Abstract
We compared headache frequency trajectories between clinical trial participants who received cognitive behavioral therapy plus amitriptyline (CBT+A) or headache education plus amitriptyline (HE+A) to determine if there was differential time course of treatment response between the groups. 135 patients (age 10–17) diagnosed with chronic migraine participated, attending 8 one-hour one-on-one CBT or HE sessions with a trained psychologist for 8 weekly sessions, 2 sessions at weeks 12 and 16, and a post-treatment visit at week 20. Participants kept daily headache diaries and completed take-home assignments between visits. Data from daily headache diaries are presented for each day and by 28-day periods. Trajectories of improvement indicate initial decrease in headache days began during the first month of treatment, for both groups, and continued to decrease throughout treatment. The CBT+A group had greater daily improvement than the HE+A group. A significantly higher proportion of the CBT+A group had a ≥50% reduction in headache days each month, and a significantly higher proportion of the CBT+A group had ≤ 4 headache days per month in months 3 through 5. Results indicate the trajectory of decrease in headache days is significantly better for patients receiving CBT+A versus HE+A.
Perspective
This article presents daily information about headache frequency over a 20-week clinical trial. Youth with chronic migraine who received cognitive behavioral therapy and amitriptyline improved faster than those in the control group. Findings provide clinicians with evidence-based expectations for treatment response over time and ways of monitoring treatment success.
Keywords: pain, pediatrics, clinical trial, headache, treatment
Introduction
Chronic migraine has an estimated prevalence of 1.75% in children and adolescents.17 Up to 69% of children and adolescents who seek care in headache specialty clinics meet diagnostic criteria for chronic migraine (which include 15 or more headache days per month, with the majority having migraine features).4,17 Youth with chronic migraine experience severe disability as a result of the condition, including decreased functioning in home, academic, and social settings.11 Given the level of disability associated with chronic migraine among youth, understanding effective treatments for the condition is a clinical priority. Cognitive Behavioral Therapy (CBT), a form of nonpharmacological treatment, has been shown to be effective in treating chronic pain among children and adolescents by modifying behavioral responses to pain, using active coping skills, such as relaxation techniques and cognitive strategies.5–8,14 The effectiveness of CBT for treating chronic migraine among youth was demonstrated in a clinical trial comparing CBT combined with Amitriptyline (CBT+A), a standard headache medication,10 with an attention control group receiving headache education and Amitriptyline (HE+A).21 Primary results from the trial indicated that CBT+A led to better outcomes in headache frequency and disability when compared to HE+A at the 20-week endpoint and over the course of a one-year follow-up period.
When determining if treatments are effective in managing chronic migraine, response to treatment is typically measured by comparing baseline headache characteristics to those experienced post-treatment, in accordance with International Headache Society (IHS) Clinical Trial Guidelines.24 These IHS guidelines specifically recommend using an absolute reduction in headache days and a ≥ 50% reduction in headache frequency per month when determining if a treatment was effective in managing the condition. While these guidelines are useful in determining the significance of treatment outcomes in clinical trials research, they lack an accurate reflection of clinical significance, particularly for patients suffering from chronic migraine, i.e., 15 or more headache days per month before treatment. Previous work by this team has expanded upon research on guidelines for successful treatment outcomes by identifying a benchmark of a reduction in headache days to less than or equal to 4 headache days per month post-treatment.16 This benchmark is a more clinically meaningful outcome within the context of a clinical trial exploring the effectiveness of CBT; however, there is currently little information about the trajectory of improvement from the first day of treatment to the end of treatment within existing interventions for chronic migraine, including CBT.
Exploring the trajectory of improvement in response to CBT in a longitudinal fashion is necessary for a determination to be made as to when optimal treatment effects occur within the course of treatment, particularly given the demonstrated effectiveness of CBT in treating youth with chronic migraine.21 It is important for CBT clinicians, treating youth with chronic migraine, to have an understanding of time points at which most patients are likely to demonstrate a clinically meaningful reduction in headache frequency within the course of treatment. In addition, importance lies in monitoring if patients are likely to maintain treatment gains, once achieved, to effectively monitor patient outcomes and discuss expectations for treatment gains with patients and families. Thus, the aim of this study was to conduct new analyses from an NIH-funded clinical trial to examine the trajectory of daily headache frequency over 20 weeks of treatment and determine if patterns of change in improvement differ between CBT+A and HE+A groups.
