Abstract
Study Design
Retrospective analysis.
Objectives
To identify multivariate interactions of respiratory function that are sensitive to spinal cord injury level and pharmacological treatment to promote strategies that increases successful liberation from mechanical ventilation.
Setting
United States regional spinal cord injury (SCI) treatment center.
Methods
Retrospective chart review of patients consecutively admitted to Santa Clara Valley Medical Center (SCVMC) between May 2013 and December 2014 for ventilator weaning with C1-5 AIS A or B SCI, < 3 months from injury and who had a tracheostomy in place. A non-linear, categorical principal component analysis (NL-PCA) was performed to test the multivariate interaction of respiratory outcomes from patients (N=36) being weaned off ventilator support after acute SCI with (N=15) or without (N=21) theophylline treatment.
Results
36 patients met inclusion criteria (2 C1, 5 C2, 11 C3, 14 C4, 4 C5). The NL-PCA returned 3 independent components that accounted for 95% of the variance in the dataset. Multivariate general linear models (GLM) hypothesis tests revealed a significant syndromic interaction between theophylline treatment and SCI level (Wilks’ Lambda, p=0.028, F(12,64)=2.116, η2=0.256, 1−β=0.838), with post-hoc testing demonstrating a significant interaction on PC1, explained by a positive correlation between improved forced vital capacity and time it took to reach 16 hours of ventilator free breathing. Thirty-three patients (92%) achieved 16 hours ventilator-free breathing (VFB), 30 (83%) achieved 24 hours VFB.
Conclusions
We suspect that some portion of the high success rate of ventilator weaning may be attributable to theophylline use in higher cervical SCI; in addition to our aggressive regimen of high volume ventilation, medication optimization, and pulmonary toilet (positive pressure treatments and mechanical insufflation-exsufflation).
Keywords: Spinal Cord Injury, tetraplegia, mechanical ventilator weaning, retrospective study, theophylline, principal component analysis
INTRODUCTION
Respiratory dysfunction remains a leading cause of morbidity and mortality after spinal cord injury (SCI) (1-3). The pathophysiology of respiratory dysfunction in SCI is multifactorial, resulting from diaphragmatic weakness, accessory muscle weakness, impaired cough, decreased surfactant production, and unopposed vagal tone leading to increased secretions and bronchospasm (1). The greatest determinant of respiratory failure after acute SCI is the level and completeness of injury relative to the phrenic nucleus at C3-C5 (3, 4). Indeed, diaphragmatic function is responsible for 65% of an individual’s forced vital capacity (4). While there have been promising results with phrenic nerve and diaphragm motor-point stimulation (5), mechanical ventilation remains the mainstay of management for patients with respiratory failure after SCI. At the time of discharge from acute hospitalization, greater than 70% of patients with complete cervical SCI at C5 and above have historically been shown to require ongoing mechanical ventilation (6). Unfortunately, mechanical ventilation is one of the most costly consequences of cervical SCI due to the associated infectious risks, social isolation, financial and caregiver burdens (1, 4, 7).
Methylxanthines such as theophylline have been used in respiratory dysfunction since the 1920s (8). The earliest published use of methylxanthines for respiratory dysfunction in SCI was about 40 years later (9). Theophylline has three primary modes of action in the treatment of pulmonary dysfunction including bronchodilation, anti-inflammation, and improved diaphragmatic contractility (10-12). In cervical SCI, theophylline has an additional proposed mechanism of improving pulmonary function, namely activation of a latent crossed phrenic pathway by adenosine receptor antagonism (13). Despite multiple promising animal studies exploring the use of theophylline in upper cervical SCI (1, 13-15), human studies in SCI are limited to case reports and one placebo-controlled study of theophylline use in chronic, non-ventilator dependent SCI where no significant benefit was observed (16-18).
In a previous publication from our SCI center, we demonstrated a comprehensive and effective pulmonary management strategy for acute cervical SCI that employs a combination of high tidal volume ventilation, high frequency percussive ventilation, and mechanical insufflation–exsufflation techniques (3). In the years since this publication, we have continued to optimize our pulmonary toilet and ventilator weaning protocols with the hope of giving our patients the best chance of enjoying a life of ventilator independence. One of the more recent additions to our protocol is the routine administration of oral theophylline during ventilator weaning. The following retrospective chart review represents an updated description of clinically important respiratory outcomes in patients with ventilator-dependent tetraplegia admitted to the Rehabilitation Trauma Center (RTC), a multidisciplinary acute SCI medicine unit in our center under the co-management of Neurocritical Care and Physiatry. We sought to test the interaction between theophylline treatment and SCI level on the multidimensional correlation of different measures of respiratory function and health to identify treatment options that will decrease the time it takes to wean patients off a mechanical ventilator.
