Abstract
Members of the Natural resistance-associated macrophage protein (NRAMP) family are evolutionarily-conserved metal ion transporters that play an essential role in regulating intracellular divalent cation homeostasis in both prokaryotes and eukaryotes. Malvolio (Mvl), the sole NRAMP family member in insects, plays a role in food choice behaviors in Drosophila and other species. However, the specific physiological and cellular processes that require the action of Mvl for appropriate feeding decisions remain elusive. Here we demonstrate that normal food choice requires Mvl function specifically in the dopaminergic system, and can be rescued by supplementing food with manganese. Collectively, our data indicate that the action of the Mvl transporter affects food choice behavior via the regulation of dopaminergic innervation of the mushroom bodies, a principle brain region associated with decision making in insects. Our studies suggest that the homeostatic regulation of the intra-neuronal levels of divalent cations plays an important role in the development and function of the dopaminergic system and associated behaviors.
Keywords: Mvl, Nramp, Slc11a, Ionomics, Dopamine, Manganese, Food choice, D. melanogaster, fruit fly, Vinegar fly
INTRODUCTION
Correct food choices are essential for the survival and fitness of all animals. However, the genetic, neuronal, and environmental factors that regulate these basal behaviors are still not well understood. Previous studies in Drosophila melanogaster indicated that flies carrying a mutation in the gene Malvolio (Mvl), the insect homolog of the evolutionary-conserved protein family of Natural Resistant Associated Macrophage Proteins (NRAMPs) divalent metal ions transporters (D’souza et al., 1999), lead to a reduced appetitive discrimination between high and low sugar-containing foods (Rodrigues et al., 1995). The effects of the Mvl mutation on food choice behavior could be rescued by supplementing food with manganese or iron (Orgad et al., 1998). In addition, studies in the Honey bee Apis mellifera showed that expression levels of Mvl in the brain differ between bees, which perform different tasks, and are associated with changes in the behavioral response threshold to sugar stimuli in bees. Similarly, feeding manganese to young, pre-foraging bees leads to a lower response threshold to sugar stimuli, and a precocious transition to foraging behavior (Ben-Shahar et al., 2004, Sovik et al., 2015). The effect of the Mvl mutation on taste behavior is not due to abnormal gustatory sensory signaling. These data suggest that the effects of Mvl mutations on food choice are mediated via central elements of the feeding decision neuronal circuit or non-neuronal tissues (Rodrigues et al., 1995). NRAMP genes are also expressed in the nervous systems of vertebrates, but their physiological roles in these tissues are not well understood (Evans et al., 2001, Ke et al., 2005, Skjorringe et al., 2015).
In addition to the genetic support for the role of Mvl in regulating feeding, other studies indicated that trace metals such as manganese and iron contribute to the regulation of organismal energy homeostasis via diverse mechanisms. For example, manganese can act as an insulin-mimetic in vitro and in vivo in mammalian models via unknown mechanisms (Nakai et al., 2005). Similarly, environmental exposure to manganese, iron and other trace metals has been implicated in the development of both Type I and Type II diabetes (Meyer & Spence, 2009), and genetic variations in the human SLC11A1 (NRAMP1) gene are associated with the development of Type I diabetes in humans (Yang et al., 2011). Independently, iron deficiency has been implicated in obesity (García et al., 2009, Mcclung & Karl, 2009). Manganese and iron are also essential for mitochondrial respiration and cellular metabolism (Chen et al., 2015b, Levi & Rovida, 2009, Mena et al., 2015, Mühlenhoff et al., 2015, Pierrel et al., 2007). Together, these studies by us and others indicate that metal transporters play an important role in regulating diverse cognitive and metabolic functions in animals, and suggest that disruptions in the function of such transporters could lead to abnormal behavioral outcomes (Ávila et al., 2014, Dusek et al., 2015, Roels et al., 2012, Sovik et al., 2015, Su et al., 2015). Consequently, here we used the power of Drosophila genetics to investigate the cellular and molecular pathways that may explain the effects of Mvl on behavioral feeding decisions and organismal metabolic homeostasis.
MATERIALS AND METHODS
Fly stocks and maintenance
Flies were reared on standard Drosophila cornmeal medium at 25°C and 60% humidity under a 12h:12h light/dark cycle. GAL4 lines, UAS-Dcr2, UAS-Mvl-RNAi, and UAS-tetanus toxin (TNT) lines were from the Bloomington Drosophila Stock Center (Bloomington, IN).
Behavioral experiments
The single-dye feeding decisions paradigm was as previously described by us and others (Lu et al., 2012, Orgad et al., 1998). In short, the “feeding error index” represents the proportion of flies with red abdomens relative to total number of flies in a single assay. Since single-dye feeding assays cannot discriminate between flies that made a correct feeding choice and flies that did not eat at all (both will have no dye in the abdomen), we also measured the effects of genotypes and treatments on the general feeding drive of flies by allowing groups of animals to feed on plates in which all wells had 10mM trehalose with the same red dye as in feeding choice assays.
Determination of the elemental composition of fly tissues by inductively coupled plasma mass spectrometry (ICP-MS)
Four independent samples of 10 pooled fly heads per genotype were analyzed for B, Na, Mg Al, P, S, K, Ca, Mn, Fe, Co, Cu, Zn, As, Se, Rb, Mg, and Cd. ICP-MS analyses followed the standard Baxter laboratory protocol as previously described (Ziegler et al., 2013).
Quantification of biogenic amines from fly brains using high pressure liquid chromatography (HPLC)
Adult fly brains were dissected in chilled PBS. Samples consisting of ten pooled brains were flash-frozen in liquid nitrogen and stored at −80°C until analysis. Biogenic amine content was extracted in 15μl of 0.2M perchloric acid containing 10pg/μl of the HPLC standard dihydroxybenzylamine (Sigma-Aldrich, St. Louis, MO, USA) as previously described (Scheiner et al., 2014, Søvik et al., 2013). The supernatant of each sample separated across an HR-80 column (ESA Biosciences, inc., Chelmsford, MA, USA) using an Agilent 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA). Levels of biogenic amines were quantified using an ESA Coulochem III electrochemical detector with an ESA 5011A analytical cell. Standard curves for octopamine, dopamine, and serotonin (Sigma-Aldrich, St. Louis, MO) were used to calculate the total amount of each amine in the tested samples.