Methods
Participants
Patients were randomized as part of a clinical trial conducted at the Cincinnati Children’s Headache Center between October 2006 and September 2012 with approval from the Cincinnati Children’s Hospital Medical Center institutional review board. Written informed consent was obtained from parents or legal guardians for all subjects enrolled in the study, and informed assent was obtained for all youth age 11 years or older. Inclusion criteria for the study included a diagnosis of chronic migraine by a board-certified headache specialist according to International Classification of Headache Disorders, 2nd Edition (ICHD-II) criteria,9 15 or more headache days per month, age 10–17 years, and a Pediatric Migraine Disability (PedMIDAS)12 score greater than 20 (indicating moderate disruption of daily activities). Exclusion criteria were medication overuse, continuous head pain throughout the 28 day baseline assessment period, current use of amitriptyline or other prophylactic migraine medication within a period equivalent to less than 5 half-lives before study screening, other chronic pain condition, such as fibromyalgia or complex regional pain syndrome II, abnormal electrocardiogram, severe orthostatic intolerance or dysregulation, documented developmental delay or impairment, severe psychiatric comorbidity, PedMIDAS greater than 140 points (indicating very severe disability), pregnancy or being sexually active without the use of a medically acceptable form of contraception, and use of disallowed medications, including opioids, antipsychotics, antimanics, barbiturates, benzodiazepines, muscle relaxants, sedatives, tramadol, or herbal products.
Study Design
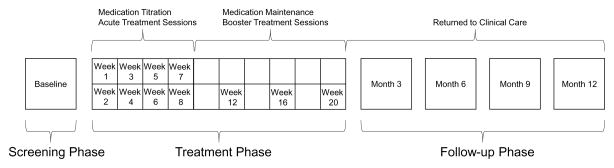
Enrolled study participants completed a baseline assessment and were instructed to keep a prospective headache diary during a 28-day screening period. Those who met the enrollment criteria after the screening period were randomized into the experimental CBT+A group or the HE+A attention control group. Treatment assignment was blinded, and both groups received the same amount of therapist contact, with only the therapist being unblinded to treatment assignment. Controlling for therapist contact emulates the purpose of pill placebo in the HE+A group.1,13,19 Participants attended 8 one-hour sessions during weeks 1 through 8, 2 “booster” sessions at weeks 12 and 16, and a post treatment visit at week 20. Sessions were conducted one-on-one. Participants who received at least one dose of treatment (completion of the first week of treatment) were considered the intent-to-treat sample and were included in the final analysis. Both groups were prescribed amitriptyline and titrated to a goal dose of 1 mg/kg/day over the initial 8-weeks of the trial. Participants were returned to standard clinical care after week 20 for medication management, and continued to receive CBT or HE booster sessions once every 3 months for a one-year follow-up period. Figure 1 details the study phases and the timing of treatment interventions.
Figure 1.
Study Phases
The study consisted of three phases, a screening phase, a treatment phase, and a follow-up phase. Visits and interventions at each phase are shown.
Outcome Measures
Subjects kept a daily headache diary throughout the screening, treatment, and follow up phases of the study (only last 28-days of each 3-month period for follow-up). Daily diaries included documentation of acute medication use, headache occurrence, headache intensity, headache duration, and associated symptoms for migraine (i.e., presence or absence of nausea, photophobia or phonophobia).
This analysis is focused on daily headache occurrence during the screening and treatment phases. Average headache frequencies during screening were analyzed to confirm that no group differences existed at the start of the treatment phase. Because the study protocol allowed for the post-treatment visit to occur within a flexible window around 20 weeks post treatment, only the first 20 weeks (140 days) of headache diaries were analyzed. Specifically, for each of the 28 baseline days, and for each of the 140 days during treatment, the proportion of participants in the CBT+A and HE+A groups that reported a headache for that day was determined (e.g., 45 of 64 participants on day X = 70%). Then the trajectory of proportion over time was statistically evaluated. Also, the proportions of participants who achieved a ≥ 50% reduction in headache days and who achieved a reduction to ≤ 4 headache days per month were calculated for each 28-day period post baseline and examined statistically for group differences (see Statistical Analysis Plan below).
