Abstract
Purpose
Long-term treatment with antidepressants can lessen the symptoms of depression, but health-related crises– such as a cancer diagnosis – may disrupt ongoing depression care. The study aims to estimate the effect of receiving a breast cancer diagnosis on antidepressant adherence among women with depression.
Methods
Using SEER-Medicare administrative claims we identified women aged 65+ with newly-diagnosed breast cancer between 2008–2011, who were diagnosed with depression and used antidepressants during the year before pre-diagnosis year. We compared antidepressant adherence among women with breast cancer to similar women without cancer using generalized estimation equations. Antidepressant adherence was estimated using the proportion of days covered one year before and after the index date.
Results
We included 1,142 women with breast cancer and pre-existing depression and 1,142 matched non-cancer patients with pre-existing depression. Mean antidepressant adherence was similar for both groups in the year before and after the index date (all around 0.71); adherence decreased by approximately 0.01 following breast cancer diagnosis in cancer group, with similar reductions among non-cancer group (p=0.19). However, substantial proportion of patients had inadequate adherence to antidepressants in the post-diagnosis period and almost 40% of patients in each group discontinued antidepressants over the study period.
Conclusions
Antidepressant adherence was not associated with receiving a breast cancer diagnosis beyond what would have been expected in a similar cohort of women without cancer; however, adherence was poor among both groups. Ensuring adequate ongoing depression care is important to improve cancer care and patient quality of life in the long term.
Keywords: adherence, breast cancer, antidepressants, psycho-oncology, supportive care, SEER-Medicare
Introduction
Depression is one of the most common mental disorders in the United States.1,2 In 2013, an estimated 16 million adults experienced major depressive disorder1 with women being 70% more likely to experience depression than men during their lifetime.3 Depression is associated with increased medical burden, poor functioning and well-being, and increased risk of morbidity and mortality.2–4 Medical conditions such as cancer could exacerbate pre-existing psychological distress,5 due to the stress of a new cancer diagnosis, treatment-induced physical health impairment, and fear of recurrence.6–8 Up to half of patients undergoing cancer treatment have experienced serious depressive symptoms.9 Nearly 50% of the women with early breast cancer could reach diagnostic threshold for depression in the year after diagnosis.10,11
Breast cancer is the most common non-cutaneous cancer for women in the U.S., with an estimated 230,000 new cases of invasive disease and 40,000 deaths in 2015.12 There is a higher incidence of breast cancer among women over the age of 65.13 These patients often face varying levels of psychological distress over the course of their breast cancer diagnosis and treatment.7,10 Cancer-related distress can interfere with quality of life, treatment decisions, and adherence to treatment.14–16
Clinical guidelines recommend the use of antidepressants17 in treating major depression among adult cancer patients.18,19 Long-term therapy with antidepressants can prevent relapse and recurrence of major depression.20–23 Without proper ongoing treatment, individuals may be at higher risk for depressive episodes.24,25 This is of substantial concern to patients with ongoing psychosocial stressors or comorbid illnesses.5,26,27 Although the benefits of depression management have been documented17–19, it is unclear whether new medical diagnoses– such as cancer– may disrupt ongoing depression care. The present study aims to examine the effect of receiving a breast cancer diagnosis on antidepressant adherence among women with depression who were using antidepressants prior to their cancer diagnosis.
Methods
Data sources
We used the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER)–Medicare linked database.28 This data source links patients from cancer registries participating in the SEER program (http://www.seer.cancer.gov/) with their fee-for-service Medicare administrative claims to provide detailed information on inpatient, outpatient and pharmacy services used by Medicare beneficiaries ages 65 and older with cancer and a 5% random sample of non-cancer patients. The SEER database is a nationally representative collection of 18 population-based cancer registries of all incident cancers from diverse geographic areas covering approximately 28% of the US population. SEER contains information on cancer incidence and survival in the U.S., as well as patient demographics, tumor characteristics, and vital status.
To estimate the association between a breast cancer diagnosis and antidepressant adherence, a non-cancer cohort of Medicare beneficiaries available in SEER-Medicare linked database was also utilized for this study. The non-cancer patients were identified from a random sample of Medicare beneficiaries residing in the SEER areas. To prevent double counting, people in the non-cancer sample who also appear in the SEER data are removed. Medicare claims are available in the same format for the same years for the non-cancer cohort as those for the cancer cohort.
