Abstract
Emerging research suggests that pain may persist longer-term for many children after major surgery, with significant impact on their health outcomes. This systematic review identified the prevalence of chronic postsurgical pain (CPSP) in children after surgery, and determined presurgical biomedical and psychosocial risk factors associated with CPSP prevalence or severity. Prospective studies assessing CPSP 3–12 months after surgery in children 6–18 years of age published in English in MEDLINE, EMBASE, PsycINFO, and Cochrane Database of Systematic Reviews since 1996 were eligible for inclusion. Of 16,084 abstracts yielded by the search, 123 full manuscripts were assessed for eligibility, and 12 studies were included in the review. Overall quality of included studies assessed using the Quality in Prognostic Studies tool was low. Based on 4 studies with a total of 628 participants across all surgery types, median prevalence of CPSP across studies was 20% (25th percentile=14.5%, 75th percentile=38%) at 12 months after surgery. Presurgical pain intensity, child anxiety, child pain coping efficacy, and parental pain catastrophizing were the only presurgical factors identified as predictive of CPSP. Biological and medical factors assessed were not associated with CPSP in any study. Well-designed studies examining prevalence and predictors of CPSP are critically needed in children.
INTRODUCTION
Close to 5 million children undergo surgery in the United States each year, and many experience significant pain and distress during the initial days and weeks of recovery. About one half of children report moderate-severe pain in the hospital after surgery5. However, relatively less is known about the short and long-term recovery of children after surgery. Children often continue to report pain for months following surgery, and emerging research suggests that pain may persist longer-term for many children.
Chronic postsurgical pain (CPSP) is defined as pain lasting greater than 3 months after surgery, that is not otherwise associated with preexisting problems or postoperative complications10. CPSP is reported in 10–50% of adults undergoing major surgery8,10. CPSP in adults is associated with higher functional disability, increased missed work days, and higher levels of depressive symptoms. Recent studies have investigated long-term outcomes in children undergoing surgery, showing that CPSP is associated with poorer health outcomes and with greater functional disability after surgery22.
The biopsychosocial model of pain is central to our understanding of factors involved in the development and maintenance of CPSP. Several presurgical risk factors for CPSP have been consistently identified in adults undergoing surgery, including biological factors (older age, female sex), medical factors (greater presurgical pain), and psychosocial factors (higher levels of presurgical anxiety and pain catastrophizing)7–10. In children, the psychosocial framework also includes the role that parents play in their child’s recovery from surgery. Specifically, parents’ cognitions and behaviors around their child’s pain directly influence their child’s experience of symptoms and can influence their child’s own pain perceptions and cognitions16,19. Studies have found that parent anxiety and sleep patterns before surgery predict pain intensity two weeks after surgery21. Both parent and child factors have also been identified as important in the development of CPSP in children4,17,22. It is imperative that we gain understanding of the biopsychosocial risk factors in order to implement targeted interventions to improve children’s recovery after surgery.
To date, the prevalence of CPSP has not been determined in children undergoing surgery. Risk factors that might precede CPSP and that are relevant for children undergoing surgery have also not been identified and summarized. Therefore, we conducted a systematic review and meta-analysis to 1) identify the prevalence of CPSP in children 3–12 months after surgery, and 2) determine presurgical biomedical (age, gender, baseline pain severity) and psychosocial risk factors (child anxiety, child pain catastrophizing, child depression, parent anxiety, and parent pain catastrophizing) associated with CPSP prevalence or severity. We hypothesized that similar rates of CPSP would be found for children and adolescents at 3–12 months after surgery as has been reported in adults. In addition, we expected to find several biopsychosocial predictors of CPSP including older child age, female sex, greater baseline pain intensity, and higher levels of presurgery emotional distress in the child and parent.
METHODS
Inclusion criteria
Types of participants
We included studies of children 6–18 years undergoing surgery. Children had to receive general or regional anesthesia at a hospital or surgery center. Diagnostic and noninvasive procedures were excluded. Studies investigating children undergoing cancer surgery (malignant) or with a neurological disability were excluded due to multiple confounding factors in these populations. Age 6 was chosen as the lower bound as the age at which children reliably self-report pain25. Studies extending beyond the eligible age limits were considered for inclusion if most participants were within the eligible age range, or if data were reported separately for children in the eligible age range.
Types of studies
We only considered studies that were published, peer-reviewed reports, written in English. Non-English studies were excluded as we did not have resources to interpret foreign language manuscripts. We considered different study designs including cross-sectional, case-series, case-control, and cohort studies that included more than 10 participants. Single case reports, retrospective studies (e.g. chart review), and intervention studies were excluded. Only studies that report pain between 3–12 months after surgery were eligible for inclusion.
Types of Outcomes
Prevalence of CPSP pain
The primary outcome was the presence of pain 3–12 months after surgery. We did not define the cutoff for pain presence but rather abstracted the definition used in each individual study. We abstracted both presence of pain as well as severity of pain. If multiple reports of pain were assessed in this period, each was extracted. If self-report was not available, we extracted parent-report or nurse-report of child pain.
