Abstract
Precursors of neurotransmitters are increasingly often investigated as potential, easily-accessible methods of neuromodulation. However, the amino-acid glutamine, precursor to the brain’s main excitatory and inhibitory neurotransmitters glutamate and GABA, remains notably little investigated. The current double-blind, randomized, placebo-controlled study provides first evidence 2.0 g glutamine administration in healthy adults affects response selection but not motor sequence learning in a serial reaction time task. Specifically, glutamine increased response selection errors when the current target response required a different hand than the directly preceding target response, which might indicate enhanced cortical excitability via a presumed increase in glutamate levels. These results suggest glutamine can alter cortical excitability but, despite the critical roles of glutamate and GABA in motor learning, at its current dose glutamine does not affect sequence learning.
Introduction
There is growing research interest in evaluating the neuromodulatory effects of exogenous administration of neurotransmitter precursors on cognition. Upon administration, precursors are assumed to be converted into their end-products, thus increasing neurotransmitter levels and consequently, influencing cognitive function. For example, tyrosine and tryptophan are two amino-acid precursors of monoamine neurotransmitters that have been demonstrated to provide neuromodulatory effects. Tyrosine is a precursor of dopamine and norepinephrine, and its administration has been shown to modulate, amongst others, working memory1, 2 and Stroop performance3 (for a review, see ref. 4). Tryptophan is a precursor of serotonin (5-HT) and its administration has been shown to affect social behaviour and mood (for reviews, see refs 5–7), as well as improve or impair cognitive function depending on the individual’s mood and stress level due to mild sedation5. While neuromodulatory effects of tyrosine and tryptophan have attracted substantial research attention, the amino-acid precursor glutamine (Gln) has not been well researched as a potential neuromodulator of cognitive function despite being the precursor of glutamate (Glu) and γ-aminobutyric acid (GABA)8, which are the main excitatory and inhibitory neurotransmitters, respectively, within the brain9. After absorption into the circulatory system, Gln is able to pass through the blood-brain barrier10 upon which it then increases Glu and GABA levels in the brain11. Glu and GABA play critical roles in shaping cortical excitability and synaptic plasticity12–17. Because of their effects on cortical excitability, levels of Glu and GABA are implicated in, amongst others, response selection and inhibition18–20, impulsivity21, and error detection and response conflict monitoring22. Notably, cortical excitation facilitates the adjustment of synaptic strength via NMDA-receptor-driven long-term potentiation (LTP)23, thereby implicating Glu and GABA in learning as well. Given that Gln administration, via central changes in Glu and GABA levels, has the potential to alter cortical excitability and thus response selection and inhibition behaviour, it seems appropriate to consider the effects of Gln administration on sequence learning. Sequenced actions heavily rely on response selection processes24 and the acquisition of sequence patterns is associated with increases in cortical excitability25. Because sequenced actions are fundamental to most everyday tasks in humans26, it is important to investigate the potential neuromodulatory effects provided by Gln administration.
Glutamate and GABA
As the primary excitatory and inhibitory neurotransmitters in the brain, higher levels of Glu and GABA respectively increase and decrease cortical excitability. It is hypothesized18–20 that increased cortical inhibition due to high GABA levels can sharpen task-relevant representations in the cortex and inhibit competing responses, thereby facilitating response selection and inhibition processes. It could then be argued that increased cortical excitation due to high Glu levels might have the opposite effect by facilitating activation of competing responses, thus increasing the time necessary to resolve response selection processes and impairing accuracy of these processes20. This model of the roles of Glu and GABA in response selection is supported by studies that directly assessed brain neurotransmitter levels using magnetic resonance spectroscopy (MRS), albeit with a particular focus on GABA. For example, individual differences in regionally-specific GABA concentration have been shown to predict motor decision speed in a saccade distractor task27, with higher levels predicting faster response initiation to a target in the face of distractors. Conversely, higher striatal GABA concentration has been associated with overall faster responses28 and higher accuracy29 in the Simon task. Furthermore, higher GABA concentration, in particular in airplane pilot trainees, has been associated with a more serial as opposed to parallel action cascading strategy, which has been argued to indicate more efficient action control30. Lastly, one study using MRS to assess the balance between Glu and GABA, rather than their individual levels, indicated a higher Glu-to-GABA ratio is associated with increased selection costs and slower reaction times in language production tasks20. In sum, there is converging support for the idea that increased GABA facilitates response selection via reduced cortical excitability, whereas increased Glu impairs response selection via heightened cortical excitability.
