Abstract
Background
Studies examining associations between circulating concentrations of C-peptide and total adiponectin, two biomarkers related to obesity and insulin secretion and sensitivity and pancreatic ductal adenocarcinoma (PDA) risk have shown inconsistent results and included limited numbers of smokers.
Methods
We examined associations of these biomarkers and high molecular weight (HMW) adiponectin with PDA, overall, and by smoking status. We conducted a pooled nested case-control analysis in 3 cohorts (Prostate, Lung, Colorectal, and Ovarian Cancer Trial, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, and Cancer Prevention Study-II), with 758 cases (435 current smokers) and 1052 controls (531 smokers) matched by cohort, age, sex, race, blood draw date and follow-up time. We used conditional logistic regression adjusted for age, smoking, diabetes, and body mass index to calculate odds ratios (OR) and 95% confidence intervals (CI).
Results
Circulating C-peptide concentration was not associated with PDA in never or former smokers, but was inversely associated with PDA in current smokers (per standard deviation OR=0.67, 95% CI 0.54, 0.84, p-interaction=0.005). HMW adiponectin was inversely associated with PDA in never smokers (OR=0.43, 95% CI 0.23, 0.81), not associated in former smokers, and positively associated in smokers (OR=1.23, 95% CI 1.04, 1.45, p-interaction=0.009). Total adiponectin was not associated with PDA in nonsmokers or current smokers.
Conclusion
Associations of biomarkers of insulin secretion and sensitivity with PDA differ by smoking status. Smoking-induced pancreatic damage may explain the associations in smokers while mechanisms related to insulin resistance explain associations in non-smokers.
Impact
Future studies of these biomarkers and PDA should examine results by smoking status.
Keywords: C-peptide, adiponectin, smoking, pancreatic cancer, epidemiology
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer death in the United States (US)(1). While cancer incidence and mortality rates have been declining in the US during the past decade, pancreatic cancer incidence and mortality rates have increased (1). The majority of pancreatic cancers are pancreatic ductal adenocarcinomas (PDA) (2). Diabetes, obesity, and smoking are known risk factors for PDA (3–5). Large epidemiologic analyses have shown obesity is associated with higher risk of PDA in nonsmokers, however this association is weaker or absent in current smokers (4–6), suggesting obesity-related mechanisms may be of greater importance in the etiology of PDA in nonsmokers.
The specific biological mechanisms responsible for the association between obesity and PDA remain unclear but may involve insulin resistance (7,8), which is strongly related to obesity (9). One hypothesized mechanism is that insulin resistance precipitates a compensatory increase in insulin secretion (7,8), that directly increases risk of PDA. Insulin is a mitogen that has growth promoting effects on PDA cells (10), and circulating insulin concentration has been associated with greater PDA of in two prospective studies (7,8). The potential importance of insulin resistance in pancreatic carcinogenesis is also supported by the consistent association between type 2 diabetes, which is typically preceded by insulin resistance (9), and PDA (11)
Insulin resistance is associated with higher circulating concentrations of C-peptide (12) and lower circulating concentrations of adiponectin (13). C-peptide and insulin are synthesized together in equimolar amounts by pancreatic β-cells but C-peptide has a longer half-life than insulin and is therefore a more stable biomarker of pancreatic endocrine function, and may be a better measure of insulin secretion over time (14). Adiponectin is secreted by adipocytes in three different sub-forms, of which high-molecular-weight (HMW) adiponectin is believed to be the primary biologic active form.(15,16) Higher adiponectin concentration is associated with both lower insulin resistance and lower adiposity (13,17). Based on their relationships with insulin resistance, high circulating concentrations of C-peptide and low circulating concentrations of adiponectin would be expected to be associated with increased PDA risk. However, results from previous studies evaluating the association of C-peptide and adiponectin concentrations with PDA are inconsistent (18–20). A possible explanation is smoking might modify the associations between these biomarkers and risk of pancreatic cancer.
We examined the association of pre-diagnostic circulating concentrations of C-peptide, adiponectin, and HMW adiponectin with PDA using a large pooled analysis of three prospective studies. Because epidemiologic evidence suggests obesity-related mechanisms for pancreatic cancer may be of greater importance in nonsmokers than in smokers (4–6,21), we were particularly interested in examining these associations by smoking status.
MATERIAL AND METHODS
Cohorts
This is a pooled nested case control study that includes data from the Alpha-Tocopherol, Beta Carotene Cancer Prevention (ATBC) study(22), the Cancer Prevention Study-II Nutrition Cohort (CPS-II)(23), and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO).(24) Details of each study have been previously described.(22–25) All individuals within their respective studies provided informed consent. Each study was approved by its local institutional review boards (IRB), specifically the Emory University IRB for CPS-II(23) and the National Cancer Institute Special Studies IRB for the PLCO and ATBC cohorts. Additionally, the PLCO study was approved by the IRBs of its 10 participating screening centers and the ATBC study was approved by the IRB at the National Public Health Institute in Finland.
Briefly, the ATBC study included approximately 29,000 Finnish male smokers, ages 50 to 69, who provided a blood sample after an overnight fast prior to randomization between 1985 and 1988 (22). The CPS-II study, enrolled in 1992–93, included a subset of approximately 40,000 men and women who provided a non-fasting blood sample between 1998 and 2001 (23). The screening arm of the PLCO study included approximately 77,000 men and women, ages 55 to 74, who provided a blood sample at enrollment between 1993 and 2001 (24). Fasting blood samples were not required in PLCO, but many participants may have been fasting at the time of blood draw because they were scheduled for a colorectal endoscopy later the same day as part of the trial protocol.
Data on lifestyle, demographics and possible confounders were collected from questionnaires at baseline from each cohort (22–25). For the present study, information was obtained from each cohort on sex, age, race, body mass index (BMI), cigarette smoking history, self-reported diabetes, diet, and alcohol use and the data were harmonized.
Case ascertainment and selection of controls
Cases were incident primary pancreatic adenocarcinomas (ICD-O-3 code C250-C259 or C25.0-C25.3, C25.7-C25.9 and ICD-9 157.0). Endocrine pancreatic tumors (C25.4, histology type, 8150, 8151, 8153, 8155, and 8240, 157.4) were excluded as the etiology of these cancers is thought to be different. Case ascertainment varied between studies but included linking participants to cancer registries (ATBC, CPS-II), self- and next-of-kin reports (PLCO, CPS-II) (23,24), and use of national death indices (ATBC, PLCO, and CPS-II) (22–25). The interval between serum collection and diagnosis was up to 22.8, 7.4 and 14.5 years for the ATBC (22), CPS-II (23) and PLCO (24) studies, respectively.
On the date of cancer case diagnosis, matched controls were alive and free from PDA. One matched control was selected for each case in CPS-II, while in ATBC and PLCO one or two controls were selected for each case. Controls were matched to cases on age at blood draw (±5 years), gender, and date of blood draw (within 30 days for ATBC and within 2–3 month blocks for PLCO and CPS-II), and race.
