Abstract
Optical reporters for cAMP represent a fundamental advancement in our ability to investigate the dynamics of cAMP signaling. These fluorescent sensors can measure changes in cAMP in single cells or in microdomains within cells as opposed to whole populations of cells required for other methods of measuring cAMP. The first optical cAMP reporters were FRET-based sensors utilizing dissociation of purified regulatory and catalytic subunits of PKA, introduced by Roger Tsien in the early 1990s. The utility of these sensors was vastly improved by creating genetically encoded versions that could be introduced into cells with transfection, the first of which was published in the year 2000. Subsequently, improved sensors have been developed using different cAMP binding platforms, optimized fluorescent proteins, and targeting motifs that localize to specific microdomains. The most common sensors in use today are FRET-based sensors designed around an Epac backbone. These rely on the significant conformational changes in Epac when it binds cAMP, altering the signal between FRET pairs flanking Epac. Several other strategies for optically interrogating cAMP have been developed, including fluorescent translocation reporters, dimerization-dependent FP based biosensors, BRET (bioluminescence resonance energy transfer)-based sensors, non-FRET single wavelength reporters, and sensors based on bacterial cAMP-binding domains. Other newly described mammalian cAMP-binding proteins such as Popdc and CRIS may someday be exploited in sensor design. With the proliferation of engineered fluorescent proteins and the abundance of cAMP binding targets in nature, the field of optical reporters for cAMP should continue to see rapid refinement in the coming years.
Keywords: PKA, Epac, cAMP signaling microdomains, fluorescent proteins, FRET
Graphical abstract

1. Introduction
This review opens with a tribute to Roger Y. Tsien, who drove not only the early development of calcium sensors, but who should also be credited for his pioneering contributions in the field of cAMP signaling. Among other things, he will be remembered for introducing the concept of cAMP imaging [1], the creation of novel cAMP analogs that are truly membrane-permeant [2], and for his very early adoption of fluorescence resonance energy transfer (FRET) in sensor design [1] [3]. His monumental gifts to our understanding of second messenger signaling (both calcium and cAMP) have been, arguably, as transformative as his discoveries in the field of fluorescent proteins, the topic for which he shared a Nobel Prize in 2008.
2. Optical reporters for cAMP- a brief history
Roger Tsien’s first effort to develop a FRET-based cAMP reporter took advantage of the cAMP-induced dissociation between regulatory and catalytic subunits of protein kinase A (PKA). Named “FlCRhR”, this ratiometric reporter utilized fluorescein-tagged PKA catalytic subunits (“FlC”) and rhodamine-labeled regulatory subunits (“RhR”) [1]. PKA normally consists of a tetrameric complex of two regulatory and two catalytic protein molecules. In Tsien’s design, binding of cAMP on the regulatory subunits of FlCRhR caused dissociation of the PKA holoenzyme, and loss of FRET. We now take for granted the concept of FRET as a means to monitor biological activities and the proximity of protein partners, but the effort led by Tsien’s group represented one of the first successful applications of the phenomenon for monitoring intracellular signaling events in real-time.
Prior to the advent of fluorescent sensors for measuring cAMP, investigators were obliged to work with populations of cells and use radioactivity or other indirect biochemical assays to probe the ensemble behavior of the sample. FlCRhR represented a major improvement by offering all the usual advantages of a fluorescent reporter, i.e. the possibility to visualize the spatial distribution of the second messenger in real-time in single live cells. FlCRhR was adopted quickly and used fruitfully to investigate the link between cAMP and diverse biological activities such as pigment granule aggregation in angelfish melanophores [4] and to examine gradients of elevated cAMP in the processes of stimulated Aplysia neurons [5]. These studies also yielded the surprising result that the resting free [cAMP] as measured by FlCRhR was much lower (< 50nM) than the high micromolar levels reported by traditional measures of cAMP, such as radioimmune assays [5]. Some investigators successfully introduced the bulky fluorophore-labeled PKA subunits into cultured mammalian cells via microinjection [6] [7], and even into single cells within brain slice preparations via perfusable patch pipettes [8]. But by and large, the requirement for invasive approaches to load the PKA holoenzyme reduced its appeal for routine use.
Development of what were, in effect, genetically-encoded versions of FlCRhR completely opened up new avenues of inquiry. Introducing the sensor into the cell became as simple as performing a routine transfection. Moreover this enabled targeting of the constructs, allowing one to interrogate cAMP in specific cellular microdomains such as organelles, and also opened the door to in vivo measurements in transgenic animals. In the year 2000, Zaccolo, De Giorgi, Pozzan, and colleagues, aided by structural knowledge of the Aequorea victoria green fluorescent protein (GFP) and PKA holoenzyme, produced the prototype for this type of genetically encoded reporter [9]. Initially utilizing enhanced blue fluorescent protein (EBFP)-labeled type 2 regulatory subunits of PKA and GFP-labeled catalytic subunits, this early version suffered the fact that GFP and EBFP were not optimal FRET pairs. Moreover, even the enhanced version of BFP was quite dim, and it bleached more readily than GFP, making it difficult to achieve a stable baseline. An improved version employing CFP and YFP FRET pairs appeared in 2002 [10]. This further increased the utility of this class of sensor, which was used widely by multiple groups [11–13]. Other refinements, e.g. those incorporating optimized linkers, and variants with different cAMP affinities, appeared shortly thereafter [14, 15].
Many of the developments in cAMP reporters were preceded by or paralleled those of the calcium sensors. The success of unimolecular Ca2+ sensors featuring a calmodulin-binding domain (“FIP-CBsm” [16]) or a calmodulin/M13-peptide (“Yellow Cameleon” [17]) in 1997 supplied the precedent for creation of FRET-based reporters encoded by a single gene. This perhaps prompted investigators to consider native cAMP-binding proteins other than PKA as platforms for unimolecular sensor design. Several labs took advantage of the substantial cAMP-dependent conformational change that occurred within the cAMP effector known as Epac (“exchange protein activated by cAMP”) to create novel single chain indicators for cAMP. In 2004, three independent groups (those of Kees Jalink, Martin Lohse, and Jin Zhang) introduced FRET sensors based on Epac [18–20]. Although the cAMP binding domains of cyclic nucleotide-gated channels (CNGCs) were similarly adopted for the construction of genetically encoded fluorescent cAMP reporters in 2005 (HCN-camps; [21]), the most widely used cAMP probes today are still versions rooted in the original designs based on Epac.
Since these early efforts, there has been a continuous evolution of genetically encoded biosensors for cyclic nucleotides (Figure 1), including new designs that have moved beyond the classical FRET paradigm for reporting cAMP levels [22–24]. In this review article we will provide a concise summary of some of the newer optical approaches for interrogating cAMP, and discuss how sensor design might be predicted to advance in the future to yield better, brighter, and more useable reporters for this ubiquitous signaling molecule.
Figure 1. Evolution of cAMP sensors.