Risk factors for CPSP pain
Biological (age and sex), medical (baseline pain severity and location), and psychosocial (presurgical child anxiety, child pain catastrophizing, child depression, child sleep patterns, parent anxiety, and parent pain catastrophizing) factors were extracted. Only risk factors that were assessed in the presurgery period were extracted and included in the analyses.
Search strategy
Cochrane Database of Systematic Reviews, MEDLINE, EMBASE, and PsycINFO were searched for peer-reviewed studies published from January 1996 to June 2016. This cutoff was chosen based on major advances in surgical and anesthetic techniques occurring in the early 1990’s making historic reports less relevant to current practice. A MEDLINE search strategy was developed first and was adapted for other search engines (see Appendix 1). Our search strategy includes terms for children/adolescents, surgery, and pain. The reference list of each included study was hand searched for additional reports potentially meeting inclusion criteria. We also conducted a citation search for each included study to identify other potential studies for inclusion.
Data collection and analysis
Study selection
One reviewer screened the abstracts to identify potential studies. A second reviewer screened 10% of all abstracts. There was a high level of agreement (99.4%) on screening. Two reviewers then assessed the full manuscripts of potentially eligible studies for inclusion in the systematic review. Disagreements were discussed and resolved with a third author.
Data extraction
One reviewer extracted data from studies meeting the inclusion criteria which was checked by a second reviewer. We extracted study characteristics (study type, sample size, surgery type), participant characteristics (age, sex, surgery type), the presence of pain 3–12 months after surgery, pain severity between 3–12 months, and presurgery biopsychosocial risk factors.
Risk of bias
Risk of bias of included studies was assessed by two reviewers using the Quality in Prognosis Studies (QUIPS) tool6. Disagreements between the two reviewers were discussed and a third reviewer arbitrated if agreement was not met. Each study was assessed on six items; study participation, study attrition, prognostic factor measurement, confounding measurement and account, outcome measurement, and analysis and reporting. Risk of bias assessments followed guidelines by Hayden and colleagues (2013). First, for study participation, we allocated a low risk of bias if there was a description of the time frame that the study recruited participants, the inclusion/exclusion criteria, the source populations, and the baseline sample. We assessed moderate risk of bias if the study partially met the requirements for low risk of bias. We allocated a high risk of bias if studies did not report details mentioned above, or if there was a low participation rate, a reported sex or age distribution which is unusual for the population being investigated, or if there was biased recruitment of the sample. We judged a study to have high risk of bias if the sample was poorly described.
We assessed low risk of bias for study attrition if authors reported participant attrition throughout the study and reasons for attrition, and included a completer vs. non-completer analysis to determine whether there were any differences. High risk of bias was given to studies not meeting this criterion.
Low risk of bias for prognostic factors was given to studies that used validated measures to investigate prognostic factors (e.g. anxiety, coping efficacy) to predict pain at follow-up. Studies that did not aim or assess prognostic factors were not evaluated on this outcome.
We judged low risk of bias for outcome measurement if a valid and reliable pain measure was used, if the authors described when the pain measurement occurred, and if outcomes were assessed consistently across participants. Measures commonly used within the population (e.g., visual analogue scale, numeric rating scale) with established psychometric properties were considered valid and reliable. Alternatively, if authors referenced a paper supporting the psychometrics of the measure within a similar population, we considered these measures as valid and reliable. Moderate or high risk of bias was allocated to studies that did not describe these details.
Low risk of bias was allocated to studies that did include confounding factors (e.g. age, and sex) in their analyses. We judged confounding factors as high risk of bias if none were controlled for in the analyses.
Finally, low risk of bias was allocated to studies reporting appropriate statistical tests for their research question. High risk of bias was given to studies that only used univariate analyses to investigate relationships between variables, or if data analyses were not appropriate for the variables assessed.
Data synthesis
Prevalence of CPSP
We aimed to describe the median, 25th percentile, and 75th percentile of prevalence of postsurgical pain between 3–12 months across studies. Only a subgroup of studies reported prevalence of pain after surgery. However, all studies within this subgroup reported prevalence of CPSP at the 12-month follow up. Therefore, we chose to summarize prevalence for this time point. Due to limited reporting of pain prevalence in the included studies, we also summarize reported pain severity at follow up. Where overlapping samples were presented in different studies, we only described the first published study, but extracted from other secondary studies if additional data were available that were not included in the primary study. For studies extracting data from registries, we highlighted where overlapping or inflated estimates may have occurred.
Subgroup analyses
Where enough data were available (at least 2 studies), we aimed to pool prevalence of CPSP by individual surgery type. In particular, we identified the following surgery types for subgroup analysis: spinal fusion, thoracotomy, laparotomy, chest wall surgery, inguinal hernia repair, amputation, and sternotomy. However, data were only available for mixed surgery samples and spinal fusion surgery. No studies meeting our inclusion criteria were available for other surgery types.
Risk factors for CPSP
Due to the lack of data, we were unable to perform meta-analysis on biopsychosocial risk factors. Therefore, we qualitatively summarized baseline biological, medical, and psychological risk factors to predict the presence or severity of CPSP (3–12 months). We were primarily interested in sex, age, presurgical pain severity, and presurgical child and parent anxiety, depression, and pain catastrophizing.
