Abstract
Aim
The objective was to determine trends in the use of chemotherapy as the initial treatment and evaluate the comparative effectiveness of initial chemotherapy versus resection of the primary tumour on survival (intention-to-treat analysis) in stage IV colorectal cancer (CRC).
Methods
This cohort study used Surveillance Epidemiology, and End Results (SEER)-Medicare (2000–2011) data, including patients ≥66 years presenting with stage IV CRC. Cox proportional hazards models and instrumental variable analysis were used to determine the association of chemotherapy versus resection of the primary tumour as the initial treatment with 2-year survival.
Results
The use of chemotherapy as the first treatment increased over time, from 26.8% in 2001 to 46.9% in 2009 (p<0.0001). The traditional Cox model showed that chemotherapy as the initial treatment was associated with the higher risk of mortality (HR, 1.35; 95% CI, 1.27–1.44). When accounting for known and unknown confounders in an instrumental variable analysis, chemotherapy as the initial treatment suggested benefit on 2-year survival (HR, 0.68; 95% CI, 0.44–1.04); however, the association did not reach statistical significance. The study findings were similar in six subgroup analysis.
Conclusions
The use of chemotherapy as the initial therapy increased substantially in the last decade. Instrumental variable analysis found that chemotherapy as the initial treatment offers similar or better 2-year survival in patients with stage IV CRC. Given the morbidity and mortality associated with colorectal resection in elderly patients, chemotherapy provides an option to patients who are not good candidates for resection.
Keywords: Colorectal cancer, comparative effectiveness research, chemotherapy, surgery, instrumental variable, selection bias
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the U.S [1]. 20 – 30% of patients present with stage IV disease, of which nearly two-thirds are considered unresectable, including inability to achieve negative margins (R0 resection) on the primary tumor and/or inability to resect all radiographically and clinically visible metastatic disease. (unable to resect all gross disease) [2]. Historically, stage IV disease was managed with surgical removal of the primary tumour to prevent complications such as obstruction, bleeding, and perforation. The introduction of oxaliplatin or irinotecan in chemotherapy regimens in the 2000s has improved tumor response and survival in stage IV disease, triggering changes in treatment paradigms [3–5].
Starting in 2006, the National Comprehensive Cancer Network (NCCN) guidelines recommend systemic therapy as the initial treatment in patients with unresectable metastatic disease [6]. For resectable stage IV disease, the NCCN recommends a multimodality approach of both systemic therapy and surgical resection of the primary tumor and metastatic disease; in this “curative” setting, the recommendations suggest systemic therapy can be delivered before or after surgery [7]. Current guidelines, however, are not based on level I evidence, and considerable debate remains regarding the best initial treatment for resectable and unresectable synchronous metastatic CRC (systemic therapy vs. resection of the primary tumour +/− metastases) [8–13].
Our goal was to evaluate population-based trends in the use of chemotherapy as the initial treatment and to compare the effectiveness of chemotherapy versus resection of the primary tumor as the initial treatment on survival (intention-to-treat analysis) in older patients with stage IV CRC and asymptomatic primary tumors. We hypothesized significant confounding by indication and, therefore, used both traditional multivariable models and instrumental variable analysis to control for unmeasured confounding.
METHODS
Data Source
We used the Surveillance Epidemiology and End Results (SEER)-Medicare linked database from 2000 to 2011. Medicare files used for this study included the Denominator file (demographics and eligibility), the Medicare Provider Analysis and Review (MedPAR) file, the Carrier claim file, and the Outpatient Standard Analytical File.