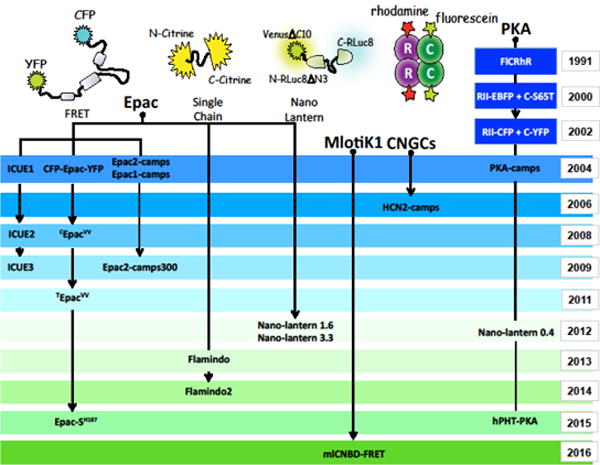
Cartoon describes the genealogy of cAMP sensors originating from PKA, Epac, CNGCs and MlotiK1.
3. Sensors based on native mammalian effectors for cAMP: PKA
Protein Kinase A was long believed to be the sole mediator of the cAMP signal in mammalian cells and there is no question of its dominant role in vertebrate cell biology. There are three mammalian genes encoding the PKA catalytic subunits (Cα, Cβ, and Cγ) and four encoding the regulatory subunits (RIα, RIβ, RIIα and RIIβ). These have differing tissue distributions, cAMP affinities, and subcellular localizations [25]. Moreover, different PKA subtypes can combine in multiple ways, generating ample diversity in the signaling outputs of the PKA hetero-tetramer. This diversity also presents an abundant choice of potential backbones for sensor design (see Table 1 for complete listing of current sensors).
Table 1.
Currently available genetically-encoded optical reporters for cAMP.
| cAMP sensor | Design | Dynamic Range | cAMP Affinity |
|---|---|---|---|
| FlCRhR | cPKA-fluorescein + rPKA-rhodamine | 50% | 88 nM |
| RII-EBFP/C-S65T | rII-PKA-EBFP + cPKA-S65T* | NA | NA |
| R-CFP/C-YFP | rPKA-CFP + cPKA-YFP (CFP-R2C2-YFP)* | ~17% | 0.3 μM |
| RR230K-CFP/C-YFP | rPKA(R230K)-CFP + cPKA-YFP (CFP-R2C2-YFP)* | ~15% | 31.3 μM |
| Epac1-camps | YFP-E157Epac1E316CFP | ~24% | 2.4 μM |
| Epac2-camps | YFP-E285Epac2E443-CFP | ~17% | 0.9 μM |
| PKA-camps | YFP-M264rIIβB-PKAA403-CFP | ~15% | 1.9 μM |
| CFP-Epac-YFP | CFP-M1Epac1P881-YFP | ~30% | 50 μM |
| CFP-Epac(δDEP-CD)-YFP | CFP-P149Epac1(T781A/F782A)P881-YFP | ~45% | 14 μM |
| ICUE1 | ECFP-V2Epac1P881-citrine | 20% | NA |
| HCN-camps | YFP-A467HCN2K638-CFP | ~20% | 6 μM |
| ΔRIIβ-CFP/Cα-YFP | 81rIIβ-PKA416-CFP + CPKAα-YFP* | 82% | NA |
| GndΔ-Epac-mRFP (H81) | GFP(A206K)-P149Epac1(T781A/F782A)P881-mRFP | 29% | NA |
| Cnd-Epac-Vd (H84) | CFP(A206K)-P149Epac1(T781A/F782A)P881-Venus | 31% | NA |
| Cnd-Epac-cp173Vd (H90) | CFP(A206K)-P149Epac1(T781A/F782A)P881-cp173Venus | 36% | NA |
| Gnd-Epac-mCherry (H94) | GFP(A206K)-P149Epac1(T781A/F782A)P881-mCherry | 23% | NA |
| CdΔ-Epac-cp173V/Vd (H96) | 1CFP225-P149Epac1(T781A/F782A)P881-cp173Venus-Venus | 36% | NA |
| ICUE2 | ECFP-E148Epac1P881-citrine | 60% | 12.5 μM |
| ICUE3 | ECFP-E148Epac1P881-cpVenus(L194) | 102% | NA |
| Epac2-camps300 | YFP-E285Epac2(K405E) E443-CFP | 80% | 0.3 μM |
| Epac2-camps300-Cit | YFP-E285Epac2(K405E) E443-citrine | 92% | NA |
| TEpacVV (H74) | mTurquoiseΔ-P149Epac1(T781A/F782A)P881-cp173Venus-Venus | 82%/84% | NA |
| Nano-lantern (cAMP0.4) | 1VenusΔC10228-4N-RLuc8ΔN3228-M245PKARIαBV381-Linker-229C-RLuc8(S257G)311 | NA | 0.4 μM |
| Nano-lantern (cAMP3.3) | 1VenusΔC10228-4N-RLuc8ΔN3228-G170Epac1(Q270E)A327-Linker-229C-RLuc8(S257G)311 | NA | 3.3 μM |
| Nano-lantern (cAMP1.6) | 1VenusΔC10228-4N-RLuc8ΔN3228-G170Epac1(Q270E)A327-229C-RLuc8(S257G)311 | 130% | 1.6 μM |
| cit-mCNBD-cer | citrine-202mCRIS353-cerulean | NA | NA |
| Flamindo | 1N-citrine(Q69M)144-157mEpac1316-146C-citrine238 | 100% | 2.1 μM |
| Flamindo2 | 1N-citrine(Q69M)144-ALKK-157mEpac1316-146C-citrine238 | 300% | 3.2 μM |
| Epac-SH187 | mTurquoise2Δ-P149Epac1(Q270E/T781A/F782A)P881-tdcp173Venus-Venus | 163.9% | NA |
| Epac-SH134 | mTurquoise2Δ- P149Epac1(Q270E/T781A/F782A)P881-cp173Venus-Venus | 86% | 4 μM |
| Epac-SH126 | mTurquoise2Δ-P149Epac1(T781A/F782A)P881-cp173Venus-Venus | 80.4% | 9.5 μM |
| pPHT-PKA | RA-cPKA + B-rPKA + Free GA** | NA | NA |
| RIα #7 | cp173Venus-245rIα-PKA381-ECFP | 38% | 37.2 nM |
| mlCNBD-FRET | Citrine-F223mlotiK1R349-cerulean-His10 | 47% | 66 nM |
(~) indicates that dynamic range was estimated from the figures of cited papers.Truncated proteins are indicated using the number of the starting amino acid and ending amino acid (for example aa#GFPaa#) when the information was available;
co-transfection of two separate constructs required;
co-transfection of three separate constructs required. Mutations, if any, are denoted in parentheses. Note that reporters available exclusively from commercial sources are not listed.
The advantage of utilizing full-length PKA holoenzyme to report a change in cAMP is that it offers a glimpse into what the native PKA “sees”, as the construct will distribute and respond to cAMP in the same way as the native protein. Of course the disadvantages are that the exogenous PKA subunits will have biological activity, and can be expected to mix and match with endogenous subunits, thereby diluting the efficiency of the FRET readout. This led to efforts to pare down the functional and interacting portions of the PKA protein. For example, an early version known as “PKA-camps” from Martin Lohse’s group featured a single cAMP-binding site, and lacked the catalytic activity and cooperative binding characteristics of the tetrameric sensors. However PKA-camps proved less responsive than the popular Epac-based reporters generated at the same time by this group [19].