RESULTS
Search results
Our search criteria were broad, producing a large number of abstracts for initial sifting. The search produced 16,084 abstracts, of which 584 were duplicates (see Figure 1). We identified and read 123 full manuscripts for inclusion, and included 13 in our final sample. Many of the studies excluded in the first sift of the abstracts were retrospective (e.g. medical chart review), examined non-surgical procedures (dental extraction), included adults, or did not assess pain within our defined time frame (e.g. only assessed in-hospital pain). Of the 13 papers that met our inclusion criteria, two papers reported on the same study17,18. Therefore, 12 studies are included in our review.
Figure 1.

PRISMA Flow Diagram
Study characteristics
The 12 studies included in our systematic review were published between 2010 and 2016. Study characteristics are presented in Table 1. There were 1835 children and adolescents included in these studies, however, there may be some duplication due to several of the studies using data from central registries. Of the 10 studies that reported a mean age, children were an average of 14.37 years old at the time of surgery. Seven studies exclusively focused on patients undergoing spine surgery, of which six included patients with scoliosis and one included patients with lumbar disk herniation. In addition, two further studies examined youth undergoing a mix of different surgeries, however, the majority of participants underwent spine surgery17,18,22. Of the remaining three studies, one examined outcomes after ilioanal pull through (a major abdominal procedure), and two small studies examined outcomes after two specific knee surgeries. All these procedures are major inpatient surgeries with the exception of the knee procedures, which are usually performed as outpatient procedures. The majority of patients in the included studies were female, which is consistent with the sex differences in rates of scoliosis.
Table 1.
Characteristics of included studies
| Study | N | Age in years Mean (range) | Sex Female/Male | Surgery type |
|---|---|---|---|---|
| Carreon et al.2 | 887 | 14.3 (10–18) | 735/152 | Spinal fusion |
| Connelly et al.4 | 50 | 14.5 (11–17) | 41/9 | Posterior spinal fusion |
| Landman et al.11 | 295 | NR (8–22)* | NR* | Spinal fusion |
| Lillehei et al.12 | 44 | 14.7 (10–18.8) | 28/16 | Ilioanal pull through |
| Lind et al.13 | 20 | 12.5 (8–16) | 11/9 | Medial patellofemoral ligament reconstruction (knee) |
| Mariconda et al.14 | 87 | 14.8 (11–22) | 77/10 | Spinal fusion |
| Nishimura et al.15 | 12 | 14.4 (12–17) | 0/12 | Osteochondral transplant (donor knee evaluated) |
| Page et al.17,18 | 83 | 13.8 (8–18) | 56/27 | Major orthopedic and general surgery |
| Pellegrino et al.20 | 33 | 15.6 (11–20) | 31/2 | Posterior spinal fusion |
| Rabbitts et al.22 | 60 | 14.7 (10–18) | 40/20 | Major orthopedic and general surgery |
| Sieberg et al.23 | 190 | 14.4 (8–21) | 137/53 | Spinal fusion |
| Stromqvist et al.24 | 74 | 17** (12–17) | 43/31 | Surgery for lumbar disk herniation |
Demographics only provided for full sample of 1433 patients and not for 295 included in the analyses
Median age reported
Eight studies were prospective cohort studies and four studies analyzed data from prospectively collected patient registries. Three of these database studies (Sieberg et al.23, Landman et al.11, and Carreon et al.2) used a registry for scoliosis surgery from the Spinal Deformity Study Group, and one used data on patients having lumbar spine surgery from the Swedish National Spine Register24. Sieberg et al.23 included 190 patients from Boston Children’s Hospital between 2003 and 2007. Landman et al.11 included 295 patients enrolled in the database from the US since 2003 (end date not provided). Carreon et al.2 included 887 patients from sites within the US and Europe, however date ranges were not provided. There is likely to be significant overlap in data among these three studies.
Risk of bias
Of the 12 studies included in the review, four were judged as having low risk of bias for study participation as they provided full descriptions of their study participants. Five were assessed as moderate risk of bias, and three were judged to have high risk of bias providing an insufficient account of recruitment and sample (see Figure 2 and Appendix 2 for risk of bias assessments). Regarding study attrition, only one study provided an adequate description of attrition and included a completer vs non-completer analysis. Five studies provided partial description of attrition, and were judged to have moderate risk of bias, and the remaining six studies did not provide descriptions of attrition and were rated as high risk of bias.
Figure 2.
