003;41:347–353. doi: 10.1002/glia.10162. [DOI] [PubMed] [Google Scholar]
- Leitch B, Szostek A, Lin R, Shevtsova O. Subcellular distribution of L-type calcium channel subtypes in rat hippocampal neurons. Neuroscience. 2009;164:641–657. doi: 10.1016/j.neuroscience.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C] raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Licata SC, Freeman AY, Pierce-Bancroft AF, Pierce RC. Repeated stimulation of L-type calcium channels in the rat ventral tegmental area mimics the initiation of behavioral sensitization to cocaine. Psychopharmacology (Berl) 2000;152:110–118. doi: 10.1007/s002130000518. [DOI] [PubMed] [Google Scholar]
- Licata SC, Pierce RC. The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J Neurochem. 2003;85:14–22. doi: 10.1046/j.1471-4159.2003.01662.x. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl) 1987;91:415–433. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- Liljequist S, Ossowska K, Grabowska-Anden M, Anden NE. Effect of the NMDA receptor antagonist, MK-801, on locomotor activity and on the metabolism of dopamine in various brain areas of mice. Eur J Pharmacol. 1991;195:55–61. doi: 10.1016/0014-2999(91)90381-y. [DOI] [PubMed] [Google Scholar]
- Liu Y, Blackwood DH, Caesar S, de Geus EJ, Farmer A, Ferreira MA, Ferrier IN, Fraser C, Gordon-Smith K, Green EK, Grozeva D, Gurling HM, Hamshere ML, Heutink P, Holmans PA, Hoogendijk WJ, Hottenga JJ, Jones L, Jones IR, Kirov G, Lin D, McGuffin P, Moskvina V, Nolen WA, Perlis RH, Posthuma D, Scolnick EM, Smit AB, Smit JH, Smoller JW, St Clair D, van Dyck R, Verhage M, Willemsen G, Young AH, Zandbelt T, Boomsma DI, Craddock N, O’Donovan MC, Owen MJ, Penninx BW, Purcell S, Sklar P, Sullivan PF. Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol Psychiatry. 2011;16:2–4. doi: 10.1038/mp.2009.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loewinger GC, Beckert MV, Tejeda HA, Cheer JF. Methamphetamine-induced dopamine terminal deficits in the nucleus accumbens are exacerbated by reward-associated cues and attenuated by CB1 receptor antagonism. Neuropharmacology. 2012;62:2192–2201. doi: 10.1016/j.neuropharm.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Logue A, Stanford K, Xu M, Zhang J, Richtand NM. Dose-response analysis of locomotor activity and stereotypy in dopamine D3 receptor mutant mice following acute amphetamine. Synapse. 2006;60:399–405. doi: 10.1002/syn.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTavish S, McPherson M, Harmer C, Clark L, Sharp T, Goodwin G, Cowen P. Antidopaminergic effects of dietary tyrosine depletion in healthy subjects and patients with manic illness. The British Journal of Psychiatry. 2001;179:356–360. doi: 10.1192/bjp.179.4.356. [DOI] [PubMed] [Google Scholar]
- Mills K, Ansah TA, Ali SF, Mukherjee S, Shockley DC. Augmented behavioral response and enhanced synaptosomal calcium transport induced by repeated cocaine administration are decreased by calcium channel blockers. Life Sci. 2007;81:600–608. doi: 10.1016/j.lfs.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E, Owen MJ, O’Donovan MC. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol Psychiatry. 2009;14:252–260. doi: 10.1038/mp.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Brodie HK, Goodwin FK, Bunney WE., Jr Regular induction of hypomania by L-dopa in “bipolar” manic-depressive patients. Nature. 1971;229:135–136. doi: 10.1038/229135a0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, Sorensen KM, Andersen PS, Nordentoft M, Werge T, Pedersen CB, Hougaard DM, Mortensen PB, Mors O, Borglum AD. CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry. 2010;15:119–121. doi: 10.1038/mp.2009.69. [DOI] [PubMed] [Google Scholar]
- O’Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita M, Watanabe Y, Taya K, Utsumi H, Hayashi T. Presynaptic L-type Ca(2)+ channels on excessive dopamine release from rat caudate putamen. Physiol Behav. 2000;68:641–649. doi: 10.1016/s0031-9384(99)00227-9. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Welge JA, Vornik LA, Hirschfeld RM, Keck PE., Jr Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67:509–516. doi: 10.4088/jcp.v67n0401. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Rajadhyaksha A, Husson I, Satpute SS, Kuppenbender KD, Ren JQ, Guerriero RM, Standaert DG, Kosofsky BE. L-type Ca2+ channels mediate adaptation of extracellular signal-regulated kinase 1/2 phosphorylation in the ventral tegmental area after chronic amphetamine treatment. J Neurosci. 