With respect to learning, studies have indirectly examined GABA and Glu by assessing the behavioural effects of a history of concussive injuries, which is thought to lead to accumulation of brain GABA and consequently stronger intracortical inhibition. This has important implications for learning, as excitation of the cortex facilitates LTP-driven learning via activation of NMDA receptors23. Consistent with reduced LTP due to higher GABA levels, previously-concussed athletes demonstrated reduced synaptic plasticity and less implicit motor sequence learning in a serial reaction time (SRT) task when compared to unconcussed teammates31. A follow-up study demonstrated that older concussed athletes had greater age-related decreases of Glu and that Glu concentration was positively related to motor sequence learning in the SRT task32. These findings are consistent with the important role of the primary motor cortex (M1) in sequence learning33 and the fact that M1 is sensitive to Glu and GABA34–36. In sum, they suggest sequence learning would benefit from increased excitation and suffer from increased inhibition of the cortex.
The present study
Despite the critical roles of Glu and GABA in response selection and cortical excitability, we are not aware of any studies with healthy adults that have focused on the effects of Gln administration on these processes. Because of this lack of previous studies and the fact that Gln is the precursor to both Glu and GABA, which are hypothesized to have opposite effects on response selection and learning, it is difficult to establish a priori the direction in which Gln administration modulates performance. This is further compounded by the aforementioned finding that not just individual Glu and GABA levels but also the relative balance between the two determines performance20 and it remains unclear in favour of which neurotransmitter Gln would modulate this balance, if at all. Nevertheless, the direction of our results can tentatively suggest how the Glu-to-GABA ratio is modulated. As indicated by the previously discussed findings, increased GABA facilitates response selection but impairs motor sequence learning. Thus, improved response selection and/or impaired learning performance following Gln administration could be indicative of an increase in GABA level (compare31). The opposite results, i.e. decrements in response selection and/or enhanced learning performance, would then be consistent with an increased Glu level (compare32).
To investigate the effect of low dose Gln (2.0 g) on sequence learning we utilized the SRT task37–39, performance on which has been related to Glu and GABA levels (e.g. refs 31, 32). It represents a simple 4-choice reaction time task and thus involves response selection, inhibition and error detection processes that may be sensitive to a Gln-induced manipulation of Glu and/or GABA levels. The response sequence can be varied randomly, in which case participants can rely solely on the stimulus for selecting the appropriate response and have a 33% chance of guessing the correct response, given that there are no immediate response repetitions. As such, random blocks are particularly stimulus-oriented. However, the task also includes blocks with an embedded, second-order conditional (SOC) sequence that allows (unconscious) anticipation of the correct response and potentially induces a shift from stimulus-based to plan-based control40. The implicit learning of the SOC sequence is typically reflected in a gradual decrease in response latency, and modulation of this decline in response latency would indicate a potential influence of Gln on motor sequence learning. Contrasting results from stimulus-oriented, random blocks with those from SOC blocks can shed light on a possible differential effect of Gln on stimulus-based versus plan-based action control.
Results
To assess the effect of Gln on processes associated with response selection and inhibition, one group of participants was administered 2.0 g Gln (N = 48) and another group received a neutral placebo (N = 43). Groups were then compared on response error percentage (REP) and mean reaction time (MRT) in the SRT task.
Response error percentage
REP results are illustrated in Fig. 1 (top panel). In the first three stimulus-oriented blocks, repeated measures ANOVA indicated a non-significant effect of Group, (p = 0.21), but a significant effect of Block, F(2, 178) = 20.6, p < 0.001, partial η 2 = 0.19 and a significant Group × Block interaction, F(2, 178) = 4.1, p = 0.02, partial η 2 = 0.04. REP for the Gln group in stimulus-oriented block 1 (M = 1.96, SD = 1.53) and block 2 (M = 3.40, SD = 2.50) was not significantly different from REP for the placebo group in block 1 (M = 2.17, SD = 2.06) and block 2 (M = 3.10, SD = 2.02) (p = 0.58, 0.53). However, in block 3 REP was significantly higher (p = 0.012) in the Gln group (M = 4.15, SD = 2.37) than the placebo group (M = 2.97, SD = 1.97). To further explore the effect of Gln on REP we divided error trials in the first three stimulus-oriented blocks according to whether current error trial and the preceding trial required the same or a switched hand to make the response. REP was then submitted to repeated measures ANOVA with the factors Group and Hand Switch (same vs. switched hand). This revealed no significant interaction between Group and Hand Switch nor a three-way interaction involving Block, both p > 0.490. An additional analysis with the factors Group and Switch Direction (left-to-right vs. right-to-left) revealed no significant interaction between Group and Switch Direction nor a three-way interaction involving Block, both p > 0.731.
Figure 1.