Study Cohort
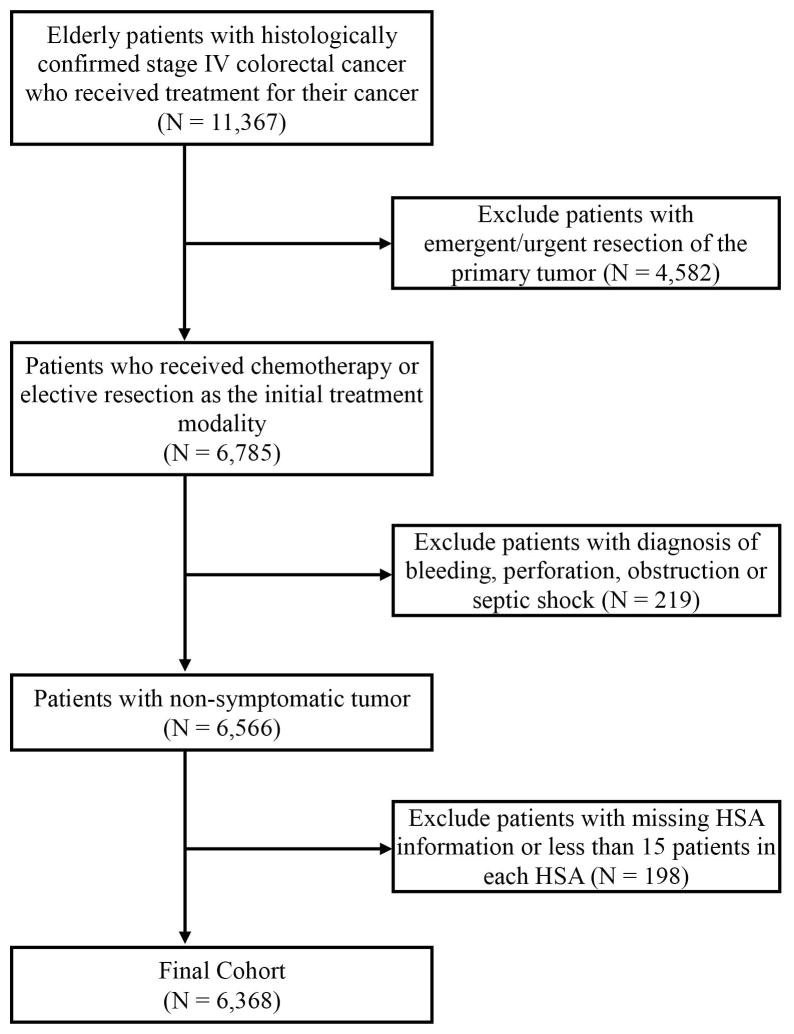
We studied older patients (age ≥66 years) with histologically confirmed stage IV CRC from 2001 to 2009 who were continuously enrolled in Medicare and received treatment (either chemotherapy or resection) for CRC. We excluded the following: 1) patients who received emergency or urgent resection of the primary tumour as identified from the MedPAR file; 2) patients with symptomatic tumour defined as those with a primary diagnosis of bleeding, perforation, obstruction or septic shock; and 3) patients with missing health service area (HSA) information or those in HSAs having less than 15 patients.
Treatment
The primary variable of interest was the use of chemotherapy or resection of the primary tumor as the initial treatment. Chemotherapy was identified using Healthcare Common Procedure Coding System (HCPCS) Codes, International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) procedure and diagnosis codes, J codes, and revenue center codes (Appendix 1). Resection of the primary tumor was identified using ICD-9-CM procedure and Current Procedural Terminology, Fourth Edition (CPT-4) codes for colorectal resections (Appendix 1). We determined the date of first chemotherapy use and/or date of resection of the primary tumor. Whichever was earlier was considered the initial treatment.
Outcome
The primary outcome measure was 2-year cancer-specific survival from the date of initial treatment as the 5-year survival rate for stage IV colorectal cancer is low (12.5%) and current chemotherapy mainly improves the 2-year survival rate [1,4]. Furthermore, the study used an intention-to-treat analysis approach to compare the initial treatment modality.
Covariates
Covariates were selected based on prior studies and clinical knowledge. We included age, sex, race/ethnicity, Medicare/Medicaid dual eligibility status, SEER region, year of diagnosis, cancer type, resection of metastatic disease (metastasectomy), Charlson comorbidity score and function-related indicators. All covariates were assessed in a year prior the cancer diagnosis date. Metastasectomy was defined as liver or pulmonary resection and identified using CPT and ICD-9-CM procedure codes (Appendix 1). Comorbidity was assessed using the Charlson comorbidity score [14,15]. To capture functional status of patients, we included the following indicators: mobility, blood transfusion, oxygen use, sepsis, malnutrition, fall-related injury and syncope [16]. Multimodality therapy (i.e., patients who received chemotherapy then resection of the primary tumor or vice versa), metastasectomy, and receipt of radiation were included as time-dependent covariates.
Descriptive Analysis
Descriptive statistics were used to describe the study cohort. A Cochran-Armitage test for trend was used to evaluate time trends. Baseline covariates between the two treatment groups were compared using standardized difference scores. Unlike t-test or chi-square test, the standardized difference is not affected by sample size and can be used to compare baseline characteristics between two groups. A standardized difference of 0.2 (or 20%) indicates a small effect size and comparability between two groups [17,18]. The Kaplan-Meier method was used to determine the unadjusted survival time curve for two treatment groups.
