Abstract
Purpose
Recently, a large randomized trial found a survival advantage among patients who received elective neck dissection in conjunction with primary surgery for clinically node-negative oral cavity cancer compared with those receiving primary surgery alone. However, elective neck dissection comes with greater upfront cost and patient morbidity. We present a cost-effectiveness analysis of elective neck dissection for the initial surgical management of early-stage oral cavity cancer.
Methods
We constructed a Markov model to simulate primary, adjuvant, and salvage therapy; disease recurrence; and survival in patients with T1/T2 clinically node-negative oral cavity squamous cell carcinoma. Transition probabilities were derived from clinical trial data; costs (in 2015 US dollars) and health utilities were estimated from the literature. Incremental cost-effectiveness ratios, expressed as dollar per quality-adjusted life-year (QALY), were calculated with incremental cost-effectiveness ratios less than $100,000/QALY considered cost effective. We conducted one-way and probabilistic sensitivity analyses to examine model uncertainty.
Results
Our base-case model found that over a lifetime the addition of elective neck dissection to primary surgery reduced overall costs by $6,000 and improved effectiveness by 0.42 QALYs compared with primary surgery alone. The decrease in overall cost despite the added neck dissection was a result of less use of salvage therapy. On one-way sensitivity analysis, the model was most sensitive to assumptions about disease recurrence, survival, and the health utility reduction from a neck dissection. Probabilistic sensitivity analysis found that treatment with elective neck dissection was cost effective 76% of the time at a willingness-to-pay threshold of $100,000/QALY.
Conclusion
Our study found that the addition of elective neck dissection reduces costs and improves health outcomes, making this a cost-effective treatment strategy for patients with early-stage oral cavity cancer.
INTRODUCTION
Oral cavity carcinoma composes approximately 3% of all cancers worldwide with an estimated incidence of more than 300,000 new cases per year.1 Surgical excision of the primary tumor represents the mainstay of treatment for patients with early-stage (T1/T2) node-negative (N0) oral cavity cancer. Patients with clinically node-negative disease have a high risk of occult nodal involvement, which can exceed 30% in select patients.2-4 Despite this high risk of occult disease, the question of whether to recommend elective neck dissection (END) for patients with clinically node-negative disease represents an area of historic controversy. The oncologic benefit of END in conjunction with primary surgery was recently demonstrated in a large randomized phase III study by D’Cruz et al5 from the Tata Memorial Centre, which compared this strategy to watchful waiting (WW). The study found that the use of END improved disease-free survival and overall survival compared with WW among patients with early-stage clinically node-negative oral cavity cancer.
The D’Cruz study found improved survival with END, although this additional procedure has the capacity to increase treatment costs and carries the risk of increased morbidity and decreased quality of life. This conundrum raises the natural question of whether END is a cost-effective strategy that should be included in standard treatment protocols. This question is particularly relevant, given the high incidence and mortality of oral cavity cancer in low- and middle-income countries in which health care decisions must often be made under heavily constrained resources.1 The purpose of this study was to perform a cost-effectiveness analysis of END at the time of primary tumor resection versus WW for patients with early-stage clinically node-negative oral cavity carcinoma. This analytic method incorporates a broad comparison of the differences in survival, cost, and quality of life between the two treatment strategies.
METHODS
Decision Model
We compared the cost effectiveness of two surgical strategies for managing the neck in patients with early-stage (T1/T2) clinically node-negative (N0) oral cavity squamous cell carcinoma: (1) primary resection with END and (2) primary resection followed by WW. We constructed a Markov model to simulate treatments, toxicities, morbidity, and survival in this patient population. The state-transition diagram in Figure 1 demonstrates how patients flow through the cost-effectiveness model. The four main health states included no evidence of disease, successfully treated with salvage therapy, multiply recurrent/metastatic disease, and death. The Markov model was based on a third-party payer’s perspective and was run by using a 1-year cycle length over a 30-year time horizon. The model was built using TreeAge Pro 2015 (TreeAge Software, Williamstown, MA).
Fig 1.