Statistical Analysis
Hierarchical linear modeling (HLM) for longitudinal data involving a binary outcome18 was performed in two steps. In the first step, the most parsimonious longitudinal trend (linear, quadratic, cubic, etc.) that best modeled average changes in the binary headache occurrence across participants during the 20-week treatment phase was selected. This was accomplished by adding a lower-order trend component to the model (e.g., adding a linear fixed effect to the model), followed immediately by a test of whether that trend component showed significant variation across participants (i.e., adding a linear slope random effect to the analysis model). The next trend component was then added to the model and tested in similar fashion. Three guidelines were observed during this process: First, if a trend component fixed effect was non-significant, but the random effect for that trend component was significant, both were retained in the analysis model. Second, if a trend component fixed effect was significant, but the random effect for that trend component was not significant, only the fixed effect was retained in the analysis model. Third, this process continued until both the fixed and random effects of a given trend component were non-significant. Treatment group differences in headache frequency were tested in a second step after the overall longitudinal changes in headache frequency were parsimoniously modeled. The missing binary headache response variable data (10.9%) was handled via multilevel (or model-based) multiple imputation with M = 100 imputed datasets used for analysis.2,18,22,23,25,26
The proportion of participants who reached the clinically meaningful benchmarks of a ≥ 50% reduction in headache frequency as compared to their baseline frequency and a reduction in headache days to ≤ 4 days per month were calculated from the diary data. The proportion of participants in each group who met each criterion was calculated for each month of treatment (5 distinct periods of 28 days) as additional measures of treatment trajectory during the treatment phase. Chi-square analyses were conducted at each time point to determine if there were significant group differences between the treatment groups at each month for each criterion; The False Discovery Rate procedure was used to control Type-1 error inflation..3 All analyses were completed using Mplus18 and SPSS Version 22 (IBM Corp). A p value of < 0.05 was considered statistically significant.
Results
Study Participants
The intent-to-treat sample consisted of 135 participants, with 64 randomized to the CBT+A group and 71 to the HE+A group. Subjects were 79% female, 89% white, aged 10 to 17 years (average 14.4 ± 2 years). All participants had ≥ 15 headache days per the 28-day screening period, with an overall baseline headache frequency of 21.1 ± 5.4 days21. Over 20,000 daily individual headache records were collected during the screening and treatment phases of the study. One participant from the CBT+A group was excluded in this secondary analysis as they did not provide diaries past the first treatment visit, resulting in a sample size of 134.
Headache Frequency
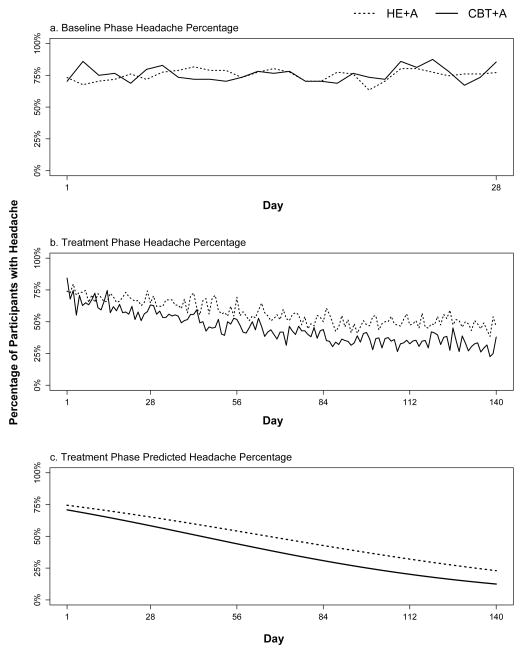
Analyses of headache frequency during the 28-day screening period prior to the start of treatment showed no significant overall changes in headache frequency (i.e., an ‘intercept-only’ longitudinal model best fit the screening data), and no significant headache frequency differences between the two treatment groups were found [See Figure 2a]. First-step analysis of the longitudinal trial data showed that the most parsimonious longitudinal headache frequency model contained: 1) significant fixed effect estimates for the intercept threshold (b = −1.84; p < 0.001; 95% CI: −2.13 to −1.15), linear slope (b = −0.02; p < 0.001; 95% CI: −0.03 to −0.01), and quadratic change term (b = 0.001, p < 0.001; 95% CI 0.001 to 0.001), and 2) significant random effects (i.e., variances) for both the intercept threshold (b = 4.345, p < 0.001; 95% CI: 2.63 to 6.05) and the linear slope (b = 0.0001, p = 0.03; 95% CI: −0.03 to −0.01). Second step analysis results showed treatment group was a significant predictor of linear slope variance (b = −0.01, p = 0.002; 95% CI: −0.02 to −0.01); the treatment group showed significantly fewer headaches over time than the control group [See Figure 2b].