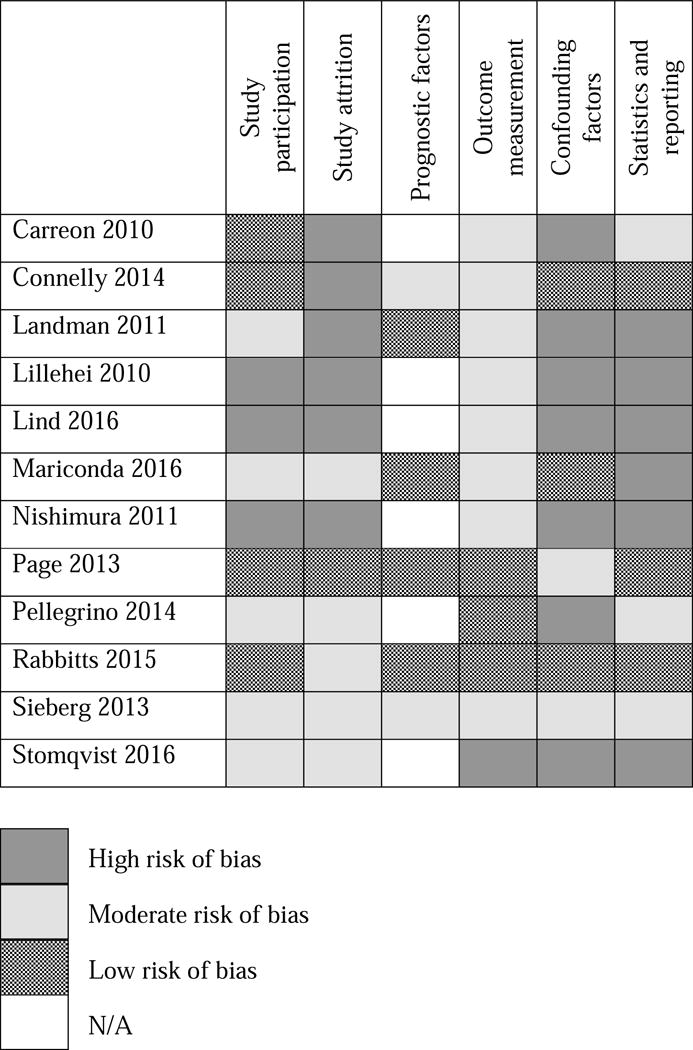
Quality in Prognosis Studies (QUIPS) Risk of bias for included studies
Six studies included prognostic factors, of which four were judged to be low risk of bias. We judged two studies to have a moderate risk of bias for this category, as either some measures were not valid or reliable, or a domain of an outcome measure was used for the prognostic factors while a different domain of the same measure was used to assess the outcome. Fourth, we assessed risk of bias for outcome measurement. We judged three studies to use valid and reliable measures of pain assessment, applied consistently across participants. Most studies did not describe when or how measurements were performed and were judged to have moderate risk of bias (n=8). We judged one study to have high risk of bias because the authors did not describe how pain was assessed or the pain measure used.
Most studies did not include confounding demographic factors in their analyses and were therefore judged to have a high risk of bias (n=7). Three studies did include confounding factors, which we judged to have low risk of bias and two studies had moderate risk of bias. Finally, three studies used appropriate statistical methods, which were reported and were judged as low risk of bias. Three studies used partially appropriate statistical methods, which we judged to have moderate risk of bias. Six studies only used univariate analyses, which were not adequate to answer the research questions proposed and so were allocated as high risk of bias.
Prevalence of chronic postsurgical pain
Four studies with a total of 628 participants across all surgery types reported prevalence of CPSP. The median prevalence of CPSP across studies was 20% (25th percentile=14.5%, 75th percentile=38%) at 12 months after surgery. Prevalence ranged widely among studies, likely due to inconsistent definitions of CPSP. Higher prevalence estimates (54%) were described for presence of any pain11, whereas lower prevalence estimates (11–22%) were found for moderate-severe pain18,22,23. Studies with higher risk of bias reported prevalence estimates (11% and 54%) which diverged most from the median prevalence across studies, whereas studies with low to moderate risk of bias reported prevalence estimates (18% and 22%) close to the median prevalence.
Spinal fusion
Two studies reported rates of pain of 11%23 and 54%11 12 months after spinal fusion. Both these studies had moderate to high risk of bias in most categories, and draw from the same database and therefore we did not calculate summary statistics for pain prevalence for this surgery type.
Mixed major surgeries
Based on two studies including mixed major surgeries, the median prevalence of CPSP after mixed major surgeries was 20%. These two prospective studies reported rates of pain of 18%22 and 22%18 at one year after major general and orthopedic surgeries. Both studies reported similar rates of pain at earlier assessments (3 and 4 months). These studies both had low to moderate risk of bias.
Chronic postsurgical pain severity
Nine studies provided summary statistics for pain severity 3–12 months after surgery, presented in Table 2. Measures used for assessing pain varied across studies. Across the three studies using a 0–10 Numeric Rating Scale (NRS), low median pain intensity was found at 12 months (Median= 1.5/10). Across the three studies using the pain domain from the Scoliosis Research Society (SRS) measure, favorable pain scores were reported on the pain domain at 12 months (Median=4.3/5, range 1–5, higher score indicates better pain outcome).
Table 2.