State transition diagram. The four main health states are represented by ovals. Patients may transition from “No evidence of disease” to “Salvaged,” “Multiply recurrent or metastatic,” or “Death.” Patients may receive adjuvant therapies after their initial surgery, including radiation with or without chemotherapy. Patients with local or regional recurrence may receive salvage surgery and radiation with or without chemotherapy. Patients with multiply recurrent or metastatic disease receive palliative chemotherapy.
Model Probabilities
We assumed that all patients entered the model with early-stage clinically node-negative oral cavity cancer and received either surgical resection of the primary oral cavity tumor and END or primary resection alone (WW). Outcomes from D’Cruz et al5 were used to model adjuvant treatments after initial surgery, patterns of disease spread, and salvage treatments after recurrence. After initial surgery, a subset of patients received adjuvant radiation therapy or adjuvant cisplatin-based chemoradiotherapy. Indications for radiotherapy are outlined in the D’Cruz trial; briefly, these included positive nodes, primary tumor depth of > 10 mm, and positive margin status. The indications for use of chemotherapy delivered concurrently with radiation were not reported by D’Cruz. It was therefore assumed that cisplatin-based chemotherapy with radiation would be administered to patients with adverse features such as extracapsular nodal spread, perineural invasion, or lymphovascular invasion. Although the absolute indications for chemoradiation in the United States differ slightly (extracapsular spread and positive margin),6,7 this limitation was considered a fair trade-off for retaining consistency with the outcomes reported in D’Cruz et al.5 Patients who developed local or regional recurrence underwent salvage surgery when possible and adjuvant treatment after salvage as described in the D’Cruz study. Patients who developed multiply recurrent disease or metastatic disease were considered incurable, received palliative chemotherapy, and remained in the multiply recurrent/metastatic health state until death.
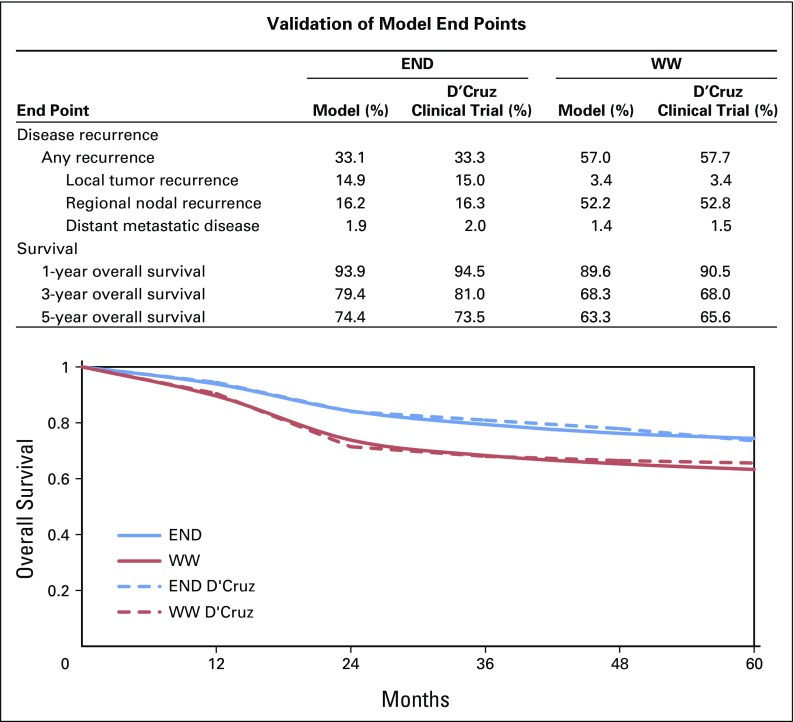
We internally validated our model through direct comparison with the D’Cruz study. Figure 2 demonstrates the results of our validation, which shows good agreement between our predicted survival and disease recurrence patterns compared with those in the D’Cruz study.
Fig 2.
Cost-effectiveness model validation results. The top panel (table) shows how our model predicts disease recurrence and overall survival compared with the D’Cruz randomized trial. The bottom panel (plot) shows how our model predicts survival compared with the D’Cruz randomized trial. END, elective neck dissection; WW, watchful waiting.