Cox Proportional Hazards Regression Analysis
First, we constructed unadjusted Cox proportional hazards regression models to determine the association of initial treatment with survival. Then, a multivariable Cox proportional hazards regression, which only controls for measured confounding, was used to evaluate the association between initial treatment and survival after adjusting for potential confounders.
Instrumental Variable Analysis
Instrumental variable analysis is a useful method to control for selection bias and unmeasured confounding in observational comparative effectiveness research studies [19, 20]. In our study there were multiple potential sources of unmeasured confounding. Patients receiving chemotherapy first may have had a greater burden of metastatic disease and been sicker than those who underwent initial surgery. A patient’s likelihood of receiving chemotherapy first may be determined by Eastern Cooperative Oncology Group (ECOG) or Karnofsky performance status (KPS). However, these variables are not captured in the SEER-Medicare data, making it difficult to estimate the true effect of treatment on the outcome. We used the percentage of those receiving initial chemotherapy within the HSA as the instrumental variable. This instrumental variable has been used in previous comparative effectiveness studies [21, 22]. It acts as a natural randomization of patients to regional HSA-based treatment groups that determine the likelihood of receiving the chemotherapy as the initial treatment [20–22]. Thus, instrumental variable analysis exploits the natural variation in treatment choice and uses that to control for unmeasured confounding [20]. A good instrument should affect treatment, should be unrelated to patient characteristics, and should be related to the outcome only through its association with treatment [19]. We used the partial F-test to confirm that the instrument is strongly correlated with the treatment. An F-statistic more than 10 suggests that the instrument is strong. To confirm that the instrument is not related to outcome through patient characteristics we evaluated the balance of covariates across the level of the instrument (below and above the median value of an instrumental variable). We used the two-stage residual inclusion (2SRI) method for instrumental variable analysis [23]. We performed the following sensitivity analyses: (i) by including colon cancer patients only, (ii) stratified analysis by age group (above and below 75 years) to determine the role of age, (iii) by time period (2001–2004 and 2005–2009), and (iv) by excluding patients who underwent resection of metastatic disease. We used Bonferroni correction method with a significance level of 0.0083 (0.05/6) to adjust for multiplicity while performing subgroup analysis.
All analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC). Statistical significance was accepted at the p<0.05 level. The Institutional Review Board at the University of Texas Medical Branch exempted the study from review.
RESULTS
Cohort Description and Baseline Characteristics
The final cohort included 6,368 patients (Figure 1). The mean age of the cohort was 76.0±6.5 years, 50.9% were female, and 85.4% were non-Hispanic white. As the initial treatment, 2,216 (34.8%) received chemotherapy and 4,152 (65.2%) underwent resection of the primary tumor. The median time from diagnosis to receipt of first treatment was 33 days (interquartile range, 21–49 days). Patients who received chemotherapy as the initial treatment were more likely to be younger, have rectal cancer as primary cancer and be less likely to undergo metastasectomy compared to those who received resection first (Table 1).