Chronic pain outcomes 3 to 12 months after surgery
| Study | Pain measure+ | Time point (months) | Pain severity M (SD, range++) | Chronic pain definition | Pain prevalence |
|---|---|---|---|---|---|
| Carreon et al.2 | SRS-22 pain domain | 12 | 4.35 (0.61) | NR | NR |
|
| |||||
| Connelly et al.4 | VAS 0–100, typical pain in past month | 3 | 13.91 (18.85, 0–70) | NR | NR |
| 6 | 11.57 (17.56, 0–82) | ||||
|
| |||||
| Landman et al.11 | SRS-22 pain item, pain in past month | 12 | NR | Mild-severe (rarely to very often) | 158/295 (53.6%) |
|
| |||||
| Lillehei et al.12 | SF-36 bodily pain domain | 12 | 87.2 (13.8) | NR | NR |
|
| |||||
| Lind et al.13 | NRS 0–10, pain when walking | 12 | 1.5 (1.3) | NR | NR |
|
| |||||
| Mariconda et al.14 | SRS-23 pain domain | 12 | 3.8 (0.6) | NR | NR |
|
| |||||
| Nishimura et al.15 | VAS 0–10, knee pain | 3 | 0.25 (0.6, 0–2) | NR | NR |
| 6 | 0 (0, 0–0) | ||||
| 12 | 0 (0, 0–0) | ||||
|
| |||||
| Page et al.17,18 | NRS 0–10, current pain intensity | 6 | 1.9 (1.3) | Moderate-severe (4 or more/10) | 14/61 (23.1%) |
| 12 | 1.6 (1.1) | 13/59 (22.0%) | |||
|
| |||||
| Pellegrino et al.20 | SRS-30 pain domain | 3 | 3.18 (0.14) | NR | NR |
| 6 | 4.04 (0.13) | ||||
| 12 | 4.30 (0.06) | ||||
|
| |||||
| Rabbitts et al.22 | NRS 0–10, daily pain assessment for 7 days | 4 | 1.2 (1.7) | Late pain recovery trajectory* | 11/60 (18.3%) |
| 12 | 1.1 (1.3) | ||||
|
| |||||
| Sieberg et al.23 | SRS-30 pain item, pain in past month | 12 | NR | Moderate-severe** | 19/169 (11%) |
|
| |||||
| Stromqvist et al.24 | SF-36 bodily pain domain | 12 | NR | NR | NR |
Time frame of pain ratings provided if reported;
Pain severity ranges were provided if reported; NR, not reported;
Trajectory based on statistical modeling of pain over 12 months;
cutoff/range not reported.
Scoliosis Research Society (SRS) uses a 1–5 scale with higher scores on pain domain indicating better pain-related quality of life; higher scores of pain item indicate lower pain frequency. Higher scores on a Visual Analogue Scale (VAS) and Numeric Rating Scale (NRS) indicate higher pain intensity. Short Form-36 (SF-36) uses a 0–100 scale, with higher scores indicating higher functioning.
Measurement of postsurgical pain severity
Of the 12 studies included in the review, 5 used standard measures of pain intensity (NRS, and Visual Analogue Scale VAS). The remaining 7 used quality of life measures (SRS-22, SRS-23, SRS-30, and Short Form-36) to assess pain. Five of these 7 studies used validated scores from the pain domains of the quality of life measures, while 2 used a single pain item from the SRS measures assessing pain frequency. The majority of the studies did not indicate the time frame assessed (i.e. current, past week, past month), or whether worst, least, usual, or provoked pain were reported.
Risk factors for chronic postsurgical pain
Three studies examined presurgical risk factors for CPSP, presented in Table 3. Two studies examined associations between biological, medical, and psychological factors and presence or severity of postsurgical pain 3–12 months after surgery4,22. One additional study examined age and sex as predictors of presence of chronic pain at 6 and 12 months after surgery18.
Table 3.
Presurgery risk factors for chronic postsurgical pain 3 to 12 months after surgery
| Study | Pre-operative Risk Factors assessed | Association with CPSP | Outcome | Analysis |
|---|---|---|---|---|
| Connelly et al.4 | Age | No | Rate of improvement in typical and highest pain over 6 months | Multivariate linear growth modeling |
| Sex | No | |||
| Baseline pain | Yes | |||
| BMI | No | |||
| Scoliosis severity | No | |||
| Time since diagnosis | No | |||
| Child anxiety | Yes | |||
| Child negative mood | No | |||
| Pain self-efficacy | Yes | |||
| Income | No | |||
|
| ||||
| Page et al.17,18 | Age | No | Presence of moderate/severe pain at 6 months and 12 months | Binary logistic regression analyses |
| Sex | No | |||
|
| ||||
| Rabbitts et al.22 | Age | No | Late pain recovery trajectory over 12 months* | Multivariate logistic regression analysis |
| Sex | No | |||
| Baseline pain | No | |||
| Child pain catastrophizing | No | |||
| Parental pain catastrophizing | Yes | |||
BMI, Body Mass Index;
Trajectory based on statistical modeling of pain over 12 months
Biological factors
Three studies examined age and sex as predictors of CPSP in children4,18,22. Contrary to hypotheses, significant relationships were not found.
Medical factors
Two studies examined associations between presurgery pain intensity and CPSP, with conflicting results. Connelly et al.4 found significant associations between presurgery pain and postoperative pain whereas Rabbitts et al.22 did not. Connelly et al.4 also examined other clinical factors including presurgery body mass index, scoliosis severity (Cobb angle), and time since scoliosis diagnosis, finding that none of these factors were associated with postsurgical pain outcomes.