PATIENTS AND METHODS
Participants
After obtaining approval by our center’s Institutional Review Board, we performed a retrospective chart analysis of consecutively admitted SCI patients to the RTC between May 2013 and November 2014. We included all traumatic spinal cord injured patients with: neurologic level of injury between C1 and C5, American Spinal Injury Association (ASIA) Impairment Scale (AIS) A or B, date of injury within 3 months of admission, history of tracheostomy, and ventilator dependence. We excluded patients who had already maintained 16 or more hours of ventilator-free breathing (VFB) at the time of admission. We documented basic demographics as well as pulmonary comorbidities such as smoking, asthma, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), lung trauma at the time of SCI, and obstructive sleep apnea (OSA). Patients were defined as having received a course of theophylline if they were treated with at least 7 consecutive days of oral theophylline during their stay.
Outcomes
We evaluated two clinically important primary outcomes including (1) the ability to wean off the ventilator for all waking hours (16 hours VFB), and (2) complete liberation from the ventilator (24 hours VFB). We examined secondary outcomes including time from injury to first attempt to breath without ventilator support (initiation of VFB), time from injury to 16 hours VFB, time from injury to 24 hours VFB, time from injury to decannulation, and change in forced vital capacity (FVC) during admission (defined as the best FVC minus the first FVC; “first FVC” was defined as the best FVC recording during the first week of admission given inconsistency of initial FVCs; “best FVC” was defined as the 95th percentile of all FVCs collected during the patient’s stay in order to exclude outliers). For statistical analysis, FVCs were normalized to cc/kg of ideal body weight. Because normal lung volumes are predicted on the basis of sex and height, ideal body weight for male patients was calculated using the formula 50 + 0.91 (centimeters of height - 152.4) and for female patients the formula was 45.5 + 0.91 (centimeters of height - 152.4) (19). Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of height measured in meters.
A respiratory therapist obtained forced vital capacities daily or twice daily. Forced vital capacity was measured by the use of a Wright Spirometer unless patients were in isolation in which case a disposable spirometer kit was used. The disposable spirometer consists of a 5 liter calibrated bag, which can be used with either mouthpiece or tracheostomy. A prior internal clinical analysis of patients in isolation showed that correction of our disposable spirometer bag values by a factor of 2/3 correlated with the Wright Spirometer values, and thus adjusted values were used for patients in isolation. Finally, we documented any adverse effects that could be attributed to theophylline administration.
Statistical plan
Due to the complex nature of SCI pathophysiology, we recognize that any single outcome will not be sufficiently powered in small patient populations to detect strong effect sizes. Knowing that univariate approaches will not fully tap into the interplay between different measures of dysfunction that are inherent in such a complex disorder, we developed an analytical workflow according to methods that have been optimized to capture the heterogeneity of this disorder (20-23). A set of physiologically meaningful outcomes were determined by the clinicians collecting the data, including BMI, first FVC, improved FVC, best FVC, time to admission, and 16 hours VFB (Figure 1A). Using these variables, a non-linear, categorical principal component analysis (NL-PCA) was applied to the data (Figure 1B-C) to determine which variables clustered together as well as their contributions to overall outcome variance. Each variable was analyzed as a categorical/ordinal measure, and a 3-factor structure was imposed on the SCI syndromic space (20, 23). Upon examining the three components, although parsimonious, they were not easily interpretable. A varimax rotation was performed by first optimally-scaling the variables using NL-PCA, and then rotating the resulting factors, using linear PCA. To evaluate the stability of results given the relatively small N, bootstrapping was conducted by repeating the NL-PCA analysis 1000 times while randomly dropping 20% of the sample to simulate a larger population. Thus, the three component NL-PCA with varimax rotation were considered parsimonious and interpretable (Figure 1D). Once we validated the components and found them to have clinically relevant face-validity, (all done blinded to treatment), we then performed hypothesis testing on the 3-dimensional (3D) PC outcome space (Figure 1E), blocking groups of patients based on SCI spinal level (C1-C5) and treatment condition (theophylline or nothing). Multivariate general linear model (GLM) was used for hypothesis testing on each set of PC scores (PC1-PC3), to test whether treatment and/or SCI level of injury significantly impacted each of the 3 multivariate outcomes generated using NL-PCA (Figure 1F). The final wave of analyses involved posthoc testing on the univariate outcomes individually (Figure 1G). Our test for normal distribution failed both the Kolmogorov-Smirnov and Shapiro-Wilk tests for normality, so we used non-parametric tests for hypothesis testing.
Figure 1. Statistical analysis workflow.