Figure 1.
Cohort Selection Diagram
Table 1.
Balance of Baseline Patient Characteristics across Treatment Groups and the Median Level of the Instrumental Variable
| Patient Treatment Status | Instrumental Variable Status (Initial Chemotherapy Rate by HSA) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Patient Characteristics | Resection of Primary Tumor as Initial Treatment N (%) |
Chemotherapy as Initial Treatment N (%) |
Absolute Standardized Difference (%) |
Below Median (≤36%) N (%) |
Above Median (>36%) N (%) |
Absolute Standardized Difference (%) |
| Number of patients | 4,152 | 2,216 | 3,169 | 3,199 | ||
|
| ||||||
| Age, mean (SD) | 76.6 (6.8) | 74.9 (6.0) | 26.6 | 75.37 (6.5) | 76.3 (6.6) | 9.3 |
|
| ||||||
| Sex | 9.8 | 4.8 | ||||
| Male | 1,966 (47.4) | 1,158 (52.3) | 1,593 (50.3) | 1,531 (47.9) | ||
| Female | 2,186 (52.6) | 1,058 (47.7) | 1,576 (49.7) | 1,668 (52.1) | ||
|
| ||||||
| Race | 4.9 | 0.1 | ||||
| White | 3,569 (86.0) | 1,866 (84.2) | 2,705 (85.4) | 2,730 (85.3) | ||
| Non-White | 583 (14.0) | 350 (15.8) | 464 (14.6) | 469 (14.3) | ||
|
| ||||||
| Medicare-Medicaid dual eligibility | 632 (15.2) | 367 (16.6) | 3.7 | 507 (16.0) | 492 (15.4) | 1.7 |
|
| ||||||
| Charlson comorbidity | 6.6 | 4.2 | ||||
| 0 | 2,437 (58.7) | 1,371 (61.9) | 1,903 (60.1) | 1,905 (59.6) | ||
| 1 | 1,060 (25.5) | 516 (23.3) | 760 (24.0) | 816 (25.5) | ||
| 2 | 388 (9.3) | 194 (8.8) | 296 (9.3) | 286 (8.9) | ||
| ≥3 | 267 (6.4) | 135 (6.1) | 210 (6.6) | 192 (6.0) | ||
|
| ||||||
| Summary Charlson comorbidity score, median (Q1, Q3) | 0 (0, 2) | 0 (0, 2) | 5.6 | 0 (0, 2) | 0 (0, 2) | 2.9 |
|
| ||||||
| Function-related indicators | ||||||
| Mobility | 176 (4.2) | 96 (4.3) | 0.5 | 136 (4.3) | 136 (4.2) | 0.2 |
| Blood transfusion | 425 (10.2) | 144 (6.5) | 13.5 | 317 (10.0) | 252 (7.9) | 7.4 |
| Oxygen use | 128 (3.1) | 89 (4.0) | 5.1 | 117 (3.7) | 100 (3.1) | 3.1 |
| Sepsis | 56 (1.3) | 23 (1.0) | 2.9 | 38 (1.2) | 42 (1.3) | 1.3 |
| Malnutrition | 119 (2.9) | 75 (3.4) | 3.0 | 96 (3.0) | 98 (3.1) | 0.2 |
| Fall-related injury | 501 (12.1) | 238 (10.7) | 4.2 | 375 (11.8) | 364 (11.4) | 1.4 |
| Syncope | 246 (5.9) | 95 (4.3) | 7.5 | 177 (5.6) | 164 (5.1) | 2.0 |
|
| ||||||
| Cancer type | ||||||
| Colon | 3,466 (83.5) | 1,478 (66.7) | 39.5 | 2,478 (78.2) | 2,466 (77.1) | 2.6 |
| Rectum | 686 (16.5) | 738 (33.3) | 691 (21.8) | 733 (22.9) | ||
|
| ||||||
| Metastasectomy | 1,047 (25.2) | 63 (2.8) | 68.1 | 624 (19.7) | 486 (15.2) | 11.9 |
|
| ||||||
| Year of diagnosis | 28.3 | 11.3 | ||||
| 2001 | 575 (13.9) | 210 (9.5) | 394 (12.4) | 391 (12.2) | ||
| 2002 | 543 (13.1) | 207 (9.3) | 348 (11.0) | 402 (12.6) | ||
| 2003 | 557 (13.4) | 263 (11.9) | 400 (12.6) | 420 (13.1) | ||
| 2004 | 542 (13.4) | 260 (11.7) | 401 (12.7) | 401 (12.6) | ||
| 2005 | 502 (12.1) | 262 (11.8) | 402 (12.7) | 362 (11.3) | ||
| 2006 | 433 (10.4) | 236 (10.7) | 321 (10.1) | 348 (10.9) | ||
| 2007 | 394 (9.5) | 256 (11.5) | 324 (10.2) | 326 (10.2) | ||
| 2008 | 328 (7.9) | 278 (12.6) | 282 (8.9) | 324 (10.1) | ||
| 2009 | 278 (6.7) | 244 (11.0) | 297 (9.4) | 225 (7.0) | ||
HSA, health service area.