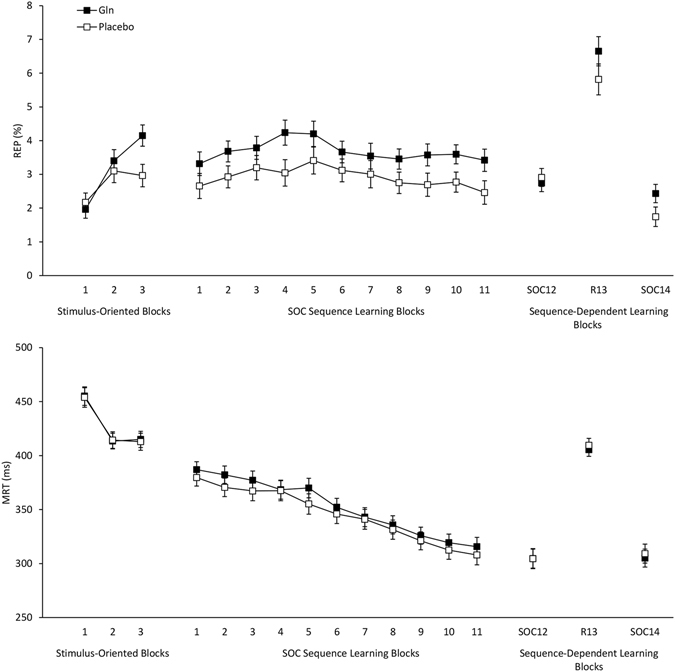
Mean REP (top panel) and MRT (bottom panel) as a function of block and group (Gln vs. placebo). ‘SOC’ refers to an SOC sequence block and ‘R’ refers to a random sequence block. Bars represent standard error of the means.
For SOC sequence blocks 1–11, repeated measures ANOVA indicated a significant effect of Group, F(1, 89) = 4.7, p = 0.03, partial η 2 = 0.05, and Block, F(10, 890) = 2.3, p = 0.01, partial η 2 = 0.03, while there was no significant Group × Block interaction, (p = 0.97). Across these blocks, the Gln group had significantly higher REP (M = 3.67, SD = 2.55) than the placebo group (M = 2.91, SD = 2.04). REP in blocks 4 and 5 was significantly higher than in block 1 while REP in block 11 was significantly lower than block 3–5 (all p < 0.05).
To further explore the effect of Gln on REP we divided error trials in SOC blocks 1–11 again according to whether the current error trial and the preceding trial required the same or a switched hand to make the response. The total number of errors per SOC block was then submitted to repeated measures ANOVA with the factors Group and Hand Switch. This revealed a significant effect of Group F(1, 89) = 4.34, p = 0.04, η 2 = 0.046 and Hand Switch, F(1, 89) = 123.1, p < 0.001, η 2 = 0.58, as well as a significant Group × Hand Switch interaction, F(1, 89) = 4.5, p = 0.037, η 2 = 0.048. Post-hoc comparisons indicated a significantly higher total amount of errors on trials requiring a switch of hands in both the Gln (M = 43.9, SD = 22.5 vs. M = 18.9, SD = 10.0) and placebo (M = 34.0, SD = 17.4 vs. M = 17.0, SD = 11.2) groups, both ps < 0.001. Importantly, the Gln group demonstrated significantly more switch hand errors (p = 0.023) than the placebo group whereas the groups did not differ on same hand errors (p = 0.406). Exploring these results yet further by dividing switch trials in those requiring a left-to-right or right-to-left switch revealed significant effects of Group, F(1, 89) = 5.4, p = 0.023, η 2 = 0.057 and Switch Direction, F(1, 89) = 55.6, p < 0.001, η 2 = 0.384, indicating significantly more errors on a left-to-right (M = 23.1, SD = 12.7) than right-to-left switch (M = 16.3, SD = 9.7), but this effect was not modulated by Gln as revealed by a nonsignificant Group × Switch Direction interaction, p = 0.658. In sum, Gln increased response errors when the hand required to carry out the target response on the current trial was not the hand used on the preceding trial and this effect was independent of the direction of the switch. To exclude the possibility this effect is driven by a group difference in amount of left-handed participants (see Table 1), we again analysed the effects of Group and Hand Switch on REP after excluding all left-handed participants. The Group × Hand Switch interaction remained significant and in the same direction, F(1, 80) = 4.4, p = 0.039, η 2 = 0.052, thereby excluding a potential confounding of the effect by group differences in handedness.
Table 1.
