Abstract
Purpose
The primary cause of death due to head and neck squamous cell carcinoma (HNSCC) is local treatment failure. The goal of this study was to examine this phenomenon using an unbiased approach.
Experimental design
We utilized HPV-negative cell lines rendered radiation resistant (RR) via repeated exposure to radiation, a panel of HPV-negative HNSCC cell lines and three cohorts of HPV-negative HNSCC tumors (n=68, 97, & 114) from patients treated with radiotherapy and subjected to genomic, transcriptomic and proteomic analysis.
Results
RR cell lines exhibited up-regulation of several proteins compared to controls, including increased activation of Axl and PI3 kinase signaling as well as increased expression of PD-L1. Additionally, inhibition of either Axl or PI3 kinase led to decreased PD-L1 expression. When clinical samples were subjected to RPPA and mRNA expression analysis in separate cohorts, PD-L1 was correlated with both Axl and PI3K signaling as well as dramatically associated with local failure following radiotherapy. This finding was confirmed examining a third cohort using immunohistochemistry. Indeed, tumors with high expression of PD-L1 had failure rates following radiotherapy of 60%, 70% and 50% compared to 20%, 25% and 20% in the PD-L1 low expression group (p=0.01, 1.9×10−3 and 9×10−4 respectively). This finding remained significant on multivariate analysis in all groups. Additionally, those patients with PD-L1 low/CD8+TILs high had no local failure or death due to disease (p=5×10−4 and p=4×10−4 respectively).
Conclusions
Taken together, our data point to a targetable Axl-PI3 kinase-PD-L1 axis that is highly associated with radiation resistance.
Keywords: PD-L1, Axl, PI3 kinase, Head and neck cancer, radiation
Introduction
Head and neck squamous cell carcinoma (HNSCC) leads to the death of more than 140,000 patients annually, primarily due to failure of local therapy, either surgery, radiotherapy or a combination of the two(1). Although advances in treatment, including the addition of cytotoxic chemotherapy to radiotherapy, have improved outcomes, approximately 30–50% of patients fail locally due to therapeutic resistance and ultimately succumb to their disease(2–4).
At present, the only effective biomarker of outcome in this tumor is the presence of human papilloma virus (HPV), which is associated with both improved response to therapy and outcome(5). Conversely, HPV-negative HNSCCs have far worse outcomes and lack clinically utilized biomarkers of response. Moreover, the addition of targeted agents to sensitize tumors to radiation in this disease has met with mixed results, with the most recent trial examining the addition of cetuximab showing no improvement over the current standard of care(2). Further targetable alterations in HNSCC potentially leading to therapeutic resistance, such as PI3 kinase and Axl activation, as well as our own work examining focal adhesion kinase expression have been identified(6–8). However, the vast majority of this work has not taken into account the interaction between tumor and host immune response.
Although radiation is thought to be locally immunosuppressive due to the high radiosensitivity of lymphocytes, results from several studies have led investigators to postulate that the immune response is required for maximal response to radiation. Treatment with radiation can lead to enhanced MHC class 1 antigen expression(9) as well as increased numbers of antigen presenting cells and interferon gamma secreting tumor infiltrating lymphocytes (TILs)(10). Further, in an in vivo model of melanoma, the response to radiation is abrogated in the absence of functional T-lymphocytes(11). In additional pre-clinical models, blockade of PD-1/PD-L1 signaling led to improved efficacy of radiation(12).
Moreover, signaling cascades related to radioresistance have also been linked to tumor immune response. For example, Hwu and colleagues have recently shown that tumor loss of PTEN in melanoma leads to increased inhibition of a T-cell mediated tumor response, with PI3 kinase inhibition at least partially reversing this phenomenon(13). Moreover, the epithelial to mesenchymal transition (EMT) has been linked to upregulation of immune checkpoints such as PD-L1(14,15). As EMT has been linked to both Axl and PI3 kinase activation as well as radiation resistance, it is possible that these signaling cascades may be driving both intrinsic radioresistance as well as inhibition of the tumor immune response necessary for radioresponse(16–18)
Despite this interesting pre-clinical data, the clinical significance of the interaction between tumor immunity and radioresponse is unclear, as are the tumor signaling events that modulate this interaction. In the current study, we utilized an integrated approach to examine this phenomenon and identified a potential modulation of PD-L1 expression via Axl-PI3K leading to clinical radioresistance and alterations in CD8+ TIL response.
Materials and Methods
Study design
The objectives of this study were two-fold. Firstly, we wished to identify novel markers of resistance to radiation treatment in HPV-negative HNSCC using a combination of HNSCC cells engineered to be resistant to radiation via repeated exposure, a large bank of HPV-negative HNSCC cell lines and multiple cohorts of pre-treatment HPV-negative HNSCC tumor specimens from patients treated uniformly (with surgery and post-operative radiation). These samples were subjected to proteomic and transcriptomic analysis using RPPA and mRNA expression array, respectively. Using this method we identified PD-L1 as highly associated with treatment failure following radiation. We validated this finding using a third similarly treated patient cohort using targeted immunohistochemical (IHC) analysis. Secondly, we wished to explore upstream signaling to PD-L1 in HPV-negative HNSCC. This was performed using a combination of clinical specimens and in vitro analysis using chemical inhibition of potential upstream regulators of PD-L1.