Study population
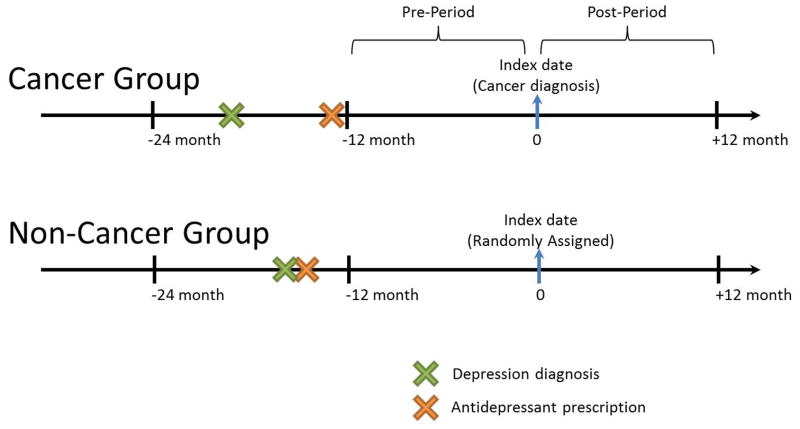
The study population consisted of women aged 65 years and older with breast cancer newly diagnosed between January 2008 and December 2011 (as index date). Since the SEER-Medicare database records the number and sequence of all primary tumors occurring over the lifetime of a patient, we ensured new diagnosis by restricting the study population to women with first primary breast cancers. A non-cancer comparison group was matched 1:1 based on age, month and year of a randomly assigned index date. Both breast cancer and the non-cancer comparison groups were required to have had a depression diagnosis (ICD-9: 311, 296.2, 296.3, 300.4)29 between −24 and −12 month prior to index date (as 0) (i.e., cancer diagnosis for cancer group or randomly assigned date for non-cancer group) and have claimed any antidepressant (e.g., selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, or other antidepressants not classified in the above categories; see Appendix Table S1 for the full list of included medications22,30) between −24 and −12 months before their index date (as 0). We considered antidepressants to be interchangeable and therefore allowed switching within and across drug classes. To ensure the completeness of Medicare claims data, patients were required to be continuously enrolled in fee-for-service Medicare Parts A, B, and D, and non-HMO for 24 months before and 12 months after their index date. Patients with end-stage renal diseases were excluded because these patients have other health care priorities. See Appendix Figure S2 for the flow chart of study population selection and Figure 1 for the definition of cancer and non-cancer groups.
Figure 1.
Antidepressant adherence
The primary outcome of interest was antidepressant adherence. Adherence was measured as the proportion of days covered (PDC)31, calculated as the number of days when medication was available over a one year period using the fill dates and days of supply for dispensed prescriptions. We measured adherence in the year prior to index date (between −12 months and index date (assuming index date as 0)) as pre-period adherence and the year after index date (between index date and 12 months) as post-period adherence (Figure 1). Adherence was calculated in two ways: (1) using a continuous measure of PDC over each year; and (2) calculating the proportion of people having 80% or more of days covered in the year. In addition to our adherence measures, we also measured the proportion of patients who experienced a treatment discontinuation over time. We considered patients to have discontinued antidepressants if they had a gap of more than 60 days within the treatment year31 and created a dichotomous indicator for this calculation.
For the above measurements, a patient’s accumulated supply was truncated at 180 days if he accumulated more than 180 days’ medication supply. This refinement aimed to address potential over-counting where combination therapies are used or when patients refill their prescriptions before finishing the drug on hand.32
Stabilized Inverse Probability of Treatment Weighting
After matching, we calculated and applied stabilized inverse probability of treatment weights to adjust for the imbalance of patient characteristics between the cancer group and non-cancer group. To do so, first we used logistic regression to estimate the propensity score by predicting treatment group assignment as a function of the following covariates: race/ethnicity, Klabunde’s adaptation of the Charlson comorbidity index (which assesses the 10 year survival/mortality risk for patients with Charlson comorbidity index included comorbidities other than cancer)33, whether a patient received financial assistance for their medications at index date, the number of office visits during pre-period (i.e., −12 to minus;2 month; the month right before the index date (i.e., 0 to −1 month) was not considered to avoid counting for increased healthcare use due to cancer), whether the last refill before the pre-period is a long days of supply refill (i.e., 90 day-of-supply), the last antidepressant drug class used during the year before the pre-period (i.e., −24 to −12 month), and pre-period adherence. Individuals were defined as having financial assistance if they received a low income subsidy (LIS) through Medicare or if they qualified for both Medicaid and Medicare (i.e., “dual-eligibles”) at their index date.
Next, using the resulting propensity score, we created inverse probability of treatment weights (IPTW)34 for each patient. These were equal to 1/p (where p is the propensity score) for patients in the cancer diagnosis group and 1/(1−p) for patients in the non-cancer group. We stabilized the propensity score weights by multiplying the IPTW weights by the marginal prevalence of the treatment they received, providing an estimate of the treatment effect in the population.
For women with breast cancer we also summarized their cancer-related characteristics including cancer stage at diagnosis (stage I–IV, according to the American Joint Committee on Cancer (AJCC) staging system), tumor grade (well differentiated, moderately differentiated, poorly differentiated, undetermined), number of positive lymph nodes, and estrogen/progesterone receptor status. Since these cancer-related variables were only available for cancer group, we did not include them in the IPTW calculation.