Measurement of biomarkers
Serum C-peptide, adiponectin, and high molecular weight (HMW) adiponectin concentrations were measured in Dr. Michael Pollak’s laboratory (Lady Davis Institute for Medical Research, Montreal Quebec, Canada) using an enzyme-linked immunosorbent assay (ELISA). C-peptide and total adiponectin were measured on 758 cases and 1052 controls (ATBC 377 cases, 493 controls; CPS-II 90 cases, 90 controls; PLCO 291 cases, 469 controls). HMW adiponectin was measured on 351 ATBC and 90 CPS-II matched case-control sets. We did not measure HMW adiponectin on PLCO participants. Total and HMW adiponectin were analyzed with reagents from R&D Systems Inc. (McKinley Place NE, Minneapolis, MN) and C-peptide was analyzed with reagents from ALPCO Diagnostics (Keewaydin Drive, Salem, NH). The same internal laboratory control sample was run in each batch. The internal control C-peptide concentrations were used as a reference to correct for differences between batches, across studies, and periods of time. Case and control samples were handled in the same manner and the laboratory was blinded to case-control status. Matched case-control samples were analyzed consecutively within batches and blinded replicate quality control samples were placed in triplicate towards the beginning and end of each batch and comprised 10% of each batch. Coefficients of variation were 11.6% for C-peptide, 9.5 % for HMW adiponectin, and 5.5% for total adiponectin.
Statistical analysis
Medians, inter-decile ranges, and proportions of selected characteristics were determined for the cases and controls overall and by each cohort (Table 1). Least square means adjusted for age, gender, and cohort for each biomarker (Table 2) were calculated for age, cohort, gender, smoking history, BMI, and diabetes using mixed linear models.
Table 1.
Baseline characteristics of cases and controls (mean and inter-decile range or number and proportion) overall and by cohorta
| Cohorts Combined | ATBC | CPS-II | PLCO | |||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
| (n = 758) | (N = 1,052) | (N = 377) | (N = 493) | (n = 90) | (N = 90) | (n = 291) | (N = 469) | |
| Age, years | ||||||||
| Blood Draw | 62 (53, 71) | 62 (53, 71) | 57 (51, 65) | 57 (52, 64) | 70 (63, 76) | 70 (63, 77) | 65 (58, 71) | 66 (58, 72) |
| Diagnosis | 71 (62, 79) | 69 (60, 77) | 73 (65, 81) | 72 (64, 80) | ||||
| Follow up, years | 8.2 (1.8, 17.2) | -- | 10.9 (2.9, 19.8) | -- | 2.9 (0.5, 5.9) | -- | 7.7 (2.0, 11.9) | -- |
| Males | 603 (79.6) | 835 (79.4) | 377 (100) | 493 (100) | 47 (52.2) | 47 (52.2) | 179 (61.5) | 295 (62.9) |
| Race | ||||||||
| White | 726 (95.8) | 1004 (95.4) | 377 (100) | 493 (100) | 87 (96.7) | 88 (97.8) | 262 (90.0) | 423 (90.2) |
| Black | 9 (1.2) | 14 (1.3) | --- | --- | --, | --- | 9 (3.1) | 14 (3.0) |
| Asian | 16 (2.1) | 25 (2.4) | --- | --- | 1 (1.1) | 1 (1.1) | 15 (5.2) | 24 (5.1) |
| Other | 7 (0.9) | 9 (0.9) | --- | --- | 2 (2.2) | 1 (1.1) | 5 (1.7) | 8 (1.7) |
| Smoking | ||||||||
| Never | 157 (20.7) | 267 (25.4) | --- | --- | 45 (50.0) | 44 (48.9) | 112 (38.5) | 223 (47.5) |
| Former | 166 (21.9) | 254 (24.1) | --- | --- | 40 (44.4) | 45 (50.0) | 126 (43.3) | 209 (44.6) |
| Current | 435 (57.4) | 531 (50.5) | 377 (100) | 493 (100) | 5 (5.6) | 1 (1.1) | 53 (18.2) | 37 (7.9) |
| BMI categoriesc | ||||||||
| Normal | 277 (36.5) | 380 (36.1) | 137 (36.3) | 186 (37.7) | 42 (46.7) | 30 (33.3) | 98 (33.7) | 164 (35.0) |
| Overweight | 332 (43.8) | 481 (45.7) | 173 (45.9) | 228 (46.2) | 35 (38.9) | 39 (43.3) | 124 (42.6) | 214 (45.6) |
| Obese | 146 (19.3) | 186 (17.7) | 67 (17.8) | 79 (16.0) | 13 (14.4) | 18 (20.0) | 66 (22.7) | 89 (19.0) |
| BMI, Kg/m2 | 26.2 (22.1, 32.0) | 26.3 (21.9, 31.7) | 26.2 (22.1, 31.7) | 26.1 (21.9, 31.1) | 25.5 (21.7, 31.3) | 26.4 (21.0, 33.7) | 26.6 (22.3, 32.6) | 26.6 (22.0, 32.2) |
| Diabetes | 72 (9.5) | 75 (7.1) | 23 (6.1) | 29 (5.9) | 13 (14.4) | 8 (8.9) | 36 (12.4) | 38 (8.1) |
| Daily intake | ||||||||
| Alcohol, g | 6.1 (0, 41.6) | 5.3 (0, 40.7) | 10.5 (0, 46.6) | 10.9 (0, 46.3) | 3.7 (0, 28.6) | 2.3 (0, 27.9) | 1.5 (0, 40.4) | 1.0 (0, 32.3) |
| Total fat, g | 88.3 (39.9, 154.9) | 88.6 (40.6, 155.7) | 116 (74.8, 170.2) | 117.2 (74.1, 172) | 52.8 (34.5, 98.2) | 54.3 (34.4, 99.8) | 62.0 (60.8, 110.3) | 62.4 (33.1, 119.2) |
| Saturated fat, g | 31.6 (12.9, 67.8) | 31.0 (12.9, 68.6) | 49.4 (28.6, 79.9) | 49.6 (27.3, 80.1) | 17.1 (11.2, 31.5) | 18.0 (10.2, 31.1) | 21.1 (10.2, 37.9) | 20.7 (10.9, 41.1) |
Continuous measures show mean (10–90th %). 441 cases and 441 controls were included in the HMW adiponectin analysis and 758 cases and 1052 controls were included in the adiponectin and C-peptide analyses
HMW = High Molecular Weight Adiponectin
BMI = Body Mass Index. BMI was calculated by dividing measured weight (kg) by height squared (m2) and categorized according to the World Health Organization obesity classifications as less than 25 (normal), 25 to 30 (overweight), and 30 kg/m2 or more (obese). BMI includes 755 cases and 1047 controls due to missing data. BMI is missing from 3 PLCO cases and 3 CPS-2 and 2 PLCO controls.
Table 2.