Costs
This analysis was performed from a third-party payer’s perspective. We included costs of all aspects of treatment and follow-up, including the cost of primary tumor resection, neck dissection, chemotherapy, and radiation, as well as the cost of remission and the cost of end-of-life care. Primary tumor resection, the added cost of neck dissection at the time of primary oral resection, the total cost of stand-alone salvage neck dissection, and the cost of radiation were estimated from primary literature (Table 1). The costs of chemotherapy concurrent with radiation and in the metastatic setting were determined from wholesale drug pricing coupled to chemotherapy administration costs available via the Health Care Utilization Project.15 The cost of end-of-life care was estimated from the literature; all those who entered the terminal health state “death” were charged this cost. All costs were adjusted to 2015 US dollars by using the Consumer Price Index and were rounded to the nearest $100. All costs and health utilities were discounted at 3% per year.
Table 1.
Parameters for Cost-Effectiveness Model

Outcome Measures
Effectiveness was measured in quality-adjusted life-years (QALYs), which reflects the product of a patient’s health utility over time. Health utility, in effect, measures quality of life and ranges from 1 (perfect health) to 0 (death). We obtained health utility scores from the literature, preferentially using prospectively acquired utility measurements whenever possible. In general, all patients had a similar starting baseline health utility score after primary tumor resection, and each patient received health utility deductions (tolls) for additional adjuvant treatment or progressive disease. Because acute toxicity in the D’Cruz trial was minimal, with little difference between treatment arms, we did not consider acute toxicity in our model. Given the long-term adverse quality-of-life impact of radiation and chemoradiation, we assumed that these health utility tolls would extend over the course of a patient’s life. Specific values of the health utilities, utility tolls, and primary literature sources used in this study are included in Table 1.
Analysis
Cost effectiveness of one treatment compared with another was measured with an incremental cost-effectiveness ratio (ICER) which represents the incremental cost per QALY gained. We considered a treatment cost effective if the ICER fell under a willingness-to-pay threshold of $100,000/QALY. It is important to note that willingness-to-pay thresholds vary by person, region, health care system, and country. We chose $100,000/QALY because this reflects a commonly used threshold in cost-effectiveness research.21 Treatments that were more effective and less costly were considered dominant, and with dominated strategies, the ICERs would be negative and are not reported.
Our main (base case) cost-effectiveness analysis used our best estimates of cost, health utilities, and transition probabilities to calculate effectiveness (QALY) and overall cost for each treatment strategy (END and WW). In addition, we performed one-way deterministic sensitivity analyses on each variable to identify factors that directly influenced cost-effectiveness. Finally, we performed a probabilistic sensitivity analysis to assess the impact of uncertainty in all transition probabilities, costs, and health utilities. The probabilistic sensitivity analysis used a Monte Carlo simulation with 100,000 iterations.22 Cost estimates were modeled with gamma distributions, which represent probability distributions that, like cost, are bound by the interval from 0 to infinity. All transition probabilities and health utilities were modeled with beta distributions, which are bound by the interval from 0 to 1. When available, the standard deviations of the individual variables were obtained from the literature. When they were not available, we used a standard deviation of 20% of the mean. We conducted separate probabilistic sensitivity analyses in which we varied the standard deviation from 10% to 40%, which did not have an impact on our results. For simplicity, we present only the results of our analysis that had a standard deviation of 20% of the mean. Base-case values for every variable along with 95% CIs for the distribution are found in Table 1.
RESULTS
Base Case Analysis
The primary cost-effectiveness analysis found that the lifetime cost of treatment using the END strategy was $63,400 compared with $69,400 for WW. Despite the increased upfront cost of END, this strategy proves less costly in the long run because of reduced costs related to using salvage therapy. When considering effectiveness, the END strategy yielded 9.20 QALYs compared with 8.78 QALYs for WW. Although upfront neck dissection leads to a slightly reduced quality of life in the END group, the improved survival and decreased recurrence rates increase the total measure of effectiveness above that of WW. Overall, END was $6,000 less costly and added 0.42 QALYs per patient compared with WW, making END a dominant strategy.