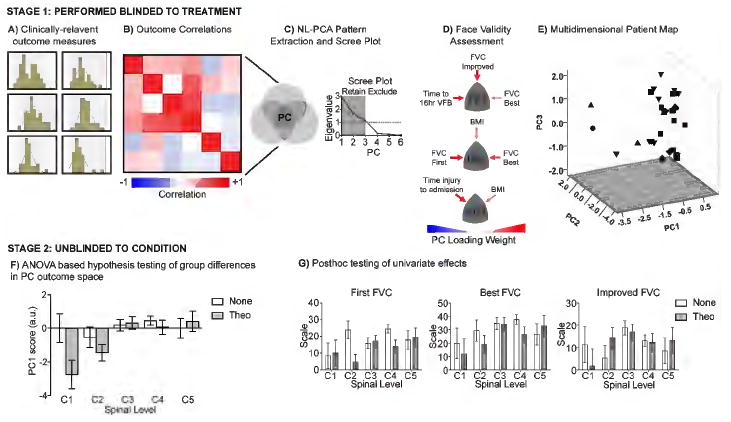
(A) Data distribution of each clinically relevant outcome was assessed to determine the type of statistical tests to use. (B) Correlation matrix of all outcomes used to perform (C) non-linear principal component analysis (NL-PCA) to identify 3 principal components (PC) based on both having eigenvalues greater than 1 whose (D) multivariate outcomes also had face validity to the clinicians that collected the data. (E) Composite PC scores from each multivariate outcome was assigned to each patient and used to map each subject into a 3D space based on their syndromic outcomes generated with NL-PCA. Workflows A-E were all conducted blinded to treatment condition. (F) Hypothesis testing was performed on multivariate outcomes (PC1-PC3) for interactions between type of treatment (group 1 vs group 2) and SCI spinal level on the multivariate space. (G) Individual outcomes tested with the same hypothesis using univariate statistics, demonstrating that only the multivariate outcomes are sufficiently powered for hypothesis testing, whereas individual outcomes separately cannot reliably test these hypotheses.
The Pearson chi-square test was used to determine whether there was a significant difference between categorical variables. For continuous variables, the Mann-Whitney U test was used. Statistical Package of Social Science (SPSS Inc., Chicago, IL, USA) was used for data processing and analysis. Histogram plots were generated using GraphPad Prism (v 7.0a), and 3D plots of PC syndromic space were generated using SPSS syntax. Overview of the statistical analysis workflow is depicted in Figure 1. Statistical significance was evaluated at a threshold of p<.05. Significant PC loadings were set to a threshold of |0.4|, based on the threshold of the PC loading, which is the same as a Pearson correlation, where the r value necessary to detect significant correlations (p<.05) depends on the degrees of freedom (df) in the dataset (N-2). The value of the loadings needed to detect significance with df=34 is |0.35| or higher, according to the Table of Critical Values for Pearson’s r, and is therefore sufficient for the current study.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
RESULTS
Patient Clinical Characteristics
A total of 40 patients with C1-5 AIS A or B SCI were admitted during the study period, however 4 patients were excluded for having already appropriately weaned off the ventilator by sustaining ≥ 16 hours/day of VFB prior to admission. Patient demographics can be seen in Table 1.
Table 1.
Patient Demographics
| N (%) | ||
|---|---|---|
| Gender | Male | 29 (81) |
| Female | 7 (19) | |
| Age | 16-30 y | 11 (31) |
| 31-45 y | 13 (36) | |
| 46-60 y | 8 (22) | |
| 61-75 y | 2 (6) | |
| >75 y | 2 (6) | |
| Mechanism of Injury | Transport | 18 (50) |
| Fall | 8 (22) | |
| Sports | 5 (14) | |
| Violence | 5 (14) | |
| Level of Injury | C1 | 2 (6) |
| C2 | 5 (14) | |
| C3 | 11 (31) | |
| C4 | 14 (39) | |
| C5 | 4 (11) | |
| Impairment Scale | A | 30 (83) |
| B | 6 (17) |
Mean time from injury to admission to the RTC was 28 days (median 25.5, range 3-54 days). Mean time to initiation of VFB from admission to the RTC was 7.7 days (median 3, range 1-43 days). Mean FVC on admission was 1180 +/- 634cc (mean +/- standard deviation, median 1042cc) and adjusted for ideal body weight 17.7 +/- 8.8 cc/kg, (median 16.6cc/kg).
Rates of successful VFB are presented in Table 2.
Table 2.