Reverse phase protein array (RPPA)
Samples from similar HPV-negative HNSCC patients treated with surgical resection followed by post-operative radiation were analyzed via RPPA as described previously(19,20). Tumor characteristics for this group are found in Supplemental Table 1. HPV-negative cell lines (48) were subjected to RPPA using the methodology described previously(8). To generate an induced radioresistance model, we subjected FADU HPV-negative HNSCC cells to 2 Gy twice a week for four weeks (FADU RR) cultured in parallel with unirradiated parental cells (FADU Parental). Cells were cultured and collected for baseline protein expression in triplicate and run in duplicate on RPPA as described previously(8). All cells were subjected to STR genotyping.
mRNA expression
Pre-treatment surgical specimens from 97 HPV-negative HNSCC patients treated with surgery and post-operative radiation were examined for mRNA expression (Supplemental Table 2). Total RNA was extracted for clinical tumors and analyzed via Illumina mRNA expression array as described previously(8). llumina HumanWG-6 V3 BeadChip human whole-genome expression arrays (Illumina, Inc., San Diego, CA) were used for RNA labeling and microarray hybridization. Following amplification and purification, each sample was hybridized for each array using standard Illumina protocols with streptavidin-Cy3 being used for detection. A total of 97 unique microarrays from 97 tumor specimens were processed for statistical analysis.
mRNA expression data from the Cancer Genome Atlas HPV-negative HNSCC cohort (n=243) was also analyzed.(21)
Tissue microarray (TMA)
A tissue microarray comprised of 114 of head and neck squamous cell carcinoma (HNSCC) was previously generated as described utilizing formalin fixed paraffin embedded (FFPE) tissue(22). All samples on the TMA represent surgical specimens of HNSCC patients treated with surgical resection followed by post-operative radiation, almost uniformly to 60 Gy. Tumor specimens were tested for p16 expression; those that were positive (n=12) were excluded from analysis (Supplemental Table 3). Immunohistochemical evaluation was performed on the TMA for all of the following markers utilizing standard techniques and the fully automated Leica Bond-Max stainers (Buffalo Grove, Illinois). The following antibodies were used: anti-PD-L1 (CD274) (clone SP142, dilution 1:10), Spring bioscience, Pleasanton CA)(23,24); anti-PD-1 (1:100), anti-CD8 (1:20), and CD4 (1:25) (Cell marque, Rocklin CA). Scoring of all immune markers were performed by an experienced head and neck pathologist (MW) and grouped and verified by a separate observer (UG). The average value was utilized between tumor replicates. For PD-L1 expression, positive staining was independently determined within the tumor cells (0–100%) via cellular morphology. For CD4 and CD8, the number of positive lymphoid cells was quantitated within the tumor (tumor infiltrating lymphocytes (TILs)) was counted. PD-1 staining was scored as either present, mixed (average of present and absent on duplicates) or absent. See Supplemental figure 1 for images of individual antibody staining.
Ion Torrent tumor sequencing
Isolated DNA was quantified by QPCR for Rnase P. Sequencing libraries were made from 10ng of DNA with a custom AmpliSeq library containing 739 amplicons designed against the entire coding regions of 17 genes (CASP8, CCND1, CDKN2A, EGFR, FAT1, FBXW7, HLA-A, KEAP1, NFE2L2, NOTCH1, NOTCH2, NSD1, PIK3CA, TGFBR2, TP53 and TP63) and the mutation hotspots (G12/13 and Q61) in HRAS according to manufacturer’s protocols. Samples were sequenced on Ion 318 chips to a depth of approximately 800×. Variants were called by using Ion Reporter software and manual filtering. HLA-A variants were ignored due to concerns about appropriate mapping.
Flow cytometry and immunoblot analysis
Flowcytometric analysis of PD-L1 expression was performed as described previously(25). Briefly, cells at 70–80% confluency were trypsinized to obtain a single cell suspension. Approximately 500,000 cells were washed with 2% FBS and re-suspended in 100ul 2% FBS and incubated with mouse anti-human PD-L1 (CD274) antibody that was directly conjugated with PE (1:200 dilution, Clone MIH1, BD Pharmingen, San Diego CA) for 30 min at 4°C. Cells were then washed in 2% FBS and analyzed using a Gallios flow cytometer, (Beckman Coulter, Brea CA) and FlowJo software (v.10.1). Prior to collection cells were treated with either Bay 80-6946 (PI3 kinase inhibitor) or SGI-7079 (Axl inhibitor) (both from Selleck Chemical, Houston TX) for 24h where indicated. Immunoblot analysis was performed as described previously(8). Commercially available siRNAs specific for either Axl or p110 PI3 kinase (GE Dharmacon, Lafayette CO) were transfected via electroporation (Nucleofector II, Amaxa, Basel, Switzerland) by using program T-001 with transfection agent T (Lonza, Visp, Switzerland). Cells were collected 48–72h post-transfection and assayed as described above. Comparisons between treatment groups were performed using Student’s t-test, with p<0.05 denoting significance.
Statistics
For all patient groups the optimal cutoff for PD-L1 group analysis was performed by generating a density plot of the staining distribution, which revealed a tri-modal distribution. The high PD-L1 expression group was defined as the upper tertile, whereas the low PD-L1 expression group represents the remaining patients. Survival curves were generated by using the method of Kaplan–Meier, with log-rank statistics used to determine significance. Univariate analysis was performed using Cox regression. Variables with a P < 0.1 were included in the multivariate model and forward stepwise Cox regression was performed. R software, SPSS statistical software (v.20), JMP Pro (v.11.2.1) and GraphPad Prism were used.
