Abstract
Aerobic exercise improves outcomes in a variety of chronic health conditions, yet the support for exercise-induced effects on chronic pain in humans is mixed. While many rodent studies have examined the effects of exercise on persistent hypersensitivity, the vast majority employed forced exercise paradigms that are known to be highly stressful. Since stress can also produce analgesic effects, we studied how voluntary exercise, known to reduce stress in healthy subjects, alters hypersensitivity, stress and swelling in a rat model of persistent hind paw inflammation. Our data indicate that voluntary exercise rapidly and effectively reduces both hypersensitivity and stress-related outcomes without altering swelling. Moreover, the level of exercise is unrelated to the analgesic and stress-reducing effects, suggesting that even modest amounts of exercise may impart significant benefit in persistent inflammatory pain states.
Keywords: Voluntary exercise, analgesia, stress, pain, inflammation, rat
Introduction
While physical activity is effective in preventing or delaying the onset of persistent pain in animal models [10; 77; 99; 107] and is associated with lower risk of chronic pain in humans [118; 124], outcomes of therapeutic exercise (i.e. exercise initiated after injury) in human chronic pain populations are mixed, mainly due to differences in type of pain, type of exercise and exercise intensity [40; 55; 67; 72; 95]. In contrast, rodent studies employing therapeutic exercise uniformly show robust analgesic effects in a variety of persistent pain models and using a variety of exercise intensities [4; 9; 11; 18–24; 34; 35; 41; 54; 63; 69; 81; 82; 93; 102; 103; 112; 132]. Importantly, none of these studies assessed stress-related outcomes such as plasma corticosterone despite employing forced running paradigms that can be highly stressful [28; 36; 48; 62; 74; 80; 88; 89; 92]. As such, while carefully conditioned forced running paradigms can activate reward centers in the rodent brain [52] and can result in beneficial physiological changes in certain contexts [51; 64; 92; 94], it is nonetheless difficult to determine if the observed analgesic effects were, at least in part, mediated by stress-induced analgesic mechanisms [15; 68]. To assess exercise-induced analgesia while minimizing the potential effects of stress, we employed a voluntary exercise approach. Voluntary exercise is highly rewarding in uninjured humans [12; 97] and rodents [8; 27; 29; 45; 57; 59; 109; 123], engaging mesolimbic reward pathway circuitry [45; 85; 126]. Voluntary exercise can also reduce plasma corticosterone [46; 48; 62], increase endogenous opioid levels [8; 32; 45; 75; 76; 86; 105], promote hippocampal neurogenesis [47; 130; 131] as well as incur other beneficial effects such as improved viral and microbial resistance as well as decreased risk of heart disease and high blood pressure, among others [39].
While no rodent studies addressing therapeutic use of voluntary exercise are available, voluntary running initiated prior to injury can prevent or delay the development of pain [10; 77; 99; 107]. Specifically, these studies show that high levels of voluntary running initiated prior to injury, achieved by housing mice with running wheels, prevents the development of hypersensitivity in a model of chronic musculoskeletal pain [77; 99; 107]; However, this approach does not address the potential therapeutic benefits of exercise initiated after injury. Moreover, housing rodents with the running wheel allows for higher levels of running behavior that may not necessarily reflect the level of exercise seen in most humans. To assess therapeutic effects of voluntary running while incorporating more modest levels of exercise, our rats were given limited access to running wheels (2 hours/day, 4 days/week, 3 weeks) following intra-articular Complete Freund’s Adjuvant (CFA), a rodent model of persistent inflammation [16; 128]. We assessed both pain- and stress-related outcomes 24–48 hours after running behavior to avoid acute stress- or reward-dependent effects. Our findings establish that voluntary exercise rapidly and effectively reduces pain- and stress-related outcomes without altering inflammation-induced swelling. Importantly, the amount of exercise was unrelated to the degree of improvement in pain- and stress-related outcomes, suggesting that modest levels of self-regulated exercise effectively improves pain and stress associated with persistent inflammatory pain.
Methods
Animals
Male Long Evans rats (Charles River) between 250–275g were used for all experiments (n=16–24/group). Rats were kept on an inverted 12hr/12hr light/dark cycle (lights out at 9am) and were only tested during the early portion of their waking cycle. All animals were housed in pairs and given free access to food and water. Each cage was randomly assigned to one of the four experimental conditions. All protocols were reviewed and approved by National Institute of Neurological Disorders and Stroke/National Institute on Deafness and other Communication Disorders Animal Care and Use Committee (NINDS/NIDCD ACUC). Experiments were in accordance with the NINDS/NIDCD ACUC and the International Association for the Study of Pain (IASP) guidelines for the care and use of experimental animals.
Peripheral inflammation
A total of 25ul of Complete Freund’s Adjuvant (CFA; 1mg/ml Mycobacterium tuberculosis, Sigma Alderich) was injected into the tibial–tarsal joint of the left hind paw in isoflurane-anesthetized (5% in O2) rats according to the method described in Butler et al. [16]. Control (sham) animals underwent the same procedure including needle insertion but without irritant injection in order to prevent potential volume-related damage to the ankle joint. Animals were immediately returned to their home cage for observation. Intra-articular CFA typically results in approximately 6–8 weeks of ankle/knee swelling and hypersensitivity [16; 84; 113], but can reach 12 weeks depending on volume and concentration of CFA injection [128]. Joint swelling and hypersensitivity typically reach maximum levels within within the first week post-injection [16; 84; 113; 121].
Experimental design and exercise paradigm
Four groups of rats were studied: (i) CFA + home cage sedentary (CFA-HC SED) that had no access to running wheels (n=24), (ii) CFA + locked wheel sedentary (CFA-LW SED) that spent 2 hours/day, 4 days/week for 3 weeks in the running wheel cages with locked wheels (n=10), (iii) CFA + Exercise (CFA-Run) that spent 2 hours/day, 4 days/week for 3 weeks in running wheel cages with functioning wheels (n=24) and (iv) Sham + Exercise (Sham-Run) that spent 2 hours/day, 4 days/week for 3 weeks in running wheel cages with functioning wheels (n=16). No statistically significant differences were found between the inflamed home cage sedentary (CFA-HC SED) and inflamed locked wheel sedentary (CFA-LW SED) groups in body weight, ankle width, weight bearing, thermal latency, plasma corticosterone or HRV, so data from these groups was pooled into a single group (CFA-SED). A total of seven cohorts of animals were tested to achieve the desired sample sizes and ensure consistent results. Each cohort of 10–12 rats was comprised of animals from each experimental group. A sample size of n=24 for the CFA-Run group was chosen in order to expand upon on the sample sizes used in other published rodent exercise studies performing linear regression analysis (i.e. n=6–18 per group) [42; 52; 116]. For the Sham group, we chose to use a smaller group size (n=16) because this was a control group that was not expected to exhibit any changes in hypersensitivity and would not be used for regression analysis. In addition, a smaller group size of n=16 would minimize animal usage while maintaining satisfactory statistical power.
Voluntary exercise was performed on stainless steel running wheels (diameter 34 cm; width 7 cm) in polycarbonate cages (48 × 31.5 × 47 cm; Bioseb, Boulogne, France). A computer connected to the wheels automatically recorded the distance travelled by each animal during the running session. Considering that peak running behavior in rats occurs in the early portion of their waking cycle [45], rats were always given access to wheels shortly after lights-out (i.e. 9:30–11:30am). Each morning, all rodents were transported in their home cage to an adjacent room containing running wheels. Rats in the exercise groups were given individual access to running wheels for 2 hours/day, 4 days/week for 3 weeks beginning 3 days after induction of CFA-induced inflammation (Fig. 1A). The total distance run per day was monitored for each rat. Rats were not pre-trained on the running wheels. No experimenters were present during the running sessions. For all animals with running wheel access, the daily running distance was measured. The time spent running was also measured for a subset of animals. For analysis, running behavior was expressed either as distance (in meters) or average velocity (in meters/sec) per time period.
FIGURE 1. Voluntary running behavior is robust and highly variable in both shams and CFA-injected rats.