Analysis
We used a difference-in-differences approach for all analyses.35 Generalized estimating equations with IPTW were used to examine the effect of receiving a breast cancer diagnosis on patient adherence to antidepressants. For continuous outcomes, PDC during the year pre- or post-index date, we used an identity link and normal distribution. For binary outcomes, including treatment discontinuation and adherence (PDC ≥ 80% (or 0.8)), we used a log link and binomial distributions. Adjusted risk ratios (aRRs) with 95% confidence intervals (CI) were estimated. Statistical significance was defined as p=0.05.
Three essential components composed our model: an indicator for a breast cancer diagnosis; an indicator for the 1-year post-diagnosis period; and an interaction term of breast cancer diagnosis and the 1-year post-diagnosis period. IPTW were used to adjust for patient level factors as mentioned in the Stabilized Inverse Probability of Treatment Weighting section.
Sensitivity Analysis
In sensitivity analyses, we included only patients receiving selective serotonin reuptake inhibitors (SSRI), as these agents are generally suggested as first-line treatment for depression and are the most widely prescribed class of antidepressants.20–22 Drug switching within SSRIs was also allowed. Second, we examined monthly PDC over the study period to take a closer look at adherence changes before and after receiving cancer diagnosis. In addition, because out-of-pocket costs could influence patient access to antidepressants, we estimated whether there were differences in adherence behaviors among women with and without financial assistance for their medications. Lastly, we evaluated differences in adherence among new and prevalent antidepressant users to account for the potential effect of longer versus shorter prior antidepressant treatment histories on the likelihood of experiencing a disruption in use as a result of a cancer diagnosis. We defined new users as patients who had at least an antidepressant fill in the period between −12 and −18 months (index date as 0) but no fill in the period between −18 and −24 months; prevalent users as patients who had at least an antidepressant fill in the period between −12 and −18 months (index date as 0) as well as in the period between −18 and −24 months. Both new and prevalent users were required to have depression diagnosis during −12 to −18 month (Appendix Figure S3).
Results
A total of 1,142 women diagnosed with both breast cancer and pre-existing depression (i.e., having at least a depression diagnosis and at least one claim an antidepressant) were identified for inclusion and assigned 1:1 to match non-cancer patients with pre-existing depression. Due to matching, breast cancer and non-cancer groups did not differ with respect to age; the average age was 75.6 years (Table 1). Imbalance in patient characteristics between groups was reduced after IPTW (e.g., comorbidity, race); standardized differences were all below 0.05. For patients with breast cancer, most women had early-stage cancer (85.3%, stage I and II) and no lymph node involvement (58.1%) at diagnosis.
Table 1.
Patient Baseline Characteristics, before and after Inverse Probability of Treatment Weighting (IPTW)
| Before IPTW | Standardized Difference | After IPTW | Standardized Difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Cancer Group
|
Non-cancer Group
|
Cancer Group
|
Non-cancer Group
|
|||||||
| N=1,142 | N=1,142 | N=1,142 | N=1,142 | |||||||
| Mean | STDa | Mean | STDa | Mean | STDa | Mean | STDa | |||
|
|
|
|
|
|||||||
| Age, yr | 75.6 | (6.7) | 75.6 | (6.7) | 0.000b | 75.6 | (6.7) | 75.6 | (6.7) | 0.000c |
| Klabunde Comorbidity Score | 1.0 | (1.3) | 1.2 | (1.4) | −0.106 | 1.1 | (1.9) | 1.1 | (2.0) | −0.041 |
| Proportion of Days Covered (PDC) | 0.71 | (0.33) | 0.71 | (0.33) | 0.005 | 0.71 | (0.47) | 0.70 | (0.47) | 0.007 |
| Numbers of Office Visit | 9.5 | (8.1) | 9.1 | (8.0) | 0.054 | 9.5 | (11.4) | 9.2 | (11.2) | 0.029 |
| N | % | N | % | Nd | % | Nd | % | |||