One-Way Sensitivity Analysis
Our cost-effectiveness model was most sensitive to assumptions about the survival benefit of END and the health utility deduction resulting from neck dissection (Fig 3). The D’Cruz study found that END reduced the risk of death by 36% (hazard ratio [HR], 0.64) compared with WW.5 Our sensitivity analysis demonstrated that if END reduced the risk of death by only 22% (HR, 0.78), then END no longer conferred an improvement in QALYs over WW (incremental effectiveness of 0.0 QALYs) and therefore END would no longer dominate WW. Furthermore, if END reduced the risk of death by 20% (HR, 0.80), then END would no longer be cost effective compared with WW at a willingness-to-pay threshold of $100,000/QALY. Our base-case analysis assumed a health utility deduction from a neck dissection of 0.072. However, if a neck dissection decreased quality of life by 0.118, then END no longer dominates WW, and if the utility deduction was 0.123 (nearly twice as bad), then END would no longer be cost effective compared with WW.
Fig 3.
One-way sensitivity analyses. The graphic shows the impact of varying individual model inputs on the cost effectiveness of elective neck dissection (END) compared with watchful waiting for early-stage oral cavity cancer. The individual plots represent (A) the impact of varying the hazard ratio for survival between END and watchful waiting, (B) the impact of varying the health utility deduction for a neck dissection, (C) the risk of recurrence for END, and (D) watchful waiting. A treatment strategy dominates another if it was less costly and more effective. A strategy was considered cost effective if the incremental cost-effectiveness ratio was under $100,000 per quality-adjusted life year.
Our cost-effectiveness model also demonstrated sensitivity to assumptions about disease recurrence. If the risk of disease recurrence in the END group increased from 33% to 38.6%, then END no longer dominated WW, and if the recurrence rate increased to 39.1%, then END would no longer be cost effective. Conversely, if the risk of disease recurrence of the WW cohort decreased from 57% to 51.8%, then END no longer dominated WW, and if the recurrence rate decreased to 51.3%, then END would not be a cost-effective strategy compared with WW. Other probability, cost, or utility variables did not have a substantial impact on the cost effectiveness of END.
Probabilistic Sensitivity Analysis
We conducted a probabilistic sensitivity analysis for END versus WW over 100,000 iterations varying all probabilities, costs, and utilities simultaneously. The results are demonstrated in Figure 4, which shows a scatterplot of the incremental costs versus incremental effectiveness from individual iterations in the probabilistic sensitivity analysis. In the probabilistic sensitivity analysis, END dominated WW in 72% of the iterations, and overall END was cost effective in more than 76% of the iterations at a willingness-to-pay threshold of $100,000/QALY. A cost-effectiveness acceptability curve demonstrating results of the probabilistic sensitivity analysis for different willingness-to-pay thresholds is included as Appendix Figure A1 (online only).
Fig 4.
Probabilistic sensitivity analysis scatter plot. This plot shows the distribution of incremental costs and incremental effectiveness of individual iterations of the probabilistic sensitivity analysis comparing the cost effectiveness of elective neck dissection (END) versus watchful waiting in early-stage oral cavity cancer. The dashed line represents a willingness-to-pay threshold of $100,000 per quality-adjusted life year (QALY). Blue and red dots represent individual iterations of the probabilistic sensitivity analysis; blue dots indicate iterations in which END was considered cost effective, and red dots indicate iterations in which END was not cost effective compared with watchful waiting.
DISCUSSION
The debate over END for patients with early-stage clinically node-negative oral cavity cancer was recently clarified by the survival benefit found in the D’Cruz randomized clinical trial conducted in India.5 This will likely influence practitioners in the United States and abroad to abandon the WW approach in favor of a more extensive elective surgery. As physicians, we often prioritize patient survival over everything else, but in today’s health care environment, one must consider cost and quality of life in the delivery of comprehensive health care. Cost-effectiveness research helps to define the value of interventions within health care by weighing the cost against potential benefits in survival and/or quality of life. With this study in early-stage oral cavity cancer, we sought to determine whether the survival benefit of END outweighed the added cost and morbidity of the procedure.
The key finding of this study is the observation that END costs less and improves effectiveness compared with WW. Decreased costs with an added procedure can be counterintuitive, although upfront END reduces the risk of recurrence and thus reduces the costs associated with treating recurrent disease. Given the high worldwide incidence of head and neck cancer, this cost-saving and quality-enhancing approach could prove particularly useful in countries with resource constraints.