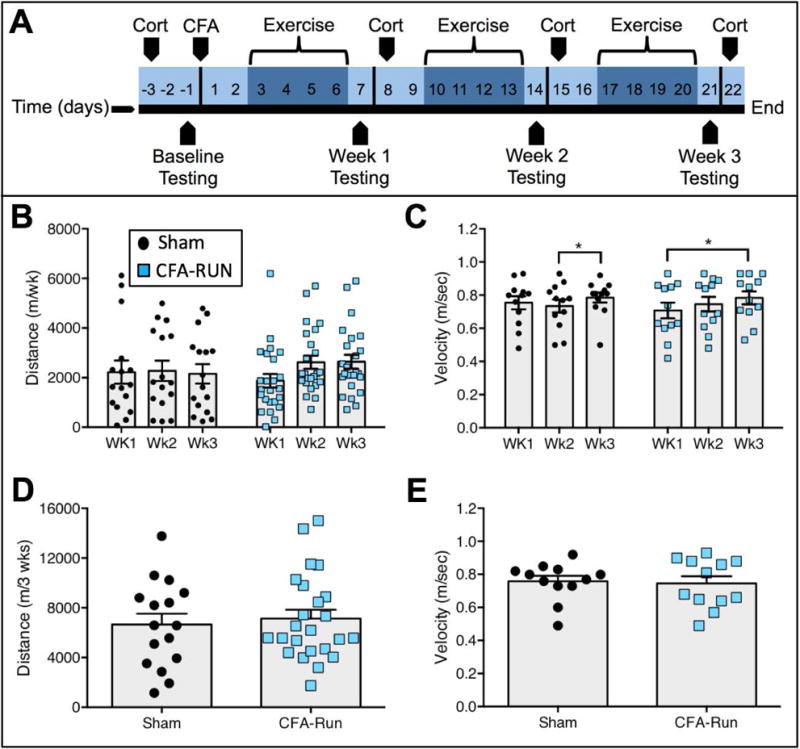
(A) Experimental time line. (B) In both sham and CFA-RUN groups, no significant differences were observed in weekly running distance. (C) In terms of average weekly velocity, the average velocity was significantly higher in week 3 than week 2 for shams, and week 3 was higher than week 1 for the CFA-RUN group. (D) No differences in the total running distance over the three-week period were observed between groups. (E) No difference in average velocity for the entire 3-week time span were found between the sham and CFA-RUN groups. n=12–24 per group.
Baseline behavioral testing occurred one day prior to CFA-injection and at post-injection days 7, 14 and 21 (Figure 1A). In all groups, post-CFA testing was always performed 24 hours after last weekly exercise of the exercise groups. Similarly, plasma corticosterone was always harvested 48 hours after last weekly exercise of the exercise groups. Access to running wheels and behavioral testing occurred under red-light conditions to avoid interfering with rodents’ nocturnal waking cycle. Rodents were habituated to all testing apparatus on three separate days prior to baseline testing. Experimenters were blinded to active or sedentary condition, but were not blinded to inflamed versus sham groups due to obvious hind paw swelling in inflamed rodents. No animals were excluded from analyses.
Hind paw swelling
After behavioral testing, rats were lightly anesthetized with isoflurane. The widest point of the ankle joint, between the medial and the lateral malleolus, was measured with a digital micrometer for both the ipsilateral and contralateral paw. Data is presented as the proportion of ipsilateral to contralateral paw width, in percent.
Corticosterone
Plasma corticosterone was assessed using an enzyme-linked immunosorbent assay (ELISA; Enzo Life Sciences kit #ADI-900-097). Rats were allowed to enter a soft folded towel where they were gently restrained. To minimize stress, rats were handled in a separate room using a new towel for each rat. A small volume of blood (20–30μl) was harvested with heparin-treated capillary tubes from the tip of the tail via a 0.5mm incision. The entire procedure lasted approximately 2 minutes. Blood samples were kept at 4°C until centrifugation (2 minutes at 16,000RPM; Iris CritSpin) to separate plasma from serum. Plasma was pipetted into individually labeled sample tubes and stored at −80 °C until ELISA processing. Plasma levels of corticosterone were assessed according to the small sample volume instructions from Enzo Life Sciences kit #ADI-900-097. Briefly, samples were brought to room temperature and 10 μl of each sample was diluted (40:1) with assay buffer. 100 μl of samples and standards were pipetted into the appropriate wells of the ELISA plate. Conjugate (50 μl) and antibody (50 μl) were added to each well and incubated in darkness for 2 hours on a shaker (500 RPM) at room temperature. The plate was then washed 3 times with washing buffer and gently tapped on an absorbent paper to remove remaining moisture. 200 μl of p-nitrophenyl phosphate substrate was added to each well and incubated in darkness for 1 hour at room temperature without shaking. 50 μl of ‘stop’ solution was then added to each well and the absorbance was immediately measured with a Wallac 1420 model plate reader with a 405nm filter. All samples, controls and standards were measured in duplicate. Plasma levels of corticosterone were expressed in picograms/milliliter (pg/ml).
Heart rate variability (HRV)
HRV reflects the change in the time intervals between successive heartbeats, an effect that is driven by various regulatory systems in order to adapt to stressors [119]. Reductions in HRV have been linked to deficits in stress adaptation that can result in overall poorer health and decreased longevity [33; 70; 71; 119; 120]. Here, HRV was assessed using a non-invasive approach whereby rats were placed on a small platform with three 5 × 5cm disposable conductive plate electrodes (ECGenie; Mouse Specifics). Rats were free to explore the platform during recording. Only traces recorded during immobility on the platform were used for analysis. The ECG signal was recorded when one forepaw and the contralateral hind paw contacted two different electrodes. Only traces between 5–30 seconds long were used for analysis. Results from multiple traces were used to calculate a mean value for each rat. All rodents were habituated to the device prior to recording in order to minimize stress and movement artefact. Electrocardiogram signals were analyzed using LabChart software (ADInstruments, Colorado, USA) to calculate standard deviation of the R-R interval (SDNN) as well as the root mean square of successive differences (RMSSD). As described elsewhere [38; 83], SDNN is considered to represent total variability in the recording and is calculated by determining the standard deviation of the R-R intervals in the given trace. RMSSD, however, is thought to reflect mainly the high frequency component of the given trace [5; 111]. RMSSD is calculated as the square root of the mean of the squares of the successive differences between adjacent R-R intervals. Similar to other groups [3], we calculated delta (Δ) scores for both SDNN and RMSSD by subtracting baseline from week 3 scores.
Weight bearing and thermal hypersensitivity measurements
Static weight bearing capacity was measured with an Incapacitance Meter (IITC Life Science, model 600). Similar to humans with a painful injury to the ankle or knee, the static weight bearing assay reflects the rodent’s unwillingness or incapacity to bear weight on the injured limb. As such, it can be considered an ethologically relevant measure of mechanical hypersensitivity within the injured joint rather than on the skin. To measure static weight bearing, we used a commercially available device (Incapacitance Meter, IITC Life Science, model 600) and employed a commonly used approach [13]. Briefly, the rat was placed on an inclined plane within a small transparent Perspex enclosure where its tail was gently held to prevent excessive movement. When the animal had finished exploring the enclosure and hind paws were stably resting on the two strain gauges, the measurement was taken, expressed as the proportion of total weight placed on the left and right hind paws. Three measurements of 5 seconds each were recorded for each animal and a mean was calculated. The entire procedure lasted no more than approximately two minutes for each animal. For analysis, weight bearing was expressed as the percent of contralateral scores.
Thermal hypersensitivity was measured using the Plantar Test device (IITC Life Science, model 400), whereby the latency to withdraw from a radiant light beam directed at the plantar aspect of the hind paw was recorded. The intensity of the radiant light source was preset to produce withdrawal latencies of 10–12 seconds in naïve rats. The glass surface was maintained at 30°C to reduce the potential influence of cold hypersensitivity on thermal responses. Rats were habituated to the testing enclosures for 30 minutes prior to testing. Each Perspex enclosure measured 10 × 20 cm with opaque side panels to prevent visual communication between rats. Three latencies were obtained for each rat with 2–3 minutes between measurements and a mean was calculated. For analysis, thermal latency was expressed as the percent of contralateral scores.
Analysis
All statistical analysis was performed with SPSS version 22 (IBM Analytics, New York). Data are represented graphically as mean ± SEM. To avoid non-normal distribution effects, logarithmic transformation of the data was performed prior to analysis. Unpaired t-tests, one-way ANOVA or two-way mixed design Repeated Measures ANOVA were used where indicated. Results of t-tests, one- and two-way ANOVA’s are displayed in Table 1. Post-hoc tests were Bonferroni multiple comparisons tests. Analysis of non-spherical data was corrected with using Greenhouse-Geisser adjustment. In all cases, p < 0.05 was considered significant. Linear regression analysis was used to assess the relationship between measures of voluntary running behavior (i.e. total distance, average velocity) in the CFA-RUN group versus either week 3 scores in weight bearing, plasma corticosterone or RMSSD or delta (Δ) scores in weight bearing, plasma corticosterone or RMSSD (calculated as baseline scores subtracted from week 3 scores). To simplify statistical analysis, running behavior was binned into either weekly or total running scores over the three post-injection weeks.