|
|
|
|
|
|||||||
| Race/Ethnicity | 0.054 | 0.029 | ||||||||
| White | 1,026 | (89.8) | 980 | (85.8) | 1,018 | (89.2) | 992 | (86.9) | ||
| Black | 61 | (5.3) | 66 | (5.8) | 65 | (5.7) | 61 | (5.4) | ||
| Others | 33 | (2.9) | 55 | (4.8) | 24 | (3.1) | 38 | (4.5) | ||
| Hispanic | 22 | (1.9) | 41 | (3.6) | 35 | (2.1) | 51 | (3.3) | ||
| LISe/Dual Eligibility | −0.189 | 0.000c | ||||||||
| Any Extra helpf | 489 | (42.8) | 596 | (52.2) | 543 | (47.5) | 543 | (47.5) | ||
| No Extra helpf | 653 | (57.2) | 546 | (47.8) | 600 | (52.5) | 600 | (52.5) | ||
| Klabunde Comorbidity Score33 | −0.189 | 0.000c | ||||||||
| 0 | 527 | (46.2) | 474 | (41.5) | 513 | (45.0) | 486 | (42.6) | ||
| 1 | 317 | (27.8) | 324 | (28.4) | 318 | (27.9) | 323 | (28.3) | ||
| 2 | 150 | (13.1) | 176 | (15.4) | 156 | (13.7) | 171 | (15.0) | ||
| 3+ | 148 | (13.0) | 168 | (14.7) | 154 | (13.5) | 161 | (14.1) | ||
| Proportion of Days Covered (PDC) | −0.189 | 0.000c | ||||||||
| 0.8 ≤ PDC | 636 | (58.1) | 663 | (55.7) | 664 | (58.1) | 634 | (55.5) | ||
| 0.6 le; PDC < 0.8 | 183 | (12.8) | 146 | (16.0) | 147 | (12.9) | 184 | (16.1) | ||
| 0.4 le; PDC < 0.6 | 102 | (8.6) | 98 | (8.9) | 96 | (8.4) | 104 | (9.1) | ||
| 0.2le; PDC < 0.4 | 56 | (6.7) | 77 | (4.9) | 77 | (6.7) | 56 | (4.9) | ||
| PDC< 0.2 | 165 | (13.9) | 158 | (14.5) | 158 | (13.8) | 164 | (14.4) | ||
| Long Last Refill before Pre-Index Date Period | 187 | (16.3) | 195 | (17.1) | 0.019 | 187 | (16.4) | 198 | (17.3) | −0.025 |
| Last Drug Class Used before Pre-Index Date Period | 0.019 | −0.025 | ||||||||
| SSRIs | 701 | (61.4) | 694 | (60.8) | 703 | (61.6) | 695 | (60.9) | ||
| SNRIs | 192 | (16.8) | 168 | (14.7) | 191 | (16.8) | 168 | (14.7) | ||
| TCAs | 81 | (7.1) | 78 | (6.8) | 81 | (7.1) | 78 | (6.8) | ||
| Others | 168 | (14.7) | 202 | (17.7) | 166 | (14.5) | 202 | (17.7) | ||
| Index Year | 0.000b | 0.000c | ||||||||
| 2008 | 181 | (15.9) | 181 | (15.9) | 182 | (15.9) | 181 | (15.8) | ||
| 2009 | 322 | (28.2) | 322 | (28.2) | 323 | (28.2) | 321 | (28.1) | ||
| 2010 | 319 | (27.9) | 319 | (27.9) | 317 | (27.9) | 319 | (27.9) | ||
| 2011 | 320 | (28.0) | 320 | (28.0) | 320 | (28.0) | 322 | (28.2) | ||
| Cancer Stage | ||||||||||
| I | 567 | (49.7) | ||||||||
| II | 406 | (35.6) | ||||||||
| III | 126 | (11.0) | ||||||||
| IV | 43 | (3.8) | ||||||||
| Cancer Grade | ||||||||||
| Well differentiated | 281 | (24.6) | ||||||||
| Moderately differentiated | 495 | (43.3) | ||||||||
| Poorly differentiated | 294 | (25.8) | ||||||||
| Undetermined | 72 | (6.3) | ||||||||
| Number of positive lymph nodes | ||||||||||
| 0 | 663 | (58.1) | ||||||||
| 1–4 | 201 | (17.6) | ||||||||
| 5+ | 78 | (6.8) | ||||||||
| Unknown | 200 | (17.5) | ||||||||
| Estrogen receptors | ||||||||||
| Positive/Borderline | 930 | (81.4) | ||||||||
| Negative | 158 | (13.8) | ||||||||
| Unknown | 54 | (4.7) | ||||||||
| Progesterone receptor | ||||||||||
| Positive/Borderline | 790 | (69.2) | ||||||||
| Negative | 298 | (26.1) | ||||||||
| Unknown | 54 | (4.7) | ||||||||
STD, standard deviation
Age and index year were matching variables before applying IPTW, thus the standardized difference is zero.
The standardized difference is less than 0.001.
The weighted values were rounded to the nearest whole number as whole person.
LIS, Low income subsidy.
Extra help was defined as qualified for full LIS, partial LIS, or Medicare Dual-eligible beneficiaries at the month of index date.
In terms of medication adherence, women who were diagnosed with cancer and those without had similar levels of antidepressant adherence at baseline as measured by the continuous proportion of days covered (PDC) (0.71 vs 0.71) (Table 2). Both cancer and non-cancer groups had decreases in their mean PDC in the follow-up period (0.01 vs 0.02), although these changes were small. In the multivariate difference-in-differences model, receiving a breast cancer diagnosis was not significantly associated with changes in the proportion of days covered by antidepressant therapy (p=0.19).