Quantification of mRNA expression levels by real-time qRT-PCR
Total RNA was extracted from pools of ~30 adult heads using the TRIzol reagent (Thermo Fisher Scientific). First strand cDNA was prepared using SuperScript II reverse transcriptase (Thermo Fisher Scientific). Gene specific assays were conducted with a SYBR Green kit on a 7500 Real-Time PCR System (Applied Biosystems). Each cDNA sample was run in triplicate PCR reactions. The housekeeping gene rp49 was used as a loading control. Relative expression data were analyzed and presented as expression fold-difference by using the ΔΔCT method, as we have previously described (Lu et al., 2012, Lu et al., 2014, Sovik et al., 2015, Zheng et al., 2014).
Immunohistochemistry
Antibody staining of dopaminergic neurons was as previously described (Lu et al., 2012, Lu et al., 2014, Zelle et al., 2013, Zheng et al., 2014). Brains were co-stained with anti-GFP (Invitrogen) and nc82 antibodies (Developmental Studies Hybridoma Bank, Iowa City, IA), and mounted using the VECTASHIELD HardSet Antifade Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA). Imaging was performed using Nikon A1 confocal microscope. Figures were generated from maximal intensity Z-stacks using the Nikon NIS-Elements software package.
Statistical analyses
Feeding behavioural data and brain ionomics were analysed with two-way Student’s t-tests (StatPlus, AnalystSoft Inc.). All values for the statistical tests are described in Tables S1–S5. Biogenic amines levels were analyzed by a linear mixed model with genotype as a categorical factor. HPLC analysis batch was controlled for in the model as well. Dry weight and glucose levels were analysed by one-way ANOVA followed by a Tukey posthoc test (P<0.05).
RESULTS
Mvl is required in neuronal tissues for normal food choice behavior
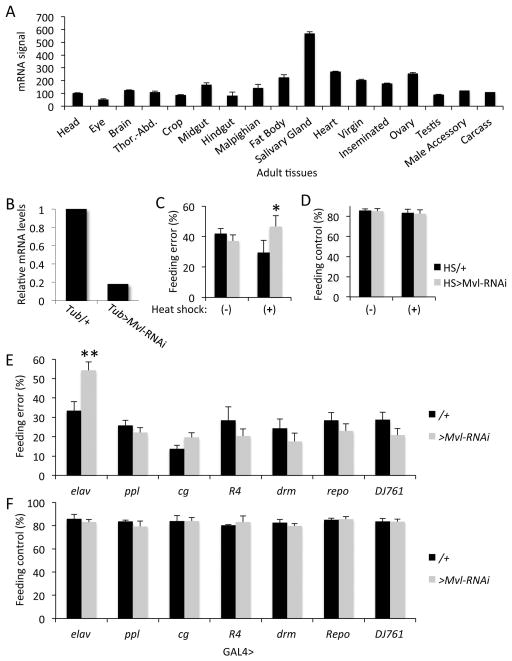
Analysis of the spatial Mvl mRNA expression data from the FlyAtlas database (Robinson et al., 2013) indicated that it is broadly expressed throughout the adult body (Fig. 1A). Therefore, to identify the specific tissues in which Mvl is required for feeding decisions, we employed a tissue-specific RNAi-based gene knockdown approach (Brand & Perrimon, 1993). To test whether the available UAS-Mvl-RNAi line can effectively knockdown endogenous mRNAs, we first demonstrated the effect of the UAS-Mvl-RNAi on the global expression of Mvl with the ubiquitous αTub84B-GAL4 line (Fig. 1B).
Fig. 1.
Tissue specific role for Mvl in feeding decisions. (A) Tissue distribution of Mvl expression in adult Drosophila. Data are from FlyAtlas (Robinson et al., 2013). Error bars represent SEM (n=4 independent microarrays). (B) Global ectopic expression of transgenic Mvl-RNAi with the tubulin GAL4 driver reduces Mvl expression. (C) Conditional Mvl gene knockdown in the adult stage with the heat-shock-induced Hsp70 GAL4 driver(‘−’, without heat shock; ‘+’, with heat shock). One-way ANOVA; *, P<0.05. (D) General feeding-drive control experiment. Genotypes and conditions as in C. (E) Tissue specific Mvl knockdown. (F) General feeding-drive control experiment. Genotypes and conditions as in E. (see Table S1 for statistics).
Previous studies indicated that the abnormal feeding phenotype in Mvl mutant flies can be rescued on a physiological timescale with manganese or iron food supplements (Orgad et al., 1998). However, because Mvl is broadly expressed throughout development (not shown), it is still impossible to know whether the behavioral phenotype of adult mutants is due, at least in part, to abnormal developmental processes. Therefore, we investigated the impact of global Mvl knockdown on feeding decisions specifically in the adult stage by expressing the UAS-Mvl-RNAi with the conditional heat-induced hsp70-GAL4 driver. We found that under heat-shock conditions, adult flies exhibited abnormal feeding behavior (Fig. 1C and Tables S1–S2). These data further suggest that the effects of the Mvl gene knockdown on behavior are not solely dependent on post-embryonic development.
Because Mvl is broadly expressed (Fig. 1A), we next used specific organ-enriched GAL4 drivers to investigate whether the effects of Mvl knockdown on food choice behavior are tissue-specific. The tissue-level RNAi screen revealed that Mvl expression in the nervous system (elav-GAL4) is necessary for normal food choice behavior (Fig. 1D). In contrast, expressing the Mvl-RNAi with GAL4 drivers enriched in the fat body (ppl), hemocytes and fat body (cg), salivary glands and fat body (R4), gut (drm), glia (repo), or everywhere outside the nervous system (DJ761), did not affect feeding decisions (Fig. 1D and Table S1). Together, these data indicate that normal feeding decisions require Mvl activity in the nervous system. We did not observe any effects of the Mvl gene knockdown on general feeding drive in any of the tested genotypes (Fig. 1F and Table S2).