2004;24:7464–7476. doi: 10.1523/JNEUROSCI.0612-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S, Bergen SE, Collins AL, Crowley JJ, Fromer M, Kim Y, Lee SH, Magnusson PK, Sanchez N, Stahl EA, Williams S, Wray NR, Xia K, Bettella F, Borglum AD, Bulik-Sullivan BK, Cormican P, Craddock N, de Leeuw C, Durmishi N, Gill M, Golimbet V, Hamshere ML, Holmans P, Hougaard DM, Kendler KS, Lin K, Morris DW, Mors O, Mortensen PB, Neale BM, O’Neill FA, Owen MJ, Milovancevic MP, Posthuma D, Powell J, Richards AL, Riley BP, Ruderfer D, Rujescu D, Sigurdsson E, Silagadze T, Smit AB, Stefansson H, Steinberg S, Suvisaari J, Tosato S, Verhage M, Walters JT, Levinson DF, Gejman PV, Laurent C, Mowry BJ, O’Donovan MC, Pulver AE, Schwab SG, Wildenauer DB, Dudbridge F, Shi J, Albus M, Alexander M, Campion D, Cohen D, Dikeos D, Duan J, Eichhammer P, Godard S, Hansen M, Lerer FB, Liang KY, Maier W, Mallet J, Nertney DA, Nestadt G, Norton N, Papadimitriou GN, Ribble R, Sanders AR, Silverman JM, Walsh D, Williams NM, Wormley B, Arranz MJ, Bakker S, Bender S, Bramon E, Collier D, Crespo-Facorro B, Hall J, Iyegbe C, Jablensky A, Kahn RS, Kalaydjieva L, Lawrie S, Lewis CM, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JT, Farh KH, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Mitchell AC, Voloudakis G, Fullard JF, Pothula VM, Tsang J, Stahl EA, Georgakopoulos A, Ruderfer DM, Charney A, Okada Y, Siminovitch KA, Worthington J, Padyukov L, Klareskog L, Gregersen PK, Plenge RM, Raychaudhuri S, Fromer M, Purcell SM, Brennand KJ, Robakis NK, Schadt EE, Akbarian S, Sklar P. A role for noncoding variation in schizophrenia. Cell Rep. 2014;9:1417–1429. doi: 10.1016/j.celrep.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryding E, Lindström M, Träskman-Bendz L. The role of dopamine and serotonin in suicidal behaviour and aggression. Progress in brain research. 2008;172:307–315. doi: 10.1016/S0079-6123(08)00915-1. [DOI] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci U S A. 2008;105:4405–4410. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Pardo M, Yohn SE, Lopez-Cruz L, SanMiguel N, Correa M. Mesolimbic Dopamine and the Regulation of Motivated Behavior. Curr Top Behav Neurosci. 2016;27:231–257. doi: 10.1007/7854_2015_383. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LG, Carbonetto P, Engel KA, Krauss KS, Barrios-Camacho CM, Palmer AA. Genetic background limits generalizability of genotype=phenotype relationships. Neuron. 2016;91:1253–1259. doi: 10.1016/j.neuron.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, Nimgaonkar VL, McQueen MB, Faraone SV, Kirby A, de Bakker PI, Ogdie MN, Thase ME, Sachs GS, Todd-Brown K, Gabriel SB, Sougnez C, Gates C, Blumenstiel B, Defelice M, Ardlie KG, Franklin J, Muir WJ, McGhee KA, MacIntyre DJ, McLean A, VanBeck M, McQuillin A, Bass NJ, Robinson M, Lawrence J, Anjorin A, Curtis D, Scolnick EM, Daly MJ, Blackwood DH, Gurling HM, Purcell SM. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Ripke S, Scott LJ, Andreassen OA, Cichon S, et al. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Ripke S, Lee PH, Neale B, Nurnberger JL, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Inazu M, Matsumiya T. Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:620–623. doi: 10.1007/s00210-002-0640-0. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Khansa MR. Repeated administration of MK-801 produces sensitization to its own locomotor stimulant effects but blocks sensitization to amphetamine. Brain Res. 1991;562:164–168. doi: 10.1016/0006-8993(91)91202-c. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimizu T, Pan JQ, Mungenast AE, Madison JM, Su S, Ketterman J, Ongur D, McPhie D, Cohen B, Perlis R, Tsai LH. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010;208:443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Bhat S, Terrillion CE, Smith RJ, Tonelli LH, Gould TD. Sex-dependent modulation of age-related cognitive decline by the L-type calcium channel gene Cacna1c (Ca 1.2) Eur J Neurosci. 2015 doi: 10.1111/ejn.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Cooper DC, White FJ. Repeated cocaine treatment decreases whole-cell calcium current in rat nucleus accumbens neurons. J Pharmacol Exp Ther. 2002;301:1119–1125. doi: 10.1124/jpet.301.3.1119. [DOI] [PubMed] [Google Scholar]