Table 2.
Effect of Breast Cancer Diagnosis on the Proportion of Days of Antidepressant Coverage Using Difference in Difference Models (N=2,284)
| Pre-Indexa | Post-Indexa | Difference in Difference | p value | |
|---|---|---|---|---|
| PDC (Mean) | 0.01 (±0.01) | 0.19 | ||
| Cancer group | 0.71 (±0.33) | 0.70 (±0.36) | ||
| Non-cancer group | 0.71 (±0.33) | 0.68 (±0.37) | ||
| PDC (Median) | ||||
| Cancer group | 0.86 (0.50–0.97) | 0.86(0.49–0.98) | ||
| Non-cancer group | 0.85 (0.53–0.97) | 0.85(0.45–0.98) |
Medication adherence was measured through PDC in both 1 year pre- and 1 year post-index date. In cancer group, the index date was the date of receiving a breast cancer diagnosis. In non-cancer group, the index date was a date randomly assigned between 2008 and 2011.
When considering the proportion of individuals who had at least 80% of days covered by antidepressant therapy, more than 40% of the patients in either group were not adherent to antidepressants during both the pre-diagnosis period (41.9% of cancer patients and 44.3% of non-cancer patients) and the post-diagnosis period (43.3% of cancer patients and 44.6 % of non-cancer patients) (Table 3). In addition, more than one-third of each group had discontinued antidepressant treatment by the end of the post-diagnosis period (39.1% of cancer patients and 38.3% of non-cancer patients). Among those who discontinued over the study period, around 80% re-initiated the therapy. Changes in the proportion of patients who were adherent or who discontinued were similar between groups (aRR=0.98, 95% CI: 0.91–1.05 and aRR=0.99, 95% CI: 0.88–1.10, respectively).
Table 3.
Effect of Breast Cancer Diagnosis on Discontinuation and Adherence to Any Antidepressant (N=2,284)
| Adherence (PDCa>=0.8) Rate
|
Discontinuationb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Index | Post-Index | aRRc | 95%CId | Pre-Index | Post-Index | aRRc | 95%CId | |
| Cancer group | 663(58.1%) | 648(56.7%) | 0.98 | 0.91–1.05 | 351(30.7%) | 447(39.1%) | 0.99 | 0.88–1.10 |
| Non-cancer group | 636(55.7%) | 633(55.4%) | Reference | 335(29.3%) | 437(38.3%) | Reference | ||
Note: P-values for both adherence and discontinuation measures were greater than 0.05; adherence: p=0.53; discontinuation: p=0.80).
PDC, proportion of days covered. We considered patients to be adherent if PDC was over 80% (or 0.8).31
A patient would be defined as treatment discontinuation if there was a gap of more than 60 days during the treatment periods.31
aRR, adjusted Relative Risk comparing pre- and post-period and between cancer and non-cancer groups.
CI, confidence interval.
In our sensitivity analysis restricted to SSRI users alone, a total of 1,560 patients were included (i.e. 780 patients in each group) (data not shown). PDC was generally lower than observed in our primary analysis, but results were consistent with the primary analysis with no significant difference in adherence when comparing cancer and non-cancer groups.
In the examination of monthly adherence over time, we found that monthly PDC appeared to be stable between the pre- and post-diagnosis periods as well as similar between the two groups. The result is consistent with our main findings (Appendix, Figure S4).
In the analysis considering the role of financial assistance we found that, among cancer patients, PDC was slightly lower among patients without financial assistance than those with financial assistance in the pre-period (Appendix Figure S5). Among cancer patients, those not receiving financial assistance had increased by 0.03 in PDC in the post-diagnosis period, as compared to those receiving financial assistance (p=0.04).
Finally, we considered how antidepressant use in the pre-diagnosis period would change for individuals who were new versus ongoing antidepressant users. We found that adherence was generally lower among new users compared to prevalent users. However, the difference between cancer and non-cancer groups, respectively, was very small (<0.02) (Appendix Figure S6).
Discussion
This study examined the association between receiving a breast cancer diagnosis and antidepressant utilization among older women with pre-existing depression. We found that receiving a breast cancer diagnosis did not disrupt antidepressant use more than what would be expected in a similar group of patients without cancer. However, antidepressant adherence was not optimal for both groups at baseline and worsened over time. A substantial proportion of the women in our sample were non-adherent and more than one-third discontinued antidepressants over the study period.