3.1 Fluorescent translocation reporters
There are several other types of cAMP biosensors derived from PKA. One of these takes advantage of membrane-anchored CFP-labeled RII subunits, which are co-expressed with YFP-labeled catalytic subunits [26, 27]. When cAMP is low, both constructs are evident at the plasma membrane because the regulatory subunits serve to sequester the catalytic subunits in this location. Elevated cAMP causes the catalytic component to break free of RII and diffuse into the cytosol. Thus cAMP can either be reported as a loss of FRET [27] or by following the localization of the CFP- and YFP-labeleld subunits [26]. The added advantage of the latter “fluorescent translocation reporters” [28] is that subplasmalemmal fluorescence can be monitored with exquisite precision using TIRF (total internal reflection fluorescence) microscopy to yield a ratiometric map of cAMP just under the plasma membrane. This approach provides an extraordinarily high signal-to-noise readout because TIRF illumination only excites fluorophores in a very thin plane (>100nm) at the interface between the glass coverslip and membrane contact sites, thereby rejecting unwanted signals from the interior of the cell. Moreover, complete dissociation of the YFP- and CFP-labeled components would be expected to yield a larger change in FRET than a unimolecular construct.
3.2 Dimerization-dependent FP (ddFP)-based biosensors-FPX
A major conceptual innovation in ratiometric sensor design known as “dimerization-dependent fluorescent protein exchange” or “FPX” [29, 30] was recently devised by Robert Campbell’s laboratory. This is a somewhat complex strategy that, for the case of the FPX cAMP reporter (hPHT-PKA), requires cotransfection with three different constructs. The first is a red fluorescent protein engineered from tdTomato fused to the PKA catalytic domain, and the second is composed of the PKA regulatory subunit fused to an exchangeable protein that interacts with and serves to complement the brightness of the nearby fluorescent protein. When cAMP is high, there is dissociation of these two components, causing the fluorescence of the un-complemented red fluorescent protein to diminish. At the same time, this makes room for a third green construct designed to exchange with the catalytic subunit and interact with the regulatory/enhancer component, thereby augmenting the green fluorescence of the third construct. In short, cAMP causes an increase in green and decrease in red fluorescence, providing ratiometric readout of cAMP with an extremely large dynamic range.
3.3 High affinity reporters based on PKA
In situ calibrations indicate that resting cAMP ranges from around 35 nM-1 μM, and reaches concentrations of 25 μM or higher when cells are strongly stimulated (e.g. following treatment with 10 μM forskolin, an activator of adenylyl cyclase) [31, 32]. Higher affinity sensors would likely be of use for resolving subtle signaling events or changes in resting values of cAMP, which, it seems can vary in different cell types [32]. High-affinity PKARIα and PKARIβ subunit-derived cAMP-binding domains have appeared in several types of sensors and cyclic AMP pathway modifiers, for example the high affinity (~400 nM) reporter based on RIα known as “Nano-lantern (cAMP0.4)”. Nano-lanterns are described further below[33].
By taking into consideration the ordering of FRET pair partners, Ohta, Nagai, Horikawa and colleagues succeeded to produce a series of novel biosensors with significantly enhanced dynamic range [34]. In particular, these investigators generated a super high affinity FRET reporter (Kd ~ 37nM) using a single cAMP-binding pocket of RIα sandwiched between circularly permuted Venus and CFP (cp173Venus and cp173CFP, respectively). The ultrasensitive readout from this construct rendered it suitable for imaging extracellular cAMP waves generated by migrating Dictyostelium discoideum, a multicellular organism that secretes cAMP for use as a chemoattractant. Consistent with predictions, these beautiful experiments directly showed that [cAMP] in the extracellular space oscillated between ~5 nM and ~100 nM with a regular period of about five minutes during migration.
Of note, a high-affinity cAMP-binding fragment from RIβ has been successfully used to create mCherry-tagged “cAMP sponges” for buffering cAMP transients in live cells [35]. This construct has been used, for example, to elucidate functional roles for cAMP in stress signaling in plant cells [36] and to evaluate intercellular cAMP communication via gap junctions [35]. Averaimo and colleagues used a combination of targeted cAMP sponge and light-activated adenylyl cyclases to control cAMP levels in the vicinity of lipid rafts in retinal ganglion cells. Their work demonstrated the critical importance of this cAMP signaling microdomain for executing ephrin-dependent retraction of axons and the pruning of ectopic axonal branches during nervous system development [37]. Recently, the Tomes lab reported on refinements to the original cAMP sponge by generating a membrane-permeant version employing a TAT sequence from HIV. Addition of 100 nM purified TAT-cAMP sponge protein to motile human sperm was sufficient to control cAMP-dependent steps in exocytosis [38], permitting separation of Ca2+- and cAMP-regulated aspects of the acrosome reaction.
4. Sensors employing the Epac backbone
As noted above, the most popular unimolecular cAMP reporters in use today rely on the native effector, Epac [39, 40]. There are two major forms of this cAMP-regulated guanine exchange factor, Epac1 and Epac2, the latter existing as three variants, Epac2A–2C. Epac2 has significantly higher affinity for cAMP than Epac1 [41]. Cyclic AMP analogs that can distinguish between PKA and Epac have been helpful in dissecting the biological targets of these respective pathways. It should be noted, however, that a potential complication of these analogs is that they can inhibit phosphodiesterases (PDEs), the enzymes that degrade cAMP, and thereby indirectly elevate cAMP [42].
Since their initial description [18–20], there have been numerous refinements to the Epac-FRET class of reporter [43, 44], centered mostly on fluorescent protein substitutions designed to enhance FRET efficiency and brightness. There has been some attempt to alter the cAMP binding properties of the sensors as well. For example, Norris et al., developed a YFP/CFP FRET sensor harboring a single point mutation (K405E) in Epac2 that increased the affinity for cAMP by at least two-fold with respect to Epac2-camps (EC50 ~320 nM vs. ~820 nM; measured as 50% of the maximum change in the YFP/CFP emission ratio) [45]. Named “Epac2-camps300”, this sensitive biosensor proved useful for measuring subtle effects of cGMP to increase cAMP in meiotic mouse oocytes to block meiotic progression. Meanwhile, Epac2-camps300 was able to detect luteinizing hormone-dependent decreases in resting cAMP that are important for initiating resumption of meiosis. An improved version of this reporter featured citrine in place of YFP as the FRET acceptor [46].
Epac-based cAMP reporters, for example, Epac1-PLN (Epac1 linked to phospholamban under the control of a cardiac-specific promoter) have been successfully used in several transgenic models, demonstrating their biocompatibility [47]. Of note, comparison of the PLN-targeted sensor and its cytosolic version revealed that the two sensors did not have equivalent cAMP sensitivities, an issue that was overcome using careful in situ calibration of the FRET signals.