Psychosocial factors
Two studies examined relationships between presurgery psychosocial factors and chronic postoperative pain4,22 finding significant relationships. Connelly et al.4 reported greater presurgery child anxiety and poorer pain coping efficacy, but not mood or family income, were associated with slower rate of decline in pain over 6 months after surgery. Rabbitts et al.22 examined both child and parental factors, finding that presurgery parental pain catastrophizing was associated with CPSP. However, child pain catastrophizing was not associated with chronic postoperative pain.
DISCUSSION
Our systematic review findings provide prevalence estimates of CPSP in children 3–12 months after surgery. The estimated median prevalence of CPSP was 20% across included studies, which is similar to estimated rates in adults. Only two of the 12 studies included in this review assessed biopsychosocial risk factors of CPSP, before surgery. Presurgical pain intensity and child and parent psychosocial factors were the only presurgical factors identified as predictive of CPSP. Biological factors (age and sex) and other medical factors assessed were not associated with CPSP in any study. To our knowledge, this is the first systematic review of prevalence and risk factors of CPSP in children.
Despite a broad search strategy, we identified few studies meeting inclusion criteria. Among the 12 included studies, only four studies reported the prevalence of CPSP and only three assessed presurgery factors as predictors of chronic pain after surgery. Baseline risk factors for CPSP were inconsistent across studies, partly due to the limited number of studies and the small sample sizes. Equivocal findings of the association between presurgery pain and CPSP in two studies may be related to differences in how pain was analyzed in the trajectory studies. Connelly et al.4 examined slope of pain scores after surgery, whereas Rabbitts et al.22 examined trajectory groups based on pattern over time. Similarly, in the only two studies examining presurgery psychosocial risk factors multiple differences in study design, risk factors, and study outcomes may account for differing findings. Connelly et al.4 examined only child psychosocial factors, identifying child anxiety and pain coping efficacy as predictors, whereas Rabbitts et al.22 examined both parent and child factors with parent catastrophizing as the only significant predictor. While Page et al.17,18 assessed psychosocial factors after surgery, findings were consistent with Rabbitts et al.22 in that parent factors and not child factors were associated with presence of CPSP. The literature is currently too small to draw any consistent conclusions as to which risk factors are most likely to be important in the transition to CPSP, however initial findings indicate psychosocial factors warrant further study. Larger sample sizes are needed to comprehensively assess presurgical risk factors, and to understand the magnitude of risk associated with these factors.
While extensive research has examined CPSP in adults, the paucity of literature in children highlights the critical need for research examining longer-term pain outcomes in children after surgery. While our search strategy began in 1996, no studies met our inclusion criteria prior to 2010, reflecting that pediatric postsurgical pain has received increasing attention in the past six years. Most studies did not describe CPSP prevalence, but rather presented mean pain severity at 3–12 month follow up time points. However, by averaging across all participants in a sample, important data on individual pain patterns are lost. For example, studies which reported both mean pain severity and prevalence of CPSP found low levels of pain across the whole sample (mean pain intensity 1.1±1.3 and 1.6±1.1 on a 0–10 NRS)18,22. However, they identified a significant portion of individuals experiencing CPSP at 12 months (18% and 22% respectively). Identifying individual patterns of clinically meaningful pain at follow up periods may enhance understanding of pain patterns in children after surgery.
The majority of studies assessing CPSP included patients undergoing spinal fusion. As a group, the field of pediatric spine surgery has taken the lead in this area, by including pain as one of the domains in the widely used health-related quality of life outcome measure (Scoliosis Research Society measures)1,2, and by establishing international registries for collection of long-term postsurgical outcomes data. This work has contributed to recognition of high rates of pain and the need for research aimed at improving pain outcomes in youth undergoing spine surgery. However the interpretation of these database studies is problematic due to a lack of clarity regarding overlapping samples between studies.
A significant barrier to summarizing research in this area is the variation in the definition used for CPSP. A wide variety of measures and cutoffs were used to assess chronic pain after surgery. Similar issues have been raised in the literature assessing adult postsurgical pain, particularly the varied duration after surgery considered as chronic. Partly this is due to the varying duration of what can be considered normal healing after surgery. Furthermore, common definitions used in adult CPSP research include exclusion of a preexisting condition or complications. However, it can be challenging to differentiate this in surgical populations. Moreover, definitions of CPSP currently do not include pain frequency, pain intensity, or interference due to pain, which are important when identifying clinically meaningful pain. The current definition of CPSP is persistent pain of greater than 3 months’ duration after surgery10. However, definitions incorporating impact on the patient’s physical, psychological, or socio-economic well-being have been proposed27. Notwithstanding these challenges, a unifying definition of CPSP across research and clinical practice is essential, which will require consistent criteria that may be derived from epidemiological studies of chronic pain prevalence using multidimensional pain and pain impact measures. Further, therapeutic clinical trials would also be facilitated by use of standard inclusion/exclusion criteria for individuals with CPSP.
A goal of our systematic review was to provide an assessment of study quality and we found that the overall quality of studies of CPSP was low. In particular, many studies did not take into account confounding variables (e.g., demographics) in their analyses, meaning we do not know whether findings could be due to other variables. The use of univariate analyses were used frequently within the included studies despite aiming to answer research questions that required more sophisticated testing. On a broader level, many of the included studies did not report transparent study details (e.g. sample description, flow diagrams of participant flow, comprehensive data analysis plans, power calculations, full statistics) that would allow for assessing the rigor of the methodology. Future studies in this area will be enhanced by use of more rigorous study designs with validated outcome assessments, which will increase transparency, allow for synthesizing data across studies, and increase interpretability of study findings. Despite the overall low quality of studies, there were three studies that scored low risk of bias on at least half of the items4,17,18,22.