Group characteristics.
| Gln | Placebo | p | |
|---|---|---|---|
| N, Total | 48 | 43 | |
| N, Male:Female | 14:34 | 13:30 | 0.912 |
| N, Right:Lefthanded | 40:8 | 42:1 | 0.022 |
| Age, years M (SD) | 20.5 (2.5) | 20.6 (2.5) | 0.904 |
| Weight, kg M (SD) | 66.1 (7.7) | 65.1 (7.6) | 0.531 |
| BMI, kg/m2 M (SD) | 21.6 (2.5) | 21.9 (2.2) | 0.632 |
| Sleep, hours M (SD) | 7.3 (1.3) | 6.8 (1.2) | 0.086 |
To assess sequence dependent learning we compared REP in the 12th SOC block to the 13th random block and the 14th SOC block. Repeated measures ANOVA indicated a significant effect of Block, F(2, 178) = 128.1, p < 0.001, partial η 2 = 0.59, while the Group main effect (p = 0.21) and the Group × Block interaction, (p = 0.16) were not significant. REP in block 13 (M = 6.25, SD = 3.02) was significantly higher than block 12 (M = 2.82, SD = 1.74, p < 0.0001) and block 14 (M = 2.11, SD = 1.90, p < 0.0001). REP in block 12 was also significantly higher (p < 0.01) than in block 14. Additional analyses revealed no significant interaction between Group and Hand Switch, F(1, 89) = 0.22, p = 0.642, or between Group and Switch Direction (left-to-right vs. right-to-left), F(1, 89) = 0.13, p = 0.722.
Mean reaction time
MRT results are presented in Fig. 1 (bottom panel). In each of three analyses, there was only a significant effect of Block in the three stimulus-oriented blocks, F(2, 178) = 89.2, p < 0.001, partial η 2 = 0.50, in SOC blocks 1–11, F(10, 890) = 104.4, p < 0.001, partial η 2 = 0.54, and when comparing SOC block 12 to random block 13 and SOC block 14, F(2, 178) = 321.4, p < 0.001, partial η 2 = 0.78. In contrast, no significant main effects of Group or Group × Block interactions were obtained (all p > 0.53). In the stimulus-oriented blocks, MRT was significantly higher in block 1 (M = 454.51, SD = 59.02) than block 2 (M = 413.86, SD = 50.19, p < 0.0001) and block 3 (M = 413.99, SD = 51.82, p < 0.0001) while MRT did not significantly differ between blocks 2 and 3 (p = 0.99). For SOC sequence learning blocks, MRT was significantly lower as learning progressed across blocks 1 to 11. For sequence-dependent learning assessment blocks, MRT in block 13 (M = 396.18, SD = 34.41) was significantly higher than block 12 (M = 299.69, SD = 56.10, p < 0.0001) and block 14 (M = 302.16, SD = 49.80, p < 0.0001). MRT in block 12 was not significantly different (p = 0.37) than MRT in block 14. None of the stimulus-oriented blocks, SOC sequence learning blocks or sequence-dependent learning blocks involved a significant Group × Hand Switch interaction, all p > 0.31, or a significant Group × Switch Direction interaction, all p > 0.63. In sum, Gln did not affect MRT in any of the SRT blocks.
Sequence recall
The frequency of participants reporting explicit awareness of a sequence pattern was not significantly different between Gln (“Yes” = 36, “No” = 12) and placebo (“Yes” = 34, “No” = 9) groups X 2(91) = 0.21, p = 0.65. The number of sequence chunks recalled did not significantly differ between Gln (M = 1.04, SD = 0.54) and placebo groups (M = 1.00, SD = 0.56, p = 0.72). Similarly, the average length of sequence chunks recalled did not significantly differ between Gln (M = 4.65, SD = 2.70) and placebo groups (M = 4.91, SD = 2.87, p = 0.66). In sum, Gln did not seem to affect measures of sequence recall.
Discussion
The present paper is one of the first to report proof-of-principle that the amino-acid Gln, the precursor of the brain’s main excitatory neurotransmitter Glu and inhibitory neurotransmitter GABA8, 9, modulates cognitive function related to response selection but not sequence learning. Specifically, Gln administration led to an overall increase in response selection errors in both stimulus-oriented and SOC sequence-learning blocks of an SRT task without affecting sequence-dependent learning or sequence recall, suggesting Gln affected primarily stimulus-based rather than plan-based control40. More specifically, Gln impaired performance when the hand required to carry out the target response differed on the current and preceding trial, indicating Gln primarily affected the laterality of response selection processes. This raises the possibility that Gln, via a presumed increase in Glu, enhanced the lateral motor activation associated with the most recent target response, which presented conflict when the next trial required the other hand to press the correct key. This notion is indirectly supported by findings on GABA and the Simon task28, 29 that suggest increased cortical inhibition benefits processing of laterality of responses. The opposite, that is an impairment in processing the laterality of responses, might then be expected when cortical inhibition is reduced by Glu. Consistent with this idea, heightened cortical excitability has been hypothesized to account for impaired response selection by facilitating activation of competing responses20. Furthermore, it is interesting to note the increase in response selection errors emerged only after the first two (stimulus-oriented) blocks, indicating Gln might have affected performance only after initial task familiarization when participants might start responding with less deliberation and instead perform more automatically. On the other hand, the Gln effect seems to have worn off near the end of the task, suggesting that at this point Gln may have been metabolized down to levels that no longer influenced behaviour or participants in the Gln condition could overcome the effect on response selection with sufficient training. It is also important to note the Gln-induced increase in response errors was not due to a speed-accuracy trade-off, as response latencies were unaffected. In sum, the present study provides first evidence indicating Gln administration can modulate cognitive-behavioural performance by enhancing cortical excitability.