Prior research has suggested that a cancer diagnosis may disrupt adherence to medications used to treat other chronic conditions, such as anti-diabetic medications36,37 and antihyperlipidemic agents.38 However, our findings were contrary to this prediction. There are several potential explanations that might explain the lack of association between receiving a cancer diagnosis and antidepressant treatment adherence. First, there could be an increased use of antidepressants for off-label or non-depression indications, such as insomnia, neuropathic pain or hot flashes, among women with breast cancer.22 Increased antidepressant use for indications aside from depression could lead to misclassification of our adherence measure. To better identify patients who were using antidepressants for depression-related indications, we limited our study population to those with a depression diagnosis using ICD-9 codes. However, individuals in our study may still use antidepressants for other reasons, such as anxiety, which cannot be reliably identified in administrative data. Alternatively, women might have discontinued antidepressants in the context of their ongoing depression treatment but might have initiated other antidepressants for cancer or treatment-related side effects. In sensitivity analysis we restricted antidepressants to SSRIs only, which are considered first-line therapy for depression and results were consistent with the primary analysis. However, off-label use may occur even for this subset of treatments and cannot be ruled out with certainty.
In addition, due to their increased interactions with health care providers, women with breast cancer may have closer monitoring for both cancer and non-cancer conditions. To reduce the potential effect, we used the number of office visits as a proxy of patient-provider interaction level. However, it is still possible that these frequent interactions with the health care system allow some patients with depression to receive improved management, masking adherence changes on average. There has also been greater awareness of depression among individuals with cancer, possibly leading to better evaluation and management of psychosocial problems through a multidisciplinary mental health treatment approach.39–44 In recent years, professional organizations have mandated routine distress screening as a standard of practice in oncology settings.44 Given this heightened awareness of the importance of depression care in oncology, the impact of the “health shock” on antidepressant adherence might have been reduced as compared with impacts on other chronic disease treatments.
Nonetheless, our findings suggest that antidepressant adherence remains suboptimal. Over the study period we found that more than 40% of the patients were considered non-adherent to their antidepressant treatments and the monthly PDC over time were only around 0.7. We also found a considerable proportion (about 40%) of patients discontinued treatment for depression over the study period. The actual reasons for their discontinuation are difficult to identify from our current data. This might reflect the patient failing to follow the physician’s recommendation or the overall burden of cancer-related adverse effects. However, the observed discontinuation does not necessarily suggest premature cessation; the patient might discontinue antidepressants because of a resolution of the symptoms that prompted treatment in the first place. Although it is possible that some women in our sample were no longer in need of antidepressant therapy, our further finding that more than 80% of these discontinued patients re-initiated antidepressant therapy after discontinuation limits the possibility. Therefore, it is important to ensure that those in need of ongoing care are closely monitored and appropriately managed since women with co-morbid cancer and depression are at increased risk for morbidity and mortality.
Prior research suggests that financial assistance, such as the Medicare Part D’s Extra Help program, which provides a low income subsidy for medications, could improve adherence to chronic or long-term therapies.45,46 However, in our subgroup analysis, we found that financial assistance was not consistently related to ongoing antidepressant utilization. This may be the result of Medicare Part D’s requirement to cover nearly all antidepressant therapies and the availability of low cost generic treatment options.
We also evaluated whether there were differential effects of receiving a cancer diagnosis on antidepressant adherence among new antidepressant users versus those receiving ongoing antidepressant therapy. We found that women who were new antidepressant users had lower adherence and, although only slightly, were more likely to have decreased adherence in the post-diagnosis period than continuing users. This might suggest that disruptions are more likely to occur among women without a stable antidepressant treatment regimen. However, we were not able to rule out the possibility that, in both new and prevalent user groups, the period of time during which women are in need of antidepressant treatment may vary.
There are important limitations to our analysis. First, we cannot control for the depression severity over time, which could be a strong confounder. Due to the limitation of our data source, we have no information to confirm whether the diagnosis of depression we observe could represent patient depression status at the time (e.g., initial depression episode, depression severity, the need for intense treatment). However, our use of a control group and difference-in-differences design helps to minimize the effect on our outcomes. Second, we cannot differentiate between cases of appropriate and inappropriate discontinuation of therapy. The observable duration of treatment from refills could provide information on patient needs of treatment; however, it might not reflect physicians’ judgement on patient actual need for treatment. In addition, we cannot directly identify the reason an antidepressant was prescribed using administrative data so some users may receive treatments for non-depression indications. Lastly, a PDC threshold of 80% (or 0.8) may not be optimal for all patients although for many patients with depression, acute treatment is not sufficient to effectively manage depression and therefore treatment should be considered as a rather long-term process.47
In conclusion, this study suggested that for patient with a pre-existing depression diagnosis and antidepressant use, receiving a breast cancer diagnosis did not disrupt antidepressant adherence beyond what would have been expected in a similar cohort of women without cancer. However, adherence declined over time in both cancer and non-cancer groups and many patients discontinued or failed to adhere to their depression treatment over time. Given the proven effectiveness of antidepressant in treating depression in cancer, it is important to ensure adequate depression care for these patients to improve cancer care and quality of life in the long term.