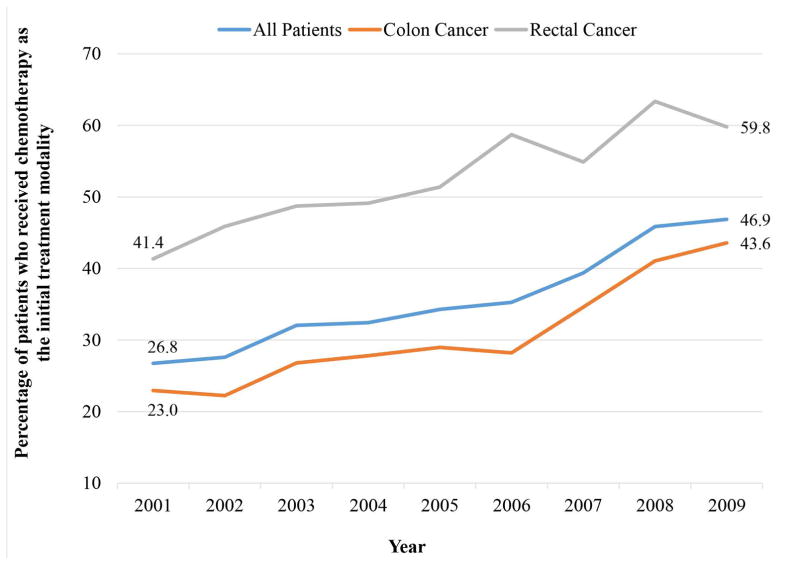
The use of chemotherapy as the initial treatment increased from 26.8% in 2001 to 46.9% in 2009 (p<0.0001). In rectal cancer, initial chemotherapy increased from 41.4% in 2001 to 59.8% in 2009 (p<0.0001); in colon cancer, it increased from 23.0% to 43.6% (p<0.0001, Figure 2). Overall, 45.1% (n=2875) of patients received multimodality therapy, with 16.4% (n=363) of patients who initially received chemotherapy having subsequent surgical resection and 60.5% (n=2,512) of patients who underwent initial resection receiving subsequent chemotherapy. Among patients who received chemotherapy as the initial treatment (n=2,216), 3.9% underwent subsequent emergency or urgent resection in the 2-year follow-up period. Patients who underwent resection of the primary tumor as the initial treatment in 2007 to 2009 (n=1,646) were more likely to be older and have higher Charlson comorbidity scores compared to patients who underwent resection in 2001 to 2003 (n=1,011); for all other characteristics, both groups were similar.
Figure 2.
Time Trends in the Use of Chemotherapy as the Initial Treatment
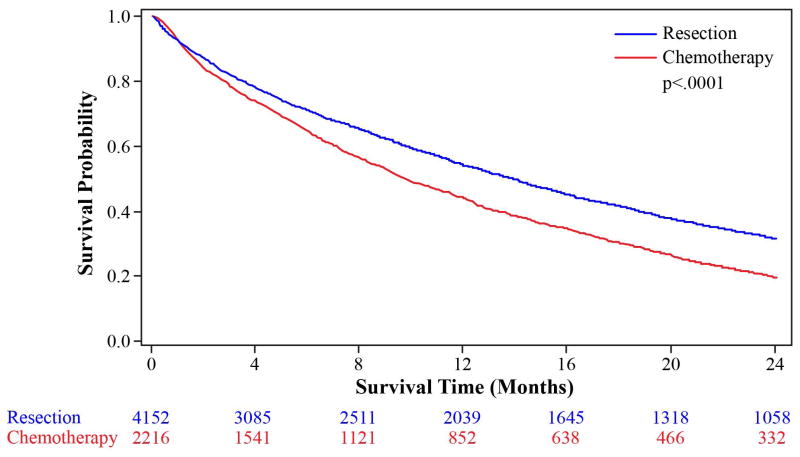
In the unadjusted Kaplan-Meier analysis, the overall 2-year survival was better in patients who received resection of the primary tumor as the first treatment compared to chemotherapy (Figure 3). Similar results were found for a subset of patients with colon cancer only (Appendix 2).
Figure 3.
Kaplan-Meier Curve for 2-Year Survival for Colorectal Cancer Patients, Stratified by the Initial Treatment
Multivariable Cox Regression
In the unadjusted analysis, chemotherapy as the initial treatment was associated with a 35% higher risk of 2-year mortality (HR, 1.35; 95% CI, 1.27–1.44). This association was attenuated to 26%; however, it remained significant, after controlling for all confounders in a multivariable Cox proportional hazards regression model (HR, 1.26; 95% CI, 1.16 –1.36) (Table 2).
Table 2.
Association of Chemotherapy as the Initial Treatment with Survival
| Chemotherapy as the Initial Treatment (Ref = Resection of the Primary Tumor as the Initial Treatment) | All Patients Hazard Ratio (95% CI) | Colon Cancer PatientsHazard Ratio (95% CI) |
|---|---|---|
| Unadjusted Cox model | 1.35 (1.27,1.44) | 1.48 (1.35,1.63) |
| Adjusted Cox modela | 1.26 (1.16,1.36) | 1.36 (1.20, 1.54) |
| Instrumental variable analysisb, c | 0.68 (0.44,1.04) | 0.78 (0.39, 1.54) |
Model was adjusted for age, gender, race, dual eligibility status, regions, Charlson comorbidity score, function related indicators (mobility, blood transfusion, oxygen use, sepsis, malnutrition, fall-related injury, syncope), cancer type, year and time-dependent covariates such as receipt of multimodality therapy, metastasectomy and radiation.