TABLE 1.
| Figure and Panel | Comparison | Statistic | Result |
|---|---|---|---|
| 1 | |||
| B | Weekly running distance | Two-way mixed design repeated measures ANOVA | Interaction: F(1.53,58.25)=1.43, p=0.247 Groups: F(1,38)=0.197, p=0.668 Time: F(1.53,58.25)=1.38, p=0.256 |
| C | Weekly average velocity | Two-way mixed design repeated measures ANOVA | Interaction: F(2,44)=1.68, p=0.199 Groups: F(1,22)=0.05, p=0.819 Time: F(2,44)=6.12, p=0.005** |
| D | Total running distance | Unpaired t-test | t(38)=0.433, p=0.668 |
| E | Total average velocity | Unpaired t - test | t(22)=0.231, p=0.819 |
| 2 | |||
| A | Weight bearing | Two-way mixed design repeated measures ANOVA | Interaction: F(5.05,179.38)=17.96, p<0.0001*** Groups: F(2,71)=83.67, p<0.0001*** Time: F(2.53,179.38)=49.79, p<0.0001*** |
| B | Plantar latency | Two-way mixed design repeated measures ANOVA | Interaction: F(5.75,140.96)=5.50, p<0.0001*** Groups: F(2,49)=29.03, p<0.0001*** Time: F(2.88,140.96)=6.55, p<0.0001*** |
| C | Body mass | Two-way mixed design repeated measures ANOVA | Interaction: F(2.66,94.33)=2.28, p=0.092 Groups: F(2,71)=1.23, p=0.300 Time: F(1.33,94.33)=245.91, p<0.0001*** |
| D | Ankle swelling | Two-way mixed design repeated measures ANOVA | Interaction: F(4.54,161.10)=17.83, p<0.0001*** Groups: F(2,71)=24.81, p<0.0001*** Time: F(2.27,161.10)=86.45, p<0.0001*** |
| E | Plasma corticosterone | Two-way mixed design repeated measures ANOVA | Interaction: F(6,204)=1.27, p=0.275 Groups: F(2,68)=2.18, p<0.0001*** Time: F(3,204)=1.92, p=0.128 |
| 3 | |||
| A | Heart rate | Two-way mixed design repeated measures ANOVA | Interaction: F(2,71)=1.36, p=0.262 Groups: F(2,71)=0.976, p=0.382 Time: F(1,71)=1.88, p=0.17 |
| B | R-R interval | Two-way mixed design repeated measures ANOVA | Interaction: F(2,71)=2.05, p=0.137 Groups: F(2,71)=0.77, p=0.465 Time: F(1,71)=98, p=0.326 |
| C | SDNN | Two-way mixed design repeated measures ANOVA | Interaction: F(2,71)=3.93, p=0.024* Groups: F(2,71)=0.283, p=0.754 Time: F(1,71)=0.31, p=0.581 |
| D | RMSSD | Two-way mixed design repeated measures ANOVA | Interaction: F(2,71)=4.29, p=0.017* Groups: F(2,71)=0.055, p=0.946 Time: F(1,71)=0.52, p=0.471 |
| E | Δ SDNN | One-way ANOVA | Groups: F(2,71)=4.36, p=0.016* |
| F | Δ RMSSD | One-way ANOVA | Groups: F(2,71)=3.92, p=0.024* |
NOTE. Results of all post hoc analyses are displayed as stars/circles in the relevant figure panel.
Results
CFA-inflamed and sham-injected rodents exhibit robust running behavior
Rats were given free access to running wheels for two hours per day, four days per week for three weeks, beginning three days post-CFA or sham injection (Fig. 1A). Most rats from both groups engaged in robust running behavior within the first week of wheel access both in terms of running distance (Fig. 1B) and average velocity (Fig. 1C). A two-way mixed design repeated measures AVOVA for weekly running distance revealed no significant group, time or interaction effects (Table 1). However, similar analysis of weekly average velocity showed a significant main effect for time (Table 1). Bonferroni post hoc analysis indicated a significant increase in average velocity between week 2 and week 3 in Shams as well as an increase between week 1 and week 3 in the CFA-RUN group (Fig. 1C). While there was substantial inter-individual variability in total running distance and total average velocity among rats in the inflamed and sham conditions, both groups exhibited similar levels of overall running behavior (Fig. 1D/E, Table 1).
Voluntary exercise attenuates CFA-induced weight bearing deficits and thermal hypersensitivity
We tested static weight bearing and thermal hypersensitivity at baseline and for each of the following three post-injection weeks. Two-way mixed design repeated measures ANOVA comparing the three treatment groups over the four time points revealed significant interaction, time and group effects (Table 1). At baseline, all groups exhibited similar weight bearing capacity (Fig. 2A). While the CFA-SED group exhibited severe weight bearing deficits for all three post-injection weeks, the CFA-RUN group showed improved weight bearing capacity from week 1. By week 3, weight bearing capacity in the CFA-RUN group was indistinguishable from sham levels, whereas the CFA-SED group remained significantly lower than the sham group (Fig. 2A).
FIGURE 2. Voluntary exercise is anti-nociceptive.

(A) While static weight bearing on the CFA-injected paw remained significantly impaired in the CFA-SED group over the course of the study, the CFA-RUN group improved from week 1 to be indistinguishable from shams by week 3. (B) In the CFA-SED group, withdrawal latencies to a radiant heat stimulus on the hind paw remained significantly lower than the sham group for the three post-CFA weeks. Thermal hypersensitivity was prevented in CFA-RUN group. (C) All groups gained weight at a similar rate, with no statistically significant differences in body mass among the groups at any time point. (D) Ankle width in both the CFA-RUN and the CFA-SED groups remained significantly wider than the sham group at all post-CFA time points. No differences between the CFA-RUN and the CFA-SED groups were observed at any time point. (E) Plasma corticosterone levels in the CFA-RUN group remained comparable to the sham group at all time points, whereas post-CFA levels were significantly higher in the CFA-SED group. Stars (*) signify significant comparisons with sham group while circles (°) represent significant comparisons with the CFA-Run group. */°p<0.05, **p<0.01, ***/°°°p<0.0001. n=16-34 per group for weight bearing and n=12–28 for plantar test.
In a subset of rats we also measured thermal withdrawal latencies to a beam of radiant light directed to the plantar aspect of the ipsilateral and contralateral paws (Fig. 2B). Two-way mixed design repeated measures ANOVA comparing treatment groups over time revealed significant interaction, time and group effects (Table 1). While rats in the CFA-SED group exhibited ongoing latency reductions lasting the full three post-injection weeks, one week of access to running wheels prevented the development of thermal hypersensitivity in the CFA-RUN group (Fig. 2B).
Voluntary exercise does not alter weight gain or swelling of the inflamed limb
Body mass increased similarly over time in all three groups (Fig. 2C), as indicated by a significant main effect for time but no group or interaction effects (Table 1). In terms of swelling of the ipsilateral ankle, intra-articular injection of CFA produced robust swelling in both the CFA-SED and CFA-RUN groups (Fig. 2D). Specifically, two-way mixed design repeated measures ANOVA comparing the three treatment groups over the four time points revealed significant interaction, time and group effects (Table 1). Post hoc Bonferroni’s multiple comparison tests revealed that ipsilateral ankle swelling was significantly greater than sham for both CFA-injected groups at all post-injection time points, with no significant differences between the two CFA-injected groups (Fig. 2D).
Voluntary exercise attenuates CFA-induced stress-related outcomes
To account for the impact of stress, we measured plasma corticosterone in the three groups at baseline and at all three post-injection weeks (Fig. 2E). Two-way mixed design repeated measures ANOVA yielded significant group effects, but no time or interaction effects (Table 1). At baseline, all groups exhibited similar levels of plasma corticosterone (Fig. 2E). However, for all three post-injection weeks, plasma corticosterone levels in the CFA-SED group were significantly higher than both the Sham group and the CFA-RUN group. Corticosterone levels for the CFA-RUN group were indistinguishable from Sham at all time points (Fig. 2E). No significant differences were observed between baseline and post-injection time points for any group.
Electrocardiographic data was collected non-invasively at baseline and at week 3 post-injection for all three groups. No significant interaction, group or time effects were found (Table 1) for either heart rate (Fig. 3A) or R-R interval (Fig. 3B). We also measured how persistent inflammation and access to exercise altered heart rate variability (HRV), measured as either standard deviation of R-R intervals (SDNN) or root mean square of successive differences (RMSSD). Two-way mixed design repeated measures ANOVA’s comparing either SDNN or RMSSD for the three groups over two time points (baseline and week 3) revealed significant interaction effects but no main effects for time or groups (Table 1). Bonferroni post hoc analysis indicated that SDNN scores decreased over time for the CFA-SED group, whereas a trend towards an increased SDNN was found for the sham group (Fig. 3C). Similarly, post hoc analyses showed that RMSSD scores increased over time for the sham group, whereas a trend for decreased RMSSD for the CFA-SED group was observed (Fig. 3D). For both SDNN and RMSSD measures, no significant group differences were observed in baseline or week 3 scores. We also calculated delta (Δ) scores for both SDNN (Fig. 3E) and RMSSD (Fig. 3F). One-way ANOVA’s comparing the three groups were significant for both SDNN and RMSSD (Table 1). Post hoc Bonferroni’s multiple comparison tests revealed a significant decrease in SDNN for the CFA-SED group compared to the Sham group (Fig. 3E). Similarly for RMSSD, post hoc testing revealed a significant decrease in RMSSD for the CFA-SED group compared to the Sham group (Fig. 3F).