The effect of neuronal Mvl knockdown on behavior can be rescued by manganese
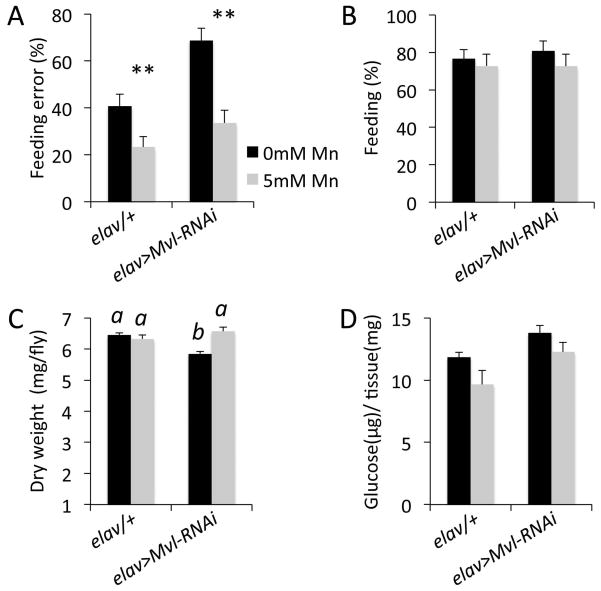
Previous work showed that manganese supplements can rescue the effect of the Mvl mutation on feeding decisions (Orgad et al., 1998). Therefore, we next asked whether the effect of neuronal-specific Mvl knockdown on feeding behavior could be rescued by a manganese supplement as well. We found that supplementing fly food with 5mM Mn2+ was sufficient to rescue the effects of the neuronal Mvl RNAi downregulation on feeding decisions (Fig 2A). However, neither genetic background nor Mn2+ treatment had an effect on the overall feeding drive of adult flies (Fig. 2B). We also found that the Mn2+ supplement rescued a small, but significant, decrease in total dry body weight observed in flies with the pan-neuronal Mvl-knockdown (Fig. 2C), but not in total glucose content (Fig. 2D), which possibly reflects the increased consumption of non-nutritive food in Mvl knockdown flies. Together, our data suggest that Mvl-dependent regulation of manganese in neuronal tissues plays a role in food choice behaviors in the fly. These data also indicate that manganese, and possibly other divalent cations, can enter cells via alternative, NRAMP-independent pathways as has been reported in other model systems (Chen et al., 2015a, Garcia-Rodriguez et al., 2015, Jenkins et al., 2016).
Fig. 2.
Manganese food supplement rescues effects of neuronal Mvl knockdown on behavior. (A) Feeding manganese rescues the effect of neuronal Mvl knock down on food choice behavior. **, t-test, t15 = 5.1628, p < 0.01. (B) General feeding-drive control experiment. Genotypes and conditions as in A. (C) Effect of neuronal Mvl knockdown and manganese treatment on total dry weight. Different letters above bars represent significantly different groups (ANOVA, p<0.05, Tukey posthoc test). (D) Effect of neuronal Mvl knockdown and manganese on glucose levels (ANOVA, NS).
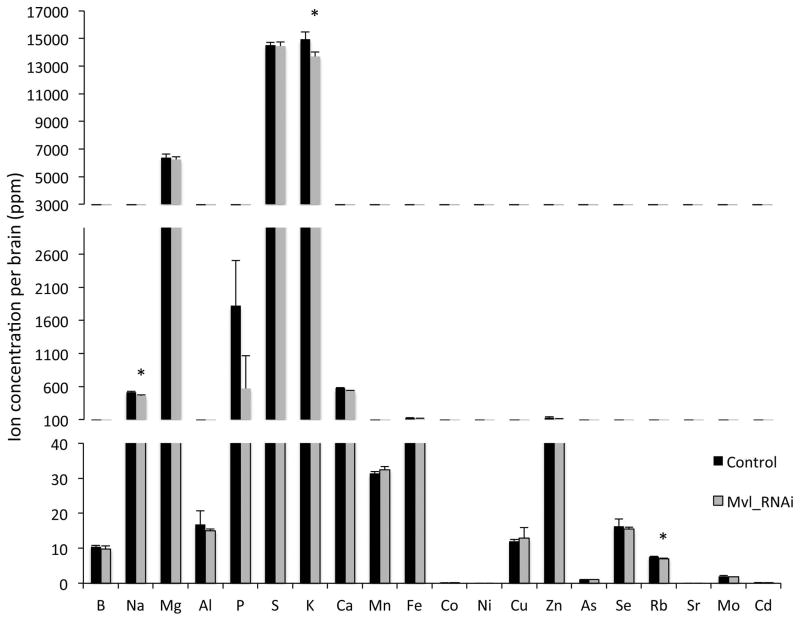
Effects of the neuronal Mvl knockdown on the head ionome
Because Mvl is a divalent metal ion transporter, we next examined the elemental composition of fly heads in elav>Mvl-RNAi flies relative to wild type controls. Surprisingly, we found that levels of Mn2+, Fe2+, and Cu2+, which are ions known to be transported by Mvl (Ben-Shahar et al., 2004, Bettedi et al., 2011, Folwell et al., 2006, Orgad et al., 1998, Southon et al., 2008), were not affected by the neuronal Mvl knockdown (Fig. 2 and Table S3). In contrast, levels of Na+, K+, and Rb+, three elements known to have similar chemical properties, showed a small but significant reduction in the Mvl knockdown animals (Fig. 3 and Table S3). These data indicate that the neuronal-specific knockdown of Mvl is not sufficient to impact the overall metal homeostasis of trace metals, possibly because levels of these metals in the hemolymph and non-neuronal tissues in the head mask neuronal-specific changes. Whether the small effects of the neuronal Mvl knockdown on the levels of other ions are directly related to the transporter activity of Mvl, or whether they represent indirect, downstream effects of the knockdown on other ionic transport pathways was not investigated further in the current study.