Adjusted mean (standard error, SE) biomarker concentrations in case and control participants by selected baseline characteristics
| C-Peptide | HMW Adiponectin | Adiponectin | ||||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | |
| Mean ng/ml (SE) | Mean ng/ml (SE) | Mean µg/mL (SE) | Mean µg/mL (SE) | Mean µg/mL (SE) | Mean µg/mL (SE) | |
| Age, yearsa, b | ||||||
| < 65 | 2.66 (0.11) | 2.73 (0.11) | 7.12 (0.43) | 7.44 (0.45) | 10.74 (0.33) | 11.79 (0.28) |
| ≥ 65 | 2.63 (0.11) | 2.89 (0.12) | 7.87 (0.51) | 7.91 (0.53) | 12.05 (0.34) | 12.01 (0.28) |
| Gender within cohortc, d | ||||||
| ATBC men | 1.78 (0.10) | 2.14 (0.10) | 5.47 (0.25) | 4.81 (0.25) | 8.25 (0.30) | 7.99 (0.25) |
| PLCO men | 3.18 (0.13) | 2.90 (0.12) | - | -- | 8.30 (0.39) | 8.37 (0.29) |
| PLCO women | 2.64 (0.16) | 2.71 (0.15) | -- | 13.3 (0.48) | 13.51 (0.36) | |
| CPS-II men | 3.84 (0.27) | 3.87 (0.30) | 3.70 (0.78) | 5.43 (0.78) | 7.98 (0.81) | 10.95 (0.74) |
| CPS-II women | 3.17 (0.27) | 3.14 (0.30) | 8.64 (0.75) | 9.76 (0.73) | 14.59 (0.80) | 16.42 (0.74) |
| Smoking within cohortc, e | ||||||
| ATBC current smoker | 1.79 (0.10) | 2.16 (0.10) | 5.38 (0.26) | 4.74 (0.26) | 8.12 (0.32) | 7.86 (0.26) |
| PLCO never or former smoker | 3.01 (0.12) | 2.79 (0.10) | -- | -- | 10.15 (0.37) | 10.44 (0.26) |
| PLCO current smoker | 2.80 (0.23) | 3.06 (0.31) | -- | -- | 11.03 (0.74) | 9.51 (0.83) |
| CPS-II never or former smoker | 3.53 (0.21) | 3.41 (0.23) | 6.37 (0.64) | 7.82 (0.63) | 11.33 (0.66) | 13.85 (0.59) |
| BMIa, b, c | ||||||
| Normal | 2.16 (0.11) | 2.22 (0.11) | 9.12 (0.43) | 8.48 (0.43) | 12.89 (0.33) | 13.35 (0.28) |
| Overweight | 2.70 (0.11) | 2.77 (0.11) | 6.12 (0.41) | 7.10 (0.42) | 10.27 (0.32) | 11.22 (0.27) |
| Obese | 3.40 (0.15) | 3.92 (0.15) | 5.22 (0.54) | 5.43 (0.53) | 9.05 (0.43) | 9.78 (0.36) |
| Diabetesa, b, c | ||||||
| No | 2.61 (0.09) | 2.70 (0.09) | 7.15 (0.37) | 7.39 (0.37) | 11.28 (0.26) | 11.88 (0.22) |
| Yes | 2.90 (0.21) | 3.74 (0.23) | 5.33 (0.83) | 5.97 (0.84) | 8.77 (0.61) | 9.76 (0.56) |
| Alcohol Intakea, b, c | ||||||
| None | 2.75 (0.17) | 2.89 (0.16) | 7.01 (0.71) | 7.38 (0.62) | 10.80 (0.52) | 11.34 (0.40) |
| >0 to 3 drinks/day | 2.65 (0.09) | 2.75 (0.10) | 6.95 (0.38) | 7.14 (0.39) | 11.08 (0.28) | 11.71 (0.24) |
| >3 drinks/day | 2.46 (0.22) | 2.64 (0.22) | 8.64 (0.73) | 7.16 (0.76) | 11.76 (0.64) | 11.75 (0.53) |
Least square means adjusted for cohort
Least square means adjusted for gender
Least square means adjusted for age
Combinations of gender and cohort are shown because the ATBC cohort included only men.
Combinations of smoking and cohort are shown because the ATBC cohort included only smokers, CPS-II current smokers not shown due to small numbers.
Overall odds ratios and 95% confidence intervals were calculated using conditional logistic regression. The biomarkers were examined as continuous variables, using sex-specific 1 standard deviation (SD) units and as quintiles (Q) based on the distribution among controls with the lowest quintile as the referent. Trend tests were based on a continuous variable for each biomarker. We evaluated potential confounding by entering individual variables into the overall model. Variables were included in the model if they changed risk estimates ≥ 10% or were established risk factors for PDA. Our final model included age, smoking status, BMI, and self-reported diabetes.
Effect modification by sex, smoking status, BMI (< 25 and ≥ 25), cohort, cancer diagnosis age (≤70 and > 70 years), diabetes, and follow-up time was evaluated with stratified analyses and tested for statistical significance with a multiplicative interaction term between the continuous biomarker variable and aforementioned categorical variables. The smoking and BMI stratified analyses used unconditional logistic regression adjusted for the matching factors (sex, cohort) beyond the full model. In the smoking stratified analyses, we excluded participants with missing BMI as there were too few subjects in this category for the models to converge.
All statistical analyses were performed using Statistical Analysis Systems (SAS) software versions 9.3 (SAS Institute, Inc., Cary, North Carolina) and statistical tests were two-tailed.
RESULTS
Overall, there were no differences between cases and controls in the distribution of BMI, but the proportion of current smokers was higher in cases than in controls. Within CPS-II and PLCO, cases more often had a history of diabetes than controls (Table 1).
Table 2 shows the adjusted mean biomarker concentration among cases and controls according to demographic and lifestyle characteristics. Females had higher HMW and total adiponectin than males. ATBC participants, all of whom provided fasting samples, had lower C-peptide compared to other cohort participants. Among cases, the adjusted mean C-peptide concentration in ATBC and current smoker PLCO participants was lower than non-smokers in PLCO and CPS-II (there were too few current smokers in CPS-II for informative analyses). Current smoker cases also had lower C-peptide and higher HMW and total adiponectin compared to current smoker controls. Among controls, current smokers had lower HMW and total adiponectin. Participants who reported diabetes had higher C-peptide and lower HMW and total adiponectin. Cases with diabetes had lower c-peptide concentrations compared to controls with diabetes.
We describe the association between the biomarkers and PDA using continuous per standard deviation unit risk estimates (Table 3). The associations for C-peptide and HMW adiponectin with PDA were modified by smoking status (P-interaction = 0.005 and 0.008, respectively). C-peptide was inversely associated with PDA (OR = 0.67, 95% CI 0.54, 0.84) in current smokers, but not in never (OR=0.97, 95% CI 0.77, 1.21) or former (OR=1.08, 95% CI 0.89, 1.29) smokers. HMW adiponectin was inversely associated with PDA among never smokers (OR=0.43, 95% CI 0.23, 0.81), positively associated in current smokers (OR=1.23, 95% CI 1.04, 1.45), and not associated among former smokers (OR=0.97, 95% CI 0.60, 1.58). Total adiponectin tended to be inversely associated with PDA among never smokers (OR=0.81, 95% CI 0.66, 1.00) but was not associated in the other groups (P-interaction=0.076). Adiponectin and HMW adiponectin were weakly correlated with c-peptide (r2 < 0.20). Mutual adjustment of the biomarkers in models did not substantially change the risk estimates. Adjusting for smoking intensity, duration, and cumulative duration did not change the association in smokers. Similar patterns of associations and interactions were observed for C-peptide and HMW adiponectin when former and never smokers were combined into a category of nonsmokers (Supplemental Table 1). Results from meta-analyses of the biomarkers were similar to that obtained from the pooled analysis (data not shown).