Take-Home Message.
Antidepressant adherence was not associated with receiving a breast cancer diagnosis beyond what would have been expected in women without cancer; however, adherence was poor among both cancer and non-cancer groups.
Given the proven effectiveness of antidepressants, it is important to ensure adequate ongoing depression care to improve cancer care and patient quality of life in the long term.
Acknowledgments
This project was supported by the National Institutes of Health (NIH) Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) K12 Program (Dusetzina). The database infrastructure used was funded by the Comparative Effectiveness Research Strategic Initiative of the North Carolina Translational and Clinical Sciences Institute (NC TraCS) supported by the NIH’s Clinical and Translational Science Award (UL1TR001111); and the UNC School of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Prior Postings and Presentations: This article has not been published previously in a peer-reviewed journal or under consideration for publication elsewhere, but a part of the results was previously presented at the 2015 annual meeting of the International Society for Pharmacoepidemiology.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. [Google Scholar]
- 2.US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute of Mental Health. [Accessed March 29, 2015];Depression. 2015 Available at: http://www.nimh.nih.gov/health/topics/depression/index.shtml.
- 4.Januzzi JL, Stern TA, Pasternak RC, et al. The influence of anxiety and depression on outcomes of patients with coronary artery disease. Arch Intern Med. 2000;160:1913–1921. doi: 10.1001/archinte.160.13.1913. [DOI] [PubMed] [Google Scholar]
- 5.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13:7–24. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler NE, Page AE, editors. Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press (US); 2008. [PubMed] [Google Scholar]
- 7.Institute of Medicine (US) and National Research Council (US) National Cancer Policy Board. Psychosocial Needs of Women with Breast Cancer. In: Hewitt M, Herdman RHJ, editors. Meeting Psychosocial Needs of Women with Breast Cancer. Washington, DC: National Academies Press (US); 2004. pp. 21–69. [PubMed] [Google Scholar]
- 8.Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1–5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3:e002317. doi: 10.1136/bmjopen-2012-002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. Natl Cancer Institute; Bethesda, MD: 2015. Available at: http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 10.Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallowfield L, Jenkins V. Psychosocial/survivorship issues in breast cancer: are we doing better? J Natl Cancer Inst. 2015;107:335. doi: 10.1093/jnci/dju335. [DOI] [PubMed] [Google Scholar]
- 12.American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: 2015. Available at: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. [Google Scholar]
- 13.Yancik R. Population Aging and Cancer : A Cross-National Concern. Cancer J. 2005;11:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Massie MJ, Holland JC. Psychological Reactions to Breast Cancer in the Pre- and Post-Surgical Treatment Period. Semin Surg Oncol. 1991;7:320–325. doi: 10.1002/ssu.2980070517. [DOI] [PubMed] [Google Scholar]
- 15.Irvine D, Brown B, Crooks D, et al. Psychosocial Adjustment in Women With Breast Cancer. Cancer. 1991;67:1097–1117. doi: 10.1002/1097-0142(19910215)67:4<1097::aid-cncr2820670438>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Stanton AL, Bower JE. Psychological adjustment in breast cancer survivors. In: Ganz PA, editor. Advances in Experimental Medicine and Biology. Vol. 862. Springer International Publishing; 2015. pp. 231–242. [DOI] [PubMed] [Google Scholar]
- 17.Holland JC, Andersen B, Breitbart WS, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management. J Natl Compr Cancer Netw. 2013;11:190–209. [Google Scholar]
- 18.Taylor D, Meader N, Bird V, et al. Pharmacological interventions for people with depression and chronic physical health problems: Systematic review and meta-analyses of safety and efficacy. Br J Psychiatry. 2011;198:179–188. doi: 10.1192/bjp.bp.110.077610. [DOI] [PubMed] [Google Scholar]
- 19.Walker J, Sawhney A, Hansen CH, et al. Treatment of depression in adults with cancer: a systematic review of randomized controlled trials. Psychol Med. 2014;44:897–907. doi: 10.1017/S0033291713001372. [DOI] [PubMed] [Google Scholar]
- 20.National Collaborating Centre for Mental Health. Depression in adults with a chronic physical health problem: Treatment and management (NICE clinical guideline 91) Leicester (UK): The British Psychological Society and The Royal College of Psychiatrists; 2010. [PubMed] [Google Scholar]
- 21.National Collaborating Centre for Mental Health. Depression the treatment and management of depression in adults (NICE clinical guideline 90) Leicester (UK): The British Psychological Society and The Royal College of Psychiatrists; 2010. [Google Scholar]