Fig. 3.
Effect of neuronal Mvl knockdown on the head ionome. The elemental metal composition of fly heads in neuronal Mvl knockdown and wild type control flies (n=4; *, p<0.05; see Table S3 for statistics).
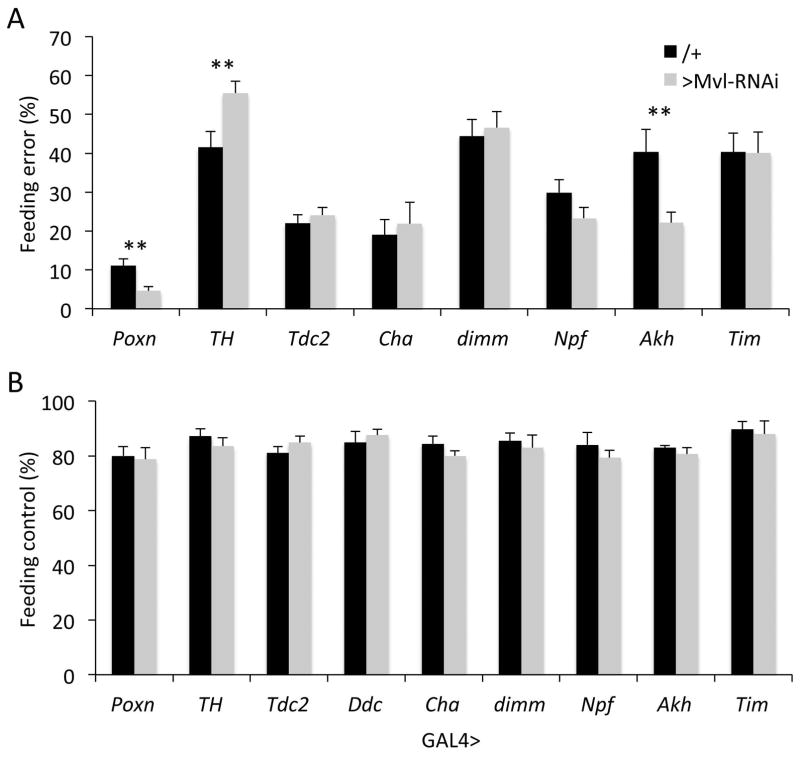
Mvl expression in the dopaminergic system is required for normal food choice behavior
Feeding decisions in all animals, including flies, are driven by complex neuronal circuits, and depend on the integration of diverse internal and external signals (Albin et al., Flood et al., 2013, Huetteroth et al., 2015b, Itskov & Ribeiro, 2013, Ledue et al., 2015, Perisse et al., 2016, Yapici et al.). To further narrow the possible neuronal populations that require Mvl function for normal feeding decisions, we next drove the Mvl-RNAi construct with GAL4 drivers that are active in specific neuronal subpopulation that have been previously reported to play a role in feeding decisions. Our screen included drivers specific to gustatory receptor (Poxn) neurons (Dahanukar et al., 2001), dopaminergic (TH) neurons (Marella et al., 2012a, Melcher & Pankratz, 2005), octopaminergic (Tdc2) neurons (Zhang et al., 2013), cholinergic (Cha) neurons (Yapici et al.), neuropeptidergic (dimm) neurons (Kahsai et al., 2010, Taghert & Nitabach, 2012, Zhang et al., 2013), Neuropeptide F-expressing (Npf) neurons (Shen & Cai, 2001), Adipokinetic hormone-expressing (Akh)neurons (Kim & Neufeld, 2015), and the circadian system (Tim)(Diangelo et al., 2011). This limited screen revealed that Mvl knockdown specifically in dopaminergic neurons (TH-GAL4; Fig. 4A and Table S4) phenocopied the abnormal food choice phenotype we observed in animals with pan-neuronal Mvl knockdown (Fig. 1E). In contrast, expression of the Mvl-RNAi in all gustatory receptor neurons (Poxn) or neurons that express the neuropeptide adipokinetic hormone (Akh), which have been previously implicated in the regulation of feeding and metabolism (Itskov & Ribeiro, 2013, Pool & Scott, 2014), showed a small but significant decrease in feeding error rates relative to wild type controls (Fig. 4A and Table S4). Unexpectedly, these data indicate that Mvl knockdown has different effects on feeding decisions in different neuronal elements of the feeding decision circuit. Regardless, Mvl gene knockdown in any of the neuronal populations we have examined did not affect the general feeding drive of adult flies, further confirming that the effects observed represents an abnormal decision making phenotype rather than simply observing the behavior of unhealthy flies (Fig. 4B and Table S5).
Fig. 4.
Effect of Mvl knockdown in specific neuronal populations. (A) Feeding choice assay. GAL4 drivers: Poxn, all gustatory receptor neurons; TH, dopaminergic neurons; Tdc2, octopaminergic neurons; Cha, cholinergic neurons; dimm, neuropeptidergic neurons; Npf, Neuropeptide F neurons; Akh, Adipokinetic hormone neurons; Tim, circadian neurons. (B) General feeding-drive control experiment. Genotypes as in A. See Tables S4 and S5 for statistics.
Effects of neuronal Mvl knockdown on behavior are not mediated via overall changes in biogenic amine synthesis
Because several of the metal ions that were previously shown to be transported by Mvl function as co-factors in neurotransmitter synthesis, and because of the effect of Mvl knockdown in dopaminergic neurons on food choice behavior, we next investigated the effects of Mvl-RNAi expression on the syntheses of biogenic amines in the fly brain. Using HPLC, we measured levels of dopamine, serotonin, and octopamine from the brains of pan-neuronal Mvl knockdown and wild type flies. Our data show that the overall brain levels of the three primary biogenic amines in the insect brain, dopamine, octopamine and serotonin (Evans, 1980, Mesce, 2002), were not affected by neuronal Mvl knockdown (Fig. 5A). These data suggest that the effects of Mvl knockdown on behavior are not a direct consequence of dramatic physiological changes in the brain biogenic amines systems.