Ventilator Weaning Outcomes
| Level of injury(n) | 16 hours VFB | 24 hours VFB | Decannulated | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| C1 (2) | 1 | 50 | 0 | 0 | 0 | 0 |
| C2 (5) | 3 | 60 | 2 | 40 | 1 | 20 |
| C3 (11) | 11 | 100 | 10 | 91 | 7 | 64 |
| C4 (14) | 14 | 100 | 14 | 100 | 13 | 93 |
| C5 (4) | 4 | 100 | 4 | 100 | 3 | 75 |
Multivariate Analysis of Health and Respiratory Function
To explore the multidimensional impact of theophylline treatment on these patients, we performed NL-PCA on respiratory outcomes from patients (N=36) attempting to be weaned off a ventilator after acute SCI with (N=15) or without (N=21) theophylline treatment (Figure 2). Varimax rotation of NL-PCA loadings revealed the presence of strong loadings on each of the three components (Figure 1D). The PC loadings can be seen in the left column of Figure 2A with bootstrap estimated variances shown in the right column. The resulting PC scores for each patient, both from the original analysis and the bootstrapped analysis, were used to create the 3D space shown in Figure 2B. Bootstrapping this data to evaluate stability (top right graphic in Figure 2C) did not show substantial wobble in the results.
Figure 2. Multivariate effects of theophylline treatment on respiratory function.
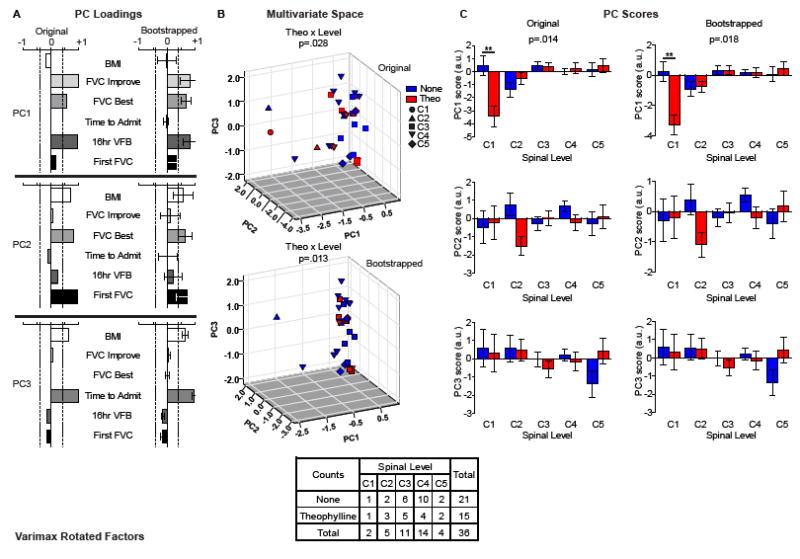
(A) NL-PCA (Original) and stability testing (Bootstrapped) of respiratory outcome patterns revealed a 3-factor structure that accounted for 95% of the variance in the dataset. Principal component 1 (PC1) accounted for 48.3% of the variance in the dataset, and represents the positive correlation between FVC best, FVC improved, and time to 16 hours VFB. After bootstrapping the sample, the error range of PC1 remained above a PC loading of 0.4 (vertical dotted lines), suggesting a stable relationship between these 3 variables. PC2 accounted for 27.8% of the variance in the dataset, and represents the positive correlation between BMI, FVC first and FVC best. Bootstrapping the sample revealed error variance in the PC loadings that fell below 0.4 for BMI and first FVC, with only best FVC remaining stable. PC3 accounted for 19.0% of the variance in the dataset, and represents the positive correlation between BMI and time from injury to admission, which remained stable after bootstrapping. (B) Multivariate hypothesis testing of PC scores for each patient (individual dots) identified a significant interaction between theophylline treatment (red color) and SCI level (different shapes) across the full 3D syndromic space that was stable after bootstrapping. (C) Posthoc hypothesis testing for each principal component (PC) showed a significant interaction between treatment and SCI level on PC1.