Model was adjusted for residuals obtained from the first stage of instrumental variable analysis and age, gender, race, dual eligibility status, regions, Charlson comorbidity score, function related indicators (mobility, blood transfusion, oxygen use, sepsis, malnutrition, fall-related injury, syncope), cancer type, year and time-dependent covariates such as receipt of multimodality therapy, metastasectomy and radiation.
F-statistics for instrumental variable = 62.4
Instrumental Variable Analyses
The percentage use of chemotherapy as the initial treatment ranged 10%–80.6% across 100 HSAs (Appendix 3). The F-statistics was 62.4, indicating that the instrument was strongly correlated with the treatment. In addition to the balance of baseline characteristics across the level of exposure, Table 1 also demonstrates the balance of baseline characteristics above and below the median value of the instrumental variable (36%). In the original cohort, substantial imbalance existed between treatment groups, suggesting strong selection bias in the receipt of initial treatment. When patient characteristics were compared using the median level of the instrumental variable, the standardized difference was attenuated (<0.20 for all variables). All variables were also well-balanced across the level of instrumental variable quintiles (Appendix 4). The instrumental variable analysis that accounted for both known and unknown confounders found that patients receiving chemotherapy as the initial treatment had lower risk of mortality (HR, 0.68; 95% CI, 0.44–1.04) compared to those undergoing resection of the primary tumor as the initial treatment; however, the association approached but did not reach statistical significance (Table 2).
Sensitivity Analyses
Results were similar to the main analysis in a subgroup of patients with colon cancer, in patients between 65 and 74 years of age, patients over 75 years of age, both time periods (2001–2004 and 2005–2009) and when we excluded patients with metastasectomy from the analysis. All sensitivity analyses results are reported in Table 2 and Appendix 5.
DISCUSSION
The study found that the use of chemotherapy as the initial treatment for older patients presenting with asymptomatic unresectable stage IV CRC has increased in the last decade. Patients receiving initial surgery vs. chemotherapy in stage IV had improved survival outcomes in unadjusted and in standard multivariate models. When accounting for measured and unmeasured confounders using an instrumental variable analysis, chemotherapy as the initial treatment showed benefit on survival compared to initial surgical resection. This association however did not achieve statistical significance. Results were similar in all six subgroup analysis.
To date, randomized controlled trials (RCT) attempting to compare survival with chemotherapy vs. surgical resection of the primary tumour as the initial treatment have had difficulty accruing patients [8]. We used observational data and an instrumental variable approach to address this question, as we hypothesized significant unmeasured confounding by indication. Traditional Cox regression showed that chemotherapy as the initial treatment was associated with worse 2-year survival compared to resection of the primary tumor. In the instrumental variable analysis, the association was not only attenuated, but direction of the association was reversed, showing improved survival for patients receiving initial chemotherapy. However, it was not statistically significant as the confidence interval included one.
Traditionally, resection of the primary tumor was preferred in patients with stage IV CRC to prevent tumor-related complications such as obstruction, perforation, and bleeding [9]. The introduction of oxaliplatin and irinotecan to chemotherapy regimens has challenged this approach.. The NCCN guidelines changed in 2006 [7]. Population-based SEER-Medicare data from 1991 to 2000 demonstrated that only 12.2% received chemotherapy as the initial treatment [24]. Our study covers a more contemporary period, and demonstrates an increasing trend with chemotherapy as the initial treatment increasing to 34.8% over the whole period, reaching 47% in 2009. A recent study based on SEER data showed a reduction in patients undergoing primary tumor resection, from 74.5% in 1998 to 57.4% in 2010. However, the median survival rate increased during the same period [25]. The improved survival may be attributed to the improved treatment due to the increased use of chemotherapy.
Despite NCCN guidelines, significant controversy remains regarding the timing and role of surgical resection and potential overuse of primary tumor resection in patients presenting with stage IV disease [9, 25]. Our study supports a meta-analysis of seven observational studies concluded that initial resection of the tumor provides only minimal palliative benefit but higher complication rates that can delay the administration of systemic chemotherapy [11]. The Cochrane collaboration found that resection of the primary tumor did not improve survival or reduce complications in asymptomatic patients with unresectable stage IV CRC managed with initial chemotherapy/radiation [8]. Another meta-analysis of eight observational studies comparing chemotherapy alone vs. chemotherapy and surgical resection and showed an improvement in the survival of patients managed with palliative resection of their primary tumor [12]. However, these studies were retrospective, small, and subject to considerable selection bias. A recent study, not included in any of the above meta-analyses, found that resecting the primary tumor in patients receiving chemotherapy for stage IV CRC did not improve overall survival in when resection of all gross disease was impossible (unresectable), supporting the use of chemotherapy as the initial treatment in unresectable patients [26].