4.1 Optimization of FRET-based probes
Apart from the Förster radius and the distance between donor and acceptor, which can be optimized in part through the judicious choice of linkers, there are many other geometric parameters that affect FRET efficiency [14, 48]. Fluorophores have a “preferred” orientation in which they interact with excitation light, and a preferred emission (dipole orientation). As mentioned above, Ohta and colleagues recently discovered that simply switching the order of donor and acceptor molecules flanking the cAMP binding motif from PKA RIα could sometimes yield a dramatic effect on FRET efficiency [34]. The orientation of a fluorescent protein can be modified through circular permutation, and this sometimes gives rise to donor and acceptor pairs with more efficient transfer of energy via FRET [49]. Circularly permuted constructs encode rearranged FPs in which the original N- and C- termini of the protein are joined together while new amino and carboxy ends are created by making cuts at permissive sites within the original body of the protein. The observation that the rigid 11-stranded GFP β-barrel enclosing the chromophore could tolerate large scale rearrangements while maintaining its fluorescence was discovered somewhat by accident. This observation was evaluated systematically by Baird and colleagues, who generated a series of circularly permuted variants in 1999 [50] that have since found wide use in sensors for Ca2+ and other analytes [49, 51]. To date, circularly permuted fluorescent proteins have been used in a relatively small number of cAMP reporters [34, 44, 52–54].
A thoughtful and comprehensive examination of FRET pairs as applied to the Epac-based probes has come from the lab of Kees Jalink [52–54]. The original first generation CFP/YFP FRET sensor described by this group in 2004 already featured modifications to the Epac moiety to prevent its catalytic activity and association with membranes [18]. Mindful of optimizing probes according to the type of imaging application (i.e. simple ratio imaging, also known as “sensitized emission” imaging, vs. Fluorescence Lifetime IMaging, or FLIM), the Jalink group has systematically tested a wide range of donor-acceptor pairs. Modifications included substitutions of CFP and YFP with brighter and more stable fluorescent species (e.g. Venus, citrine, GFP, RFP and mTurquoise), incorporation of fluorophores with more favorable Förster radius, use of circularly permuted constructs to alter dipole orientation (e.g. cp173Venus), and inclusion of tandem acceptors (e.g. cp173Venus-Venus) that are envisioned to permit some extra latitude in capturing donor energy.
In FLIM microscopy there is more emphasis placed on the characteristics of the donor because this is the component that determines the lifetime properties [55]. Several of the newer third and fourth generation reporters utilizing mTurquoise or mTurquoise2 as FRET donors proved equally useful in ratiometric imaging or FLIM applications [53, 54]. Of note, some of the fourth generation probes also feature non-emitting “dark acceptors” that were shown to dramatically enhance the FLIM readout. These also provide some additional flexibility with respect to multicolor FLIM because only one color is imaged.
4.2 Nano-lantern cAMP sensors
A completely different approach to ratiometric cAMP sensor design was introduced several years ago by Takeharu Nagai and colleagues [33]. They described a series of indicators for Ca2+, ATP, and cAMP based on “Nano-lanterns”, chimeric constructs that rely on BRET (bioluminescence resonance energy transfer) between a particularly efficient luciferase engineered from the sea pansy Renilla (RLuc8, so named because it harbors eight mutations) and the bright yellow fluorescent protein, Venus.
For the case of the cAMP reporter known as “Nano-lantern (cAMP)”, cyclic nucleotide binding domains based on Epac or PKA were sandwiched within the RLuc8 portion of the sensor so that ligand binding caused a change in luminescence. This was then transferred through BRET to the Venus moiety, resulting in increased fluorescence. Three usable reporters with varying affinities (3.3 μM, 1.6 μM, and 0.4μM) for cAMP were described. The 1.6 μM affinity version was used to image intracellular cAMP signals in Dictyostelium, complementing the work noted above using high affinity PKA-based reporters to image extracellular cAMP signals in this organism [34].
The advantages of this strategy are multiple. It obviates the need for external (sometimes damaging) illumination of the specimen. In effect, the luminescent emission at 530nm provides the excitation energy to illuminate Venus, which is much brighter than the direct luminescence signal from RLuc8. This dramatically amplifies the brightness of the reporter, while reducing background from autofluorescence. Not having to excite the sample also extends its compatibility with optogenetic tools. Disadvantages include the fact that the system requires the continuous presence of an exogenous cofactor (coelenterazine h), but unlike firefly luciferase, RLuc from the sea pansy Renilla does not require endogenous ATP or Mg2+ to catalyze oxidation reaction of its substrate.
Additional tuning of cofactors (i.e. those with different spectral properties, membrane permeability and more efficient luminescence) are expected to give rise to future refinements for this class of indicator [56]. The Nagai group also recently reported on additional FP variants of the nano-lantern series [57], based on the FRET acceptors mTurquoise2 (cyan), mNeonGreen (green), Venus (yellow), mKusabiraOrange2 (orange), and tdTomato (red). The greatly expanded color palette of this series was exploited to generate novel organelle markers that enabled simultaneous imaging in multiple compartments and enhanced Ca2+ reporters. If extended to the cAMP sensors, this would in principle allow five-color imaging of cAMP in different subcellular compartments or cell types in intact tissues, and possibly in live animals.
4.3 Non-FRET single-wavelength reporters: “Flamindos”
In the calcium signaling world, a very large number of single polypeptide reporters including pericams, CatchER, CEPIAs, GECOs, Camgaroos, and GCaMPs have been engineered through careful placement of a Ca2+-sensing motif in or around the β–barrel of a fluorescent protein, usually a circularly permuted FP, e.g. at the new N- or C-terminus (recently reviewed in [51]). Ligand binding causes alteration in the fluorophore structure and concomitant changes in fluorescence intensity. Reminiscent of this strategy as applied to the citrine-based Ca2+ sensor Camgaroo-2, a series of single wavelength reporters for cAMP known as “Flamindos” were recently introduced by Kitaguchi, Miyawaki and colleagues [58]. These sensors experience a loss of citrine fluorescence upon cAMP binding. These authors used Flamindo to examine the effects of Ca2+ influx on the Ca2+-activated adenylyl cyclase isoform AC1 in insulin secreting cells. Considering the success enjoyed by the single fluorescent protein-based biosensors for Ca2+, this simplified design strategy appears to be a promising avenue for further development.
5. Sensors utilizing bacterial cAMP-binding domains
Cyclic AMP is a stable molecule that has enjoyed great success as a mediator of intracellular (and extracellular) signaling in nearly all kingdoms of life [59]. An incredibly rich diversity of cAMP-binding proteins is found in nature, for example transcriptional regulators that directly bind cAMP to control responsiveness to nutrients and other environmental factors in bacteria, and transmembrane receptors for extracellular cAMP in organisms such as Dictyostelium discoideum. Of note, light-activated adenylyl cyclases (e.g. “bPAC” from the filamentous bacterial species Beggiatoa) [60, 61] are now being increasingly tapped for use as optogenetic tools to modulate cAMP levels.
A completely novel FRET-based biosensor from the lab of Dagmar Wachten named “mlCNBD-FRET” [62] incorporates a new design built around a cyclic-nucleotide binding domain (CNBD) of a bacterial channel known as MlotiK1. This cAMP-binding fragment is sandwiched between cerulean and citrine. A notable feature of the sensor is its heightened (nanomolar) sensitivity to cAMP (Kd for cAMP ~ 66nM), however, one caveat is that it is also modestly sensitive to cGMP (Kd ~500nM). A null-point titration was used to determine a resting cAMP concentration in HEK cells of around 35nM. These investigators also used mlCNBD-FRET to perform extremely challenging measurements of cAMP in live sperm.