FIGURE 3. Voluntary exercise prevents CFA-induced reductions in heart rate variability.
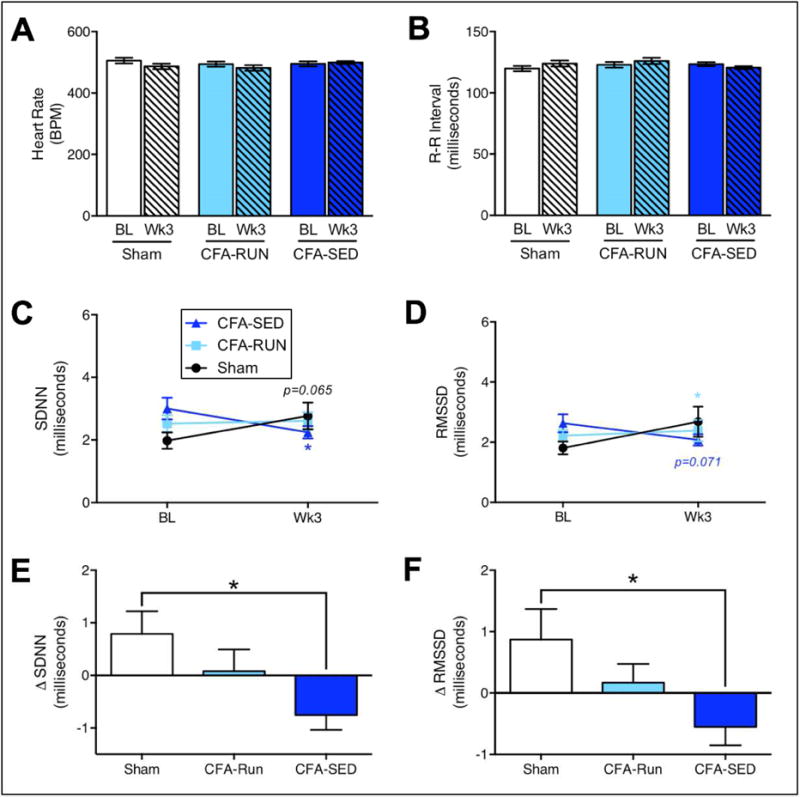
(A/B) Neither heart rate nor R-R interval were significantly changed by CFA or access to running wheels. (C) However, a significant decrease in SDNN for the CFA-SED group along with a trend toward increased SDNN for the sham group were found. (D) Similarly, a trend toward a decrease in RMSSD for the CFA-SED group along with a significant increase in SDNN for the sham group were found. Delta scores for SDNN and RMSSD showed that CFA-induced decreases in both SDNN (E) and RMSSD (F) were mitigated in the CFA-RUN group. Stars (*) signify significant comparisons with sham group. */p<0.05, n=16–34.
The level of voluntary running is unrelated to the amount of analgesia or stress-reduction
We observed clear group-level benefits of voluntary exercise on both pain- and stress-related outcomes, but no effects on ankle swelling, suggesting that the beneficial effects of exercise seen here may not be related to peripheral inflammatory processes that maintain swelling. Considering that intense exercise can yield short-term opioid-dependent euphoria (i.e. ‘runners high’) [12] and analgesia [66], we hypothesized that rats exhibiting the highest level of running behavior would also have the greatest improvement in pain- and stress-related outcomes. Therefore, in the inflamed exercise group (CFA-RUN) we assessed the relationship between the level of exercise and outcomes including weight bearing, plasma corticosterone and RMSSD. Using linear regression analysis, we found no significant relationship between total running distance and weight bearing scores (Fig. 4A), plasma corticosterone levels (Fig. 4B) or RMSSD measurements (Fig. 4C) from week 3. Similarly, we found no significant relationship between average running velocity and weight bearing scores (Fig. 4D), plasma corticosterone levels (Fig. 4E) or RMSSD measurements (Fig. 4F) from week 3. We also assessed how either total running distance or average velocity relate to the delta (Δ) scores between baseline and week 3 for weight bearing capacity (Fig. 4G/H), plasma corticosterone (Fig. 4I/J) and RMSSD (Fig. 4K/L). No significant correlations were found except for a positive correlation between Δ Corticosterone and average velocity (Figure 4J), where increased overall velocity was associated with increased plasma corticosterone levels at week 3 post-CFA.
FIGURE 4. Running measures are unrelated to degree of anti-nociception or reduction in stress-related outcomes.
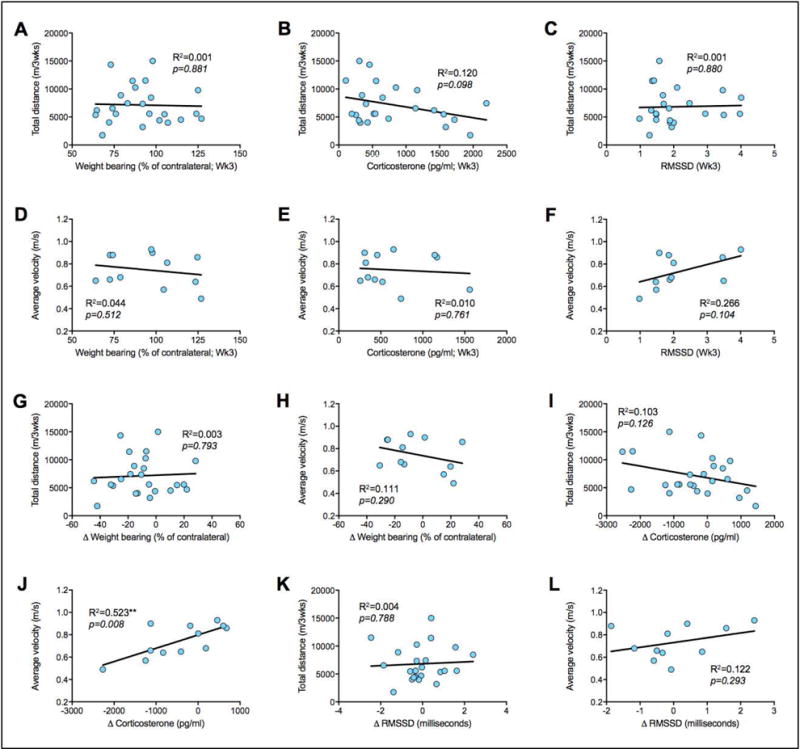
In the CFA-RUN group, linear regression analysis shows that total running distance is unrelated to week 3 weight bearing (A), plasma corticostewrone (B) and RMSSD (C). Similarly, average velocity is unrelated to week 3 weight bearing (D), plasma corticostewrone (E) and RMSSD (F). Change in weight bearing between baseline and week 3 is not associated with either total running distance or average velocity (G/H). Similarly, change in corticosterone level between baseline and week 3 is not associated with total running distance (I), but is associated with average velocity (J). Change in RMSSD between baseline and week 3 is not associated with either total running distance or average velocity (K/L). **p<0.01, n=12–24.
Discussion
In this study, we have shown that regular but modest voluntary wheel running reduces CFA-induced pain- and stress-related outcomes without altering body weight gain or peripheral inflammation-induced swelling of the hind paw. Furthermore, we demonstrate that while the level of voluntary running is highly variable among individual rodents, the amount of running is unrelated to either the degree of analgesia or stress-reduction in physically active CFA-inflamed rats.
Weight bearing and thermal latency
To better reflect the human weight bearing deficits commonly seen after lower extremity injury or arthritis, our main outcome variable was static weight bearing, or the proportion of body weight that the rodent can bear on its inflamed leg. While sedentary inflamed rats experienced ongoing deficits in weight bearing over the three-week period, inflamed rats engaging in voluntary running exhibited a steady improvement in weight bearing capacity of the ipsilateral paw, achieving basal levels by three weeks post-CFA. In studies using forced exercise paradigms, while not all groups describe complete exercise-induced reversal of hypersensitivity to von Frey stimulation, most produce progressive improvements over spans of a few days to more than five weeks [9; 11; 18–24; 35; 54; 63; 69; 82; 93; 102; 103; 112; 132]. Strikingly, development of the CFA-induced thermal hypersensitivity observed in the sedentary inflamed group was prevented by only one week of voluntary running. The eight studies examining exercise-induced changes in thermal hypersensitivity all employed neuropathic pain models, generally describing progressive attenuation or delayed onset of thermal hypersensitivity [18–20; 23; 35; 63; 81; 112]. However, considering the differences between voluntary and forced exercise paradigms, the wide variability in exercise intensities used, the different pain models, and species/strain differences, it is difficult to directly compare study outcomes.