Table 3.
OR and 95% CI for pancreatic cancer by quintile of baseline serum c-peptide, HMW adiponectin, and adiponectin overall and by smoking status
| By Smoking Statusb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overalla |
Never Smoker |
Former Smoker |
Current Smoker |
|||||||
| Cases / Controls |
Crude OR 95% CI |
OR 95% CI | Cases / Controls |
OR 95% CI | Cases / Controls |
OR 95% CI | Cases / Controls |
OR 95% CI | P interaction | |
| C Peptide | ||||||||||
| Q1c | 174 / 210 | 1.00 (ref) | 1.00 (ref) | 19 / 40 | 1.00 (ref) | 21 / 28 | 1.00 (ref) | 134 / 139 | 1.00 (ref) | |
| Q2 | 141 / 210 | 0.81 (0.61, 1.09) | 0.80 (0.59, 1.08) | 32 / 51 | 1.39 (0.68, 2.86) | 18 / 43 | 0.53 (0.23, 1.18) | 91 / 116 | 0.80 (0.55, 1.16) | |
| Q3 | 163 / 211 | 0.93 (0.69, 1.26) | 0.88 (0.64, 1.21) | 32 / 46 | 1.39 (0.67, 2.86) | 42 / 51 | 1.04 (0.50, 2.15) | 88 / 114 | 0.73 (0.50, 1.07) | |
| Q4 | 147 / 210 | 0.89 (0.66, 1.19) | 0.82 (0.59, 1.12) | 36 / 56 | 1.20 (0.58, 2.49) | 28 / 54 | 0.65 (0.31, 1.39) | 83 / 98 | 0.74 (0.49, 1.11) | |
| Q5 | 133 / 211 | 0.77 (0.56, 1.05) | 0.67 (0.47, 0.94) | 38 / 71 | 0.90 (0.43, 1.86) | 55 / 76 | 0.85 (0.41, 1.78) | 38 / 64 | 0.43 (0.26, 0.73) | |
| P- trend | - | 0.34 | 0.091 | 0.78 | 0.44 | 0.0001 | ||||
| Per SD | 758 / 1,052 | 0.95 (0.85, 1.06) | 0.91 (0.81, 1.02) | 157 / 264 | 0.97 (0.77, 1.21) | 164 / 252 | 1.08 (0.89, 1.29) | 434 / 531 | 0.67 (0.54, 0.84) | 0.005 |
|
HMW Adiponectin |
||||||||||
| Q1d | 71 / 87 | 1.00 (ref) | 1.00 (ref) | 9 / 7 | 1.00 (ref) | 4 / 5 | 1.00 (ref) | 58 / 74 | 1.00 (ref) | |
| Q2 | 107 / 89 | 1.50 (0.99, 2.29) | 1.44 (0.93, 2.21) | 12 / 3 | 4.31 (0.73, 25.3) | 10 / 11 | 1.35 (0.24, 7.76) | 85 / 75 | 1.48 (0.92, 2.37) | |
| Q3 | 76 / 87 | 1.05 (0.67, 1.63) | 1.03 (0.65, 1.64) | 7 / 8 | 0.34 (0.07, 1.79) | 6 / 5 | 1.89 (0.26, 13.9) | 63 / 74 | 1.15 (0.70, 1.88) | |
| Q4 | 72 / 89 | 0.97 (0.62, 1.52) | 1.00 (0.62, 1.61) | 11 / 11 | 0.29 (0.06, 1.42) | 10 / 12 | 1.26 (0.21, 7.56) | 51 / 66 | 1.06 (0.63, 1.79) | |
| Q5 | 115 / 89 | 1.54 (1.00, 2.37) | 1.60 (1.00, 2.56) | 6 / 14 | 0.11 (0.02, 0.65) | 10 / 10 | 1.35 (0.20, 9.13) | 99 / 63 | 2.28 (1.37, 3.79) | |
| P-trend | - | 0.39 | 0.24 | 0.002 | 0.92 | 0.01 | ||||
| Per SD | 441 / 441 | 1.06 (0.93, 1.21) | 1.09 (0.94, 1.27) | 45 / 43 | 0.43 (0.23, 0.81) | 40 / 43 | 0.97 (0.60, 1.58) | 356 / 352 | 1.23 (1.04, 1.45) | 0.008 |
| Adiponectin | ||||||||||
| Q1e | 161 / 210 | 1.00 (ref) | 1.00 (ref) | 27 / 42 | 1.00 (ref) | 31 / 45 | 1.00 (ref) | 102 / 122 | 1.00 (ref) | |
| Q2 | 154 / 210 | 1.02 (0.75, 1.37) | 1.08 (0.79, 1.46) | 32 /49 | 1.05 (0.54, 2.07) | 29 / 51 | 0.84 (0.43, 1.63) | 92 / 109 | 1.03 (0.69, 1.52) | |
| Q3 | 145 / 211 | 0.94 (0.69, 1.28) | 0.97 (0.70, 1.34) | 36 / 46 | 1.33 (0.68, 2.59) | 31 / 43 | 0.99 (0.51, 1.93) | 77 / 121 | 0.78 (0.52, 1.17) | |
| Q4 | 142 / 210 | 0.93 (0.68, 1.26) | 1.01 (0.73, 1.40) | 30 / 54 | 0.86 (0.44, 1.71) | 30 / 59 | 0.81 (0.42, 1.56) | 82 / 97 | 1.04 (0.69, 1.58) | |
| Q5 | 156 / 211 | 1.00 (0.74, 1.36) | 1.07 (0.77, 1.48) | 32 / 73 | 0.61 (0.31, 1.22) | 43 / 54 | 1.16 (0.61, 2.21) | 81 / 82 | 1.17 (0.75, 1.82) | |
| P-trend | - | 0.67 | 0.95 | 0.05 | 0.32 | 0.72 | ||||
| Per SD | 758 / 1,052 | 0.98 (0.89, 1.08) | 1.00 (0.90, 1.11) | 157 / 264 | 0.81 (0.66, 1.00) | 164 / 252 | 1.10 (0.91, 1.33) | 434 / 531 | 1.03 (0.88, 1.21) | 0.08 |
The overall results were calculated using conditional logistic regression adjusting for age (continuous). The crude model adjusts for the matching factors age (categorical), race, sex, and study cohort (ATBC, PLCO, CPS2) adherent in the study design. The multivariable model further adjusts for age (continuous), diabetes (yes/no), BMI (<25, 25-<30, 30+, unknown), and smoking (never, former quit 15+ years, former quit <15 years, current).
The smoking stratified analyses were done using unconditional logistic regression because the match needed to be broken to stratify by smoking. The multivariable models adjust for age, cohort (ATBC, PLCO, CPS2), sex, diabetes (yes/no), BMI (<25, 25-<30, 30), and smoking (former smoking models: former quit 15+ years, former quit <15 years). These models exclude unknown BMI. Interaction significance calculated using a −2 log likelihood test with 2 degrees of freedom comparing a model with an interaction term with smoking status and one without any interaction terms.