Whereas Gln seems to have affected response selection processes in both stimulus-oriented (random) and SOC blocks, its effects did not seem to extend to sequence-dependent learning. This might tentatively indicate that LTP-driven plasticity was not altered despite the presumed increase in cortical excitation. For now we can only speculate on the reason for this selectivity. First, it might be our Gln dose of 2.0 g was too low to induce changes in synaptic plasticity. Previous studies that mainly focused on Gln’s positive effects on gastrointestinal function administered daily doses of 10 g or more41–43, which exceeds the average daily intake of 3–6 g Gln44 and is 5 or more times our dose of 2.0 g. It remains unclear whether a higher dose could lead to a more pronounced and longer-lasting cognitive response, hence future studies might systematically vary Gln dose to clarify this issue. Second, Gln might have affected response selection but not sequence-dependent learning because of regional specificity, in line with reports of regionally-specific effects of GABA12, 21, 27. Although this remains speculative, perhaps Gln at a dose of 2.0 g primarily affected response conflict and impulsivity in the prefrontal cortex without affecting sequence-dependent learning mediated by motor area M133. Future studies could employ MRS to assess whether Gln has dissociable effects on Glu and GABA levels in different regions. Third, in the present study three random, stimulus-oriented blocks were always presented before twelve SOC blocks. This order of presentation might have predisposed participants to stimulus-based rather than plan-based control and discouraged sequence learning in SOC blocks, limiting the possibility of finding an effect of Gln on plan-based control. Hence, whereas this study presented the random blocks after Gln administration and immediately before starting the SOC blocks, future studies may wish to present the first random blocks before Gln administration to render them more as task familiarization, or systematically vary the order in which the blocks are presented.
The lack of previous studies with Gln and cognitive-behavioural performance made it difficult to predict a priori the direction in which Gln would enhance performance. However, the present results may form a basis for novel hypotheses that can be tested in the future and thereby provide converging evidence that Gln modulates performance via increased cortical excitability. For example response inhibition, i.e. the ability to withhold prepotent responses, seems to benefit from increased cortical inhibition due to increased GABA levels45, 46. Similarly, higher GABA levels have been associated with less impulsivity21. This suggests the opposite, that is an impairment in response inhibition and increase in impulsivity, might occur when cortical excitability is enhanced due to Gln-induced increases in Glu levels. Hence a study showing Gln reduces response inhibition efficiency and/or increases impulsivity would converge on the idea Gln enhances cortical excitability.
The present study employed a between-subjects design because asking participants whether they noticed a response sequence in the SRT task is likely to affect subsequent task performance. Because of the between-subjects design, one might argue our results are simply due to baseline group differences in response selection efficiency rather than due to the Gln administration. Although we argue this is unlikely with our sample size that is larger than in most amino-acid precursor studies, this is not to say such alternative explanations of our data are impossible. Therefore, future studies may wish to exclude the possibility of pre-existing group differences by i) using tasks that allow for a within-subjects design, ii) assessing baseline response selection performance and iii) taking pre and post-administration measurements of cortical excitability, for example by using motor-evoked potentials (MEP) to assess both pre-existing differences and Gln-induced changes in cortical excitability. If MEP measurements confirm enhanced excitability of the cortex after Gln administration and if this change would correlate with the individual frequency of response selection errors, that would provide strong support for the mechanism of action hypothesized in the present study.
It may also be interesting for future studies to consider the role of individual differences in the balance between cortical Glu and GABA levels, as variability in this balance rather than the individual neurotransmitter levels has previously been shown to predict individual differences in response selection efficiency20. Although the present study suggests Gln administration at a group level enhanced this balance in favour of Glu, it seems plausible individual response to Gln might be predicted by the pre-existing Glu/GABA balance, with Gln perhaps having more pronounced or even opposite effects in individuals with a balance highly in favour of Glu or GABA.
Lastly, it is important to consider that the effect of Gln reported here seems to apply to sequential motor control but not sequence learning, as Gln affected performance similarly in the stimulus-oriented and SOC blocks of the SRT task. However, the present version of the SRT task includes relatively few stimulus-oriented blocks, which limits the assessment of sequential motor control separately from sequence learning. To disentangle and separately investigate these processes, future research should aim to balance the amount of stimulus-oriented and SOC blocks47.