NL-PC scores were assigned to each patient to test the hypotheses about treatment group and injury SCI level on multidimensional outcomes of respiratory function, based on the time between injury to admission at Santa Clara Valley Medical Center (SCVMC), time to 16 hours of VFB, FVC (measured as first assessment, best assessment, and improvement), and BMI. Multivariate general linear models (GLM) were used to test for significance of treatment and SCI level on each PC. NL-PCA returned 3 independent components with eigenvalues greater than 1 (Figure 1C) that accounted for 95% of the variance in the dataset. PC1 accounted for 48.3% of the variance in the dataset, with an eigenvalue of 2.90. Loadings indicate that PC1 represents the positive cross-correlation between best FVC, improvement in FVC, and time to 16 hours VFB (Figure 2A top panel, original, PC1). After bootstrapping the sample, the error range of PC1 remained above a PC loading of 0.4, suggesting that it reflects a stable multidimensional outcome metric (Figure 2A top panel, bootstrapped, PC1). Base on this, we have named PC1 as improved vital capacity and latency to ventilator weaning. PC2 accounted for 27.8% of the variance in the dataset, with an eigenvalue of 1.67, and represents the positive correlation between BMI, first FVC and best FVC (Figure 2A middle panel, original, PC2). Bootstrapping the sample revealed error variance in the PC loadings that fell below 0.4 for BMI and first FVC, with only best FVC remaining stable (Figure 2A middle panel, bootstrapped, PC2). Based on this, we have named PC2 as general health and best vital capacity. PC3 accounted for 19.0% of the variance in the dataset, with an eigenvalue of 1.14, and represents the positive correlation between BMI and time from injury to admission (Figure 2A bottom panel, original, PC3), which remained stable after bootstrapping (Figure 2A bottom panel, bootstrapped, PC3). Based on this, we have named PC3 as health and latency to hospital. GLM hypothesis tests on the full outcome space described by the PC1-3 score axes revealed a significant multidimensional interaction between theophylline treatment and SCI level (Wilks’ Lambda, F(12,64)=2.115, p=0.028, η2=0.256, 1−β=0.837) (Figure 2B top panel, original), which remained stable after bootstrapping (Wilks’ Lambda, F(12,64)=2.381, p=0.013, η2=0.278, 1−β=0.886) (Figure 2B bottom panel, bootstrapped). Posthoc testing on each PC on its own (Figure 2C) found a significant interaction between theophylline treatment and SCI level on PC1 (F(4,26)=3.828, p=0.014, η2=0.371, 1−β=0.830) (Figure 2C top panel, original), which remained stable after bootstrapping (F(4,26)=3.631, p=0.018, η2=0.358, 1−β=0.807) (Figure 2C top panel, bootstrapped). The effect of theophylline on PC2 and PC3 did not reach significance, (both F(4,26) < 1.90, p>0.05, η2<0.25, 1−β<0.55) (Figure 2C middle and bottom panel, original and bootstrapped). Effects sizes were calculated from the hypotheses tests of the interaction between treatment and SCI level on the PC scores generated using the NL-PCA. Significant effect sizes were only able to be detected when calculated from the PC scores, and not the individual univariate outcomes.
The interpretation of this analysis is that the use of theophylline explained 25.6% of the variability within the model with a low likelihood of type 1 error (< 2.8%) and a high statistical power of 83.7%. The top left panel in Figure 2C (label PC 1 score) shows a large effect size of theophylline (red bars) which trends from strongly negative to weakly positive across the PC 1 space. The high degree of variability in the NL-PCA may suggest that there are responders and non-responders, which make treatment effects difficult to detect at the univariate level (Figure 3).
Figure 3. Univariate effects of theophylline treatment on clinically relevant variables of respiratory function.
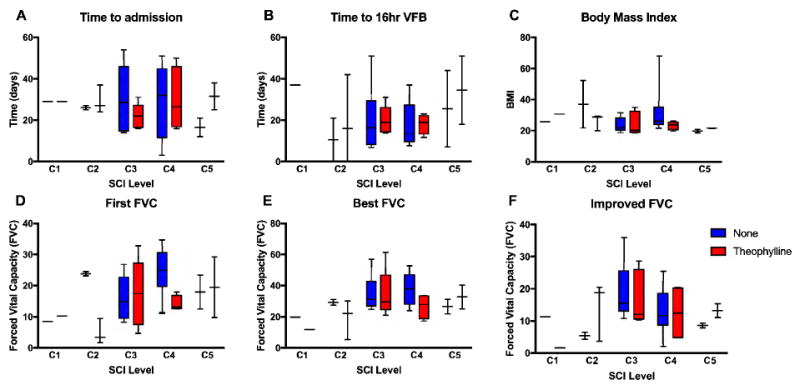
Box and whisker plots illustrating the full range (boxes) and median values for measures of respiratory function, grouped to test the interaction between SCI level (C1-C5) and theophylline treatment for (A) time to admission after SCI, time to 16 hours of (B) VFB, (C) BMI, (D) first FVC, (E) best FVC and (F) improved FVC. No significant interactions between SCI level and treatment condition were found for any of the measures individually.