6. Other mammalian cAMP-binding motifs as potential platforms in sensor design: Popdc and CRIS
Apart from the vast wealth of potential cyclic nucleotide-binding motifs derived from bacteria, there are still untapped high-affinity mammalian cAMP-binding proteins, such as the “Popeye domain containing proteins” (Popdc) [63]. This interesting family of proteins (of which there are three members, Popdc1–3) was initially identified in cardiac and skeletal muscle. Popdc2 is a three-pass membrane-spanning protein localized to the plasma membrane. It harbors a small extracellular domain and an intracellular tail that binds cAMP with an affinity similar to that of PKA. Popdc proteins can interact with and modulate the function of ion channels in the plasma membrane in a cAMP-dependent fashion, thereby affecting muscle contractility. It can therefore be considered a bona fide cAMP effector. Measurements of FRET between Popdc and its interacting proteins indicative of cAMP-dependent conformational changes [64] [65] suggest the potential for Popdc proteins to be exploited to generate new cyclic nucleotide reporters.
Of note, the Wachten laboratory recently reported on a sperm-specific cAMP binding protein known as CRIS (cyclic nucleotide receptor involved in sperm function), which was shown to control sperm development and flagellar movement, and hence, male fertility [66]. The cyclic nucleotide-binding domain of CRIS was isolated and then sandwiched between the fluorescent proteins citrine and cerulean. When expressed in HEK cells, this construct was shown to undergo FRET upon elevation of cAMP, although it was reported that the intracellular distribution and responsiveness of the expressed protein was not consistent. It seems some refinement will be required if CRIS is to form the basis of a new class of cAMP biosensor, and Wachten’s results indicate the potential for such a development exists.
7. Using optical reporters to probe cAMP microdomains
Of course one of the main drivers behind the development of optical approaches for imaging cAMP has to do with the desire to visualize localized signaling events or cAMP microdomains in live cells. This concept has been discussed extensively [24, 67] so will be covered only briefly here.
Cyclic AMP is much more diffusible than Ca2+, thus there has been some level of healthy skepticism in the signaling community over the existence of meaningful standing concentration gradients of cAMP in cells with simple geometry. However, it is apparent that compartmentalization of the various cAMP effectors (e.g. PKA, Epac and others), as well as other geometric considerations [68] promote non-homogeneity in the signal. In addition, spatial differences exist in the localization of the ten distinct isoforms of adenylyl cyclase (the enzymes that generate cAMP) and the 20-plus phosphodiesterases (the enzymes that degrade cAMP). These disparities create local sources and sinks for cAMP that translate into differential signaling efficiencies in discrete microdomains, for example in lipid rafts [37]. It’s also worth noting that cellular structures with a high surface-to-volume ratio, such as neuronal projections will be expected to have more cAMP-generating machinery in the plasma membrane for a given volume of cytosol, thus accounting for the appearance of higher cAMP production in those locales [5, 46].
A significant number of targeted constructs have been generated to monitor cAMP in subcellular compartments and organelles, for example in mitochondria, which are impermeant to cAMP generated in the cytosol, and host an independent cAMP signaling circuit contained within the matrix [24, 69, 70]. Another potential cAMP microdomain of interest is the primary cilium, which contains specific adenylyl cyclases and cell surface receptors coupled to cAMP production [71] [62]. Measuring cAMP in this tiny organelle (which occupies 1/10,000th of the cell volume) is challenging because its small size makes it difficult to optically isolate the cilium from the rest of the cell body (Figure 2). Another consideration with tiny organelles such as primary cilia is that there is the misleading optical appearance that the sensor is well localized, but simply because of its small size and geometry, it may actually have the same sensor concentration as the plasma membrane. Geneva and colleagues recently analyzed this issue and concluded that the fluorescence intensity in the cilium must be at least three times greater than in the plasma membrane to actually be considered as concentrated [72]. Moreover, targeting sequences can change properties of sensors, so one must exercise caution in over-interpreting subtle differences when comparing signals from differently targeted probes. This illustrates just a few of the challenges in measuring signaling molecules within organellar compartments, including cilia.
Figure 2. Measurement of cAMP using a biosensor targeted to the primary cilium in NIH-3T3 cells.
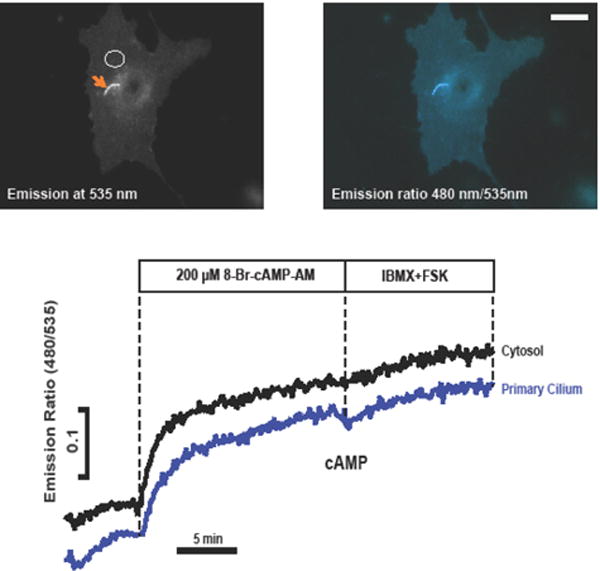
This example illustrates some of the difficulties in measuring cAMP using organelle-targeted probes. The reporter, ICUE3-SSTR3 (a kind gift of Mark von Zastrow [71]) is based on a fusion between the ciliary GPCR, somatostatin receptor 3 (SSTR3) and ICUE3, an improved cAMP sensor engineered by Jin Zhang’s lab [44]. The fluorescence appears to be quite concentrated within the cilium (arrow), but in this particular case it is actually only twice as great as the non-targeted probe in the plasma membrane. By the criteria of Geneva and colleagues [72], the probe, in spite of appearances, is likely no more concentrated in the cilium than in the plasma membrane (see text for details). Ratiometric FRET measurements show that both ciliary and extra-ciliary regions respond to the membrane permeable cAMP analog, 8-Br-cAMP-AM, with a very small additional increase following addition of the PDE inhibitor IBMX and AC activator forskolin. FRET measurements were performed as described previously [76](Scale bar: 10 μm).
8. Wish list and future directions
The cyclic nucleotide-signaling motif is used extensively throughout nature, suggesting the existence of numerous as of yet undiscovered cAMP-binding proteins that can potentially be exploited for sensor design. Among these there may exist new proteins with smaller gene sizes, higher cAMP specificity, faster kinetics (including favorable off-rates), and a wider range of affinities for cAMP.
In the meantime, continued efforts to optimize existing optical reporters remains a high priority. A systematic comparison of current genetically-encoded sensors would also be useful for determining which approach is best suited for a given application. Note that here we have not discussed other types of indirect readouts for cAMP, such as commercially-available cAMP assays, PKA activity reporters (AKARs), biosensors based on cAMP-dependent gene expression, luminescent cAMP bioassays, or Ca2+ permeable cyclic nucleotide-gated channels used in combination with Ca2+- or voltage-sensitive dyes (recently reviewed by Paramonov [23]).