- 22.American Psychiatric Association. Treatment of Patients With Major Depressive Disorder. 3. Arlington, VA: 2010. [Google Scholar]
- 23.Keller MB, Boland RJ. Implications of failing to achieve successful long-term maintenance treatment of recurrent unipolar major depression. Biol Psychiatry. 1998;44:348–360. doi: 10.1016/S0006-3223(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 24.Simons AD, Angell KL, Monroe SM, et al. Cognition and life stress in depression: Cognitive factors and the definition, rating, and generation of negative life events. J Abnorm Psychol. 1993;102:584–591. http://dx.doi.org/10.1037/0021-843X.102.4.584. [PubMed] [Google Scholar]
- 25.Iosifescu DV, Bankier B, Fava M. Impact of medical comorbid disease on antidepressant treatment of major depressive disorder. Curr Psychiatry Rep. 2004;6:193–201. doi: 10.1007/s11920-004-0064-2. [DOI] [PubMed] [Google Scholar]
- 26.Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- 27.Viguera AC, Baldessarini RJ, Friedberg J. Discontinuing antidepressant treatment in major depression. Harv Rev Psychiatry. 1998;5:293–306. doi: 10.3109/10673229809003578. [DOI] [PubMed] [Google Scholar]
- 28.National Cancer Institute. [Accessed May 20, 2015];SEER-Medicare Linked Database. 2015 Available at: http://healthcaredelivery.cancer.gov/seermedicare/
- 29.Townsend L, Walkup JT, Crystal S, et al. A systematic review of validated methods for identifying depression using administrative data. Pharmacoepidemiol Drug Saf. 2012;21:163–173. doi: 10.1002/pds.2310. [DOI] [PubMed] [Google Scholar]
- 30.WHO Collaborating Centre for Drug Statistics Methodology. [Accessed May 4, 2015];The Anatomical Therapeutic Chemical (ATC) classification and the Defined Daily Dose (DDD) system. 2015 Available at: http://www.whocc.no/
- 31.Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: A proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 32.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15:457–464. [PMC free article] [PubMed] [Google Scholar]
- 33.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/S0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 34.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Wooldridge JM. Introductory Econometrics: A Modern Approach. 5. Mason, OH: South-Western Cengage Learning; 2012. [Google Scholar]
- 36.Zanders MMJ, Haak HR, van Herk-Sukel MPP, et al. Impact of cancer on adherence to glucose-lowering drug treatment in individuals with diabetes. Diabetologia. 2015;58:951–60. doi: 10.1007/s00125-015-3497-8. [DOI] [PubMed] [Google Scholar]
- 37.Calip GS, Hubbard RA, Stergachis A, et al. Adherence to oral diabetes medications and glycemic control during and following breast cancer treatment. Pharmacoepidemiol Drug Saf. 2015;24:75–85. doi: 10.1002/pds.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calip GS, Boudreau DM, Loggers ET. Changes in adherence to statins and subsequent lipid profiles during and following breast cancer treatment. Breast Cancer Res Treat. 2013;138:225–233. doi: 10.1007/s10549-013-2424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinberger MI, Roth AJ, Nelson CJ. Untangling the complexities of depression diagnosis in older cancer patients. Oncologist. 2009;14:60–6. doi: 10.1634/theoncologist.2008-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker J, Hansen CH, Martin P, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. The Lancet Psychiatry. 2014;1:343–350. doi: 10.1016/S2215-0366(14)70313-X. [DOI] [PubMed] [Google Scholar]
- 41.Brown LF, Kroenke K, Theobald DE, et al. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology. 2010;19:734–741. doi: 10.1002/pon.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Fitzgerald P, Rodin G. Evidence-based treatment of depression in patients with cancer. J Clin Oncol Oncol. 2012;30:1187–96. doi: 10.1200/JCO.2011.39.7372. [DOI] [PubMed] [Google Scholar]
- 43.Fann JR, Ell K, Sharpe M. Integrating psychosocial care into cancer services. J Clin Oncol. 2012;30:1178–1186. doi: 10.1200/JCO.2011.39.7398. [DOI] [PubMed] [Google Scholar]
- 44.Holland JC. Distress screening and the integration of psychosocial care into routine oncologic care. J Natl Compr Cancer Netw. 2013;11:687–689. doi: 10.6004/jnccn.2013.0202. [DOI] [PubMed] [Google Scholar]
- 45.Biggers A, Neuner J, Smith E, et al. Medicare Part D low-income subsidy and disparities in breast cancer treatment. J Clin Oncol. 2014;32 abstr 2. [Google Scholar]
- 46.Yala SM, Duru OK, Ettner SL, et al. Patterns of prescription drug expenditures and medication adherence among medicare part D beneficiaries with and without the low-income supplement. BMC Health Serv Res. 2014;14:665. doi: 10.1186/s12913-014-0665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17:265–71. doi: 10.1097/00004850-200211000-00001. [DOI] [PubMed] [Google Scholar]