In subgroup analysis, we found improved but not significant survival benefit of chemotherapy as the initial treatment among patients aged 65 to 74 years. These results were in accordance with some previous studies that also found improved survival in patients younger than 75 years [27, 28]. In addition, more patients in the younger group (14% vs. 11%) underwent surgery after chemotherapy, possibly explaining the survival benefit in this group. These patients are likely to represent a selected group of patients with good tumor response who opted for more aggressive therapy and suggests an additional survival benefit with removal of the primary tumor even in the palliative setting.
In clinical practice, the choice of initial treatment may be influenced by disease burden, frailty, and patient preference. Chemotherapy first offers several advantages. Initial chemotherapy eliminates the immediate risk of operative complications that prevent receipt of systemic chemotherapy [9, 11]. Resection can then be reserved for patients who respond to chemotherapy and would most likely benefit from this approach. A prospective multicenter phase II trial, RCTs, systematic reviews and meta-analysis have all demonstrated that chemotherapy is a safe initial treatment [12, 13, 29, 30]. In patients treated with chemotherapy-first, the rate of complications ranged 6–29% [11]. In our study, less than 4% of patients who received chemotherapy-first underwent subsequent emergency or urgent resection.
The results from an instrumental variable analysis differed significantly compared to the Cox model. Use of instrumental variable analysis changed the direction of the association. Cox regression models cannot control for unmeasured confounding, which was controlled for in the instrumental variable analysis. This may explain the change in hazards ratio. Instrumental variable analysis makes strong assumptions and some are not empirically verifiable. There is really no way to know which of these answers is “correct”; violation of assumptions may produce biased estimates. However, in this study, instrument was strongly correlated with treatment (F=62.4) and covariates were well-balanced by the level of instrumental variable.
Our study has several limitations. We cannot measure KPS or ECOG, burden of disease, or whether chemotherapy was given with intent to downstage and resect. However our instrumental variable analysis was designed to overcome some of this selection bias and unmeasured confounding. Our approach is unique in that we compared outcomes on an intent-to-treat basis, based on initial treatment strategy regardless of receipt of the other modality. This is important, as patients who initially undergo surgery often do not get chemotherapy secondary to surgical complications. Our analysis supports this observation. Patients who had surgery and no chemotherapy had the worst survival. Likewise, given the controversy regarding the need for resection in stage IV disease, this approach included patients who did and did not undergo surgery after chemotherapy. It is possible that patients who responded to chemotherapy got aggressive treatment with surgical resection, while those who progressed did not. This scenario would explain the change in the direction and magnitude of the HR when we used the instrumental variable and controlled for selection bias. The instrumental variable captures “practice style” in the geographic region and is unlikely to be related to patient survival [19–22]. The study results are applicable to Medicare patients only. We did not do a subgroup analysis on rectal cancer patients due to low sample size. While we tried to exclude patients with symptomatic tumors, patients may not be excluded if symptoms are not recorded correctly in the Medicare data.
In conclusion, the use of chemotherapy as the initial treatment has increased in the past decade. In patients with stage IV CRC, chemotherapy as the initial treatment offers similar or better 2-year survival compared to the primary resection of the tumor, with higher survival benefit among patients younger than 75 years. The goal of therapy is to optimize outcomes. Given the morbidity and mortality associated with colorectal resection in elderly patients, chemotherapy provides an option to patients who are not good candidates for resection. In patients with a large burden of disease or those who progress, surgery can be avoided.
Supplementary Material
What does this paper add to the literature?
The use of chemotherapy as the initial treatment for stage IV colorectal cancer has increased in the past decade. Chemotherapy as the initial treatment offers similar or better 2-year survival compared to the primary resection of the tumour.
Acknowledgments
Funding: Cancer Prevention Research in Texas Grant #RP140020-P03, Clinical and Translational Science Award #UL1TR000071, NIH T-32 Grant # 5T32DK007639, and AHRQ Grant #1R24HS022134.
The authors thank Sarah Toombs Smith, PhD, Science Editor and Assistant Professor in the Sealy Center on Aging, University of Texas Medical Branch at Galveston, for her editorial assistance.
Footnotes
Conflicts of interests: None
Presentation: The paper was presented as an oral presentation at the 31st International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE), Boston, MA.