C-Peptide gender specific quintiles: male (Q1≤ 1.22, Q2=1.22–1.70, Q3=1.71–2.27, Q4=2.27–3.40, Q5≥3.41 ng/mL) and female (Q1≤1.19, Q2=1.20–1.87, Q3=1.88–2.74, Q4=2.77–4.02, Q5≥4.06 ng/mL), and standard deviations (SD) for male (1.94 ng/mL) and females (2.10 ng/mL). P-trend based on SD.
HMW adiponectin gender specific quintiles: male (Q1≤1838, Q2=1841–3243, Q3=3252–4552, Q4=4577–6409, Q5≥6439 ng/mL) and female (Q1≤4165, Q2=4261–8207, Q3=8765–10601, Q4=10624–16955, Q5≥18465 ng/mL) and standard deviations (SD): male (3966 ng/mL) and female (6659 ng/mL). P-trend based on SD.
Adiponectin gender specific quintiles: male (Q1≤4991, Q2=4993–6509, Q3=6509–8265, Q4=8265–10618, Q5≥10634 ng/mL) and female (Q1≤8781, Q2=8844–11477, Q3=1147–14772, Q4=14772–19579, Q5≥19579 ng/mL) and standard deviation (SD): male (4183 ng/mL) and female (6665 ng/mL). P-trend based on SD.
Given the distribution of smokers across studies (ATBC participants were all current smokers, and most CPS-II and PLCO participants were nonsmokers), the associations with C-peptide (P-interaction =0.0023) and HMW adiponectin (P-interaction =0.024) were also modified by cohort (Table 4). As expected, the associations between each of the biomarkers and PDA in the ATBC study were similar to the associations observed among all current smokers. Analyses restricted to current smokers in PLCO and CPS-II showed similar pattern of associations (n=57 cases, 38 controls: C-peptide OR=0.89, 95%CI: 0.58, 1.35 and total adiponectin OR=1.51, 95% CI: 0.90, 2.53), as that of C-peptide and HMW adiponectin in the ATBC men, although confidence intervals were wide due to small numbers.
Table 4.
OR and 95% CI for pancreatic cancer by quintile of baseline serum c-peptide, HMW adiponectin, and adiponectin by cohort
| By Cohort |
|||||||
|---|---|---|---|---|---|---|---|
| ATBC |
CPSII |
PLCO |
|||||
| Cases / Controls |
OR 95% CI | Cases / Controls |
OR 95% CI | Cases / Controls |
OR 95% CI | P interaction | |
| C Peptide | |||||||
| Q1b | 120 / 125 | 1.00 (ref) | 8 / 10 | 1.00 (ref) | 46 / 65 | 1.00 (ref) | |
| Q2 | 84 / 109 | 0.85 (0.58–1.26) | 9 / 8 | 1.62 (0.36–7.29) | 48 / 93 | 0.66 (0.39–1.13) | |
| Q3 | 76 / 106 | 0.72 (0.47–1.11) | 16 / 16 | 1.10 (0.29–4.18) | 71 / 89 | 0.99 (0.59–1.67) | |
| Q4 | 73 / 86 | 0.87 (0.56–1.33) | 22 / 20 | 1.49 (0.38–5.85) | 52 / 104 | 0.65 (0.38–1.13) | |
| Q5 | 24 / 57 | 0.37 (0.20–0.67) | 35 / 36 | 1.24 (0.35–4.37) | 74 / 118 | 0.77 (0.45–1.33) | |
| p-trend | 0.0001 | 0.83 | 0.66 | ||||
| Per SD | 377 / 493 | 0.63 (0.48–0.81) | 90 / 90 | 0.96 (0.70–1.33) | 291 / 469 | 1.04 (0.89–1.21) | 0.0023 |
|
HMW Adiponectin |
|||||||
| Q1c | 57 / 74 | 1.00 (ref) | 14 / 13 | 1.00 (ref) | |||
| Q2 | 84 / 74 | 1.61 (0.99–2.60) | 23 / 15 | 0.84 (0.26–2.70) | |||
| Q3 | 62 / 74 | 1.16 (0.70–1.92) | 14 / 13 | 0.56 (0.13–2.33) | |||
| Q4 | 50 / 66 | 1.07 (0.63–1.82) | 22 / 23 | 0.50 (0.14–1.78) | |||
| Q5 | 98 / 63 | 2.35 (1.38–4.00) | 17 / 26 | 0.33 (0.09–1.22) | |||
| p-trend | 0.017 | 0.029 | |||||
| Per SD | 351 / 351 | 1.23 (1.03–1.46) | 90 / 90 | 0.66 (0.45–0.98) | 0.024 | ||
| Adiponectin | |||||||
| Q1d | 90 / 117 | 1.00 (ref) | 13 / 9 | 1.00 (ref) | 58 / 84 | 1.00 (ref) | |
| Q2 | 85 / 100 | 1.14 (0.76–1.71) | 12 / 13 | 0.57 (0.15–2.09) | 57 / 97 | 1.13 (0.67–1.90) | |
| Q3 | 68 / 110 | 0.81 (0.52–1.26) | 19 / 11 | 0.74 (0.20–2.75) | 58 / 90 | 1.19 (0.70–2.05) | |
| Q4 | 74 / 89 | 1.06 (0.68–1.65) | 17 / 20 | 0.45 (0.12–1.71) | 51 / 101 | 1.13 (0.66–1.93) | |
| Q5 | 60 / 77 | 1.05 (0.65–1.69) | 29 / 37 | 0.32 (0.09–1.13) | 67 / 97 | 1.50 (0.88–2.55) | |
| p-trend | 0.66 | 0.023 | 0.048 | ||||
| Per SD | 377 / 493 | 0.96 (0.81–1.14) | 90 / 90 | 0.69 (0.50–0.97) | 291 / 469 | 1.17 (1.00–1.37) | 0.069 |
The cohort stratified analyses were calculated using conditional logistic regression. Models adjusts for the matching factors age, race, sex, and cohort (ATBC, PLCO, CPS2) inherent in study design. The multivariable model further adjusts for age (continuous), diabetes (yes/no), BMI (<25, 25-<30, 30+, unknown), and smoking (never, former quit 15+ years, former quit <15 years, current). The p-values for c-peptide and adiponectin were calculated using a −2 log likelihood test with 2 degrees of freedom comparing a model with interaction terms for cohort to a model without any interaction terms. For HMW adiponectin, a 1 degree of freedom test was used.
C-Peptide gender specific quintiles: male (Q1≤1.22, Q2=1.22–1.70, Q3=1.71–2.27, Q4=2.27–3.40, Q5≥3.41 ng/mL) and female (Q1≤1.19, Q2=1.20–1.87, Q3=1.88–2.74, Q4=2.77–4.02, Q5≥4.06 ng/mL), and standard deviations (SD) for male (1.94 ng/mL) and females (2.10 ng/mL). P-trend based on SD.
HMW adiponectin gender specific quintiles: male (Q1≤1838, Q2=1841–3243, Q3=3252–4552, Q4=4577–6409, Q5≥6439 ng/mL) and female (Q1≤4165, Q2=4261–8207, Q3=8765–10601, Q4=10624–16955, Q5≥18465 ng/mL) and standard deviations: male (3966 ng/mL) and female (6659 ng/mL). P-trend based on SD.