Univariate Analysis of Health and Respiratory Function
Univariate hypothesis testing of individual outcomes, including time to admit, time to achieve 16 hours of VFB, BMI, first FVC, best FVC, and improvement in FVC are presented in Figure 3, based on interaction between theophylline treatment and SCI level. Rates of successful ventilator weaning by risk factor are presented in Table 3 (values presented are P values derived from Pearson chi-square). Success in ventilator liberation was strongly correlated with level of injury for 16 hours VFB (P = 0.0082) and 24 hours VFB (P = 0.0003). Likewise, first FVC was strongly correlated with achievement of 24 hours of ventilator free breathing (P = 0.0110). Gender and age were moderately associated with weaning success with p = 0.0309 for 16 hours VFB and P = 0.0383 for 24 hours VFB respectively. Achievement of 24 hours of VFB approached significance for mechanism of injury (P = 0.0700), absence of pleural effusion (P = 0.0878, absence of unilateral hemi-diaphragm elevation (P =0.1314). In this study, the rates of successful VFB were not significantly correlated with impairment scale (limited to AIS A or B at enrollment), bronchoscopy prior to admission, asthma, smoking, obstructive sleep apnea, COPD, history of pneumothorax, or body mass index.
Table 3.
Predictors of Ventilator Free Breathing
| Risk Factor | 16 hours VFB (P value) | 24 hours VFB (P value) |
|---|---|---|
| Gender | 0.0309 | 0.3464 |
| Age | 0.3470 | 0.0383 |
| Mechanism of Injury | 0.1170 | 0.0700 |
| Level of Injury | 0.0082 | 0.0003 |
| Impairment Scale | 0.4185 | 0.8415 |
| Bronchoscopy Prior to Admission | 0.5465 | 0.3711 |
| Asthma | 0.6608 | 0.5152 |
| Smoking | 0.7598 | 0.6502 |
| OSA | 0.5854 | 0.4185 |
| COPD | 0.6608 | 0.5152 |
| Pneumothorax | 1.0000 | 1.0000 |
| Hemi-diaphragm | 0.3091 | 0.1314 |
| Pleural Effusion | 0.2498 | 0.0878 |
Fourteen patients were treated with oral theophylline at a dose of 200-300mg/day split into twice or three times daily dosing for 7 or more days (mean 22 days, median 20 days). Among those treated for greater than 7 days, theophylline was discontinued in 6 cases due to adverse events including loose stool (N = 2), increased anxiety (N= 2), acute interstitial nephritis (N = 1; not confirmed to be related to theophylline) and concern of increased risk of arrhythmia in one patient with pre-existing cardiac disease. These cases were all statistically analyzed as having been treated with theophylline.
Our univariate analysis of theophylline’s impact on ventilator weaning rates was underpowered to determine effect and did not reach statistical significance. Further analysis suggested that the use of oral theophylline could be a factor in our relatively high ventilator weaning rates. Only when assessed in a multivariate space, combined with stability testing for potential outlier influences, are any potential treatment effects detected. Importantly, we also found oral theophylline to be safe at the low doses described above.
DISCUSSION
This descriptive study provides the first known data on achievable rates of partial and complete ventilator weaning after motor complete high cervical SCI. We propose that daytime ventilator independence (16 hours of VFB) is a clinically important and attainable goal for high cervical SCI patients who might otherwise fail to be completely liberated from mechanical ventilation. Indeed, there are both SCI and non-SCI-related reasons why a person may have difficulty weaning off the ventilator at night. We found that our cohort demographics are consistent with national trends in age, gender, and mechanism of injury. Our analysis revealed that 100% of patients with C3-C5 and 50-60% of C1-2 SCI were able to achieve daytime ventilator independence. For those with SCI, weaning from mechanical ventilation is likely to facilitate improved social participation and decreased costs and caregiver burden (24, 25).
The findings presented here also offer an important update to expected rates of complete (24 hour of VFB) ventilator weaning. Table 4 presents our results in comparison to previously published rates of successful ventilator weaning, as defined as 24 hours VFB (3, 26). The reasons for our improved rates of ventilator weaning are likely multiple including increased implementation of non-invasive ventilation strategies, positive pressure treatments, and consistent use of mechanical insufflation-exsufflation in the multidisciplinary respiratory care of these patients at our center, and possibly the use of theophylline.
Table 4.
Comparison of Successful Ventilator Weaning Rates Reported in Literature
| Study (first author and year) | Successful ventilator weaning for given level of SCI (% weaned with AIS A or B) | ||||
|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | |
| Chiodo 2008 | NR | 0 | 25 | 77 | 50 |
| Wong 2012 | 0 | 0 | 75 | 91 | NR |
| Current Data (Table 2) | 0 | 40 | 91 | 100 | 100 |
NR = not reported
Our study has multiple limitations inherent in the fact that this is a retrospective analysis with a small sample size. As a retrospective study, there was inherent bias in the selection of patients who received theophylline compared to those who did not. There was a change in practice at our center in December of 2013 after which theophylline was routinely administered to patients with high cervical injuries. Patients who received theophylline had increased rates of smoking, decreased FVC on admission and were generally those with less preservation of diaphragmatic innervation based on level of injury and zone of partial preservation.