To conclude, the present study is the first to investigate Gln administration in healthy adults in relation to response selection and sequence learning performance. Results show Gln impairs response selection but does not alter sequence learning, suggesting an increase in cortical excitability without affecting synaptic plasticity. As such, despite the critical roles of Glu and GABA in motor learning, this study finds no evidence for an effect of their precursor on sequence learning performance, but does present first evidence that Gln modulates sequential motor control processes.
Methods
Participants
A total of 91 students from Leiden University were recruited to participate for money or course credit. Using a double blind, placebo-controlled design, participants were randomly assigned to receive either Gln (N = 48) or a neutral placebo (N = 43). See Table 1 for group characteristics. The groups did not differ in terms of gender distribution, age, weight, BMI, or hours of sleep the night before the study, but the Gln group did contain significantly more left-handed individuals.
Study participation eligibility criteria were based on previous studies on neuromodulation from our lab1, 48–50. Specifically, interested individuals were screened for cardiac, hepatic, renal, neurologic or psychiatric disorders, and medication (except oral or implanted contraceptives) or recreational drug use, and those who reported any of these conditions were not eligible to participate. Females were only eligible to participate if they used either oral or implanted hormonal contraception, to reduce the impact of fluctuating estrogen-dopamine interactions on our results51–53.
Prior to participation informed consent was obtained from all participants. The study conformed to the ethical standards of the Declaration of Helsinki and the protocol was approved by the local ethical committee (Leiden University, Institute for Psychological Research).
Glutamine administration
All doses were prepared and coded by a researcher not involved in running the study, to blind the experiment leader to the administered dose. Participants received either 2.0 g Gln or 2.0 g microcrystalline cellulose, a neutral placebo, dissolved in 400 mL of orange juice. Given the lack of prior studies on Gln administration and cognition, this dose was based on previous studies with tyrosine in our lab that showed reliable effects1, 54, 55. The dose of 2.0 g is safe and less than the normal daily intake of approximately 3–6 g Gln from protein44 and far less than in studies on Gln and gastrointestinal function that administered 10 g or more daily41–43.
Serial reaction time task
To assess response selection and sequence learning participants performed a standard SRT task37–39 presented using E-Prime 2.0 software (Psychology Software Tools, Inc., Pittsburgh, PA, USA). In this task four horizontally-aligned empty squares were presented in the centre of the screen. On each trial one of the squares turns red and the participant must press a corresponding button on the QWERTY keyboard (from left to right: V, B, N, M) using the index and middle fingers of the left (V, B) and right (N, M) hand. An error sound is presented if the wrong button is pressed, along with the Dutch words “Verkeerde toets!” (“Wrong button!”). Reaction time (RT) is measured in milliseconds as the latency in the key press to the stimulus and if RT exceeds 3,000 ms, the Dutch words “Te langzaam!” (“Too slow!”) are presented. Following the response, the four empty squares appear for a 50 ms response-stimulus interval before the next stimulus is presented. Participants were instructed that accuracy and response speed were equally important in the task. Participants first completed three random sequence blocks, then twelve SOC sequence learning blocks in which responses followed a 12-item SOC sequence (VBVNMBNVMNBM56), which was cycled through ten times in each block. To determine sequence dependence or the serial effect as opposed to general practice effects, a random-ordered block was inserted followed by a final block that re-introduced the SOC sequence. The sudden introduction of a random response sequence likely interferes with the anticipation of responses in a plan-based action control style, requiring an abrupt shift to stimulus-oriented control. Hence RT and response errors are expected to sharply increase in the random block57 but performance is expected to recover when the sequence is reintroduced in the final SOC block. All blocks contained 120 trials and after completion of each block performance feedback indicating number of errors and mean RT was presented followed by a 30 s rest interval. Following completion of the final block, participants were asked to respond “Yes” or “No” to a question that asked if they noticed a pattern in the responses at any point of the task to determine explicit awareness of the serial sequence. When answering “Yes”, participants were then asked to use the response keys to produce one cycle of the 12-item sequence as a recall test.
Procedure
Participants entered the lab between 9:00 and 10:00 to be tested individually, having fasted overnight (compare1, 48). Informed consent was obtained, after which they consumed 2.0 g of either Gln or placebo dissolved in 400 mL orange juice. Afterwards, apples and oranges were offered to prevent strong hunger. Although Gln has been shown to increase in vitro rat brain Glu and GABA levels as soon as 15 minutes11, in line with previous studies on precursors of neurotransmitters, testing commenced precisely one hour after Gln or placebo administration to allow time for plasma and brain Gln levels to increase (compare1, 48, 55). Participants then performed the SRT task, which took approximately 30 minutes. Lastly, participants were debriefed on the nature and hypotheses of the study and were compensated and thanked for their participation.