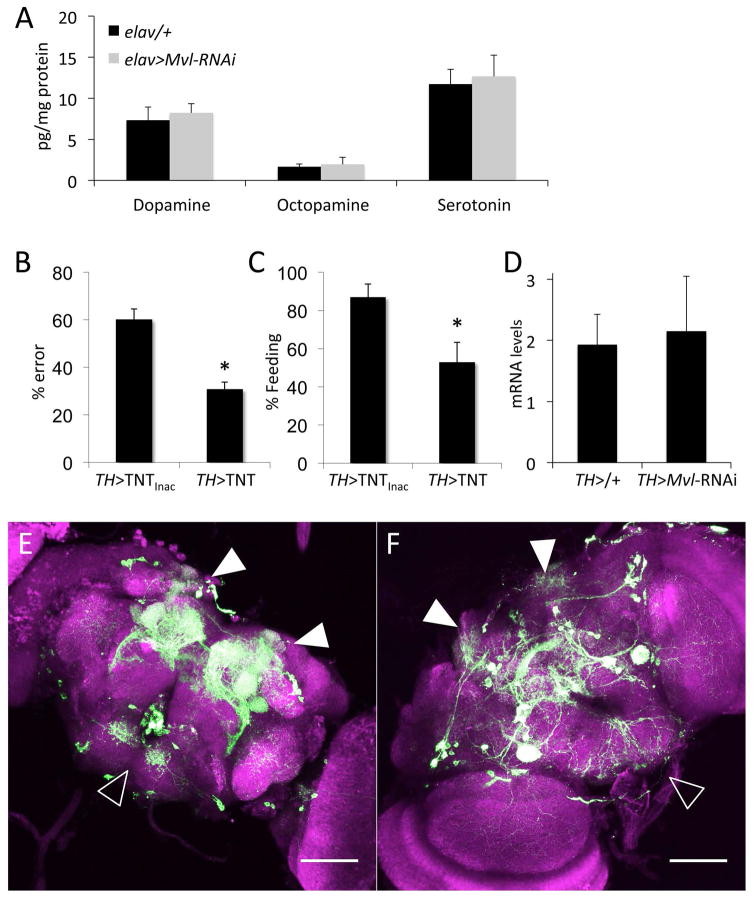
Fig. 5.
Effects of Mvl knockdown on the dopaminergic system. (A) HPLC analyses of biogenic amines levels. Dopamine, t34=−0.9463, NS; Octopamine, t34=−0.8967, NS; Serotonin, t31=−0.4124, NS. (B) Effect of blocking dopaminergic synaptic release with the ectopic expression of the tetanus toxin (TNT) on feeding errors (t21 = 4.8833, p < 0.001). (C) Effect of blocking dopaminergic synaptic release with the ectopic expression of the tetanus toxin on feeding rate (t11 = 2.668, p = 0.02187). (D) Effect of dopaminergic Mvl knockdown mRNA expression levels of ple (TH>Mvl-RNAi). (E) Dopaminergic projection patterns in wild type brains (TH-GAL4>UAS-CD8::GFP). (F) Effect of dopaminergic Mvl knockdown on dopaminergic neuronal projection patterns (TH-GAL4>UAS-CD8::GFP//UAS-Mvl-RNAi). In E and F, solid arrowheads mark innervations of the mushroom bodies, and empty arrowhead mark innervations of the suboesophageal ganglion (SOG).
Since Mvl knockdown in dopaminergic neurons does not seem to affect overall dopamine synthesis, we next asked whether the effects of dopaminergic Mvl knockdown are due to a reduced dopamine release. Previous studies indicated that disruptions of the dopaminergic signaling pathway can lead to a general low appetite (hypophagia) (Krashes et al., 2009, Riemensperger et al., 2011). In agreement with the earlier findings, we found that blocking dopamine release by the ectopic expression of the tetanus toxin (TNT) with the dopaminergic TH-GAL4 driver resulted in general hypophagia (Fig. 5B–C), which is unlike the phenotype we observed in Mvl knockdown animals. We also found that the mRNA levels of ple (the gene encoding TH) were not affected by the expression of the Mvl-RNAi in the dopaminergic system. Together, these data further indicate that the effects of Mvl knockdown in the dopaminergic system on food choice behavior are not likely to be directly mediated via modulation of dopamine synthesis or release (Fig. 5D).
Since Mvl activity does not seem to directly affect dopamine synthesis or release, we also examined the effects of the Mvl knockdown on dopaminergic neuron morphology. As in others animals, the dopaminergic system in the fly is complex and heterogeneous. Previous studies have identified the protocerebral anterior medial (PAM) neuronal cluster, which innervates the mushroom bodies, as the primary modulatory pathways for the integration of food-rewards in long-term memory (Huetteroth et al., 2015a, Liu et al., 2012). Independently, the activity of TH-VUM, a single dopaminergic neuron that innervates the subesophageal ganglion (SOG) taste center, was shown to regulate the appetitive acceptance of sugar rewards (Marella et al., 2012b). To test whether Mvl knockdown affected dopaminergic morphology, we expressed a membrane tethered GFP (UAS-CD8::GFP) with the TH-GAL4 line, with or without the UAS-Mvl-RNAi. These studies revealed that that Mvl knockdown animals exhibit a dramatic reduction in the dopaminergic innervation of both the mushroom bodies and the SOG (Figs. 5F and S2B). By contrast, control animals show extensive innervation of the mushroom bodies in the protocerebrum and TH-VUM signal in the SOG (Figs. 5E and S2A). To further establish a causal link between the action of Mvl in the dopaminergic system and food-choice behaviors, we also attempted to study dopaminergic neuronal morphology and food choice behavior in flies carrying the previously published Mvl97f allele (D’souza et al., 1999, Orgad et al., 1998, Rodrigues et al., 1995). However, in our hands, the observed phenotype of flies carrying this allele in two different genetic backgrounds was weak and inconsistent (not shown). Nevertheless, our findings suggest that Mvl activity in dopaminergic neurons play a critical role in modulating synaptic connectivity in the dopaminergic system, which possibly affects specific neuronal pathways associated with food choice decisions.