New probes will surely capitalize on the incredible developments in the realm of engineered fluorescent proteins [73]. EGFP has been the standard for brightness for many years, but new FPs like mNeon green and mClover are eclipsing EGFP in performance [73]. Apart from having greater intrinsic brightness (inherent properties of the FP that depend on the extinction coefficient and fluorescence quantum yield), the effective (practical) brightness can differ between probes depending on efficiency of gene expression (i.e. codon optimization), protein folding, chromophore maturation, and the ability of the FP to resist degradation. Brighter, more stable, constructs will be in demand because fewer sensor molecules are needed to achieve photon detection above background, minimizing buffering and off-target biological effects from the ligand binding domains of the sensor. It has been noted that one of the factors accounting for the widespread success of fluorescent Ca2+ indicators is that they are introduced against a large background of endogenous Ca2+ buffers [51], so even relatively high concentrations of the fluorophore (as much as 100 μM) contribute little to the perturbation of Ca2+ signaling dynamics.
A greater color palette would also be highly desirable. Almost all cAMP reporters are based on green or cyan/yellow variants, with few usable red variants to enable multicolor labeling experiments. More than one investigator has succumbed to the temptation of mixing the Ca2+ indicator fura-2 (excitation 340nm/380nm; emission 510nm) with sensors for cAMP utilizing CFP/YFP FRET pairs, giving rise to spurious “oscillations” in cAMP that reflect bleed-through of the fura-2 emission into the FRET channel [11]. Such problems of spectral separation would be eliminated with the newer FPs that now span the rainbow from violet to far red and beyond (e.g. new infrared FPs such as smURFP) [73].
Investigators in the Ca2+ signaling field also have at their disposal of number of specialized measurement tools that have not yet been adapted for measuring cyclic nucleotides. For example, Fosque et al. recently introduced “CaMPARI” (Calcium-Modulated PhotoActivatable Ratiometric Integrator)[74]. This calcium sensor is based on a mutated version of the bright green photoconvertible fluorescent protein known as mEos, which is sandwiched between the classical CaM/M13 Ca2+-binding module. CaMPARI has the unique property that the permanent green-to-red photoconversion that takes place when mEos is excited with 405 nm excitation light occurs only when the sensor domain is bound to Ca2+. Therefore one can generate a “snapshot” of regions where Ca2+ was elevated in the instant the 405nm light pulse was applied. This permits leisurely post-hoc imaging of a large areas (for example a complex neuronal circuit) using relatively slow high-resolution image acquisition approaches, even in fixed tissues. This method was used to retrospectively map active brain regions in response to specific stimuli in swimming zebrafish, mice, and fruit flies. CaMPARI for cAMP (“cAMP-ARI?”) and other signaling molecules may someday become reality.
Another area in which the cAMP field could take their cues from the Ca2+ signaling field regards optical reporters compatible with advanced measurement techniques such as super-resolution microscopy [75], which could potentially provide a cellular map of cAMP at nanometer resolution. With some notable exceptions, imaging of cAMP in live organisms, including brains and other organs of freely moving animals, has also not achieved the same level of advancement as with the newer generation of genetically encoded calcium indicators. These are goals that can be realized in the near future, however, especially considering the rapid pace at which those labs dedicated to cAMP sensor design continue to move the field forward to produce brighter, more versatile reporters with ever larger dynamic ranges.
Highlights.
More than 35 distinct genetically-encoded biosensors for cAMP have been developed since the first descriptions of this class of optical reporter in the year 2000.
These reporters are based on cyclic nucleotide binding motifs from diverse cAMP binding proteins, including PKA, Epac, and bacterial proteins.
Epac-based FRET sensors remain the most popular tools to investigate cAMP signaling dynamics.
Newly discovered cAMP binding motifs and enhanced fluorescent proteins will likely be adopted to build cAMP probes with better performance in the future.
Acknowledgments
We gratefully acknowledge support from the Harvard Catalyst (NIH 1UL1 TR001102-01; to AMH), NIH/NIDCR (1R21DE025921-01; to AMH), and the Medical Research Service of the Veteran’s Administration (VA-ORD 1 I01 BX000968-01; to AMH). We thank Mark von Zastrow (University of California at San Francisco) for the kind gift of the SSTR3-ICUE3 plasmid.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- PKA
protein kinase A
- FRET
fluorescence resonance energy transfer
- Epac
exchange protein activated by cAMP
- PDE
phosphodiesterase
- AKAR
A-kinase activity reporters
- BRET
bioluminescence resonance energy transfer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991;349:694–697. doi: 10.1038/349694a0. [DOI] [PubMed] [Google Scholar]
- 2.Schultz C, Vajanaphanich M, Genieser HG, Jastorff B, Barrett KE, Tsien RY. Membrane-permeant derivatives of cyclic AMP optimized for high potency, prolonged activity, or rapid reversibility. Molecular pharmacology. 1994;46:702–708. [PubMed] [Google Scholar]
- 3.Tsien RY, Bacskai BJ, Adams SR. FRET for studying intracellular signalling. Trends in cell biology. 1993;3:242–245. doi: 10.1016/0962-8924(93)90124-j. [DOI] [PubMed] [Google Scholar]
- 4.Sammak PJ, Adams SR, Harootunian AT, Schliwa M, Tsien RY. Intracellular cyclic AMP not calcium, determines the direction of vesicle movement in melanophores: direct measurement by fluorescence ratio imaging. The Journal of cell biology. 1992;117:57–72. doi: 10.1083/jcb.117.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- 6.Liu CY, Jamaleddin AJ, Zhang H, Christofi FL. FlCRhR/cyclic AMP signaling in myenteric ganglia and calbindin-D28 intrinsic primary afferent neurons involves adenylyl cyclases I, III and IV. Brain research. 1999;826:253–269. doi: 10.1016/s0006-8993(99)01269-x. [DOI] [PubMed] [Google Scholar]
- 7.Civitelli R, Bacskai BJ, Mahaut-Smith MP, Adams SR, Avioli LV, Tsien RY. Single-cell analysis of cyclic AMP response to parathyroid hormone in osteoblastic cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1994;9:1407–1417. doi: 10.1002/jbmr.5650090912. [DOI] [PubMed] [Google Scholar]
- 8.Vincent P, Brusciano D. Cyclic AMP imaging in neurones in brain slice preparations. Journal of neuroscience methods. 2001;108:189–198. doi: 10.1016/s0165-0270(01)00393-4. [DOI] [PubMed] [Google Scholar]
- 9.Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nature cell biology. 2000;2:25–29. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