Analysis
For each participant, REP was determined based on the number of error trials (incorrect key press) as a percentage of the total number of trials in each block. MRT was then calculated for each participant and each block, after removing error trials as well as trials with outlier RT (1.2%) based on RT that was more than 3 standard deviations above the individual’s overall mean for correct trials. Participant REP and MRT were then submitted to separate repeated measures ANOVA that i) compared performance between Gln and placebo Groups in the first three stimulus-oriented Blocks, ii) compared sequence learning between Gln and placebo Groups in SOC Blocks 1–11, and iii) compared sequence-dependent learning in Gln and placebo Groups across Blocks 12 (SOC), 13 (random) and 14 (SOC). Although others39, 57 proposed averaging performance over SOC Blocks 12 and 14 before comparison with random Block 13, performance in Block 14 might still suffer from interference by the previous random block. As such, we argue that comparing all three blocks can provide a clearer picture of how performance is affected by the introduction of the random block. Explicit sequence awareness frequency was compared between Gln and placebo groups using Χ2 analysis. Sequence recall was analysed based on a response chunking approach58–60. The presence of chunks of the training SOC sequence was determined for each participant with chunk lengths ranging between 4 to 12 items. The probability of entering the smallest chunk length, a 4-item chunk, by chance was calculated as 11% (0.33 × 0.33) given that there are no consecutive repetitions in the SOC sequence structure61. To identify any matches between the participant’s recalled sequence and the target sequence, the participant’s sequence was divided into chunks made up of between 4 and 12 items. These chunks could commence with any sequence item, with the condition that the end of the sequence could not be extended to the initial sequence items since the participant’s chunk needed to be contiguous. The target sequence was also divided into chunk lengths of between 4 and 12 items, however, here these chunks could start with any item in the sequence and continue on to include items at the beginning of the sequence. Continuing the chunks past the end of the SOC sequence reflects the repeating nature of the sequence, meaning the participant could have treated the commencement of a chunk at any point of the repeated sequence. Performance on chunk recall of the sequence was based on the number of matched chunks and mean length of the matched chunks for each participant. Only the longest chunk was recorded as a match and matched chunks were only recorded once in the event the participant repeated the same chunk. As the 12-item sequence recall allows for 9 possible 4-item chunks, a participant would be expected to recall approximately 1 valid 4-item chunk by chance (9 × 0.11). Participant’s recalled chunk count and mean chunk length were separately submitted to ANOVA for Group comparisons. All repeated measures analyses use Greenhouse-Geisser correction when the sphericity assumption was violated and all post-hoc comparisons use Fisher’s LSD adjustment. For all tests a significance threshold of 0.05 was adopted.
Random ordering of responses in the first three stimulus-oriented blocks and random Block 13 could have introduced group differences in the number of reversal trials resulting in MRT performance artefacts47, 56. A reversal trial occurs when the third trial of any three consecutive trails involves the same target response as the first trial (e.g., VBV47). With respect to the number of reversal trials in the first three stimulus-oriented blocks, there was a non-significant effect of Group (p = 0.21) and a non-significant Group × Block interaction (p = 0.13). In addition, the number of reversal trials in Block 13 did not significantly differ between Groups (p = 0.12). In stimulus-oriented blocks, MRT was significantly longer in reversal trials than in non-reversal trials, F(1, 89) = 145.8, p < 0.0001, partial η 2 = 0.62, however, there was a non-significant Group × Reversal Trial interaction (p = 0.68) and a non-significant Group × Reversal Trial × Block interaction (p = 0.61). In SOC blocks, MRT was significantly longer in reversal trials than in non-reversal trials, F(1, 89) = 24.5, p < 0.0001, partial η 2 = 0.22, however, there was again a non-significant Group × Reversal Trial interaction (p = 0.56) and a non-significant Group × Reversal Trial × Block interaction (p = 0.89). This indicates any group differences in these blocks are not confounded by differences in the number of reversal trials.