Limitations of this review
There are some limitations of our review that should be considered when interpreting the findings. First, we did not extract studies that included broad quality of life measures without explicitly referring to assessment of ‘pain’ in the abstract. It is possible that health-related quality of life measures that assess bodily pain were not included in our review. Second, an overall small number of studies met inclusion criteria. Many studies identified in our search did not meet our inclusion criteria regarding study design, participants, and pain assessment at 3–12 months. There are additional studies that included mixed samples of children or older adolescents as well as adults but unfortunately child outcomes could not be extracted separately. Third, some of our planned analyses could not be conducted due to the heterogeneity of pain measures used and limited available data reported. For example, we were not able to examine differences by surgery type, which may have contributed to the variability in results.
Implication for practice and research
Our review highlights several areas that can be addressed through future research and practice. First, reporting standards of postsurgical studies should be improved. Studies should report on basic sample characteristics, refusal rate, attrition, and provide a clear description of the data analysis plan. Confounding variables should be considered, and statistics should be reported according to established guidelines (e.g. STROBE26). This will increase transparency and quality of research conducted. At minimum, age and sex should be included in analyses assessing postsurgical pain in youth. Second, standardized and developmentally appropriate pain measures should be agreed upon and used across practice and research. The Scoliosis Research Society questionnaires were used in some studies included here, which is useful for children undergoing scoliosis surgery. However, the pain domain includes questions that overlap (e.g., pain in the last month, pain in the last 6 months, and pain at rest), and does not provide an assessment of pain intensity. In other studies, varying pain intensity visual analogue scales using different anchors were used. This heterogeneity across studies means that meta-analyses are difficult to perform and produce wider confidence intervals. We recommend using the visual analogue scale (0–100) or a numeric rating scale 0–10 which are both reliable and valid in children older than six years of age3,25, with anchors ‘worst pain possible’ and ‘no pain’. In addition, postsurgical studies that include both adults and children should report outcomes separately by age group.
With regards to practice, our review demonstrates that pain lasting for longer than three months occurs in a sizeable proportion of youth undergoing major surgery and there are identifiable baseline risk factors that may predict child postsurgical pain. However, despite these data demonstrating the persistence of postsurgical pain, there are currently few resources available for providing pain management and support to children and families after surgery. Further research is needed to replicate findings of the risk factors identified here, and testing of additional risk factors. Additional resources and interventions should be developed and implemented for these youth who report persistent pain after surgery. Further, while our review did not assess pain reported after 12 months postsurgery, it is likely that these children report pain beyond this marker and potentially into adulthood.
CONCLUSIONS
In summary, this systematic review identified similar rates of CPSP in children as have been reported in adults. However, the findings are limited by the paucity and poor quality of studies. Well-designed studies examining prevalence and predictors of chronic pain after surgery are critically needed in children.
Supplementary Material
Perspective.
In this systematic review, the median prevalence of chronic postsurgical pain in children was 20% across studies. Presurgical pain intensity, and child and parent psychosocial factors predicted CPSP. Additional resources and interventions are needed for youth who report persistent pain after surgery.
Median prevalence of CPSP across studies was 20% at 12 months postsurgery
Presurgical pain intensity and child and parent psychosocial factors predicted CPSP
Studies examining prevalence and predictors of CPSP are critically needed in children
Acknowledgments
The authors thank Susan Klawansky, MLS, AHIP of Seattle Children’s Library & Information Commons for consultation on search design, and assistance conducting the search. This study was supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award No. K23HD078239 (PI: Jennifer A. Rabbitts); Tonya M. Palermo is supported by NIH K24HD060068.