Results
PD-L1 is upregulated in HNSCC cells with acquired radioresistance and modulated by Axl and PI3 kinase in vitro
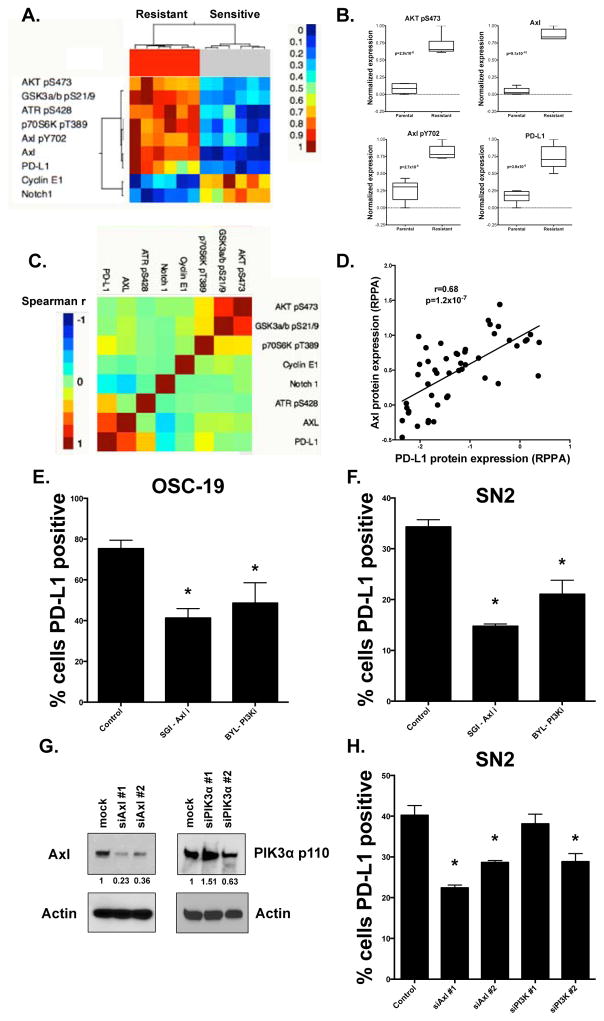
As a first step toward identifying pathways associated with radioresistance, we exposed an HPV-negative HNSCC cell line to repeated 2 Gy doses of RT over a 4 week period, yielding a line that was significantly more radioresistant (FADU RR) than the parental cell from which it was derived (Supplemental Figure 2). We then examined protein expression in these cells compared to the parental cell line (FADU parental) using RPPA. We observed Axl and p-Axl as well as markers of PI3K/AKT/mTOR pathway activation (phosphoAKT, phosphoGSK, and phosphoP70S6Kinase), and PD-L1 among the top proteins upregulated in FADU RR cells compared to parental line at an FDR of 0.1 (Figure 1A & B). We confirmed the overexpression of several of these proteins via immunoblot and flow cytometry (Supplemental Figure 3A & B).
Figure 1. PD-L1 expression is upregulated by Axl-PI3K signaling in an induced model of radioresistance.
A) Unsupervised hierarcherical cluster analysis of proteins and phospho-proteins from RPPA significantly different (FDR 0.05) between parental (FADU P) and induced radioresistant (FADU RR) cell lines (performed in triplicate). B) Protein expression from RPPA between FADU P and FACU RR for selected proteins. p-values are for comparisons between FADU P and FADU RR for each protein. C & D) Correlation matrix for selected protein expression data from RPPA in 48 HPV-negative cell lines (C), with the correlation between PD-L1 and Axl expression from this data shown in D. E & F) Flow cytometric analysis of PD-L1 positivity in OSC-19 HNSCC cells treated with vehicle, SGI-7079 (Axl inhibitor, 750 nM) or Bay 80-6946 (PI3 kinase inhibitor 5 μM) for 24 hours. G) Immunoblot following transfection of siRNA specific for either Axl or PI3 kinase p110, showing knockdown of Axl in both siRNAs utilized and p110 in one of the two siRNAs used. H) Flow cytometic analysis of PD-L1 positivity shows inhibition of PD-L1 expression with effective siRNA-mediated knock down of either Axl or PI3 kinase. This was not observed using siRNA construct against p110 that did not lead to p110 inhibition. *-indicates p<0.05 compared to control.
To further explore potential interactions between these proteins in vitro, we examined RPPA data from 48 HPV-negative HNSCC cell lines (Figure 1C). We observed significant correlation clustering between known downstream targets of PI3 kinase (Figure 1C). We also observed that Axl was highly correlated with PD-L1 expression (Figure 1C & D; Spearman’s r 0.68, p= 1.16×10−7). Although, previously EMT and activation of PI3 kinase have been linked to radioresistance, the involvement of Axl and PD-L1 as well as their connection to one another and to radioresistance is unclear. To explore this we examined PD-L1 expression via flow cytometry in OSC-19 HNSCC cells, which have high baseline expression of PD-L1 based on RPPA data (Figure 1E, Supplemental Figure 4A & B). Indeed 73% of these cells had baseline detectable expression of PD-L1 in vitro. We then treated these cells with either a chemical inhibitor of Axl or PI3 kinase. Either inhibitor led to substantially decreased PD-L1 expression compared to vehicle control, providing evidence for direct regulation of PD-L1 levels by both Axl and the PI3K pathway (Figure 1E). A similar phenomenon was observed in another HPV negative HNSCC cell line (SN2, Figure 1F). Moreover, inhibition of either Axl or PI3 kinase using siRNA led to decreased PD-L1 expression in SN2 cells (Figure 1G & H).