- 10.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 11.Gerbino A, Ruder WC, Curci S, Pozzan T, Zaccolo M, Hofer AM. Termination of cAMP signals by Ca2+ and G(alpha)i via extracellular Ca2+ sensors: a link to intracellular Ca2+ oscillations. The Journal of cell biology. 2005;171:303–312. doi: 10.1083/jcb.200507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardone RA, Bagorda A, Bellizzi A, Busco G, Guerra L, Paradiso A, Casavola V, Zaccolo M, Reshkin SJ. Protein kinase A gating of a pseudopodial-located RhoA/ROCK/p38/NHE1 signal module regulates invasion in breast cancer cell lines. Molecular biology of the cell. 2005;16:3117–3127. doi: 10.1091/mbc.E04-10-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dal Molin F, Tonello F, Ladant D, Zornetta I, Zamparo I, Di Benedetto G, Zaccolo M, Montecucco C. Cell entry and cAMP imaging of anthrax edema toxin. The EMBO journal. 2006;25:5405–5413. doi: 10.1038/sj.emboj.7601408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lissandron V, Terrin A, Collini M, D’Alfonso L, Chirico G, Pantano S, Zaccolo M. Improvement of a FRET-based indicator for cAMP by linker design and stabilization of donor-acceptor interaction. Journal of molecular biology. 2005;354:546–555. doi: 10.1016/j.jmb.2005.09.089. [DOI] [PubMed] [Google Scholar]
- 15.Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circulation research. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- 16.Romoser VA, Hinkle PM, Persechini A. Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators. The Journal of biological chemistry. 1997;272:13270–13274. doi: 10.1074/jbc.272.20.13270. [DOI] [PubMed] [Google Scholar]
- 17.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 18.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO reports. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. The Journal of biological chemistry. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 20.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circulation research. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 22.Willoughby D, Cooper DM. Live-cell imaging of cAMP dynamics. Nature methods. 2008;5:29–36. doi: 10.1038/nmeth1135. [DOI] [PubMed] [Google Scholar]
- 23.Paramonov VM, Mamaeva V, Sahlgren C, Rivero-Muller A. Genetically-encoded tools for cAMP probing and modulation in living systems. Frontiers in pharmacology. 2015;6:196. doi: 10.3389/fphar.2015.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pendin D, Greotti E, Lefkimmiatis K, Pozzan T. Exploring cells with targeted biosensors. The Journal of general physiology. 2017;149:1–36. doi: 10.1085/jgp.201611654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilouz R, Lev-Ram V, Bushong EA, Stiles TL, Friedmann-Morvinski D, Douglas C, Goldberg G, Ellisman MH, Taylor SS. Isoform-specific subcellular localization and function of protein kinase A identified by mosaic imaging of mouse brain. eLife. 2017;6 doi: 10.7554/eLife.17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyachok O, Isakov Y, Sagetorp J, Tengholm A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature. 2006;439:349–352. doi: 10.1038/nature04410. [DOI] [PubMed] [Google Scholar]
- 27.Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, Elvassore N, Prinz A, Herberg FW, Houslay MD, Zaccolo M. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. The Journal of cell biology. 2006;175:441–451. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tengholm A, Idevall-Hagren O. Imaging sub-plasma membrane cAMP dynamics with fluorescent translocation reporters. Methods in molecular biology. 2015;1294:85–101. doi: 10.1007/978-1-4939-2537-7_7. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y, Li J, Enterina JR, Shen Y, Zhang I, Tewson PH, Mo GC, Zhang J, Quinn AM, Hughes TE, Maysinger D, Alford SC, Zhang Y, Campbell RE. Ratiometric biosensors based on dimerization-dependent fluorescent protein exchange. Nature methods. 2015;12:195–198. doi: 10.1038/nmeth.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Y, Lai T, Campbell RE. Red fluorescent proteins (RFPs) and RFP-based biosensors for neuronal imaging applications. Neurophotonics. 2015;2:031203. doi: 10.1117/1.NPh.2.3.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koschinski A, Zaccolo M. A novel approach combining real-time imaging and the patch-clamp technique to calibrate FRET-based reporters for cAMP in their cellular microenvironment. Methods in molecular biology. 2015;1294:25–40. doi: 10.1007/978-1-4939-2537-7_3. [DOI] [PubMed] [Google Scholar]
- 32.Borner S, Schwede F, Schlipp A, Berisha F, Calebiro D, Lohse MJ, Nikolaev VO. FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells. Nature protocols. 2011;6:427–438. doi: 10.1038/nprot.2010.198. [DOI] [PubMed] [Google Scholar]
- 33.Saito K, Chang YF, Horikawa K, Hatsugai N, Higuchi Y, Hashida M, Yoshida Y, Matsuda T, Arai Y, Nagai T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nature communications. 2012;3:1262. doi: 10.1038/ncomms2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohta Y, Kamagata T, Mukai A, Takada S, Nagai T, Horikawa K. Nontrivial Effect of the Color-Exchange of a Donor/Acceptor Pair in the Engineering of Forster Resonance Energy Transfer (FRET)-Based Indicators. ACS chemical biology. 2016;11:1816–1822. doi: 10.1021/acschembio.6b00221. [DOI] [PubMed] [Google Scholar]
- 35.Lefkimmiatis K, Moyer MP, Curci S, Hofer AM. “cAMP sponge”: a buffer for cyclic adenosine 3′, 5′-monophosphate. PloS one. 2009;4:e7649. doi: 10.1371/journal.pone.0007649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabetta W, Vannini C, Sgobba A, Marsoni M, Paradiso A, Ortolani F, Bracale M, Viggiano L, Blanco E, de Pinto MC. Cyclic AMP deficiency negatively affects cell growth and enhances stress-related responses in tobacco Bright Yellow-2 cells. Plant molecular biology. 2016;90:467–483. doi: 10.1007/s11103-016-0431-5. [DOI] [PubMed] [Google Scholar]
- 37.Averaimo S, Assali A, Ros O, Couvet S, Zagar Y, Genescu I, Rebsam A, Nicol X. A plasma membrane microdomain compartmentalizes ephrin-generated cAMP signals to prune developing retinal axon arbors. Nature communications. 2016;7:12896. doi: 10.1038/ncomms12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucchesi O, Ruete MC, Bustos MA, Quevedo MF, Tomes CN. The signaling module cAMP/Epac/Rap1/PLCepsilon/IP3 mobilizes acrosomal calcium during sperm exocytosis. Biochimica et biophysica acta. 2016;1863:544–561. doi: 10.1016/j.bbamcr.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 39.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 40.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacological reviews. 2013;65:670–709. doi: 10.1124/pr.110.003707. [DOI] [PubMed] [Google Scholar]
- 42.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nature methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 43.Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. The Journal of biological chemistry. 2008;283:2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 44.DiPilato LM, Zhang J. The role of membrane microdomains in shaping beta2-adrenergic receptor-mediated cAMP dynamics. Molecular bioSystems. 2009;5:832–837. doi: 10.1039/b823243a. [DOI] [PubMed] [Google Scholar]
- 45.Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro LR, Gervasi N, Guiot E, Cavellini L, Nikolaev VO, Paupardin-Tritsch D, Vincent P. Type 4 phosphodiesterase plays different integrating roles in different cellular domains in pyramidal cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6143–6151. doi: 10.1523/JNEUROSCI.5851-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sprenger JU, Perera RK, Steinbrecher JH, Lehnart SE, Maier LS, Hasenfuss G, Nikolaev VO. In vivo model with targeted cAMP biosensor reveals changes in receptor-microdomain communication in cardiac disease. Nature communications. 2015;6:6965. doi: 10.1038/ncomms7965. [DOI] [PubMed] [Google Scholar]