In contrast to studies using exercise as a therapeutic tool, a number of studies have used voluntary running behavior as a diagnostic tool in persistent pain states [25; 43; 60; 61; 115; 127]. In studies using CFA-induced inflammation, wheel running in rodents pre-trained with running wheels is reduced for approximately 3 days post-injury [25; 60; 61]. To avoid initiating exercise in this window of reduced activity, access to running wheels in our study was granted three days after CFA-injection. As such, while we did not measure running behavior immediately after injury, no differences between sham and CFA-RUN groups were observed at later time points, which appears to be consistent with studies indicating relatively brief running deficits after CFA [25; 60; 61; 115].
Stress-related outcomes
Plasma corticosterone is well known to reflect hypothalamic-pituitary-adrenal (HPA) axis neuroendocrine responses to stressors, whereas HRV reflects the drive by various regulatory systems to adapt to stressors via fluctuations in the time intervals between successive heartbeats [119]. While there is as yet no broad consensus on the optimal measure of HRV, higher levels of HRV are generally associated with improved health and stress adaptability, whereas reductions are associated with deficits in stress adaptation that can result in overall poorer health and decreased longevity [33; 70; 71; 119; 120]. Here, we analyzed electrocardiographic data as both SDNN and RMSSD, well-characterized measures that are understood to reflect total variability and the high frequency component of HRV, respectively [5; 111]. While preventative (pre-injury) initiation of voluntary wheel running has recently been demonstrated to produce beneficial results on mechanical hypersensitivity and HRV measures in a mouse model of chronic musculoskeletal pain [99], our findings demonstrate that voluntary exercise initiated therapeutically (i.e. after injury) also prevent the CFA-induced decreases in HRV. These findings are consistent with human studies showing that persistent pain reduces HRV [87], while aerobic exercise improves HRV in healthy individuals [2; 31; 56; 73; 78; 100; 114] as well as during persistent pain states [101](but also see [6]).
In addition to HRV, we also measured plasma corticosterone levels in the three groups. Similar to the elevated plasma cortisol levels seen in human chronic pain populations [122], our sedentary CFA-injected rats exhibited increased plasma corticosterone levels compared to the sham group. In CFA-injected rats with access to running wheels, however, we observed significantly lower corticosterone levels that were comparable to sham levels. While no studies were found assessing voluntary exercise-induced effects on plasma corticosterone in persistent pain states, forced exercise increases plasma corticosterone levels in models of traumatic brain jury [48] and brain ischemia [62], whereas voluntary exercise prevents corticosterone increases in these models as well as preventing stress-induced increases in plasma corticosterone [46] and other stress hormones [89]. As such, our results support a beneficial role for voluntary exercise-related stress and cardiac health benefits in persistent pain states.
Peripheral Inflammation
While some groups have found that aerobic exercise can decrease swelling around the arthritic joint [90] and that reduced body weight can attenuate osteoarthritic joint pain in humans [50; 96], our findings show that a relatively moderate voluntary exercise paradigm altered neither body weight gain nor ankle swelling compared to the sedentary inflamed group. Of course, while ankle swelling does not reflect the full complement of peripheral inflammatory processes, it underscores the potential independence of pain and swelling in peripheral inflammatory pain states. Indeed, while both swelling and hypersensitivity can be dose-dependently reduced with non-steroidal anti-inflammatory drugs (NSAIDs; [49; 91]), hypersensitivity can also be attenuated independently from swelling [14; 37; 125]. Therefore, while ankle swelling alone should not be considered to be a definitive measure of the state of peripheral inflammation, it suggests that the processes underlying ongoing swelling may be unaffected by voluntary exercise, in contrast to pain- and stress-related behaviors. Clearly, studies addressing specific peripheral inflammatory processes are required.
Variability in running behavior
Some rats are more inclined to engage in high level running while others remain relatively low level runners [1; 30; 117]. We, too, observed substantial variability in voluntary running behavior in our rats. Many groups have exploited these inter-individual differences, creating breeding lines of high and low runners that were found to have different dopaminergic [85; 126] and opioidergic tone [79], as well as plasma corticosterone responses to running [126]. Considering that high intensity aerobic exercise increases endogenous opioid-induced euphoria [12] and analgesia [66], we expected that high-running inflamed rats would experience greater benefit in stress- and pain-related outcomes. Instead, with one exception our data indicates that the level of running is unrelated to weight bearing, plasma corticosterone and HRV in a persistent inflammatory pain state. Firstly, in terms of the significant positive correlation that we observed between average velocity and the change in plasma corticosterone, this finding is neither surprising nor contradictory to our suggestion that voluntary exercise benefits stress in persistent pain states. Specifically, while we found an association between changes in plasma corticosterone and running velocity, overall corticosterone levels were significantly lower in the CFA-RUN group compared to the CFA-SED group. Moreover, vigorous exercise can acutely increase cortisol levels in humans [53] as well as promote longer term cortisol increases in endurance athletes [106]. Importantly, despite higher cortisol levels, regular exercise and high cardiorespiratory fitness are nonetheless associated with reduced risk for cardiovascular diseases [17]. As such, while some of the faster running rats exhibited increased levels of plasma corticosterone, corticosterone decreased for most of the group. In terms of the overall lack of association between running behavior and other outcomes, it is possible that inter-individual differences in factors such as stress susceptibility and/or sensitivity to reward play a role. Indeed, reward sensitivity is not the same for everyone; response to reward and/or predictive cues varies among individuals [7; 26; 104] in a dopamine-dependent manner [129]. In fact, while the level of voluntary wheel running correlates with gray matter volume in the rat motor cortex [116] as well as with hippocampal neurogenesis and spatial learning in mice [98], the amount of running does not correlate with NAc ΔFosB/FosB labeling [45], conditioned place preference for the exercise chamber [8], stress resistance [44] or opioid sensitivity [58; 108; 110]. Therefore, it appears that while certain brain functions (i.e. spatial and/or motor learning) are clearly related to the amount of exercise, the relationship between reward/stress processing and exercise is more complex.
Limitations
It must be noted that a few limitations influence our interpretation of the observed results. Firstly, while we did not observe exercise-induced changes in ankle swelling, it cannot be said that ankle swelling reflects all inflammatory processes occurring in the periphery. Since we did not measure specific inflammatory mediators in the hind paw, we cannot conclude that our effects were independent of peripheral inflammatory effects. In addition, it is possible that exercise-induced changes in ankle swelling required more time to become apparent. Another important note involves the lack of association between running intensity and pain- or stress-related outcomes–a finding that appears to contrast human studies showing running intensity-related beneficial effects [67]. While it is difficult to compare rodent running with human running behavior, it must be noted that the average self-regulated running velocity in our rodents was relatively low compared to running velocity seen in other rodent studies [65; 92]. As such, it is possible that the intensity-related benefits seen in human studies are related to running intensities well above those seen in our rats. Alternatively, while higher intensity-running may be beneficial for acute pain thresholds, it may have little or no effect in persistent pain states. Additional experiments are required to address these issues. Lastly, we are unable to address potential gender-related effects of voluntary exercise due to our exclusive use of male rats.
Conclusion
In this study, we addressed the question of whether or not voluntary exercise can be analgesic in the absence of stressors such as forced running. We demonstrated that indeed, voluntary exercise improves pain-related behavior independently of running intensity. While we did not identify a specific mechanism underlying these outcomes, the observed improvement in stress-related measures clearly shows that the analgesia was not due to increased stress, such as the stress that may be produced in forced running paradigms. As such, our study provides valuable evidence supporting not only an analgesic but also a stress-reducing role for voluntary exercise in chronic pain states that cannot necessarily be said of studies using forced exercise paradigms. While further work is required to define the mechanisms contributing to voluntary exercise-induced analgesia, our findings suggest that consistent, self-regulated levels of exercise may be more relevant to health improvement in persistent pain states than standardized activity goals.
Perspective.
Modest levels of voluntary exercise reduce pain- and stress-related outcomes in a rat model of persistent inflammatory pain, independently of the amount of exercise. As such, consistent, self-regulated activity levels may be more relevant to health improvement in persistent pain states than standardized exercise goals.
Highlights.
In rats, intra-articular CFA produces pain, stress and ankle swelling.
Voluntary exercise reduces pain and stress, but not ankle swelling.
Inflamed rats voluntarily exercise as much as uninflamed sham rats.
The amount of exercise is unrelated to the level of reduction in pain and stress.
Consistent, self-regulated exercise may be more relevant than standardized activity goals.