PD-L1 expression is associated with Axl and PI3 kinase signaling
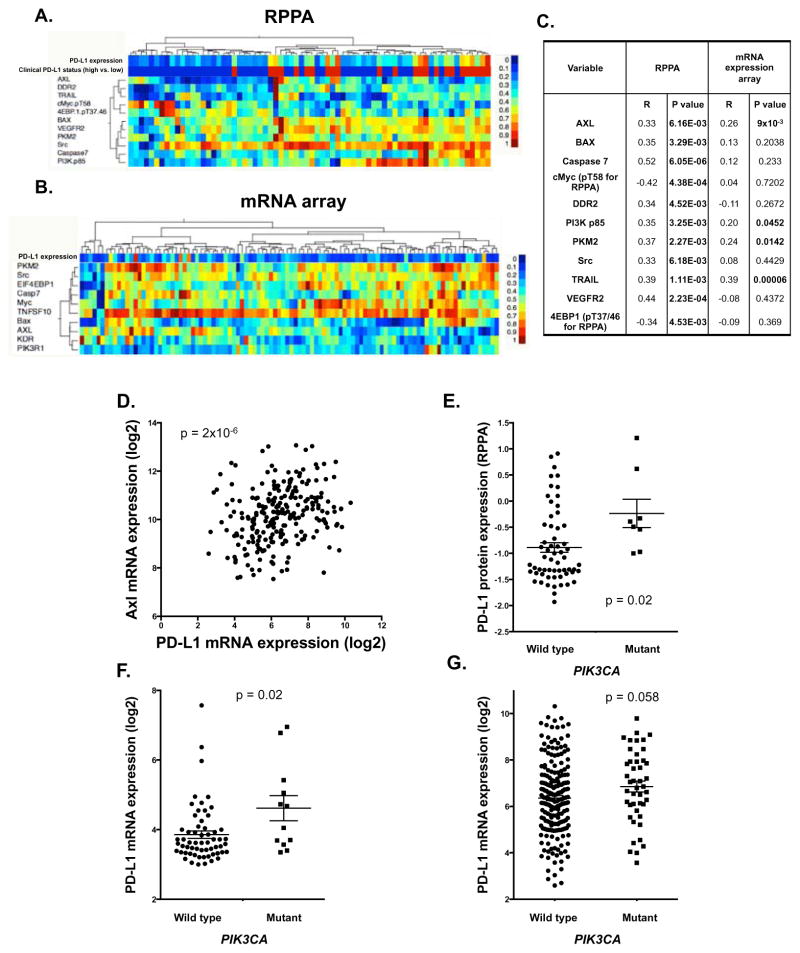
To determine whether these in vitro observations could be validated clinically, and to further investigate the mechanism by which PD-L1 is upregulated in HPV-negative HNSCC, we examined tumors from a total of 68 patients with locally advanced HPV-negative HNSCC treated with surgery and post-operative radiotherapy via RPPA and evaluated for possible association with PD-L1 expression. Proteins and phospho-proteins significantly correlated with PD-L1 with an FDR of 0.1 are shown in Figure 2A & C. These findings confirmed the clinical association between PD-L1, Axl, and the PI3K pathway.
Figure 2. PD-L1 expression is associated with Axl and PI3 kinase signaling.
A) Hierarchical cluster analysis examining protein expression from clinical RPPA data correlated with PD-L1 (FDR=0.1). B) Hierarchical cluster analysis of mRNA expression of targets identified in (A). (C) Spearman r and significance values from RPPA and mRNA array for correlation with PD-L1. D). Correlation between Axl and PD-L1 mRNA expression in the HPV-negative Head and Neck TCGA cohort (Spearman r=0.3, p=2×10−6). E–G) PD-L1 expression levels in PIK3CA wild type and mutant tumors from the RPPA cohort (E)(p=0.02), mRNA cohort (F) (p=0.02), and TCGA mRNA cohort (G) (p=0.058).
To expand upon this finding, we assayed mRNA expression in a second cohort of 97 HNSCC patients similarly treated with surgery and post-operative radiation. We then examined mRNA expression of proteins associated with PD-L1 expression on RPPA and correlated their mRNA expression with PD-L1 mRNA expression (Figure 2B & C). Several molecules were correlated with PD-L1 expression at the proteomic and mRNA level, including Axl, the p85 subunit of PI3 kinase, pyruvate kinase muscle isozyme (PKM) and TRAIL. Moreover, in the TCGA HPV-negative Head and Neck cohort, Axl mRNA expression was significantly correlated with PD-L1 mRNA expression (Spearman r=0.3, p=2×10−6; Figure 2D).