The driving force behind the model’s prediction comes from a few key variables, notably the decreased quality of life associated with any neck dissection and the survival benefit derived from the END approach. Our one-way sensitivity analysis demonstrates that the reduced health utility from a neck dissection would need to double before END was no longer cost effective compared with WW. The model’s sensitivity to the survival benefit of neck dissection deserves further consideration. Although the addition of a neck dissection improved survival in the D’Cruz study, other factors influencing the risk of occult disease in the neck might influence the value of a neck dissection. For example, deeper tumor thickness in the oral tongue correlates with increased risk of occult metastatic nodal disease.23 Subset analysis in the D’Cruz study demonstrated survival advantage of END only in patients with invasion of > 3 mm, although the number of patients with invasion of ≤ 3 mm was limited. Regardless, one could hypothesize that patients with minimal depth of invasion might stand to benefit less from END, which would tend to push cost effectiveness away from the END strategy. In addition, the use of sensitive imaging such as positron emission tomography and/or computed tomography in staging or in follow-up might help further select patients at higher or lower risk of recurrence, which could influence the cost effectiveness of END.
Limitations in this cost-effectiveness study arise primarily from the quality of inputs used to inform our cost-effectiveness model. Ideally, all model inputs would come from high-level evidence such as prospective randomized trials, as well as prospective costing and health utility assessments. Although the D’Cruz randomized trial provided a large fraction of our transition probabilities, other model inputs such as costs and health utilities came from more heterogeneous sources. Health utilities in our study arose from different ascertainment techniques such as standard gamble,17 the European Quality of Life Five Dimensions (EuroQol-5D) questionnaire,20 or expert opinion.19 Similarly, costs of various components of treatment and follow-up came from an array of sources. Varying health utilities and costs could influence our estimation of cost effectiveness; however, our model was relatively insensitive to variation in these parameters, which suggests that our results and conclusions would change only if our estimated utilities and costs were dramatically inaccurate. A final limitation relates to our choice of perspective for this cost-effectiveness analysis. Considering a more expansive societal perspective would involve including indirect medical costs such as lost wages from being unable to go to work, reduced productivity, and other indirect costs patients might encounter. Including these costs in our cost-effectiveness model could influence our results, although given that patients in the WW group experienced more disease recurrence and had shorter median survival times, we suspect that considering the societal perspective would only skew cost-effectiveness further toward END.
In conclusion, our study demonstrates that the addition of END for patients with early-stage clinically node-negative oral cavity cancer reduces lifetime costs and improves health outcomes making this a cost-effective treatment strategy.
Appendix
Fig A1.
Cost-effectiveness acceptability curve. This plot represents the results of a probabilistic sensitivity analysis (for details, see Methods) comparing the cost-effectiveness of elective neck dissection (END) versus watchful waiting (WW) in early-stage oral cavity cancer. QALY, quality-adjusted life year.
Footnotes
The views expressed in this article are the authors’ only and are not official positions of the University of California, San Diego.
Supported by Grants No. 1TL1 TR001443 and KL2 TR00099 from the National Institutes of Health.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Joseph R. Acevedo, Katherine E. Fero, Bayard Wilson, Charles S. Coffey, James D. Murphy
Collection and assembly of data: Joseph R. Acevedo, Katherine E. Fero, Bayard Wilson, Charles S. Coffey, James D. Murphy
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cost-Effectiveness Analysis of Elective Neck Dissection in Patients With Clinically Node-Negative Oral Cavity Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Joseph R. Acevedo
No relationship to disclose
Katherine E. Fero
No relationship to disclose
Bayard Wilson
No relationship to disclose
Assuntina G. Sacco
Research Funding: VentiRx Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Pfizer
Other Relationship: Pfizer
Loren K. Mell
No relationship to disclose
Charles S. Coffey
No relationship to disclose
James D. Murphy
No relationship to disclose
REFERENCES
- 1. Ferlay J, Soerjomataram I, Ervik M, et al: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase. No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr.