DISCUSSION
In recent years, studies of the dopaminergic system across animal phyla have indicated that it plays a conserved, important role in regulating cognitive processes associated with decision making in general, and feeding decisions in particular (Barron et al., 2015, De Bivort & Van Swinderen, 2016, Khani & Rainer, 2016, Stopper & Floresco, 2015). Consequently, the highly conserved cellular and biochemical properties of the dopaminergic system across mammals and invertebrates indicate that mechanistic insights gained here are likely relevant to neuronal and behavioral functions of metal transporters in many other animal species, including humans.
We have previously demonstrated that chronic feeding of wild type flies and honey bees with manganese increased the overall brain levels of dopamine, and adversely affected bee foraging decisions (Sovik et al., 2015). We have interpreted these published data to suggest that the effects of environmental exposure to manganese were mediated via a direct effect on the dopaminergic synthesis pathway. Therefore, we originally expected that the effects of knocking down Mvl, an established manganese transporter, on feeding behavior will be associated with changes in dopaminergic levels as well. Our finding that the adverse behavioral outcomes of dopaminergic Mvl knockdown are not associated with overall changes in dopamine levels could be possibly explained by the insensitivity of currently available assays to very small changes in dopamine levels. Alternatively, these data could indicate that the interaction between the dopaminergic system and metal ion homeostasis is more complex than we originally expected, and may independently affect brain functions at both the developmental and physiological time scales. Nonetheless, our finding that Mvl knockdown in dopaminergic neurons changes their innervation patterns further supports this alternative interpretation.
Previous studies demonstrated that the dopaminergic system in the fly brain is remodeled at the pupal stage during metamorphosis (Budnik & White, 1988). However, we still do not know whether the effect of Mvl knockdown on the morphology and innervation patterns of dopaminergic neurons is due to its requirement at the pupal neuronal remodeling stage, or whether it reflects an interaction between Mvl activity and post-pupal neuronal plasticity. In addition, although the focus of the current study is on the interaction between Mvl and the dopaminergic system, we cannot exclude a possible effect of Mvl knockdown on the morphology of other neuronal classes, and therefore, on other, dopamine-independent behavioral phenotypes.
We found that knocking down Mvl expression specifically in the dopaminergic system is sufficient to phenocopy the pan-neuronal knockdown phenotype. We interpret these data to suggest that, in agreement with previous studies, the dopaminergic system acts as a “master switch”, which determines the probability that an animal will respond behaviorally to an appetitive stimulus. The concept of a dopaminergic “master switch” is further supported by the contrary effects of Mvl knockdown specifically in the gustatory sensory system and Npf-expressing neurons, which resulted in an increase in the appetitive preference for sugar relative to control animals. However, to fully understand how Mvl-dependent changes in dopaminergic innervation affect feeding-related decision making behaviors will require the identification and characterization of the relevant neuronal elements that receive inputs from Mvl-expressing dopaminergic neurons. Nevertheless, these data suggest that Mvl, and its possible effects on metal ion homeostasis, play independent roles in different elements of the neuronal circuit associated with feeding decisions.
Data presented here strongly support the idea that metal ion homeostasis in specific neuronal circuits could have a dramatic impact on neuronal functions and behavior. The conserved biochemical properties of the molecules involved in metal ion homeostasis across distant phylogenetic clades indicate that insights gained in the current studies are likely conserved in other animal species, including humans.
Supplementary Material
Acknowledgments
This study was funded by a Children’s Discovery Institute grant MD-II-2009-170 to JGD and YB-S, NIH R21NS089834 to YB-S. We thank members of the Ben-Shahar laboratory for their helpful comments on earlier versions of the manuscript. We thank Ivan Baxter and Greg Ziegler of the USDA-ARS Ionomic Profiling Facility at the Donald Danforth Plant Science Center for assistance with elemental analysis.
References
- Albin Stephanie D, Kaun Karla R, Knapp J-M, Chung P, Heberlein U, Simpson Julie H. A Subset of Serotonergic Neurons Evokes Hunger in Adult Drosophila. Current Biology. 25:2435–2440. doi: 10.1016/j.cub.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Ávila D, Puntel R, Folmer V, Rocha J, dos Santos A, Aschner M. Manganese Neurotoxicity. In: Kostrzewa RM, editor. Handbook of Neurotoxicity. Springer; New York: 2014. pp. 843–864. [Google Scholar]
- Barron AB, Gurney KN, Meah LF, Vasilaki E, Marshall JA. Decision-making and action selection in insects: inspiration from vertebrate-based theories. Front Behav Neurosci. 2015;9:216. doi: 10.3389/fnbeh.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar Y, Dudek NL, Robinson GE. Phenotypic deconstruction reveals involvement of manganese transporter malvolio in honey bee division of labor. J Exp Biol. 2004;207:3281–3288. doi: 10.1242/jeb.01151. [DOI] [PubMed] [Google Scholar]
- Bettedi L, Aslam MF, Szular J, Mandilaras K, Missirlis F. Iron depletion in the intestines of Malvolio mutant flies does not occur in the absence of a multicopper oxidase. J Exp Biol. 2011;214:971–978. doi: 10.1242/jeb.051664. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Budnik V, White K. Catecholamine-containing neurons in Drosophila melanogaster: Distribution and development. The Journal of Comparative Neurology. 1988;268:400–413. doi: 10.1002/cne.902680309. [DOI] [PubMed] [Google Scholar]
- Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, Aschner M. Manganese homeostasis in the nervous system. J Neurochem. 2015a;134:601–610. doi: 10.1111/jnc.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Chakraborty S, Peres TV, Bowman AB, Aschner M. Manganese-induced neurotoxicity: from C. elegans to humans. Toxicology Research. 2015b;4:191–202. doi: 10.1039/C4TX00127C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza J, Cheah PY, Gros P, Chia W, Rodrigues V. Functional complementation of the malvolio mutation in the taste pathway of Drosophila melanogaster by the human natural resistance-associated macrophage protein 1 (Nramp-1) J Exp Biol. 1999;202:1909–1915. doi: 10.1242/jeb.202.14.1909. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- de Bivort BL, van Swinderen B. Evidence for selective attention in the insect brain. Curr Opin Insect Sci. 2016;15:9–15. doi: 10.1016/j.cois.2016.02.007. [DOI] [PubMed] [Google Scholar]
- DiAngelo JR, Erion R, Crocker A, Sehgal A. The central clock neurons regulate lipid storage in Drosophila. Plos One. 2011;6:e19921. doi: 10.1371/journal.pone.0019921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek P, Roos PM, Litwin T, Schneider SA, Flaten TP, Aaseth J. The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements. 2015;31:193–203. doi: 10.1016/j.jtemb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Evans C, Harbuz M, Ostenfeld T. Nramp1 is expressed in neurones and is associated with behavioural and immune responses to stress. Neurogenetics. 2001;3:69–78. doi: 10.1007/s100480100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PD. Biogenic Amines in the Insect Nervous System. In: Berridge MJ, JET, Wigglesworth VB, editors. Advances in Insect Physiology. Academic Press; 1980. pp. 317–473. [Google Scholar]
- Flood TF, Iguchi S, Gorczyca M, White B, Ito K, Yoshihara M. A single pair of interneurons commands the Drosophila feeding motor program. Nature. 2013;499:83–87. doi: 10.1038/nature12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folwell JL, Barton CH, Shepherd D. Immunolocalisation of the D. melanogaster Nramp homologue Malvolio to gut and Malpighian tubules provides evidence that Malvolio and Nramp2 are orthologous. J Exp Biol. 2006;209:1988–1995. doi: 10.1242/jeb.02193. [DOI] [PubMed] [Google Scholar]
- García OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutrition reviews. 2009;67:559–572. doi: 10.1111/j.1753-4887.2009.00228.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez N, Manzano-Lopez J, Munoz-Bravo M, Fernandez-Garcia E, Muniz M, Wellinger RE. Manganese redistribution by calcium-stimulated vesicle trafficking bypasses the need for P-type ATPase function. J Biol Chem. 2015;290:9335–9347. doi: 10.1074/jbc.M114.616334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huetteroth W, Perisse E, Lin S, Klappenbach M, Burke C, Waddell S. Sweet Taste and Nutrient Value Subdivide Rewarding Dopaminergic Neurons in Drosophila. Current Biology. 2015a;25:751–758. doi: 10.1016/j.cub.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huetteroth W, Perisse E, Lin S, Klappenbach M, Burke C, Waddell S. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr Biol. 2015b;25:751–758. doi: 10.1016/j.cub.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskov PM, Ribeiro C. The dilemmas of the gourmet fly: the molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front Neurosci. 2013;7:12. doi: 10.3389/fnins.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J, Papkovsky DB, Dmitriev RI. The Ca2+/Mn2+-transporting SPCA2 pump is regulated by oxygen and cell density in colon cancer cells. Biochem J. 2016;473:2507–2518. doi: 10.1042/BCJ20160477. [DOI] [PubMed] [Google Scholar]
- Kahsai L, Kapan N, Dircksen H, Winther AM, Nassel DR. Metabolic stress responses in Drosophila are modulated by brain neurosecretory cells that produce multiple neuropeptides. Plos One. 2010;5:e11480. doi: 10.1371/journal.pone.0011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Chang YZ, Duan XL, Du JR, Zhu L, Wang K, Yang XD, Ho KP, Qian ZM. Age-dependent and iron-independent expression of two mRNA isoforms of divalent metal transporter 1 in rat brain. Neurobiol Aging. 2005;26:739–748. doi: 10.1016/j.neurobiolaging.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Khani A, Rainer G. Neural and neurochemical basis of reinforcement-guided decision making. J Neurophysiol. 2016;116:724–741. doi: 10.1152/jn.01113.2015. [DOI] [PubMed] [Google Scholar]
- Kim J, Neufeld TP. Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nat Commun. 2015;6:6846. doi: 10.1038/ncomms7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDue EE, Chen YC, Jung AY, Dahanukar A, Gordon MD. Pharyngeal sense organs drive robust sugar consumption in Drosophila. Nat Commun. 2015;6:6667. doi: 10.1038/ncomms7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Rovida E. The role of iron in mitochondrial function. Biochimica et biophysica acta. 2009;1790:629–636. doi: 10.1016/j.bbagen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- Lu B, LaMora A, Sun Y, Welsh MJ, Ben-Shahar Y. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 2012;8:e1002587. doi: 10.1371/journal.pgen.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Zelle KM, Seltzer R, Hefetz A, Ben-Shahar Y. Feminization of pheromone-sensing neurons affects mating decisions in Drosophila males. Biology Open. 2014:152–160. doi: 10.1242/bio.20147369. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012a;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Mann K, Scott K. Dopaminergic Modulation of Sucrose Acceptance Behavior in Drosophila. Neuron. 2012b;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JP, Karl JP. Iron deficiency and obesity: The contribution of inflammation and diminished iron absorption. Nutrition reviews. 2009;67:100–104. doi: 10.1111/j.1753-4887.2008.00145.x. [DOI] [PubMed] [Google Scholar]
- Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. Plos Biol. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena NP, Urrutia PJ, Lourido F, Carrasco CM, Núñez MT. Mitochondrial iron homeostasis and its dysfunctions in neurodegenerative disorders. Mitochondrion. 2015;21:92–105. doi: 10.1016/j.mito.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Mesce KA. Metamodulation of the biogenic amines: second-order modulation by steroid hormones and amine cocktails. Brain Behav Evol. 2002;60:339–349. doi: 10.1159/000067793. [DOI] [PubMed] [Google Scholar]