We then investigated whether activation of the PI3K pathway by PIK3CA mutation was also associated with an upregulation of PD-L1 (specific mutations for each cohort in Supplemental Table 4). Targeted sequencing for PIK3CA was performed in 52 HNSCC tumors for which tissue was available. Interestingly, both PD-L1 protein expression (Figure 2E, p=0.02) and mRNA expression (Figure 2F, p=0.02) were significantly greater in PIK3CA mutants versus wild type. We examined PD-L1 mRNA expression in the TCGA HPV negative cohort and PD-L1 mRNA trended toward higher expression levels in PIK3CA mutants (Figure 2G; p=0.058). Thus in three separate HPV-negative HNSCC cohorts, Axl-PI3 kinase signaling was associated with PD-L1 expression.
RPPA identifies PD-L1 as a candidate biomarker of treatment failure following radiation
To determine the clinical significance of a potential Axl-PI3 kinase-PD-L1 axis, a univariate analysis of loco-regional recurrence of disease (LRR) following radiation was performed for each protein on RPPA utilizing the Cox proportional hazards model. The results of this analysis are shown in Supplemental Table 5.
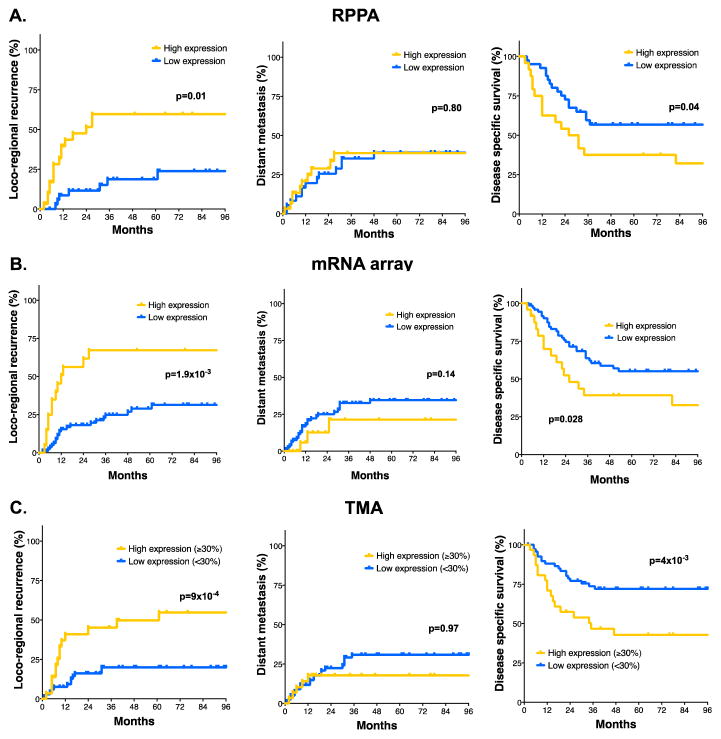
One of the most significant proteins associated with LRR was PD-L1 (p=0.0034). On further analysis, including clinical variables, PD-L1 expression remained significantly associated with LRR (Table 1, p=3.2×10−4). Further, 3 year LRR rate in patients with tumors expressing high levels of PD-L1 was 60%, compared to 20% in the low PD-L1 group (Figure 3A, p=0.01). PD-L1 expression was also significantly associated with disease specific survival (DSS, p=0.04), but had no association with distant metastasis (DM, p=0.8).
Table 1.
| Univariate analysis of locoregional recurrence (LRR)
| ||||
|---|---|---|---|---|
| Variable | Comparison | RPPA p-value | mRNA array p-value | IHC p-value |
| Tumor stage | T1–3 vs. T4 | 0.261 | 0.229 | 0.654 |
| Nodal stage | N0–2a vs. >N2a | 0.0987 | 0.129 | 0.001 |
| Site | 0.611 | 0.208 | 0.869 | |
| PD-L1 | Continuous | 0.003 | 4.4×10−4 | 0.003 |
| PD-1 | 0.758 | |||
| CD8 | 0.224 | |||
| CD4 | 0.626 | |||
| Multivariate analysis of locoregional recurrence (LRR)
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RPPA | mRNA array | IHC | ||||||||
| Variable | Comparison | HR | 95%CI | p-value | HR | 95%CI | p-value | HR | 95%CI | p-value |
| Nodal stage | N0–2a vs. >N2a | 3.54 | 1.3–9.5 | 0.012 | 2.05 | 0.99–4.2 | 0.05 | 3.05 | 1.3–7.2 | 0.011 |
| PD-L1 | Continuous | 2.83 | 1.6–5.0 | 3.2×10−4 | 1.95 | 1.4–2.8 | 1.6×10−4 | 1.02 | 1.002–1.02 | 0.021 |
Figure 3. PD-L1 expression is highly associated with LRR.
A–C) Time to LRR, Time to DM or Disease specific survival in the RPPA (A) and mRNA expression (B) and TMA cohorts (C) by high versus low PD-L1 expression (See Materials and Methods for cutoff selection).