Adiponectin gender specific quintiles: male (Q1≤4991, Q2=4993–6509, Q3=6509–8265, Q4=8265–10618, Q5≥10634 ng/mL) and female (Q1≤8781, Q2=8844–11477, Q3=1147–14772, Q4=14772–19579, Q5≥19579 ng/mL) and standard deviation: male (4183 ng/mL) and female (6665 ng/mL). P-trend based on SD.
There were no interactions (P-interaction >0.05) by sex, BMI, diabetes (data not shown) or follow-up time (Supplemental Table 2) except by age at cancer diagnosis for the C-peptide association (P-interaction=0.02) which was explained by a slightly younger age at diagnosis among smokers. There were no significant interactions of the associations between the biomarkers by follow-up time (p-values >0.05). For example, similar inverse associations were observed for c-peptide early as later during follow-up [per SD, < 5 years, OR=0.84, 95% CI 0.68, 1.03; ≥ 5, OR=0.93, 95% CI 0.80, 1.07]. The positive association for HMW adiponectin remained during follow-up that occurred more than 5 years after blood was drawn [per SD, ≥ 5 years OR=1.21, 95% CI 1.01, 1.45].
Four-knot cubic splines were used to model a nonlinear relation between C-peptide, total and HMW adiponectin, and PDA (26). The tests for nonlinearity (P-values > 0.30) were consistent with a linear relationship.
DISCUSSION
In this large prospective study, smoking status modified the associations between pre-diagnostic concentrations of HMW adiponectin, total adiponectin, C-peptide, and PDA. In never smokers, total and HMW adiponectin were associated with lower PDA risk, while no association was observed with C-peptide. In current smokers, HMW adiponectin was associated with greater risk and C-peptide was associated with lower risk. To the best of our knowledge, this is the largest study to examine the interaction of the association between prediagnostic C-peptide and HMW adiponectin with pancreatic cancer by smoking status.
Results from two previous prospective studies evaluating the association of C-peptide concentrations with PDA were inconsistent (27,28). In a pooled analysis of four prospective cohorts in the U.S., C-peptide was associated with increasing risk with PDA overall (P-trend=0.005), but this increase was driven by never smokers (Q4 vs. Q1, OR=3.13 95% CI: 1.30,7.54, P-trend<0.001) whereas no association was observed among ever smokers.(27) This pooled study also reported 4-fold C-peptide association in participants who were non-fasting but no association among those who were fasting (27). The European Prospective Investigation into Cancer and Nutrition (EPIC) study reported no association between pre-diagnostic C-peptide and PDA, regardless of smoking or fasting state (28). Both of these studies included fewer current smokers than our present study and neither reported C-peptide results in current smokers alone (27,28).
Other prospective analyses have reported positive PDA cancer associations with circulating insulin and proinsulin, two biomarkers directly related to C-peptide (7,8). Although insulin and C-peptide both reflect beta-cell function, C-peptide has negligible extraction by the liver, and, compared to insulin, has a longer half-life, and is less influenced by fasting status (14). Hence, C-peptide concentration more reliably reflects β-cell function than a single measurement of insulin (14,29). One study also examined the insulin to proinsulin ratio as a measure of β-cell function and found no association with PDA but did not report results in current smokers (8).
We hypothesize that the inverse association between C-peptide and PDA in smokers is due to lower C-peptide levels being a marker for smoking-induced pancreatic damage. Specifically, among smokers, those with the most extensive smoking-induced pancreatic inflammation and damage may have both the lowest C-peptide concentration, due to the destruction of insulin secreting pancreatic beta cells, and the highest risk of developing PDA (Figure). This hypothesis is supported by experimental rodent studies showing inhalation of tobacco smoke causes pancreatic inflammation and damage (30,31). Human studies provide additional support for this hypothesis. While most smokers will not develop clinically apparent chronic pancreatitis, heavy smoking is associated with chronic pancreatitis (32,33), which is associated with both low C-peptide concentrations (34–36) and PDA(3). In support of this notion, we observe lower C-peptide concentrations among cases in our study who are current smokers compared to cases who are non-smokers and respective smoker and non-smoker controls (Table 2). Active smoking is also consistently and positively associated with diabetes (37).
Figure 1.
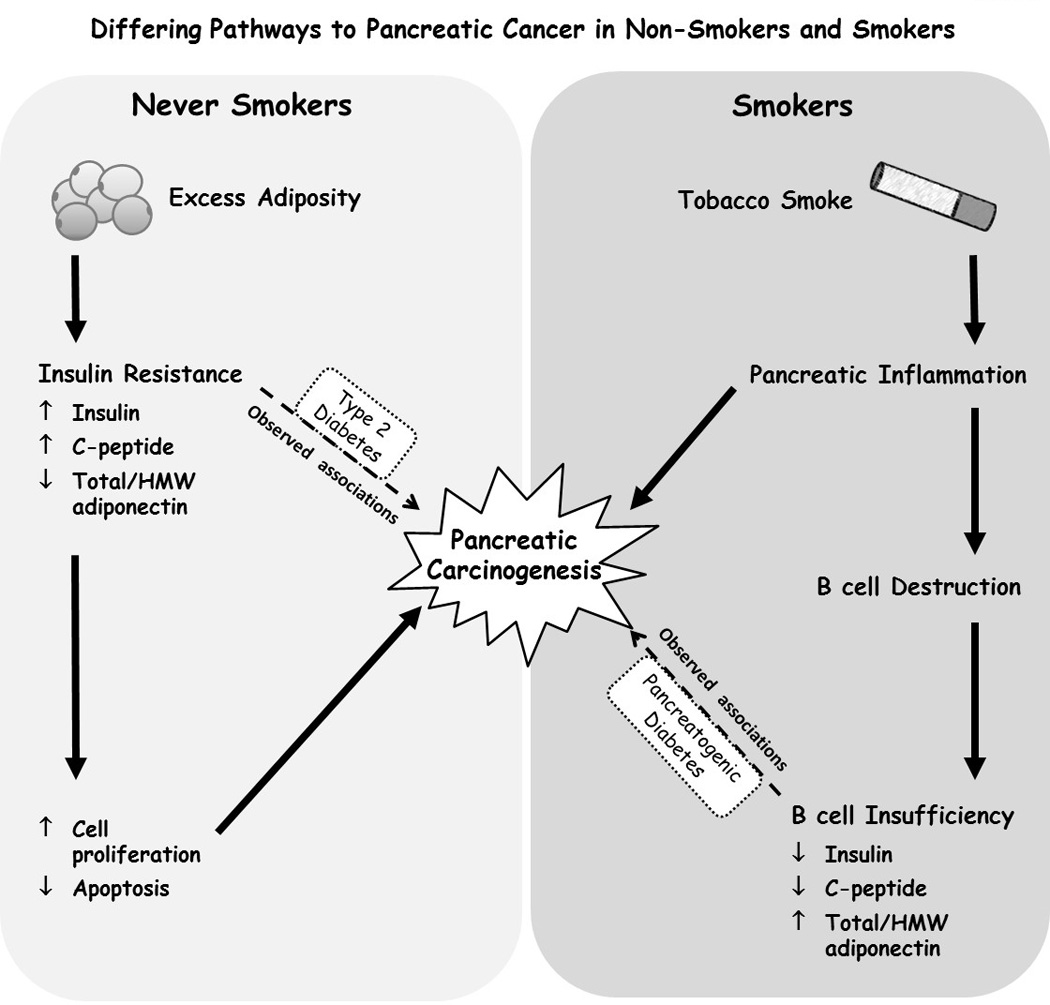
Differing pathways to pancreatic cancer in non-smokers and smokers (36,42)
The inverse association that we observed between total and HMW adiponectin and PDA in never smokers, but not among former or current smokers, is consistent with results from a nested case control study within the EPIC cohort (18). The EPIC study investigators reported an inverse association for increasing total adiponectin (Q4 vs. Q1, OR=0.52, 95% CI: 0.27, 1.01, P-trend=0.03) in never smokers and positive associations in former and current smokers (p-interaction=0.09) (18). A pooled nested case-control study from five cohorts showed higher prediagnostic adiponectin concentration was associated with lower risk of PDA overall and among both never smokers and ever smokers (current and former smokers combined) (20). In a previous analysis using earlier follow-up data from the ATBC male smokers population, increasing total adiponectin was associated with slightly lower risk of PDA overall, but only when adjusted for C-peptide concentrations (19). The inverse associations with total and HMW adiponectin that we observed in never smokers is also indirectly supported by similar inverse associations of these biomarkers with excess body weight and type 2 diabetes (Figure)(13) two known risk factors for PDA (4,5,11).