- Meyer Ja, Spence DM. A perspective on the role of metals in diabetes: past findings and possible future directions. Metallomics: integrated biometal science. 2009;1:32. [Google Scholar]
- Mühlenhoff U, Hoffmann B, Richter N, Rietzschel N, Spantgar F, Stehling O, Uzarska MA, Lill R. Compartmentalization of iron between mitochondria and the cytosol and its regulation. European Journal of Cell Biology. 2015;94:292–308. doi: 10.1016/j.ejcb.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Nakai M, Sekiguchi F, Obata M, Ohtsuki C, Adachi Y, Sakurai H, Orvig C, Rehder D, Yano S. Synthesis and insulin-mimetic activities of metal complexes with 3-hydroxypyridine-2-carboxylic acid. Journal of Inorganic Biochemistry. 2005;99:1275–1282. doi: 10.1016/j.jinorgbio.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Orgad S, Nelson H, Segal D, Nelson N. Metal ions suppress the abnormal taste behavior of the Drosophila mutant malvolio. J Exp Biol. 1998;201:115–120. doi: 10.1242/jeb.201.1.115. [DOI] [PubMed] [Google Scholar]
- Perisse E, Owald D, Barnstedt O, Talbot CB, Huetteroth W, Waddell S. Aversive Learning and Appetitive Motivation Toggle Feed-Forward Inhibition in the Drosophila Mushroom Body. Neuron. 2016;90:1086–1099. doi: 10.1016/j.neuron.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrel F, Cobine Pa, Winge DR. Metal Ion availability in mitochondria. Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine. 2007;20:675–682. doi: 10.1007/s10534-006-9052-9. [DOI] [PubMed] [Google Scholar]
- Pool AH, Scott K. Feeding regulation in Drosophila. Curr Opin Neurobiol. 2014;29:57–63. doi: 10.1016/j.conb.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemensperger T, Isabel G, Coulom H, Neuser K, Seugnet L, Kume K, Iche-Torres M, Cassar M, Strauss R, Preat T, Hirsh J, Birman S. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc Natl Acad Sci U S A. 2011;108:834–839. doi: 10.1073/pnas.1010930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SW, Herzyk P, Dow JA, Leader DP. FlyAtlas: database of gene expression in the tissues of Drosophila melanogaster. Nucleic Acids Res. 2013;41:D744–750. doi: 10.1093/nar/gks1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues V, Cheah PY, Ray K, Chia W. malvolio, the Drosophila homologue of mouse NRAMP-1 (Bcg), is expressed in macrophages and in the nervous system and is required for normal taste behaviour. EMBO J. 1995;14:3007–3020. doi: 10.1002/j.1460-2075.1995.tb07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels HA, Bowler RM, Kim Y, Claus Henn B, Mergler D, Hoet P, Gocheva VV, Bellinger DC, Wright RO, Harris MG, Chang Y, Bouchard MF, Riojas-Rodriguez H, Menezes-Filho JA, Tellez-Rojo MM. Manganese exposure and cognitive deficits: a growing concern for manganese neurotoxicity. Neurotoxicology. 2012;33:872–880. doi: 10.1016/j.neuro.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner R, Toteva A, Reim T, Søvik E, Barron AB. Differences in the phototaxis of pollen and nectar foraging honey bees are related to their octopamine brain titers. Frontiers in Physiology. 2014;5:1–8. doi: 10.3389/fphys.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, Cai HN. Drosophila neuropeptide F mediates integration of chemosensory stimulation and conditioning of the nervous system by food. Journal of Neurobiology. 2001;47:16–25. doi: 10.1002/neu.1012. [DOI] [PubMed] [Google Scholar]
- Skjorringe T, Burkhart A, Johnsen KB, Moos T. Divalent metal transporter 1 (DMT1) in the brain: implications for a role in iron transport at the blood-brain barrier, and neuronal and glial pathology. Front Mol Neurosci. 2015;8:19. doi: 10.3389/fnmol.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southon A, Farlow A, Norgate M, Burke R, Camakaris J. Malvolio is a copper transporter in Drosophila melanogaster. J Exp Biol. 2008;211:709–716. doi: 10.1242/jeb.014159. [DOI] [PubMed] [Google Scholar]
- Søvik E, Cornish JL, Barron AB. Cocaine tolerance in honey bees. Plos One. 2013;8:e64920. doi: 10.1371/journal.pone.0064920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovik E, Perry CJ, LaMora A, Barron AB, Ben-Shahar Y. Negative impact of manganese on honeybee foraging. Biology letters. 2015:11. doi: 10.1098/rsbl.2014.0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB. Dopaminergic circuitry and risk/reward decision making: implications for schizophrenia. Schizophr Bull. 2015;41:9–14. doi: 10.1093/schbul/sbu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Chen K, Zou Y, Shen Y, Xia B, Liang G, Lv Y, Wang F, Huang D, Yang X. Chronic exposure to manganese sulfate leads to adverse dose-dependent effects on the neurobehavioral ability of rats. Environ Toxicol. 2015 doi: 10.1002/tox.22161. [DOI] [PubMed] [Google Scholar]
- Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JHM, Downes K, Howson JMM, Nutland S, Stevens HE, Walker NM, Todd Ja. Evidence of association with type 1 diabetes in the SLC11A1 gene region. BMC medical genetics. 2011;12:59. doi: 10.1186/1471-2350-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N, Cohn R, Schusterreiter C, Ruta V, Vosshall Leslie B. A Taste Circuit that Regulates Ingestion by Integrating Food and Hunger Signals. Cell. 165:715–729. doi: 10.1016/j.cell.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelle KM, Lu B, Pyfrom SC, Ben-Shahar Y. The genetic architecture of degenerin/epithelial sodium channels in Drosophila. G3. 2013;3:441–450. doi: 10.1534/g3.112.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Branch A, Shen P. Octopamine-mediated circuit mechanism underlying controlled appetite for palatable food in Drosophila. Proc Natl Acad Sci U S A. 2013;110:15431–15436. doi: 10.1073/pnas.1308816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Valakh V, DiAntonio A, Ben-Shahar Y. Natural antisense transcripts regulate the neuronal stress response and excitability. eLife. 2014;3:e01849. doi: 10.7554/eLife.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G, Terauchi A, Becker A, Armstrong P, Hudson K, Baxter I. Ionomic Screening of Field-Grown Soybean Identifies Mutants with Altered Seed Elemental Composition. The Plant Genome. 2013:6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.