Acknowledgments
This research was supported by the Intramural Research Program of the National Center for Complementary and Integrative Health (NCCIH/NIH). We thank Latoya Hyson, Scott Thompson, Graham Pitcher and the NIH Section on Instrumentation for their valuable advice and technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Afonso VM, Eikelboom R. Relationship between wheel running, feeding, drinking, and body weight in male rats. Physiol Behav. 2003;80:19–26. doi: 10.1016/s0031-9384(03)00216-6. [DOI] [PubMed] [Google Scholar]
- 2.Albinet CT, Boucard G, Bouquet CA, Audiffren M. Increased heart rate variability and executive performance after aerobic training in the elderly. Eur J Appl Physiol. 2010;109:617–624. doi: 10.1007/s00421-010-1393-y. [DOI] [PubMed] [Google Scholar]
- 3.Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res. 2007;3:16. doi: 10.1186/1746-6148-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Balocchi R, Cantini F, Varanini M, Raimondi G, Legramante JM, Macerata A. Revisiting the potential of time-domain indexes in short-term HRV analysis. Biomed Tech (Berl) 2006;51:190–193. doi: 10.1515/BMT.2006.034. [DOI] [PubMed] [Google Scholar]
- 6.Bardal EM, Roeleveld K, Mork PJ. Aerobic and cardiovascular autonomic adaptations to moderate intensity endurance exercise in patients with fibromyalgia. J Rehabil Med. 2015;47:639–646. doi: 10.2340/16501977-1966. [DOI] [PubMed] [Google Scholar]
- 7.Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86:1736–1740. doi: 10.1016/j.apmr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Benson C, Paylor JW, Tenorio G, Winship I, Baker G, Kerr BJ. Voluntary wheel running delays disease onset and reduces pain hypersensitivity in early experimental autoimmune encephalomyelitis (EAE) Exp Neurol. 2015;271:279–290. doi: 10.1016/j.expneurol.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Bobinski F, Martins DF, Bratti T, Mazzardo-Martins L, Winkelmann-Duarte EC, Guglielmo LG, Santos AR. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neuroscience. 2011;194:337–348. doi: 10.1016/j.neuroscience.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 12.Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, Valet M, Berthele A, Tolle TR. The runner’s high: opioidergic mechanisms in the human brain. Cereb Cortex. 2008;18:2523–2531. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- 13.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 14.Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, Fehrenbacher JC, Fitz SD, Khanna M, Park CK, Schmutzler BS, Cheon BM, Due MR, Brustovetsky T, Ashpole NM, Hudmon A, Meroueh SO, Hingtgen CM, Brustovetsky N, Ji RR, Hurley JH, Jin X, Shekhar A, Xu XM, Oxford GS, Vasko MR, White FA, Khanna R. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(+) channel complex. Nat Med. 2011;17:822–829. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Butler SH, Godefroy F, Besson JM, Weil-Fugazza J. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48:73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- 17.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290:3092–3100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 18.Chen YW, Chiu CC, Hsieh PL, Hung CH, Wang JJ. Treadmill training combined with insulin suppresses diabetic nerve pain and cytokines in rat sciatic nerve. Anesth Analg. 2015;121:239–246. doi: 10.1213/ANE.0000000000000799. [DOI] [PubMed] [Google Scholar]
- 19.Chen YW, Hsieh PL, Chen YC, Hung CH, Cheng JT. Physical exercise induces excess hsp72 expression and delays the development of hyperalgesia and allodynia in painful diabetic neuropathy rats. Anesth Analg. 2013;116:482–490. doi: 10.1213/ANE.0b013e318274e4a0. [DOI] [PubMed] [Google Scholar]
- 20.Chen YW, Li YT, Chen YC, Li ZY, Hung CH. Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve. Anesth Analg. 2012;114:1330–1337. doi: 10.1213/ANE.0b013e31824c4ed4. [DOI] [PubMed] [Google Scholar]
- 21.Chen YW, Tzeng JI, Lin MF, Hung CH, Wang JJ. Forced treadmill running suppresses postincisional pain and inhibits upregulation of substance P and cytokines in rat dorsal root ganglion. J Pain. 2014;15:827–834. doi: 10.1016/j.jpain.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Chuganji S, Nakano J, Sekino Y, Hamaue Y, Sakamoto J, Okita M. Hyperalgesia in an immobilized rat hindlimb: effect of treadmill exercise using non-immobilized limbs. Neurosci Lett. 2015;584:66–70. doi: 10.1016/j.neulet.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 23.Cobianchi S, Casals-Diaz L, Jaramillo J, Navarro X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol. 2013;240:157–167. doi: 10.1016/j.expneurol.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Cobianchi S, Marinelli S, Florenzano F, Pavone F, Luvisetto S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience. 2010;168:273–287. doi: 10.1016/j.neuroscience.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Res Cogn Brain Res. 2005;25:851–861. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Collier G, Hirsch E. Reinforcing properties of spontaneous activity in the rat. J Comp Physiol Psychol. 1971;77:155–160. doi: 10.1037/h0031588. [DOI] [PubMed] [Google Scholar]
- 28.Contarteze RV, Manchado F de B, Gobatto CA, De Mello MA. Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp Biochem Physiol A Mol Integr Physiol. 2008;151:415–422. doi: 10.1016/j.cbpa.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 30.D’Anci KE, Gerstein AV, Kanarek RB. Long-term voluntary access to running wheels decreases kappa-opioid antinociception. Pharmacol Biochem Behav. 2000;66:343–346. doi: 10.1016/s0091-3057(00)00245-8. [DOI] [PubMed] [Google Scholar]
- 31.De Meersman RE. Heart rate variability and aerobic fitness. Am Heart J. 1993;125:726–731. doi: 10.1016/0002-8703(93)90164-5. [DOI] [PubMed] [Google Scholar]
- 32.de Oliveira MS, da Silva Fernandes MJ, Scorza FA, Persike DS, Scorza CA, da Ponte JB, de Albuquerque M, Cavalheiro EA, Arida RM. Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Res Bull. 2010;83:278–283. doi: 10.1016/j.brainresbull.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am J Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- 34.Detloff MR, Quiros-Molina D, Javia AS, Daggubati L, Nehlsen AD, Naqvi A, Ninan V, Vannix KN, McMullen MK, Amin S, Ganzer PD, Houle JD. Delayed Exercise Is Ineffective at Reversing Aberrant Nociceptive Afferent Plasticity or Neuropathic Pain After Spinal Cord Injury in Rats. Neurorehabil Neural Repair. 2016;30:685–700. doi: 10.1177/1545968315619698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houle JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp Neurol. 2014;255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Doak GJ, Sawynok J. Formalin-induced nociceptive behavior and edema: involvement of multiple peripheral 5-hydroxytryptamine receptor subtypes. Neuroscience. 1997;80:939–949. doi: 10.1016/s0306-4522(97)00066-3. [DOI] [PubMed] [Google Scholar]
- 38.Farraj AK, Haykal-Coates N, Winsett DW, Hazari MS, Carll AP, Rowan WH, Ledbetter AD, Cascio WE, Costa DL. Increased non-conducted P-wave arrhythmias after a single oil fly ash inhalation exposure in hypertensive rats. Environ Health Perspect. 2009;117:709–715. doi: 10.1289/ehp.0800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleshner M. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc Sport Sci Rev. 2005;33:120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Focht BC. Effectiveness of exercise interventions in reducing pain symptoms among older adults with knee osteoarthritis: a review. J Aging Phys Act. 2006;14:212–235. doi: 10.1123/japa.14.2.212. [DOI] [PubMed] [Google Scholar]
- 41.Gong X, Jiang J, Zhang M. Exercise preconditioning reduces neonatal incision surgery-induced enhanced hyperalgesia via inhibition of 38 mitogen-activated protein kinase and IL-1beta, TNF-alpha release. Int J Dev Neurosci. 2016;52:46–54. doi: 10.1016/j.ijdevneu.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, Galer EL, Walker FR, Greenwood BN, Maier SF, Fleshner M, Watkins LR. Prior voluntary wheel running attenuates neuropathic pain. Pain. 2016;157:2012–2023. doi: 10.1097/j.pain.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grace PM, Loram LC, Christianson JP, Strand KA, Flyer-Adams JG, Penzkover KR, Forsayeth JR, van Dam AM, Mahoney MJ, Maier SF, Chavez RA, Watkins LR. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenwood BN, Loughridge AB, Sadaoui N, Christianson JP, Fleshner M. The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behav Brain Res. 2012;233:314–321. doi: 10.1016/j.bbr.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gregoire CA, Bonenfant D, Le Nguyen A, Aumont A, Fernandes KJ. Untangling the influences of voluntary running, environmental complexity, social housing and stress on adult hippocampal neurogenesis. PLoS One. 2014;9:e86237. doi: 10.1371/journal.pone.0086237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griesbach GS, Tio DL, Vincelli J, McArthur DL, Taylor AN. Differential effects of voluntary and forced exercise on stress responses after traumatic brain injury. J Neurotrauma. 2012;29:1426–1433. doi: 10.1089/neu.2011.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gris G, Merlos M, Vela JM, Zamanillo D, Portillo-Salido E. S1RA, a selective sigma-1 receptor antagonist, inhibits inflammatory pain in the carrageenan and complete Freund’s adjuvant models in mice. Behav Pharmacol. 2014;25:226–235. doi: 10.1097/FBP.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 50.Gudbergsen H, Boesen M, Lohmander LS, Christensen R, Henriksen M, Bartels EM, Christensen P, Rindel L, Aaboe J, Danneskiold-Samsoe B, Riecke BF, Bliddal H. Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthritis Cartilage. 2012;20:495–502. doi: 10.1016/j.joca.2012.02.639. [DOI] [PubMed] [Google Scholar]