Gene expression confirms PD-L1 as a biomarker of LRR
To further investigate the association between PD-L1 and radiation treatment failure in HPV-negative HNSCC, we performed univariate analysis for LRR in our mRNA expression data. Using Beta-Uniform Mixture (BUM) models for multiple testing adjustment, 8 genes were associated with LRR using a false discovery rate (FDR) of 0.1. The most striking finding was the high degree of significance of both PD-L1 (p= 6.09×10−05) and PD-L2 (p= 1.10×10−05) in predicting LRR following radiation in this patient population (Supplemental Table 6). Indeed, these two ligands were the 3rd and 8th most significant genes in the entire array associated with LRR. On multivariate analysis, including clinical variables, PD-L1 remained significantly associated with LRR (Table 1, p= 1.6×10−4). 3 year LRR rates were 70% in patients with high levels of PD-L1 mRNA expression, compared to 25% in the low PD-L1 group (Figure 3B, p=1.9×10−3).
PD-L1 staining is predictive of LRR
To independently validate the connection between radiation treatment failure and PD-L1 expression, we generated a tissue microarray (TMA) of tumor samples collected from patients with locally advanced HPV-negative HNSCC treated uniformly with surgery and post-operative radiotherapy. In these specimens, PD-L1 was found to be expressed at high levels in the tumor. PD-1, CD4 positive and CD8 positive immune cells were also observed and quantitated. The distribution of scores is shown in Supplemental Figure 5. On univariate analysis, only tumor PD-L1 expression (Table 1, p=0.003) and nodal stage (p=0.001) were associated with LRR in this population. On multivariate analysis, both nodal stage (p=0.011) and tumor PD-L1 expression (p=0.021) remain associated with LRR. Three year LRR rates were 50% in the PD-L1 high group compared to 20% in the PD-L1 low group (p=9×10−4, Figure 3C).
PD-L1 is predictive of LRR in HPV-negative HNSCC independent of p53 status
Prior studies have identified p53 mutations as a marker of treatment failure in HPV-negative HNSCC(26,27). Thus, any prospective biomarker of radiation treatment failure in this population should account for p53 status. To this end, targeted sequencing was performed to determine p53 status in available tumors from the previously analyzed TMA (n=52). PD-L1 expression remained associated with LRR in either a p53 wild type or mutant context (p=0.002 and p=0.014 respectively; Supplemental Figure 6A). Moreover, while the number of tumors with high PD-L1 expression was slightly lower in p53 wild type tumors compared to p53 mutant tumors (20% of patients versus 35% of patients), PD-L1 expression overall was similar in both p53 wild type and mutant tumors (Supplemental Figure 6B, p=0.3)), suggesting that the effect of PD-L1 on LRR is p53-independent.
PD-L1 expression and CD8 positive TILs modulate outcome following radiation
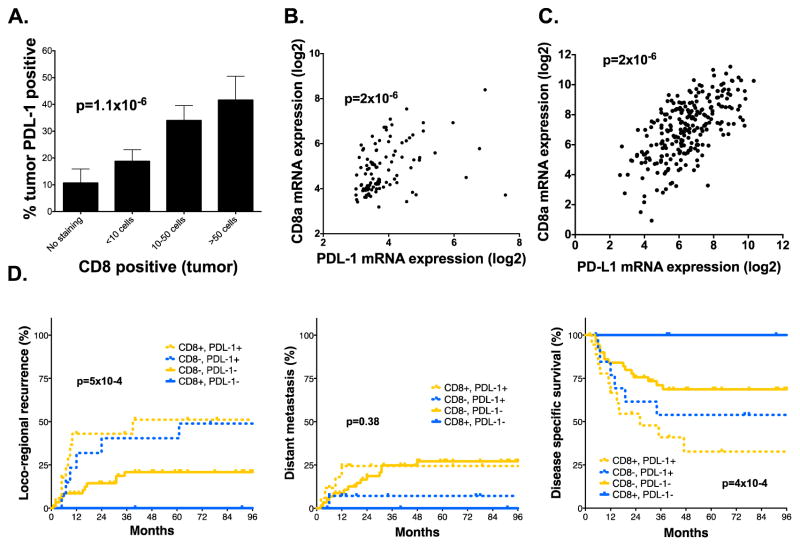
To further investigate the functional consequences of PD-L1 expression in HNSCC tumors, we also examined the correlation with subpopulations of tumor infiltrating lymphocytes (TILs) and other immune cell markers (Supplemental Table 7). A highly significant correlation was observed between CD8 positive TILs and tumor PD-L1 expression (Spearman r=0.45, p=1.1×10−6, Figure 4A). Moreover, we also analyzed our mRNA expression data and found that CD8a mRNA expression was highly correlated with PD-L1 mRNA expression (Spearman r=0.45, p=2×10−6; Figure 4B). This finding was confirmed in the HNSCC HPV-negative TCGA cohort (Figure 4C).
Figure 4. The relationship between PD-L1 and CD8 immune infiltrate.
A) Correlation between tumor PDL-1 staining and CD8 positive immune infiltrate (Spearman r=0.451, p=1.1×10−6) on TMA. B & C) The correlation between PD-L1 and CD8a mRNA expression from our institutional cohort (B) (Spearman r=0.451, p=2×10−6) as well as correlation data for PD-L1 and CD8a in the HPV-negative Head and Neck TCGA cohort (C) (CD8a, Spearman r=0.547, p=2.1×10−45) (CD8b, Spearman r=0.444, p=1×10−28). D) Subset analysis of patients from the TMA cohort examining the relationship between CD8 and PD-L1 positivity on Time to LRR, Time to DM or Disease specific survival.