- 48.Machado M, Pantano S. Structure-based, in silico approaches for the development of novel cAMP FRET reporters. Methods in molecular biology. 2015;1294:41–58. doi: 10.1007/978-1-4939-2537-7_4. [DOI] [PubMed] [Google Scholar]
- 49.Fritz RD, Letzelter M, Reimann A, Martin K, Fusco L, Ritsma L, Ponsioen B, Fluri E, Schulte-Merker S, van Rheenen J, Pertz O. A versatile toolkit to produce sensitive FRET biosensors to visualize signaling in time and space. Science signaling. 2013;6:rs12. doi: 10.1126/scisignal.2004135. [DOI] [PubMed] [Google Scholar]
- 50.Baird GS, Zacharias DA, Tsien RY. Circular permutation and receptor insertion within green fluorescent proteins. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11241–11246. doi: 10.1073/pnas.96.20.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyawaki A, Niino Y. Molecular spies for bioimaging–fluorescent protein-based probes. Molecular cell. 2015;58:632–643. doi: 10.1016/j.molcel.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 52.van der Krogt GN, Ogink J, Ponsioen B, Jalink K. A comparison of donor-acceptor pairs for genetically encoded FRET sensors: application to the Epac cAMP sensor as an example. PloS one. 2008;3:e1916. doi: 10.1371/journal.pone.0001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klarenbeek JB, Goedhart J, Hink MA, Gadella TW, Jalink K. A mTurquoise-based cAMP sensor for both FLIM and ratiometric read-out has improved dynamic range. PloS one. 2011;6:e19170. doi: 10.1371/journal.pone.0019170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K. Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity. PloS one. 2015;10:e0122513. doi: 10.1371/journal.pone.0122513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raspe M, Klarenbeek J, Jalink K. Recording intracellular cAMP levels with EPAC-based FRET sensors by fluorescence lifetime imaging. Methods in molecular biology. 2015;1294:13–24. doi: 10.1007/978-1-4939-2537-7_2. [DOI] [PubMed] [Google Scholar]
- 56.Saito K, Nagai T. Recent progress in luminescent proteins development. Current opinion in chemical biology. 2015;27:46–51. doi: 10.1016/j.cbpa.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki K, Kimura T, Shinoda H, Bai G, Daniels MJ, Arai Y, Nakano M, Nagai T. Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nature communications. 2016;7:13718. doi: 10.1038/ncomms13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitaguchi T, Oya M, Wada Y, Tsuboi T, Miyawaki A. Extracellular calcium influx activates adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells. The Biochemical journal. 2013;450:365–373. doi: 10.1042/BJ20121022. [DOI] [PubMed] [Google Scholar]
- 59.Gancedo JM. Biological roles of cAMP: variations on a theme in the different kingdoms of life. Biological reviews of the Cambridge Philosophical Society. 2013;88:645–668. doi: 10.1111/brv.12020. [DOI] [PubMed] [Google Scholar]
- 60.Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, Gartner W, Petereit L, Efetova M, Schwarzel M, Oertner TG, Nagel G, Hegemann P. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. The Journal of biological chemistry. 2011;286:1181–1188. doi: 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M. Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. The Journal of biological chemistry. 2010;285:41501–41508. doi: 10.1074/jbc.M110.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukherjee S, Jansen V, Jikeli JF, Hamzeh H, Alvarez L, Dombrowski M, Balbach M, Strunker T, Seifert R, Kaupp UB, Wachten D. A novel biosensor to study cAMP dynamics in cilia and flagella. eLife. 2016;5 doi: 10.7554/eLife.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schindler RF, Brand T. The Popeye domain containing protein family–A novel class of cAMP effectors with important functions in multiple tissues. Progress in biophysics and molecular biology. 2016;120:28–36. doi: 10.1016/j.pbiomolbio.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Froese A, Breher SS, Waldeyer C, Schindler RF, Nikolaev VO, Rinne S, Wischmeyer E, Schlueter J, Becher J, Simrick S, Vauti F, Kuhtz J, Meister P, Kreissl S, Torlopp A, Liebig SK, Laakmann S, Muller TD, Neumann J, Stieber J, Ludwig A, Maier SK, Decher N, Arnold HH, Kirchhof P, Fabritz L, Brand T. Popeye domain containing proteins are essential for stress-mediated modulation of cardiac pacemaking in mice. The Journal of clinical investigation. 2012;122:1119–1130. doi: 10.1172/JCI59410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brand T, Poon KL, Simrick S, Schindler RF. The Popeye Domain Containing Genes and cAMP Signaling. Journal of cardiovascular development and disease. 2014;1:121–133. doi: 10.3390/jcdd1010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krahling AM, Alvarez L, Debowski K, Van Q, Gunkel M, Irsen S, Al-Amoudi A, Strunker T, Kremmer E, Krause E, Voigt I, Wortge S, Waisman A, Weyand I, Seifert R, Kaupp UB, Wachten D. CRIS-a novel cAMP-binding protein controlling spermiogenesis and the development of flagellar bending. PLoS genetics. 2013;9:e1003960. doi: 10.1371/journal.pgen.1003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaccolo M, Di Benedetto G, Lissandron V, Mancuso L, Terrin A, Zamparo I. Restricted diffusion of a freely diffusible second messenger: mechanisms underlying compartmentalized cAMP signalling. Biochemical Society transactions. 2006;34:495–497. doi: 10.1042/BST0340495. [DOI] [PubMed] [Google Scholar]
- 68.Richards M, Lomas O, Jalink K, Ford KL, Vaughan-Jones RD, Lefkimmiatis K, Swietach P. Intracellular tortuosity underlies slow cAMP diffusion in adult ventricular myocytes. Cardiovascular research. 2016;110:395–407. doi: 10.1093/cvr/cvw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lefkimmiatis K, Leronni D, Hofer AM. The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. The Journal of cell biology. 2013;202:453–462. doi: 10.1083/jcb.201303159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Benedetto G, Scalzotto E, Mongillo M, Pozzan T. Mitochondrial Ca(2)(+) uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell metabolism. 2013;17:965–975. doi: 10.1016/j.cmet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Marley A, Choy RW, von Zastrow M. GPR88 reveals a discrete function of primary cilia as selective insulators of GPCR cross-talk. PloS one. 2013;8:e70857. doi: 10.1371/journal.pone.0070857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geneva II, Tan HY, Calvert PD. Untangling ciliary access and enrichment of two rhodopsin-like receptors using quantitative fluorescence microscopy reveals cell-specific sorting pathways. Molecular biology of the cell. 2016 doi: 10.1091/mbc.E16-07-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez EA, Campbell RE, Lin JY, Lin MZ, Miyawaki A, Palmer AE, Shu X, Zhang J, Tsien RY. The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins. Trends in biochemical sciences. 2017;42:111–129. doi: 10.1016/j.tibs.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fosque BF, Sun Y, Dana H, Yang CT, Ohyama T, Tadross MR, Patel R, Zlatic M, Kim DS, Ahrens MB, Jayaraman V, Looger LL, Schreiter ER. Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science. 2015;347:755–760. doi: 10.1126/science.1260922. [DOI] [PubMed] [Google Scholar]
- 75.Lambert TJ, Waters JC. Navigating challenges in the application of superresolution microscopy. The Journal of cell biology. 2017;216:53–63. doi: 10.1083/jcb.201610011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nature cell biology. 2009;11:433–442. doi: 10.1038/ncb1850. [DOI] [PubMed] [Google Scholar]