Figure 2.
Headache Proportions – Baseline and Treatment Phases
a. Proportion of participants with headache on each day of screening. b. Proportion of participants with headache on each day of treatment c. Predicted proportion of participants with headache on each day of treatment.
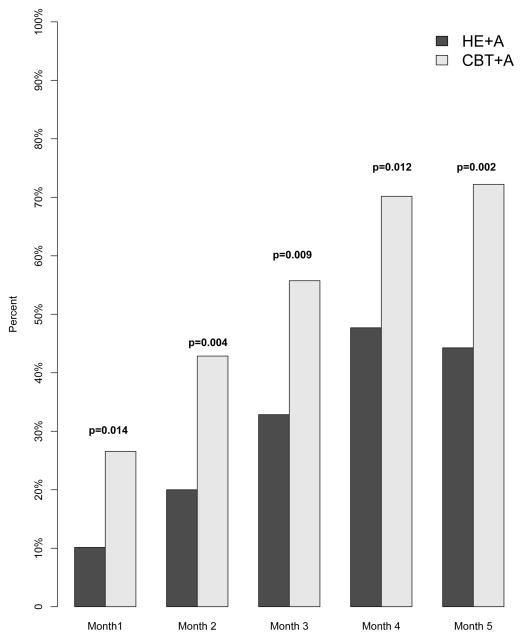
The CBT+A group had a greater proportion of participants reach the clinically meaningful benchmark of a 50% or greater reduction in headache days during each month of treatment (Month 1: CBT+A 27%, HE+A 10% p = 0.014; Month 2: CBT+A 43%, HE+A 20% p = 0.004; Month 3: CBT+A 56%, HE+A 33% p = 0.009; Month 4: CBT+A 70%, HE + A 48% p = 0.012; Month 5: CBT+A 72%, HE+A 44% p = 0.002) [See Figure 3].
Figure 3.
Percentage of Subjects with a ≥ 50% Reduction in Headache Frequency
Proportion of participants who reached the clinically meaningful endpoint of ≥ 50% reduction in headache days for each month of treatment.
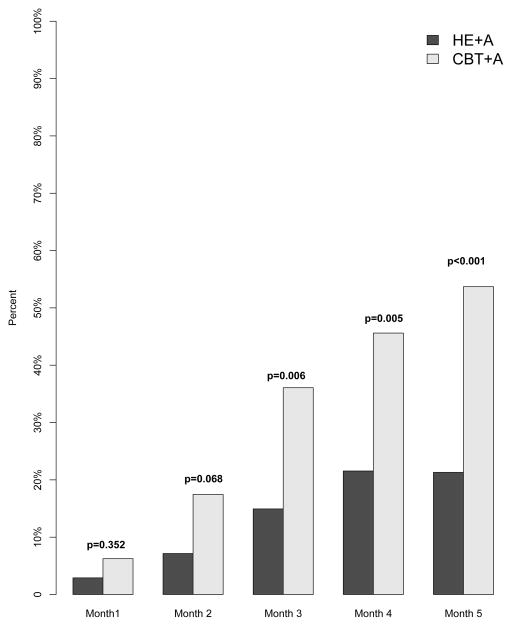
The CBT+A group had a significantly greater proportion of participants reach ≤ 4 headache days per month for months 3, 4, and 5 (Month 1: CBT+A 6%, HE+A 3% p = 0.352; Month 2: CBT+A 17%, HE+A 7% p = 0.068; Month 3: CBT+A 36%, HE+A 15% p = 0.006; Month 4: CBT+A 46%, HE + A 22% p = 0.005; Month 5: CBT+A 54%, HE+A 21% p < 0.001) [See Figure 4].