- 51.Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115:289–296. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrera JJ, Fedynska S, Ghasem PR, Wieman T, Clark PJ, Gray N, Loetz E, Campeau S, Fleshner M, Greenwood BN. Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. Eur J Neurosci. 2016;43:1190–1202. doi: 10.1111/ejn.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31:587–591. doi: 10.1007/BF03345606. [DOI] [PubMed] [Google Scholar]
- 54.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 55.Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622–636. doi: 10.1002/art.38290. [DOI] [PubMed] [Google Scholar]
- 56.Jurca R, Church TS, Morss GM, Jordan AN, Earnest CP. Eight weeks of moderate-intensity exercise training increases heart rate variability in sedentary postmenopausal women. Am Heart J. 2004;147:e21. doi: 10.1016/j.ahj.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 57.Kagan J, Berkun M. The reward value of running activity. J Comp Physiol Psychol. 1954;47:108. doi: 10.1037/h0058877. [DOI] [PubMed] [Google Scholar]
- 58.Kanarek RB, Gerstein AV, Wildman RP, Mathes WF, D’Anci KE. Chronic running-wheel activity decreases sensitivity to morphine-induced analgesia in male and female rats. Pharmacol Biochem Behav. 1998;61:19–27. doi: 10.1016/s0091-3057(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 59.Kanarek RB, Marks-Kaufman R, D’Anci KE, Przypek J. Exercise attenuates oral intake of amphetamine in rats. Pharmacol Biochem Behav. 1995;51:725–729. doi: 10.1016/0091-3057(95)00022-o. [DOI] [PubMed] [Google Scholar]
- 60.Kandasamy R, Calsbeek JJ, Morgan MM. Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. J Neurosci Methods. 2016;263:115–122. doi: 10.1016/j.jneumeth.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kandasamy R, Calsbeek JJ, Morgan MM. Analysis of inflammation-induced depression of home cage wheel running in rats reveals the difference between opioid antinociception and restoration of function. Behav Brain Res. 2017;317:502–507. doi: 10.1016/j.bbr.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ke Z, Yip SP, Li L, Zheng XX, Tong KY. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS One. 2011;6:e16643. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim YJ, Byun JH, Choi IS. Effect of Exercise on micro-Opioid Receptor Expression in the Rostral Ventromedial Medulla in Neuropathic Pain Rat Model. Ann Rehabil Med. 2015;39:331–339. doi: 10.5535/arm.2015.39.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kinni H, Guo M, Ding JY, Konakondla S, Dornbos D, 3rd, Tran R, Guthikonda M, Ding Y. Cerebral metabolism after forced or voluntary physical exercise. Brain Res. 2011;1388:48–55. doi: 10.1016/j.brainres.2011.02.076. [DOI] [PubMed] [Google Scholar]
- 65.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 66.Koltyn KF. Analgesia following exercise: a review. Sports Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. [DOI] [PubMed] [Google Scholar]
- 67.Koltyn KF. Exercise-induced hypoalgesia and intensity of exercise. Sports Med. 2002;32:477–487. doi: 10.2165/00007256-200232080-00001. [DOI] [PubMed] [Google Scholar]
- 68.Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wohr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Korb A, Bonetti LV, da Silva SA, Marcuzzo S, Ilha J, Bertagnolli M, Partata WA, Faccioni-Heuser MC. Effect of treadmill exercise on serotonin immunoreactivity in medullary raphe nuclei and spinal cord following sciatic nerve transection in rats. Neurochem Res. 2010;35:380–389. doi: 10.1007/s11064-009-0066-x. [DOI] [PubMed] [Google Scholar]
- 70.Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277:H2233–2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- 71.Laing ST, Gluckman TJ, Weinberg KM, Lahiri MK, Ng J, Goldberger JJ. Autonomic effects of exercise-based cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2011;31:87–91. doi: 10.1097/HCR.0b013e3181f1fda0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laubenstein S, Beissner K. Exercise and Movement-based Therapies in Geriatric Pain Management. Clin Geriatr Med. 2016;32:737–762. doi: 10.1016/j.cger.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Lazoglu AH, Glace B, Gleim GW, Coplan L. Exercise and heart rate variability. Am Heart J. 1996;131:825–826. doi: 10.1016/s0002-8703(96)90294-x. [DOI] [PubMed] [Google Scholar]
- 74.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 75.Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72:355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- 76.Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces cross-tolerance to the rewarding effect of morphine. Pharmacol Biochem Behav. 2002;72:101–105. doi: 10.1016/s0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- 77.Leung A, Gregory NS, Allen LA, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain. 2016;157:70–79. doi: 10.1097/j.pain.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levy WC, Cerqueira MD, Harp GD, Johannessen KA, Abrass IB, Schwartz RS, Stratton JR. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol. 1998;82:1236–1241. doi: 10.1016/s0002-9149(98)00611-0. [DOI] [PubMed] [Google Scholar]
- 79.Li G, Rhodes JS, Girard I, Gammie SC, Garland T., Jr Opioid-mediated pain sensitivity in mice bred for high voluntary wheel running. Physiol Behav. 2004;83:515–524. doi: 10.1016/j.physbeh.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Li JY, Kuo TB, Yen JC, Tsai SC, Yang CC. Voluntary and involuntary running in the rat show different patterns of theta rhythm, physical activity, and heart rate. J Neurophysiol. 2014;111:2061–2070. doi: 10.1152/jn.00475.2013. [DOI] [PubMed] [Google Scholar]
- 81.Lopez-Alvarez VM, Modol L, Navarro X, Cobianchi S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain. 2015;156:1812–1825. doi: 10.1097/j.pain.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 82.Luan S, Wan Q, Luo H, Li X, Ke S, Lin C, Wu Y, Wu S, Ma C. Running exercise alleviates pain and promotes cell proliferation in a rat model of intervertebral disc degeneration. Int J Mol Sci. 2015;16:2130–2144. doi: 10.3390/ijms16012130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mabe AM, Hoover DB. Structural and functional cardiac cholinergic deficits in adult neurturin knockout mice. Cardiovasc Res. 2009;82:93–99. doi: 10.1093/cvr/cvp029. [DOI] [PubMed] [Google Scholar]
- 84.Martindale JC, Wilson AW, Reeve AJ, Chessell IP, Headley PM. Chronic secondary hypersensitivity of dorsal horn neurones following inflammation of the knee joint. Pain. 2007;133:79–86. doi: 10.1016/j.pain.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 85.Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T, Jr, Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav Brain Res. 2010;210:155–163. doi: 10.1016/j.bbr.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McLachlan CD, Hay M, Coleman GJ. The effects of exercise on the oral consumption of morphine and methadone in rats. Pharmacol Biochem Behav. 1994;48:563–568. doi: 10.1016/0091-3057(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 87.Meeus M, Goubert D, De Backer F, Struyf F, Hermans L, Coppieters I, De Wandele I, Da Silva H, Calders P. Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: a systematic review. Semin Arthritis Rheum. 2013;43:279–287. doi: 10.1016/j.semarthrit.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Mello PB, Benetti F, Cammarota M, Izquierdo I. Effects of acute and chronic physical exercise and stress on different types of memory in rats. An Acad Bras Cienc. 2008;80:301–309. doi: 10.1590/s0001-37652008000200008. [DOI] [PubMed] [Google Scholar]
- 89.Melo SF, Lunz W, Fontes EP, Dias CM, Carneiro MA, Jr, Moura AG, Del Carlo RJ, Natali AJ. Different levels of Hsp72 in female rat myocardium in response to voluntary exercise and forced exercise. Arq Bras Cardiol. 2009;93:456–462. doi: 10.1590/s0066-782x2009001100004. [DOI] [PubMed] [Google Scholar]
- 90.Minor MA, Hewett JE, Webel RR, Anderson SK, Kay DR. Efficacy of physical conditioning exercise in patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1989;32:1396–1405. doi: 10.1002/anr.1780321108. [DOI] [PubMed] [Google Scholar]
- 91.Mirshafiey A, Cuzzocrea S, Rehm B, Mazzon E, Saadat F, Sotoude M. Treatment of experimental arthritis with M2000, a novel designed non-steroidal anti-inflammatory drug. Scand J Immunol. 2005;61:435–441. doi: 10.1111/j.1365-3083.2005.01594.x. [DOI] [PubMed] [Google Scholar]