In contrast to results in never or former smokers, the positive association between HMW adiponectin and PDA in current smokers may be due to high levels of HMW adiponectin being a marker of pancreatic damage among smokers, as we hypothesized above for low levels of C-peptide (Figure). This might occur if adipocytes increase production of HMW adiponectin to increase insulin sensitivity in order to compensate for smoking induced impairment of β-cell function in smokers. Compensatory adiponectin synthesis has been suggested as a mechanism to explain the substantially elevated levels of adiponectin, particularly HMW adiponectin, in type 1 diabetes (38,39) a condition characterized by highly impaired β-cell function. HMW adiponectin might also be a more sensitive marker for body adiposity than BMI.
Strengths of our study include its prospective design, large number of cases, and long follow-up. The biomarkers were measured in blood collected years prior to cancer diagnosis and there was not an interaction by follow-up time that reduces the likelihood of reverse causation. Our study has internal validity and no control selection bias: the cases arose from the same cohorts from which the controls were selected. We used confirmed PDA cases, reducing the potential of misclassification of the outcome. We have more current and former smokers than previous studies which provided us with more power to observe interactions by smoking (18,20,27,28).
The ATBC cohort primarily drove the observed interactions by smoking status, as most smokers in our analysis were ATBC participants and all nonsmokers were PLCO or CPS-II participants. While biomarker associations among smokers in the PLCO and CPS-II cohorts appeared consistent with those observed in ATBC men, there were relatively few smokers in these cohorts. We therefore cannot preclude the possibility that the interactions by smoking status we observed were due to effect modification by some characteristic of ATBC participants other than smoking. ATBC participants were male, younger and had less self-reported diabetes and similar BMI at baseline compared to CPS-II and PLCO participants. Our results require cautious interpretation and future studies including substantial numbers of smokers are needed for confirmation. However, we view smoking status as the most likely reason for differing associations given that smoking is a known risk factors for PDA, all ATBC participants were heavy smokers, and interactions between smoking and obesity have been previously reported (4–6). Previous studies have shown stronger positive associations between BMI and pancreatic cancer in non-smokers and either weak positive or no association in current smokers (4–6,40,41). We did not measure biomarkers repeatedly over time and a single measure may not reflect long-term concentrations. Residual confounding by smoking is possible, however careful adjustment for smoking, namely smoking intensity, duration, and cumulative duration among the current smokers, did not change risk estimates. Finally, our results may not be generalizable to groups beyond middle aged and older white populations.
In conclusion, our results support the hypothesis that associations between prediagnostic C-peptide and HMW adiponectin concentrations and PDA differ by smoking status. High levels of smoking-induced pancreatic damage may explain the associations that we observe for these biomarkers in smokers. Although speculative, our findings with C-peptide and HMW adiponectin in smokers may reflect pathophysiology similar to that observed in diabetes due to exocrine pancreatic disease which has been recently recognized to play a role in PDA (Figure) (42). Patients with pancreatogenic diabetes have pancreatic glandular inflammation, sometimes in the absence of pancreatitis symptoms, and low insulin secretion, while having increased peripheral insulin sensitivity (42). Our results suggest that the etiologic pathways leading to PDA may differ between smokers and nonsmokers. Further research examining markers of pancreatic endocrine function and insulin sensitivity in relation to PDA cancer should carefully stratify analyses by smoking status.
Supplementary Material
Acknowledgments
Financial Support:
This work was supported by the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, National Cancer Institute, the Extramural Research Program of the National Institutes of Health, Division of Cancer Control and Population Sciences, National Cancer Institute, and the American Cancer Society. Additionally, this research was supported by U.S. Public Health Service contracts N01-CN-45165, N01-RC-45035, N01-RC-37004 and HHSN261201000006C from the National Cancer Institute, Department of Health and Human Services.
The authors gratefully acknowledge Dr. Barry Graubard with help on the statistical analyses and Victoria Fisher and Jennifer Loukissas for the help with the figure.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interests.