Figure 4.
Percentage of Subjects with a Reduction to ≤ 4 Headache Days
Proportion of participants who reached the clinically meaningful endpoint of a reduction to ≤ 4 headache days for each month of treatment.
Discussion
Prior research has demonstrated the effectiveness of CBT in reducing headache frequency among youth with chronic migraine, showing that participants in a CBT+A treatment group had fewer headache days after 20 weeks when compared to those in the HE+A control group.21 However, guidelines of clinically meaningful outcomes set forth by IHS may not adequately capture the expected timing of “treatment success” for all patients based on treatment trajectories and baseline headache frequencies. Thus, the current study examined the daily course of CBT treatment response to determine rates of improvement in headache days among participants, as well as improvement at each month, using the benchmarks of a ≥ 50% reduction in headache frequency and a reduction to ≤ 4 headache days per month. Results of these analyses indicate that initial decreases in headache frequency began as early as the first month of treatment for both groups and continued to decrease over the course of the treatment phase, with the CBT+A group achieving a faster rate of headache day reduction. These results further support the use of CBT as a frontline treatment for chronic migraine among children and adolescents. 5–8,14
This novel approach to modeling treatment outcomes in chronic headache in children and adolescents provides a richer context than the traditional IHS recommended clinical trial outcomes. While establishing a clear, a priori defined pre-to-post comparison is vital to proper study design, the experience during treatment can be overlooked if the focus remains solely on baseline and post treatment results. Using daily diary data allowed precise tracking of changes in headache frequency that clearly illustrates and supports the conclusions of the parent trial. The diaries also recorded information about associated headache symptoms, such as photophobia and phonophobia, but the expression of these symptoms did not differ by group (data not presented)15. Even though less frequent, headaches experienced throughout the trial were often accompanied with migraine-type features. When looking at the results aggregated by month, the trend of improvement holds. Both groups show improvement over time, with the CBT+A group showing greater improvement each month, with further reductions in headache frequency and a greater number of participants surpassing the greater than 50% reduction and reduction to ≤ 4 headache days per month benchmarks.
The potential for patients engaged in CBT for chronic migraine to exhibit improvement in headache frequency at such early stages in treatment has a wide range of potential clinical implications. Importantly, the findings from this study provide a framework for all clinicians working with youth with chronic migraine to use while making treatment recommendations, setting patient and family treatment expectations, and monitoring treatment outcomes for individual patients. For example, if a patient receiving CBT+A is not showing improvement after the first month of treatment, this may indicate that the type and course of treatment for that particular patient may need to be adjusted, requiring additional follow-up visits with their headache specialist. Alternatively, if a patient is showing improvement similar to the expected trajectory, that individual may not need to return for care within a headache specialty clinic as frequently as other patients who are not successful with CBT+A. There are also research implications for both drug and non-drug intervention studies. For example, a recent trial of amitriptyline, topiramate, and placebo for pediatric migraine over 24 weeks offers an opportunity to examine treatment response over time.20
Although the current study lends further support to the effectiveness of CBT in treating youth with chronic migraine, the mechanism by which CBT leads to reduction in headache days warrants further study. Group differences in frequency are observed early on in treatment, with the combination of CBT and amitriptyline leading to a faster trajectory of improvement starting as early as week two when compared to the HE+A control condition. At present, the relative contributions of medication versus non-pharmacologic treatment are unknown since the trial did not include a medication-only arm or a placebo pill arm. A better understanding of why CBT leads to improved outcomes, the mechanisms of CBT that produce the changes in outcomes, and the extent to which combined treatments could lead to better overall outcomes is necessary to provide significant advancement in patient care among youth with chronic migraine.
Conclusion
CBT+A continues to be considered a first line treatment for youth with chronic migraine, with results from the current study indicating that the longitudinal trajectory of decreases in headache frequency is significantly better for patients in a CBT+A condition, as compared to an HE+A attention control group. These findings provide insight into the course of when youth with chronic migraine achieve clinically meaningful outcomes. Additionally, these results provide clinicians who care for youth with migraine with a better understanding of expectations for rate of treatment gains and ways of monitoring treatment success over the course of care. This can facilitate decision making by care providers and adjustment to the patient’s regimen for those not showing success at expected time points.