To examine the association between CD8 infiltrate, PD-L1, and response to radiation, we established groups stratified by CD8 positive (≥10 cells) and/or tumor PD-L1 positive (≥30% of tumor positive) patients based on IHC (Figure 4D). Interestingly, PD-L1 remained predictive of outcome irrespective of CD8 positive immune infiltrate, however patients that were CD8 positive, but PD-L1 negative (n=12) had the best outcome compared to other patients, with no local failures (p=5×10−4) and no death due to disease observed (4×10−4).
Discussion
Radioresistance remains a major cause of treatment failure and mortality in HNSCC, and there is a significant unmet need for new markers and therapeutic targets to combat it. Here, using an integrative analysis starting with preclinical models, we identify that Axl, the PI3K/Akt pathway, and PD-L1 are all associated with radioresistance. Furthermore, Axl and the PI3K/AKT pathway are highly associated with, and appear to modulate, PD-L1 in HNSCC cells. Validating these associations clinically, we show that PD-L1 expression in resected tumors is associated with Axl and PI3K/Akt expression in HPV-negative HNSCC tumors, and is associated with loco-regional recurrence following radiotherapy in multiple patient cohorts, across multiple evaluation platforms.
Our initial studies were guided by the marked upregulation of PI3K, Axl and PD-L1 expression in an acquired in vitro radiation resistance model. This is particularly interesting, in that this model was created in the absence of an immune response. Thus, it appears that the pathways upregulated during the acquisition of in vitro intrinsic radioresistance, lead to a potential for immune evasion even without the contribution of the selective pressure of an immune-competent microenvironment. The association between Axl, PI3K and PD-L1 observed in this model was consistent in a broad panel of cell lines as well as in multiple cohorts of clinical specimens examined via genomic, transcriptomic and proteomic analysis. Moreover, inhibition of either PI3K or Axl led to significant reductions in PD-L1 expression under basal conditions.
The signaling events leading to PD-L1 expression, and ultimately tumor immune evasion, are far from clear. Although recent work has suggested that EGFR mRNA expression is associated with PD-L1 in HNSCC(28), in our RPPA cohort, neither EGFR protein expression or phosphorylation were associated with PD-L1 expression (Supplemental Table 5). Thus, the signaling pathways regulating PD-L1 expression remain to be explored.
One possible clue to the regulation of PD-L1 in these tumors may be found in the interaction between epithelial to mesenchymal transition (EMT) and tumor immune response. Our group has observed upregulation of immune checkpoint markers, including PD-L1, in the context of the EMT phenotype in head and neck as well as non-small cell lung cancer(14,29). Further speaking to the connection between PD-L1 and EMT, it has been shown recently that upregulation of several genes associated with EMT, including Axl, are associated with resistance to PD-1 therapy in melanoma(30). Potential interactions between Axl and the PI3K pathway have previously been identified(31) and there is some evidence that the PI3K signaling network could control PD-L1 expression, albeit a definite link is still missing (32). These data, as well as the work presented herein, point to a functional link between PI3K activation and Axl leading to higher levels of PD-L1 expression, both in vitro as well as clinically.
Once expressed on the cancer cell, the relationship between PD-L1 and response to radiation is also not well explored in HPV-negative HNSCC. We found PD-L1 to be highly enriched in HPV-negative HNSCC cells engineered to be radioresistant via continued radiation challenge, while our clinical studies identified a dramatic association between PD-L1 and failure following radiotherapy via unbiased screening of both protein expression via RPPA as well as mRNA expression array as well as a validation via IHC. In all patient cohorts, high baseline expression of PD-L1 was associated with dramatically increased risk of LRR. Interestingly this was in the absence of alterations in the rate of distant metastasis in three different cohorts of patients, which argues for a primary interaction in this disease site between radiotherapy outcome and immune response.
In regards to the interplay between the host immune response and tumor PD-L1 expression, it is known that CD8(+) T cells can induce PD-L1 up-regulation in multiple tumor types both in vitro and in vivo (33,34). Consistent with that observation, we found that CD8(+) T cell infiltration at the time of surgical resection was significantly correlated with tumor PD-L1 expression in multiple patient cohorts. Thus, in this tumor type, PD-L1 overexpression may be at least partially induced as opposed to a completely intrinsic phenomenon, at least in the context of an intact tumor. This study cannot comment on the expression of PD-L1 in the setting of microscopic disease remaining following resection, nor changes in PD-L1 expression during radiotherapy, however based upon the strong association between resected tumor PD-L1 expression and outcome following radiotherapy it appears that PD-L1 expression and T-cell infiltrate at the time of surgical resection does indeed affect response following radiotherapy. It is possible that the population of CD8(+) T cells within PD-L1 positive tumors represents exhausted T cells instead of anti-tumorigenic cytotoxic infiltrate(35,36). The mechanism for this relationship between potentially exhausted T cells and tumor PD-L1 is unclear at this point. It is possible that infiltration of tumor parenchyma by cytotoxic CD8(+) T cells and consequent production of IFNγ would induce PD-L1 upregulation (33,37,38), via upregulation of PI3K and Axl, which in turn could lead to T-cell exhaustion (39,40).
In conclusion, in the current study we identified and validated PD-L1 as a significant biomarker of treatment failure in HPV-negative HNSCC following radiotherapy using both screening approaches as well as targeted IHC. This phenomenon appears to be linked to Axl/PI3 kinase signaling within the tumor. These findings provide a strong rationale for the combination of immune checkpoint blockade and radiation in this setting, as well as potentially utilizing Axl or PI3 kinase blockade to affect both tumor radiosensitization and immune response.