REFERENCES
- 1.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rishi A, Goggins M, Wood LD, Hruban RH. Pathological and molecular evaluation of pancreatic neoplasms. Seminars in oncology. 2015;42(1):28–39. doi: 10.1053/j.seminoncol.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duell EJ. Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Molecular carcinogenesis. 2012;51(1):40–52. doi: 10.1002/mc.20786. [DOI] [PubMed] [Google Scholar]
- 4.Genkinger JM, Kitahara CM, Bernstein L, Berrington de Gonzalez A, Brotzman M, Elena JW, et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Annals of Oncology. 2015;26(11):2257–2266. doi: 10.1093/annonc/mdv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stolzenberg-Solomon RZ, Schairer C, Moore S, Hollenbeck A, Silverman DT. Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. The American journal of clinical nutrition. 2013;98(4):1057–1065. doi: 10.3945/ajcn.113.058123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bethea TN, Kitahara CM, Sonderman J, Patel AV, Harvey C, Knutsen SF, et al. A pooled analysis of body mass index and pancreatic cancer mortality in african americans. Cancer Epidemiol Biomarkers Prev. 2014;23(10):2119–2125. doi: 10.1158/1055-9965.EPI-14-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294(22):2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 8.Wolpin BM, Bao Y, Qian ZR, Wu C, Kraft P, Ogino S, et al. Hyperglycemia, insulin resistance, impaired pancreatic beta-cell function, and risk of pancreatic cancer. J Natl Cancer Inst. 2013;105(14):1027–1035. doi: 10.1093/jnci/djt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 10.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. ProcNutrSoc. 2001;60(1):91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 11.Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Annals of surgical oncology. 2014;21(7):2453–2462. doi: 10.1245/s10434-014-3625-6. [DOI] [PubMed] [Google Scholar]
- 12.Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabetic Medicine. 2013;30(7):803–817. doi: 10.1111/dme.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishida K, Funahashi T, Shimomura I. Adiponectin as a routine clinical biomarker. Best practice & research Clinical endocrinology & metabolism. 2014;28(1):119–130. doi: 10.1016/j.beem.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Bonser AM, Garcia-Webb P, Harrison LC. C-Peptide Measurement: Methods and Clinical Utility. Critical Reviews in Clinical Laboratory Sciences. 1984;19(4):297–352. doi: 10.3109/10408368409165766. [DOI] [PubMed] [Google Scholar]
- 15.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. Journal of Biological Chemistry. 2004;279(13):12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 16.Zhu N, Pankow JS, Ballantyne CM, Couper D, Hoogeveen RC, Pereira M, et al. High-Molecular-Weight Adiponectin and the Risk of Type 2 Diabetes in the ARIC Study. Journal of Clinical Endocrinology & Metabolism. 2010;95(11):5097–5104. doi: 10.1210/jc.2010-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SS, Shin HJ, Ding EL, van Dam RM. Adiponectin Levels and Risk of Type 2 Diabetes A Systematic Review and Meta-analysis. Jama-Journal of the American Medical Association. 2009;302(2):179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 18.Grote VA, Rohrmann S, Dossus L, Nieters A, Halkjaer J, Tjonneland A, et al. The association of circulating adiponectin levels with pancreatic cancer risk: a study within the prospective EPIC cohort. IntJ Cancer. 2012;130(10):2428–2437. doi: 10.1002/ijc.26244. [DOI] [PubMed] [Google Scholar]
- 19.Stolzenberg-Solomon RZ, Weinstein S, Pollak M, Tao Y, Taylor PR, Virtamo J, et al. Prediagnostic adiponectin concentrations and pancreatic cancer risk in male smokers. Am J Epidemiol. 2008;168(9):1047–1055. doi: 10.1093/aje/kwn221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, et al. A Prospective Study of Plasma Adiponectin and Pancreatic Cancer Risk in Five US Cohorts. Journal of the National Cancer Institute. 2013;105(2):95–103. doi: 10.1093/jnci/djs474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. NEnglJMed. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 22.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Annals of epidemiology. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 23.Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort. Cancer. 2002;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 24.Hayes RB, Sigurdson A, Moore L, Peters U, Huang WY, Pinsky P, et al. Methods for etiologic and early marker investigations in the PLCO trial. Mutation research. 2005;592(1–2):147–154. doi: 10.1016/j.mrfmmm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clinical Trials. 2000;21(6):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 26.Durrleman S, Simon R. Flexible regression-models with cubic splines. Statistics in Medicine. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 27.Michaud DS, Wolpin B, Giovannucci E, Liu S, Cochrane B, Manson JE, et al. Prediagnostic Plasma C-Peptide and Pancreatic Cancer Risk in Men and Women. Cancer Epidemiology Biomarkers & Prevention. 2007;16(10):2101–2109. doi: 10.1158/1055-9965.EPI-07-0182. [DOI] [PubMed] [Google Scholar]
- 28.Grote VA, Rohrmann S, Nieters A, Dossus L, Tjonneland A, Halkjaer J, et al. Diabetes mellitus, glycated haemoglobin and C-peptide levels in relation to pancreatic cancer risk: a study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Diabetologia. 2011;54(12):3037–3046. doi: 10.1007/s00125-011-2316-0. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro ET, Tillil H, Rubenstein AH, Polonsky KS. Peripheral insulin parallels changes in insulin secretion more closely than C-peptide after bolus intravenous glucose administration. The Journal of clinical endocrinology and metabolism. 1988;67(5):1094–1099. doi: 10.1210/jcem-67-5-1094. [DOI] [PubMed] [Google Scholar]
- 30.Prokopczyk B, Hoffmann D, Bologna M, Cunningham AJ, Trushin N, Akerkar S, et al. Identification of Tobacco-Derived Compounds in Human Pancreatic Juice. Chemical Research in Toxicology. 2002;15(5):677–685. doi: 10.1021/tx0101088. [DOI] [PubMed] [Google Scholar]
- 31.Wittel U, Hopt U, Batra S. Cigarette smoke-induced pancreatic damage—experimental data. Langenbecks Arch Surg. 2008;393(4):581–588. doi: 10.1007/s00423-007-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye X, Lu G, Huai J, Ding J. Impact of smoking on the risk of pancreatitis: a systematic review and meta-analysis. PloS one. 2015;10(4):e0124075. doi: 10.1371/journal.pone.0124075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greer JB, Thrower E, Yadav D. Epidemiologic and Mechanistic Associations Between Smoking and Pancreatitis. Current treatment options in gastroenterology. 2015;13(3):332–346. doi: 10.1007/s11938-015-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonora E, Rizzi C, Lesi C, Berra P, Coscelli C, Butturini U. Insulin and C-peptide plasma levels in patients with severe chronic pancreatitis and fasting normoglycemia. Digestive diseases and sciences. 1988;33(6):732–736. doi: 10.1007/BF01540438. [DOI] [PubMed] [Google Scholar]
- 35.von Tirpitz C, Glasbrenner B, Mayer D, Malfertheiner P, Adler G. Comparison of different endocrine stimulation tests in nondiabetic patients with chronic pancreatitis. Hepato-gastroenterology. 1998;45(22):1111–1116. [PubMed] [Google Scholar]
- 36.Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)--are we neglecting an important disease? EurJ InternMed. 2013;24(3):203–206. doi: 10.1016/j.ejim.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 37.The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 38.Leth H, Andersen KK, Frystyk J, Tarnow L, Rossing P, Parving HH, et al. Elevated levels of high-molecular-weight adiponectin in type 1 diabetes. Journal of Clinical Endocrinology & Metabolism. 2008;93(8):3186–3191. doi: 10.1210/jc.2008-0360. [DOI] [PubMed] [Google Scholar]
- 39.Maahs DM, Ogden LG, Snell-Bergeon JK, Kinney GL, Wadwa RP, Hokanson JE, et al. Determinants of Serum Adiponectin in Persons with and without Type 1 Diabetes. American Journal of Epidemiology. 2007;166(6):731–740. doi: 10.1093/aje/kwm125. [DOI] [PubMed] [Google Scholar]
- 40.Meinhold CL, Berrington de Gonzalez A, Albanes D, Weinstein SJ, Taylor PR, Virtamo J, et al. Predictors of fasting serum insulin and glucose and the risk of pancreatic cancer in smokers. Cancer Causes Control. 2009;20(5):681–690. doi: 10.1007/s10552-008-9281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genkinger JM, Spiegelman D, Anderson KE, Bernstein L, van den Brandt PA, Calle EE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. International journal of cancer Journal international du cancer. 2011;129(7):1708–1717. doi: 10.1002/ijc.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui Y, Andersen DK. Diabetes and pancreatic cancer. Endocrine-related cancer. 2012;19(5):F9–F26. doi: 10.1530/ERC-12-0105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