Highlights.
CBT+A can significantly reduce days with headache within a couple of months
Decreases in headache days were seen as early as the 1st month of treatment
Over 70% of CBT+A participants reduced their headaches days by 50% or more
Within 5 months, over ½ of CBT+A participants had only 1 headache per week or less
Doctors and patients can set goals for treatment expectations using these finding
Acknowledgments
Funding - This work was supported by the National Institutes of Health [grant numbers R01NS050536; T32DK063929; UL1TR000077]; The authors have no conflicts of interest to report that are related to this project.
Trial Registration: clinicaltrials.gov Identifier: NCT00389038
John Kroner, Dr. Scott Powers, and Dr. James Peugh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Part of this analysis was completed as a requirement for completion of a Master’s Degree.
The authors would like to acknowledge the following individuals: Gloria Yeomans-Maldonado, BA, Ting Sa, BS, Christie Crosby, BS, Joseph Rausch, PhD, Daniel Stroman, BA, Wendy Lopez, PsyD, Anne Lynch-Jordan, PhD, Lori Crosby, PsyD, Brandon Aylward, PhD, Lisa Clifford, PhD, Sandra Cortina, PhD, Stacy Flowers, PsyD, Elizabeth Kuhl, PhD, Irina Parkins, PhD, Megan Benoit Ratcliff, PhD, Stephanie Spear Filigno, PhD, Jessica Valenzuela, PhD, Laura Bimbo Smith, PhD, Ann Segers, BS, Paula Manning, BS, Judy Bush, BS, Polly Vaughan, BS, PNP, Shannon White, PhD, Joanne Kacperski, MD, Leigh Ann Chamberlin, MEd, Maxx Somers, MS, Megan Crawford Cohen, PhD, Stephanie Sullivan, BS, Samantha Morgan, BS, Kim Barnett, BS, Erin Brannon, MS, Jen Hembree, AS, Kerry Simmons, BS, Leslie Korbee, BS, and Judy Bean, PhD (all from Cincinnati Children’s Hospital). Other than the grant funding from the National Institutes of Health that supported the work of Drs. Aylward, Clifford, Kuhl, Ratcliff, Filigno, Valenzuela, Smith, Cohen, and Bean, Mss. Chamberlin, Sullivan, Morgan, Barnett, Brannon, Hembree, Simmons, and Korbee, and Mr. Somers, there was no other compensation for each person listed as contributors. We also thank the Cincinnati Children’s Headache Center, Divisions of Behavioral Medicine and Clinical Psychology and Neurology, and the Research Foundation Office for Clinical and Translational Research.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00389038
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrasik F, Powers SW, McGrath PJ. Methodological considerations in research with special populations: children and adolescents. Headache. 2005;45:520–5. doi: 10.1111/j.1526-4610.2005.05104.x. [DOI] [PubMed] [Google Scholar]
- 2.Asparouhov T, Muthén B. Multiple imputation with Mplus. MPlus Web Notes. 2010 [Google Scholar]
- 3.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 4.Bigal ME, Lipton RB, Tepper SJ, Rapoport AM, Sheftell FD. Primary chronic daily headache and its subtypes in adolescents and adults. Neurology. 2004;63:843–7. doi: 10.1212/01.wnl.0000137039.08724.18. [DOI] [PubMed] [Google Scholar]
- 5.Eccleston C, Palermo TM, de CWAC, Lewandowski A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2012;12:CD003968. doi: 10.1002/14651858.CD003968.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eccleston C, Palermo TM, Williams AC, Lewandowski A, Morley S. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2009:CD003968. doi: 10.1002/14651858.CD003968.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Eccleston C, Palermo TM, Williams AC, Lewandowski Holley A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2014;5:CD003968. doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eccleston C, Yorke L, Morley S, Williams AC, Mastroyannopoulou K. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2003:CD003968. doi: 10.1002/14651858.CD003968. [DOI] [PubMed] [Google Scholar]