References
- 1. [Accessed June 1, 2015];SEER Stat Fact Sheets: Colon and Rectum Cancer. http://seer.cancer.gov/statfacts/html/colorect.html.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 4.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 5.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology - Colon and Rectal Cancer – version 1. 2006. [Google Scholar]
- 7.National Comprehensive Network Guidelines (NCCN) [Accessed March 21, 2016];Clinical Practice Guidelines in Oncology - version 2. 2006 http://www.nccn.org/professionals/physician_gls/recently_updated.asp.
- 8.Cirocchi R, Trastulli S, Abraha I, et al. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst Rev. 2012;8:CD008997. doi: 10.1002/14651858.CD008997.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YW, Kim IY. The Role of Surgery for Asymptomatic Primary Tumors in Unresectable Stage IV Colorectal Cancer. Ann Coloproctol. 2013;29(2):44–54. doi: 10.3393/ac.2013.29.2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damjanov N, Weiss J, Haller DG. Resection of the primary colorectal cancer is not necessary in nonobstructed patients with metastatic disease. Oncologist. 2009;14(10):963–969. doi: 10.1634/theoncologist.2009-0022. [DOI] [PubMed] [Google Scholar]
- 11.Scheer MG, Sloots CE, van der Wilt GJ, Ruers TJ. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Ann Oncol. 2008;19(11):1829–1835. doi: 10.1093/annonc/mdn398. [DOI] [PubMed] [Google Scholar]
- 12.Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. 2010;34(4):797–807. doi: 10.1007/s00268-009-0366-y. [DOI] [PubMed] [Google Scholar]
- 13.Verhoef C, de Wilt JH, Burger JW, Verheul HM, Koopman M. Surgery of the primary in stage IV colorectal cancer with unresectable metastases. Eur J Cancer. 2011;47(Suppl 3):S61–66. doi: 10.1016/S0959-8049(11)70148-4. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Chrischilles E, Schneider K, Wilwert J, et al. Beyond comorbidity: expanding the definition and measurement of complexity among older adults using administrative claims data. Med Care. 2014;52(Suppl 3):S75–84. doi: 10.1097/MLR.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 17.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. SAS Global Forum 2012 [Google Scholar]
- 18.Tritchler D. Interpreting the standardized difference. Biometrics. 1995;51(1):351–353. [PubMed] [Google Scholar]
- 19.Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19(6):537–554. doi: 10.1002/pds.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rassen JA, Brookhart MA, Glynn RJ, Mittleman MA, Schneeweiss S. Instrumental variables I: instrumental variables exploit natural variation in nonexperimental data to estimate causal relationships. J Clin Epidemiol. 2009;62(12):1226–1232. doi: 10.1016/j.jclinepi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earle CC, Tsai JS, Gelber RD, Weinstein MC, Neumann PJ, Weeks JC. Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol. 2001;19(4):1064–1070. doi: 10.1200/JCO.2001.19.4.1064. [DOI] [PubMed] [Google Scholar]
- 22.McDowell BD, Chapman CG, Smith BJ, Button AM, Chrischilles EA, Mezhir JJ. Pancreatectomy predicts improved survival for pancreatic adenocarcinoma: results of an instrumental variable analysis. Ann Surg. 2015;261(4):740–745. doi: 10.1097/SLA.0000000000000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27(3):531–543. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Temple LK, Hsieh L, Wong WD, Saltz L, Schrag D. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22(17):3475–3484. doi: 10.1200/JCO.2004.10.218. [DOI] [PubMed] [Google Scholar]
- 25.Hu CY, Bailey CE, You YN, et al. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surgery. 2015;150(3):245–251. doi: 10.1001/jamasurg.2014.2253. [DOI] [PubMed] [Google Scholar]
- 26.Yun JA, Huh JW, Park YA, et al. The role of palliative resection for asymptomatic primary tumor in patients with unresectable stage IV colorectal cancer. Dis Colon Rectum. 2014;57(9):1049–1058. doi: 10.1097/DCR.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 27.Kordatou Z, Kountourakis P, Papamichael D. Treatment of older patients with colorectal cancer: a perspective review. Therapeutic Advances in Medical Oncology. 2014;6(3):128–140. doi: 10.1177/1758834014523328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Steenbergen LN, Elferink MA, Krijnen P, et al. Improved survival of colon cancer due to improved treatment and detection: a nationwide population-based study in The Netherlands 1989–2006. Ann Oncol. 2010;21(11):2206–2212. doi: 10.1093/annonc/mdq227. [DOI] [PubMed] [Google Scholar]
- 29.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24(25):4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.