- 2.Peng KA, Chu AC, Lai C, et al. Is there a role for neck dissection in T1 oral tongue squamous cell carcinoma? The UCLA experience. Am J Otolaryngol. 2014;35:741–746. doi: 10.1016/j.amjoto.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haddadin KJ, Soutar DS, Oliver RJ, et al: Improved survival for patients with clinically T1/T2, N0 tongue tumors undergoing a prophylactic neck dissection. Head Neck 21:517-525, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg JS, El Naggar AK, Mo V, et al. Disparity in pathologic and clinical lymph node staging in oral tongue carcinoma. Implication for therapeutic decision making. Cancer. 2003;98:508–515. doi: 10.1002/cncr.11526. [DOI] [PubMed] [Google Scholar]
- 5.D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373:521–529. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 6.Pfister DG, Spencer S, Brizel DM, et al. Head and neck cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13:847–855. doi: 10.6004/jnccn.2015.0102. http://www.ncbi.nlm.nih.gov/pubmed/26150579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 8.Matoscevic K, Graf N, Pezier TF, et al. Success of salvage treatment: A critical appraisal of salvage rates for different subsites of HNSCC. Otolaryngol Head Neck Surg. 2014;151:454–461. doi: 10.1177/0194599814535183. [DOI] [PubMed] [Google Scholar]
- 9.Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26:5518–5523. doi: 10.1200/JCO.2007.15.0102. [DOI] [PubMed] [Google Scholar]
- 10.Rivera F, García-Castaño A, Vega N, et al. Cetuximab in metastatic or recurrent head and neck cancer: The EXTREME trial. Expert Rev Anticancer Ther. 2009;9:1421–1428. doi: 10.1586/era.09.113. [DOI] [PubMed] [Google Scholar]
- 11.Gourin CG, Frick KD. National trends in oropharyngeal cancer surgery and the effect of surgeon and hospital volume on short-term outcomes and cost of care. Laryngoscope. 2012;122:543–551. doi: 10.1002/lary.22447. [DOI] [PubMed] [Google Scholar]
- 12.Garcia A, Palmer BJA, Parks NA, et al. Routine prophylactic central neck dissection for low-risk papillary thyroid cancer is not cost-effective. Clin Endocrinol (Oxf) 2014;81:754–761. doi: 10.1111/cen.12506. [DOI] [PubMed] [Google Scholar]
- 13.Kohler RE, Sheets NC, Wheeler SB, et al. Two-year and lifetime cost-effectiveness of intensity modulated radiation therapy versus 3-dimensional conformal radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2013;87:683–689. doi: 10.1016/j.ijrobp.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 14. Brockstein BE, Vokes EE, Eisbruch A: Locally advanced squamous cell carcinoma of the head and neck: Approaches combining chemotherapy and radiation therapy. http://www.uptodate.com.
- 15. Healthcare Cost and Utilization Project (HCUP): Nationwide HCUP databases. http://www.hcup-us.ahrq.gov/databases.jsp.
- 16. Vermorken JB, Mesia R, Rivera F, et al: Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116-1127, 2008 . [DOI] [PubMed]
- 17. doi: 10.1002/hed.23930. de Almeida JR, Moskowitz AJ, Miles BA, et al: Cost-effectiveness of transoral robotic surgery versus (chemo)radiotherapy for early T classification oropharyngeal carcinoma: A cost-utility analysis. Head Neck 38:589-600, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Enomoto LM, Schaefer EW, Goldenberg D, et al. The cost of hospice services in terminally ill patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2015;141:1066–1074. doi: 10.1001/jamaoto.2015.2162. [DOI] [PubMed] [Google Scholar]
- 19.Sher DJ, Tishler RB, Annino D, et al. Cost-effectiveness of CT and PET-CT for determining the need for adjuvant neck dissection in locally advanced head and neck cancer. Ann Oncol. 2010;21:1072–1077. doi: 10.1093/annonc/mdp405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. doi: 10.1111/coa.12502. Govers TM, Schreuder WH, Klop WM, et al: Quality of life after different procedures for regional control in oral cancer patients: Cross-sectional survey. Clin Otolaryngol 41:228-233, 2016 . [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite RS, Meltzer DO, King JT, Jr, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 22.Doubilet P, Begg CB, Weinstein MC, et al. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 23.Huang SH, Hwang D, Lockwood G, et al. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: A meta-analysis of reported studies. Cancer. 2009;115:1489–1497. doi: 10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]