- 9.Headache Classification Subcommittee of the International Headache S. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 10.Hershey AD, Powers SW, Bentti AL, Degrauw TJ. Effectiveness of amitriptyline in the prophylactic management of childhood headaches. Headache. 2000;40:539–49. doi: 10.1046/j.1526-4610.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 11.Hershey AD, Powers SW, Bentti AL, LeCates S, deGrauw TJ. Characterization of chronic daily headaches in children in a multidisciplinary headache center. Neurology. 2001;56:1032–7. doi: 10.1212/wnl.56.8.1032. [DOI] [PubMed] [Google Scholar]
- 12.Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034–9. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- 13.Holroyd KA, Powers SW, Andrasik F. Methodological issues in clinical trials of drug and behavior therapies. Headache. 2005;45:487–92. doi: 10.1111/j.1526-4610.2005.05100.x. [DOI] [PubMed] [Google Scholar]
- 14.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, Passo MH, Schikler KN, Hashkes PJ, Spalding S, Lynch-Jordan AM, Banez G, Richards MM, Lovell DJ. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum. 2012;64:297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroner J. Associated Symptoms of Chronic Migraine in Children and Adolescents. University of Cincinnati; 2015. [Google Scholar]
- 16.Kroner JW, Hershey AD, Kashikar-Zuck SM, LeCates SL, Allen JR, Slater SK, Zafar M, Kabbouche MA, O’Brien HL, Shenk CE, Rausch JR, Kroon Van Diest AM, Powers SW. Cognitive Behavioral Therapy plus Amitriptyline for Children and Adolescents with Chronic Migraine Reduces Headache Days to </=4 Per Month. Headache. 2016;56:711–6. doi: 10.1111/head.12795. [DOI] [PubMed] [Google Scholar]
- 17.Lipton RB, Manack A, Ricci JA, Chee E, Turkel CC, Winner P. Prevalence and burden of chronic migraine in adolescents: results of the chronic daily headache in adolescents study (C-dAS) Headache. 2011;51:693–706. doi: 10.1111/j.1526-4610.2011.01885.x. [DOI] [PubMed] [Google Scholar]
- 18.Muthén B, Muthén L. Mplus software. 1998–2015. [Google Scholar]
- 19.Penzien DB, Andrasik F, Freidenberg BM, Houle TT, Lake AE, 3rd, Lipchik GL, Holroyd KA, Lipton RB, McCrory DC, Nash JM, Nicholson RA, Powers SW, Rains JC, Wittrock DA. Guidelines for trials of behavioral treatments for recurrent headache, first edition: American Headache Society Behavioral Clinical Trials Workgroup. Headache. 2005;45(Suppl 2):S110–32. doi: 10.1111/j.1526-4610.2005.4502004.x. [DOI] [PubMed] [Google Scholar]
- 20.Powers SW, Coffey CS, Chamberlin LA, Ecklund DJ, Klingner EA, Yankey JW, Korbee LL, Porter LL, Hershey AD Investigators C. Trial of Amitriptyline, Topiramate, and Placebo for Pediatric Migraine. N Engl J Med. 2016 doi: 10.1056/NEJMoa1610384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers SW, Kashikar-Zuck SM, Allen JR, LeCates SL, Slater SK, Zafar M, Kabbouche MA, O’Brien HL, Shenk CE, Rausch JR, Hershey AD. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA. 2013;310:2622–30. doi: 10.1001/jama.2013.282533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer JL. Multiple imputation with PAN. 2001. [Google Scholar]
- 23.Schafer JL, Yucel RM. Computational strategies for multivariate linear mixed-effects models with missing values. Journal of computational and Graphical Statistics. 2002;11:437–457. [Google Scholar]
- 24.Tfelt-Hansen P, Pascual J, Ramadan N, Dahlof C, D’Amico D, Diener HC, Hansen JM, Lanteri-Minet M, Loder E, McCrory D, Plancade S, Schwedt T. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia. 2012;32:6–38. doi: 10.1177/0333102411417901. [DOI] [PubMed] [Google Scholar]
- 25.Van Buuren S. Multiple imputation of multilevel data. Handbook of advanced multilevel analysis. 2011:173–196. [Google Scholar]
- 26.Van Buuren S. Flexible imputation of missing data. CRC press; 2012. [Google Scholar]