Due to our low numbers, our univariate analysis may have been underpowered to demonstrate significance between ventilator weaning and impairment scale, bronchoscopy prior to admission, asthma, smoking, obstructive sleep apnea, COPD, history of pneumothorax, or body mass index. We sought to overcome this limitation using principal component analysis and bootstrapping.
NL-PCA is a form of unsupervised machine learning in which the goal is to extract as much variance in a dataset with the fewest components. Using a PCA revealed potentially clinically relevant patterns of neurological plasticity in the respiratory function of acute cervical SCI patients treated with theophylline. The present results suggest that advanced analytics can help overcome the limited power of univariate testing performed in prior studies. However their deployment in low N cohorts risks ‘overfitting’, potentially limiting the external validity of the findings. Our bootstrapping approach partially mitigates this risk by simulating a larger cohort, lending preliminary support for the idea that theophylline may improve ventilator weaning.
CONCLUSIONS
This study demonstrates a higher rate of successful ventilator weaning in cervical SCI than previously described, using a regimen of high volume ventilation, medication optimization, non-invasive ventilation, and aggressive pulmonary toilet (positive pressure treatments, and mechanical insufflation-exsufflation) at our center. Age, gender, level of injury, and initial FVC were each found to be significantly associated with achieving VFB. Our initial univariate analysis was underpowered to identify statistically meaningful effects of treatments. However, based on advanced analytics, we suspect that some of our patients’ success in ventilator weaning may be attributable to theophylline administration, and that theophylline may have a SCI level-dependent effect on successful ventilator liberation.
The impact of different interventions to improve respiratory function after traumatic SCI remains poorly understood. Even highly anticipated clinical trials such as the recently published use of high vs. standard tidal volumes for ventilator weaning have been unable demonstrate efficacy because of limited sample size (27). In order to overcome sample size limitations typical of spinal cord injury clinical trials, we propose that a large multi-center prospective study is needed to fully evaluate a uniform ventilator weaning protocol with or without the use of adjunctive agents such as oral theophylline
Acknowledgments
This research was supported in part by funding from DoD/CDMRP SCIRP Translational Partnership Award Number: W81XWH-13-1-0297, NIH/NINDS R01NS067092, and Wings for Life Foundation.
Footnotes
STATEMENT OF ETHICS
The authors certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
References
- 1.Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007;30(4):319–30. doi: 10.1080/10790268.2007.11753947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance. 2015 [Internet] Available from: https://http://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts2015.pdf.
- 3.Wong SL, Shem K, Crew J. Specialized respiratory management for acute cervical spinal cord injury∷ a retrospective analysis. Topics in spinal cord injury rehabilitation. 2012;18(4):283–90. doi: 10.1310/sci1804-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlly M, Shem K. Respiratory management during the first five days after spinal cord injury. J Spinal Cord Med. 2007;30(4):309–18. doi: 10.1080/10790268.2007.11753946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posluszny JA, Onders R, Kerwin AJ, Weinstein MS, Stein DM, Knight J, et al. Multicenter review of diaphragm pacing in spinal cord injury: successful not only in weaning from ventilators but also in bridging to independent respiration. J Trauma Acute Care Surg. 2014;76(2):303–9. doi: 10.1097/TA.0000000000000112. discussion 9-10. [DOI] [PubMed] [Google Scholar]
- 6.Como JJ, Sutton ER, McCunn M, Dutton RP, Johnson SB, Aarabi B, et al. Characterizing the need for mechanical ventilation following cervical spinal cord injury with neurologic deficit. J Trauma. 2005;59(4):912–6. doi: 10.1097/01.ta.0000187660.03742.a6. discussion 6. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 8.Schultze-Werninghaus G, Meier-Sydow J. The clinical and pharmacological history of theophylline: first report on the bronchospasmolytic action in man by S. R. Hirsch in Frankfurt (Main) 1922. Clin Allergy. 1982;12(2):211–5. doi: 10.1111/j.1365-2222.1982.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 9.Segal JL, Brunnemann SR, Gordon SK, Eltorai IM. The absolute bioavailability of oral theophylline in patients with spinal cord injury. Pharmacotherapy. 1986;6(1):26–9. doi: 10.1002/j.1875-9114.1986.tb03446.x. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ. Theophylline. Am J Respir Crit Care Med. 2013;188(8):901–6. doi: 10.1164/rccm.201302-0388PP. [DOI] [PubMed] [Google Scholar]