Supplementary Material
Translational Relevance.
Loco-regional failure is the primary cause of death in head and neck cancer (HNSCC). Treatment of locally advanced HNSCC is typically composed of radiation in combination with either surgical resection and/or cytotoxic chemotherapy. Although human papilloma virus (HPV) positive HNSCC is sensitive to radiation, HPV negative tumors are comparatively resistant to this treatment. Thus, understanding potential, clinically targetable drivers of radioresistance in HPV negative tumors is necessary to improve survival.
In the current study we identified PD-L1 as significantly associated with loco-regional failure following radiation in multiple cohorts of HPV-negative tumors. Interestingly, PD-L1 expression appears to be at least partly driven by Axl-PI3 kinase signaling. These data provide further support to incorporate agents that target PD-1/PD-L1 in combination with radiation in this patient population as well as provide potential selection criteria for these trials. Moreover our data suggest that, in addition to direct anti-tumor effects, targeting either Axl or PI3 kinase may provide a benefit in regards to tumor immune response.
Acknowledgments
This work was supported by following grants from the National Cancer Institute (National Institutes of Health): R01 CA 168484-022 (JH), HNSCC SPORE 5 P50CA070907-16 (JH and JM), CCSG 5 P30 CA01667239 (JH), R01 CA 168485 (RM) & R21 CA 182964 (HS). This work was also supported by the Cancer Prevention Institute of Texas (RP150293) (HS) and the Center for Radiation Oncology Research (Seed Grant) (HS and CP).
The Center for Radiation Oncology Research (Seed Grant) (HS and CP) was partially funded by the Center for Radiation Oncology Research through generous support from Mr. and Mrs. William Dr. Rollnick.
Footnotes
The authors report no conflict of interest.
Bibliography
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–50. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harari PM, Harris J, Kies MS, Myers JN, Jordan RC, Gillison ML, et al. Postoperative Chemoradiotherapy and Cetuximab for High-Risk Squamous Cell Carcinoma of the Head and Neck: Radiation Therapy Oncology Group RTOG-0234. JCO. 2014;32:2486–95. doi: 10.1200/JCO.2013.53.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS, Rosenthal DI, Soulieres D, et al. Randomized Phase III Trial to Test Accelerated Versus Standard Fractionation in Combination With Concurrent Cisplatin for Head and Neck Carcinomas in the Radiation Therapy Oncology Group 0129 Trial: Long-Term Report of Efficacy and Toxicity. JCO. 2014;32:3858–67. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Coan JP, et al. AXL Is a Logical Molecular Target in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2015;21:2601–12. doi: 10.1158/1078-0432.CCR-14-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bussink J, van der Kogel AJ, Kaanders JHAM. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–96. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 8.Skinner HD, Giri U, Yang L, Woo SH, Story MD, Pickering CR, et al. Proteomic profiling identifies PTK2/FAK as a driver of radioresistance in HPV negative head and neck cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty MK, Wansley E, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6:202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou Y, Diao L, Parra Cuentas ER, Denning WL, Chen L, Fan YH, et al. Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ock C-Y, Kim S, Keam B, Kim M, Kim TM, Kim J-H, et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget. 2016;7:15901–14. doi: 10.18632/oncotarget.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19:279–90. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Ahn Y-H, Chen Y, Tan X, Guo L, Gibbons DL, et al. ZEB1 sensitizes lung adenocarcinoma to metastasis suppression by PI3K antagonism. J Clin Invest. 2014;124:2696–708. doi: 10.1172/JCI72171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett. 2013;341:63–72. doi: 10.1016/j.canlet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:860–77. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moeller BJ, Yordy JS, Williams MD, Giri U, Raju U, Molkentine DP, et al. DNA repair biomarker profiling of head and neck cancer: Ku80 expression predicts locoregional failure and death following radiotherapy. Clin Cancer Res. 2011;17:2035–43. doi: 10.1158/1078-0432.CCR-10-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34:409–18. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–61. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skinner HD, Sandulache VC, Ow TJ, Meyn RE, Yordy JS, Beadle BM, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res. 2012;18:290–300. doi: 10.1158/1078-0432.CCR-11-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, et al. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNγ That Induce PD-L1 Expression in Head and Neck Cancer. Cancer Res. 2016;76:1031–43. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mak MP, Tong P, Diao L, Cardnell RJ, Gibbons DL, William WN, et al. A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin Cancer Res. 2016;22:609–20. doi: 10.1158/1078-0432.CCR-15-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dufies M, Jacquel A, Belhacene N, Robert G, Cluzeau T, Luciano F, et al. Mechanisms of AXL overexpression and function in Imatinib-resistant chronic myeloid leukemia cells. Oncotarget. 2011;2:874–85. doi: 10.18632/oncotarget.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atefi M, Avramis E, Lassen A, Wong DJL, Robert L, Foulad D, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20:3446–57. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi F, Shi M, Zeng Z, Qi R-Z, Liu Z-W, Zhang J-Y, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–96. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 34.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S-J, Jang B-C, Lee S-W, Yang Y-I, Suh S-I, Park Y-M, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–62. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 38.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–9. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 40.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.