- 92.Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1321–1329. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- 93.Morimoto A, Winaga H, Sakurai H, Ohmichi M, Yoshimoto T, Ohmichi Y, Matsui T, Ushida T, Okada T, Sato J. Treadmill running and static stretching improve long-lasting hyperalgesia, joint limitation, and muscle atrophy induced by cast immobilization in rats. Neurosci Lett. 2013;534:295–300. doi: 10.1016/j.neulet.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 94.O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 95.O’Connor SR, Tully MA, Ryan B, Bleakley CM, Baxter GD, Bradley JM, McDonough SM. Walking exercise for chronic musculoskeletal pain: systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96:724–734 e723. doi: 10.1016/j.apmr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 96.Powell A, Teichtahl AJ, Wluka AE, Cicuttini FM. Obesity: a preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. Br J Sports Med. 2005;39:4–5. doi: 10.1136/bjsm.2004.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J Exp Biol. 2012;215:1331–1336. doi: 10.1242/jeb.063677. [DOI] [PubMed] [Google Scholar]
- 98.Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- 99.Sabharwal R, Rasmussen L, Sluka KA, Chapleau MW. Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain. Pain. 2016;157:387–398. doi: 10.1097/j.pain.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sandercock GR, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc. 2005;37:433–439. doi: 10.1249/01.mss.0000155388.39002.9d. [DOI] [PubMed] [Google Scholar]
- 101.Sanudo B, Carrasco L, de Hoyo M, Figueroa A, Saxton JM. Vagal modulation and symptomatology following a 6-month aerobic exercise program for women with fibromyalgia. Clin Exp Rheumatol. 33:S41–45. [PubMed] [Google Scholar]
- 102.Shankarappa SA, Piedras-Renteria ES, Stubbs EB., Jr Forced-exercise delays neuropathic pain in experimental diabetes: effects on voltage-activated calcium channels. J Neurochem. 2011;118:224–236. doi: 10.1111/j.1471-4159.2011.07302.x. [DOI] [PubMed] [Google Scholar]
- 103.Sharma NK, Ryals JM, Gajewski BJ, Wright DE. Aerobic exercise alters analgesia and neurotrophin-3 synthesis in an animal model of chronic widespread pain. Phys Ther. 2010;90:714–725. doi: 10.2522/ptj.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shoaib M, Spanagel R, Stohr T, Shippenberg TS. Strain differences in the rewarding and dopamine-releasing effects of morphine in rats. Psychopharmacology (Berl) 1995;117:240–247. doi: 10.1007/BF02245193. [DOI] [PubMed] [Google Scholar]
- 105.Sisti HM, Lewis MJ. Naloxone suppression and morphine enhancement of voluntary wheel-running activity in rats. Pharmacol Biochem Behav. 2001;70:359–365. doi: 10.1016/s0091-3057(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 106.Skoluda N, Dettenborn L, Stalder T, Kirschbaum C. Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology. 2012;37:611–617. doi: 10.1016/j.psyneuen.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 107.Sluka KA, O’Donnell JM, Danielson J, Rasmussen LA. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol (1985) 2013;114:725–733. doi: 10.1152/japplphysiol.01317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith MA, Lyle MA. Chronic exercise decreases sensitivity to mu opioids in female rats: correlation with exercise output. Pharmacol Biochem Behav. 2006;85:12–22. doi: 10.1016/j.pbb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 109.Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology (Berl) 2003;168:426–434. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- 111.Sollers JJ, 3rd, Buchanan TW, Mowrer SM, Hill LK, Thayer JF. Comparison of the ratio of the standard deviation of the R-R interval and the root mean squared successive differences (SD/rMSSD) to the low frequency-to-high frequency (LF/HF) ratio in a patient population and normal healthy controls. Biomed Sci Instrum. 2007;43:158–163. [PubMed] [Google Scholar]
- 112.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T., Jr Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Staton PC, Wilson AW, Bountra C, Chessell IP, Day NC. Changes in dorsal root ganglion CGRP expression in a chronic inflammatory model of the rat knee joint: differential modulation by rofecoxib and paracetamol. Eur J Pain. 2007;11:283–289. doi: 10.1016/j.ejpain.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 114.Stein PK, Ehsani AA, Domitrovich PP, Kleiger RE, Rottman JN. Effect of exercise training on heart rate variability in healthy older adults. Am Heart J. 1999;138:567–576. doi: 10.1016/s0002-8703(99)70162-6. [DOI] [PubMed] [Google Scholar]
- 115.Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011;98:35–42. doi: 10.1016/j.pbb.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sumiyoshi A, Taki Y, Nonaka H, Takeuchi H, Kawashima R. Regional gray matter volume increases following 7days of voluntary wheel running exercise: a longitudinal VBM study in rats. Neuroimage. 2014;98:82–90. doi: 10.1016/j.neuroimage.2014.04.075. [DOI] [PubMed] [Google Scholar]
- 117.Tarr BA, Kellaway LA, St Clair Gibson A, Russell VA. Voluntary running distance is negatively correlated with striatal dopamine release in untrained rats. Behav Brain Res. 2004;154:493–499. doi: 10.1016/j.bbr.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 118.Teichtahl AJ, Urquhart DM, Wang Y, Wluka AE, O’Sullivan R, Jones G, Cicuttini FM. Physical inactivity is associated with narrower lumbar intervertebral discs, high fat content of paraspinal muscles and low back pain and disability. Arthritis Res Ther. 2015;17:114. doi: 10.1186/s13075-015-0629-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 120.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 121.Uematsu T, Sakai A, Ito H, Suzuki H. Intra-articular administration of tachykinin NK(1) receptor antagonists reduces hyperalgesia and cartilage destruction in the inflammatory joint in rats with adjuvant-induced arthritis. Eur J Pharmacol. 2011;668:163–168. doi: 10.1016/j.ejphar.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 122.Van Uum SH, Sauve B, Fraser LA, Morley-Forster P, Paul TL, Koren G. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 2008;11:483–488. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- 123.Vargas-Perez H, Borrelli E, Diaz JL. Wheel running use in dopamine D2L receptor knockout mice. Neurosci Lett. 2004;366:172–175. doi: 10.1016/j.neulet.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 124.Vierola A, Suominen AL, Lindi V, Viitasalo A, Ikavalko T, Lintu N, Vaisto J, Kellokoski J, Narhi M, Lakka TA. Associations of Sedentary Behavior, Physical Activity, Cardiorespiratory Fitness, and Body Fat Content With Pain Conditions in Children: The Physical Activity and Nutrition in Children Study. J Pain. 2016;17:845–853. doi: 10.1016/j.jpain.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 125.Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren W, Stoehr N, Pagano A, Flor PJ, Vranesic I, Lingenhoehl K, Johnson EC, Varney M, Urban L, Kuhn R. Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function. I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology. 2001;40:1–9. doi: 10.1016/s0028-3908(00)00113-1. [DOI] [PubMed] [Google Scholar]
- 126.Waters RP, Renner KJ, Pringle RB, Summers CH, Britton SL, Koch LG, Swallow JG. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol Behav. 2008;93:1044–1054. doi: 10.1016/j.physbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Whitehead RA, Lam L, Sun MS, Sanchez J, Noor S, Vanderwall AG, Petersen TR, Martin HB, Milligan ED. Chronic Sciatic Neuropathy in Rat Reduces Voluntary Wheel-Running Activity With Concurrent Chronic Mechanical Allodynia. Anesth Analg. 2016 doi: 10.1213/ANE.0000000000001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wilson AW, Medhurst SJ, Dixon CI, Bontoft NC, Winyard LA, Brackenborough KT, De Alba J, Clarke CJ, Gunthorpe MJ, Hicks GA, Bountra C, McQueen DS, Chessell IP. An animal model of chronic inflammatory pain: pharmacological and temporal differentiation from acute models. Eur J Pain. 2006;10:537–549. doi: 10.1016/j.ejpain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 129.Yacubian J, Sommer T, Schroeder K, Glascher J, Kalisch R, Leuenberger B, Braus DF, Buchel C. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci U S A. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yau SY, Lau BW, Zhang ED, Lee JC, Li A, Lee TM, Ching YP, Xu AM, So KF. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience. 2012;222:289–301. doi: 10.1016/j.neuroscience.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 131.Yau SY, Li A, Zhang ED, Christie BR, Xu A, Lee TM, So KF. Sustained running in rats administered corticosterone prevents the development of depressive behaviors and enhances hippocampal neurogenesis and synaptic plasticity without increasing neurotrophic factor levels. Cell Transplant. 2014;23:481–492. doi: 10.3727/096368914X678490. [DOI] [PubMed] [Google Scholar]
- 132.Yoon H, Thakur V, Isham D, Fayad M, Chattopadhyay M. Moderate exercise training attenuates inflammatory mediators in DRG of Type 1 diabetic rats. Exp Neurol. 2015;267:107–114. doi: 10.1016/j.expneurol.2015.03.006. [DOI] [PubMed] [Google Scholar]