- 11.Wanke T, Merkle M, Zifko U, Formanek D, Lahrmann H, Grisold W, et al. The effect of aminophylline on the force-length characteristics of the diaphragm. Am J Respir Crit Care Med. 1994;149(6):1545–9. doi: 10.1164/ajrccm.149.6.8004311. [DOI] [PubMed] [Google Scholar]
- 12.Aubier M, Murciano D, Viires N, Lecocguic Y, Pariente R. Respiratory muscle pharmacotherapy. Bull Eur Physiopathol Respir. 1984;20(5):459–66. [PubMed] [Google Scholar]
- 13.Nantwi KD, Goshgarian HG. Adenosinergic mechanisms underlying recovery of diaphragm motor function following upper cervical spinal cord injury: potential therapeutic implications. Neurol Res. 2005;27(2):195–205. doi: 10.1179/016164105X21977. [DOI] [PubMed] [Google Scholar]
- 14.Bae H, Nantwi KD, Goshgarian HG. Recovery of respiratory function following C2 hemi and carotid body denervation in adult rats: influence of peripheral adenosine receptors. Exp Neurol. 2005;191(1):94–103. doi: 10.1016/j.expneurol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Nantwi KD, El-Bohy A, Goshgarian HG. Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol. 1996;140(1):53–9. doi: 10.1006/exnr.1996.0114. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson GT, Khanchandani N, Lattin CD, Goshgarian HG. Clinical Effects of Theophylline on Inspiratory Muscle Drive in Tetraplegia. Neurorehabilitation and Neural Repair. 1999:191–7. [Google Scholar]
- 17.Bascom AT, Lattin CD, Aboussouan LS, Goshgarian HG. Effect of acute aminophylline administration on diaphragm function in high cervical tetraplegia: a case report. Chest. 2005;127(2):658–61. doi: 10.1378/chest.127.2.658. [DOI] [PubMed] [Google Scholar]
- 18.Tzelepis GE, Bascom AT, Safwan Badr M, Goshgarian HG. Effects of theophylline on pulmonary function in patients with traumatic tetraplegia. J Spinal Cord Med. 2006;29(3):227–33. doi: 10.1080/10790268.2006.11753878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. The American review of respiratory disease. 1981;123(6):659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson AR, Irvine KA, Gensel JC, Nielson JL, Lin A, Ly J, et al. Derivation of multivariate syndromic outcome metrics for consistent testing across multiple models of cervical spinal cord injury in rats. PLoS One. 2013;8(3):e59712. doi: 10.1371/journal.pone.0059712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson AR, Stuck ED, Nielson JL. Syndromics: a bioinformatics approach for neurotrauma research. Transl Stroke Res. 2011;2(4):438–54. doi: 10.1007/s12975-011-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielson JL, Guandique CF, Liu AW, Burke DA, Lash AT, Moseanko R, et al. Development of a database for translational spinal cord injury research. J Neurotrauma. 2014;31(21):1789–99. doi: 10.1089/neu.2014.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielson JL, Paquette J, Liu AW, Guandique CF, Tovar CA, Inoue T, et al. Topological data analysis for discovery in preclinical spinal cord injury and traumatic brain injury. Nature communications. 2015;6:8581. doi: 10.1038/ncomms9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasgupta A, Rice R, Mascha E, Litaker D, Stoller JK. Four-year experience with a unit for long-term ventilation (respiratory special care unit) at the Cleveland Clinic Foundation. Chest. 1999;116(2):447–55. doi: 10.1378/chest.116.2.447. [DOI] [PubMed] [Google Scholar]
- 25.Tsara V, Serasli E, Voutsas V, Lazarides V, Christaki P. Burden and coping strategies in families of patients under noninvasive home mechanical ventilation. Respiration; international review of thoracic diseases. 2006;73(1):61–7. doi: 10.1159/000087460. [DOI] [PubMed] [Google Scholar]
- 26.Chiodo AE, Scelza W, Forchheimer M. Predictors of ventilator weaning in individuals with high cervical spinal cord injury. J Spinal Cord Med. 2008;31(1):72–7. doi: 10.1080/10790268.2008.11753984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenton JJ, Warner ML, Lammertse D, Charlifue S, Martinez L, Dannels-McClure A, et al. A comparison of high vs standard tidal volumes in ventilator weaning for individuals with sub-acute spinal cord injuries: a site-specific randomized clinical trial. Spinal cord. 2015 doi: 10.1038/sc.2015.145. [DOI] [PubMed] [Google Scholar]