Disclosures: This study was funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award No. K23HD078239 (PI: Jennifer A. Rabbitts); Tonya M. Palermo is supported by NIH K24HD060068.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Authors have no conflicts of interest
References
*indicates that study was an included study in the systematic review
- 1.Asher M, Min Lai S, Burton D, Manna B. Scoliosis research society-22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine (Phila Pa 1976) 2003;28:70–3. doi: 10.1097/00007632-200301010-00016. [DOI] [PubMed] [Google Scholar]
- 2*.Carreon LY, Sanders JO, Diab M, Sucato DJ, Sturm PF, Glassman SD, Spinal Deformity Study G The minimum clinically important difference in Scoliosis Research Society-22 Appearance, Activity, And Pain domains after surgical correction of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2010;35:2079–83. doi: 10.1097/BRS.0b013e3181c61fd7. [DOI] [PubMed] [Google Scholar]
- 3.Castarlenas E, Jensen MP, von Baeyer CL, Miro J. Psychometric Properties of the Numerical Rating Scale to Assess Self-Reported Pain Intensity in Children and Adolescents: A Systematic Review. Clin J Pain. 2016 doi: 10.1097/AJP.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 4*.Connelly M, Fulmer RD, Prohaska J, Anson L, Dryer L, Thomas V, Ariagno JE, Price N, Schwend R. Predictors of postoperative pain trajectories in adolescent idiopathic scoliosis. Spine. 2014;39:E174–81. doi: 10.1097/BRS.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 5.Groenewald CB, Rabbitts JA, Schroeder DR, Harrison TE. Prevalence of moderate-severe pain in hospitalized children. Paediatr Anaesth. 2012;22:661–8. doi: 10.1111/j.1460-9592.2012.03807.x. [DOI] [PubMed] [Google Scholar]
- 6.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 7.Hinrichs-Rocker A, Schulz K, Jarvinen I, Lefering R, Simanski C, Neugebauer EA. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) - a systematic review. Eur J Pain. 2009;13:719–30. doi: 10.1016/j.ejpain.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9:723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H, Edwards RR, Brennan T. Persistent Postsurgical Pain: Pathogenic Mechanisms and Preventive Strategies, Pain 2014. In: Srinivasa RN, Sommer CL, editors. Refresher Courses, 15th World Congress of Pain. Washington, D.C: IASP Press; 2014. [Google Scholar]
- 11*.Landman Z, Oswald T, Sanders J, Diab M, Spinal Deformity Study G Prevalence and predictors of pain in surgical treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2011;36:825–9. doi: 10.1097/BRS.0b013e3181de8c2b. [DOI] [PubMed] [Google Scholar]
- 12*.Lillehei CW, Masek BJ, Shamberger RC. Prospective study of health-related quality of life and restorative proctocolectomy in children. Dis Colon Rectum. 2010;53:1388–92. doi: 10.1007/DCR.0b013e3181e8efc5. [DOI] [PubMed] [Google Scholar]
- 13*.Lind M, Enderlein D, Nielsen T, Christiansen SE, Fauno P. Clinical outcome after reconstruction of the medial patellofemoral ligament in paediatric patients with recurrent patella instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:666–71. doi: 10.1007/s00167-014-3439-x. [DOI] [PubMed] [Google Scholar]
- 14*.Mariconda M, Andolfi C, Cerbasi S, Servodidio V. Effect of surgical correction of adolescent idiopathic scoliosis on the quality of life: a prospective study with a minimum 5-year follow-up. Eur Spine J. 2016 doi: 10.1007/s00586-016-4510-8. [DOI] [PubMed] [Google Scholar]
- 15*.Nishimura A, Morita A, Fukuda A, Kato K, Sudo A. Functional recovery of the donor knee after autologous osteochondral transplantation for capitellar osteochondritis dissecans. Am J Sports Med. 2011;39:838–42. doi: 10.1177/0363546510388386. [DOI] [PubMed] [Google Scholar]
- 16.Noel M, Rabbitts JA, Tai GG, Palermo TM. Remembering pain after surgery: a longitudinal examination of the role of pain catastrophizing in children’s and parents’ recall. Pain. 2015;156:800–8. doi: 10.1097/j.pain.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Page MG, Campbell F, Isaac L, Stinson J, Katz J. Parental risk factors for the development of pediatric acute and chronic postsurgical pain: a longitudinal study. J Pain Res. 2013;6:727–41. doi: 10.2147/JPR.S51055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Page MG, Stinson J, Campbell F, Isaac L, Katz J. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res. 2013;6:167–80. doi: 10.2147/JPR.S40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: an integrative approach. Pain. 2005;119:1–4. doi: 10.1016/j.pain.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 20*.Pellegrino LN, Avanzi O. Prospective evaluation of quality of life in adolescent idiopathic scoliosis before and after surgery. J Spinal Disord Tech. 2014;27:409–14. doi: 10.1097/BSD.0b013e3182797a5e. [DOI] [PubMed] [Google Scholar]
- 21.Rabbitts JA, Groenewald CB, Tai GG, Palermo TM. Presurgical psychosocial predictors of acute postsurgical pain and quality of life in children undergoing major surgery. J Pain. 2015;16:226–34. doi: 10.1016/j.jpain.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Rabbitts JA, Zhou C, Groenewald CB, Durkin L, Palermo TM. Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain. 2015;156:2383–9. doi: 10.1097/j.pain.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Sieberg CB, Simons LE, Edelstein MR, DeAngelis MR, Pielech M, Sethna N, Hresko MT. Pain prevalence and trajectories following pediatric spinal fusion surgery. J Pain. 2013;14:1694–702. doi: 10.1016/j.jpain.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Stromqvist F, Stromqvist B, Jonsson B, Gerdhem P, Karlsson MK. Lumbar disc herniation surgery in children: outcome and gender differences. Eur Spine J. 2016;25:657–63. doi: 10.1007/s00586-015-4149-x. [DOI] [PubMed] [Google Scholar]
- 25.von Baeyer CL. Children’s self-reports of pain intensity: scale selection, limitations and interpretation. Pain Res Manag. 2006;11:157–62. doi: 10.1155/2006/197616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45:247–51. doi: 10.1016/j.ypmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Werner MU, Kongsgaard UE. I. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113:1–4. doi: 10.1093/bja/aeu012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