Acknowledgements
We would like to thank Dr. Alberto Pelanda for his valuable advice on the use of glutamine. This work was supported by a research grant from the Netherlands Organization for Scientific Research (NWO; www.nwo.nl) awarded to L.S.C. (Vidi grant: #452-12-001). The NWO had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author Contributions
Authors B.J.J. and L.S.C. designed the study and wrote the protocol. Author M.A.I. undertook the statistical analysis. Author B.J.J. wrote the first draft of the manuscript and authors M.A.I. and L.S.C. edited the manuscript. All authors contributed to and have approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Colzato LS, Jongkees BJ, Sellaro R, Hommel B. Working memory reloaded: tyrosine repletes updating in the N-back task. Front. Behav. Neurosci. 2013;7:200. doi: 10.3389/fnbeh.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas JR, Lockwood PA, Singh A, Deuster PA. Tyrosine improves working memory in a multitasking environment. Pharmacol. Biochem. Behav. 1999;64:495–500. doi: 10.1016/S0091-3057(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 3.Deijen JB, Orlebeke JF. Effect of tyrosine on cognitive function and blood pressure under stress. Brain Res. Bull. 1994;33:319–23. doi: 10.1016/0361-9230(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 4.Jongkees BJ, Hommel B, Kühn S, Colzato LS. Effect of tyrosine supplementation on clinical and healthy populations under stress or cognitive demands—a review. J. Psychiatr. Res. 2015;70:50–57. doi: 10.1016/j.jpsychires.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Silber BY, Schmitt JAJ. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci. Biobehav. Rev. 2010;34:387–407. doi: 10.1016/j.neubiorev.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Steenbergen L, Jongkees BJ, Sellaro R, Colzato LS. Tryptophan supplementation modulates social behavior: a review. Neurosci. Biobehav. Rev. 2016;64:346–358. doi: 10.1016/j.neubiorev.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Young SN. The effect of raising and lowering tryptophan levels on human mood and social behaviour. Philos. Trans. R. Soc. 2013;368:20110375. doi: 10.1098/rstb.2011.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U. The glutamine-glutamate/GABA cycle: Function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem. Res. 2015;40:402–409. doi: 10.1007/s11064-014-1473-1. [DOI] [PubMed] [Google Scholar]
- 9.Petroff OAC. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- 10.Lee WJ, Hawkins RA, Viña JR, Peterson DR. Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am. J. Physiol. 1998;274:C1101–C1107. doi: 10.1152/ajpcell.1998.274.4.C1101. [DOI] [PubMed] [Google Scholar]
- 11.Bowyer JF, Lipe GW, Matthews JC, Scallet AC, Davies DL. Comparison of glutamine-enhanced glutamate release from slices and primary cultures of rat brain. Ann. N. Y. Acad. Sci. 1995;765:72–85. doi: 10.1111/j.1749-6632.1995.tb16562.x. [DOI] [PubMed] [Google Scholar]
- 12.Boy F, et al. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr. Biol. 2010;20:1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J. Neurophysiol. 2006;95:1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J. Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr. Biol. 2011;21:480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J. Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziemann U, et al. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Munakata Y, et al. A unified framework for inhibitory control. Trends Cogn. Sci. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder HR, et al. Neural inhibition enables selection during language processing. PNAS. 2010;107:16483–16488. doi: 10.1073/pnas.1002291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de la Vega A, et al. Individual differences in the balance of GABA to glutamate in PFC predict the ability to select among competing options. J. Cogn. Neurosci. 2014;26:2490–2502. doi: 10.1162/jocn_a_00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boy F, et al. Dorsolateral prefrontal y-aminobutric acid in men predicts individual differences in rash impulsivity. Biol. Psychiatry. 2011;70:866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Veen V, Carter CS. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clin. EEG Neurosci. 2006;37:330–335. doi: 10.1177/155005940603700411. [DOI] [PubMed] [Google Scholar]
- 23.Ziemann U, Siebner HR. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul. 2008;1:60–66. doi: 10.1016/j.brs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Deroost N, Soetens E. The role of response selection in sequence learning. Q. J. Exp. Psychol. 2006;59:449–456. doi: 10.1080/17470210500462684. [DOI] [PubMed] [Google Scholar]
- 25.Lin CH, et al. Brain-behavior correlates of optimizing learning through interleaved practice. Neuroimage. 2011;56:1758–1772. doi: 10.1016/j.neuroimage.2011.02.066. [DOI] [PubMed] [Google Scholar]
- 26.Clegg BA, DiGirolamo GJ, Keele SW. Sequence learning. Trends Cogn. Sci. 1998;2:275–281. doi: 10.1016/S1364-6613(98)01202-9. [DOI] [PubMed] [Google Scholar]
- 27.Sumner P, Edden RAE, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat. Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 28.Dharmadhikari S, et al. Striatal and thalamic GABA level concentrations play differential roles for the modulation of response selection processes by proprioceptive information. Neuroimage. 2015;120:36–42. doi: 10.1016/j.neuroimage.2015.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haag L, et al. Interrelation of resting state functional connectivity, striatal GABA levels, and cognitive control processes. Hum. Brain Mapp. 2015;36:4383–4393. doi: 10.1002/hbm.22920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yildiz A, et al. Feeling safe in the plane: neural mechanisms underlying superior action control in airplane pilot trainees–a combined EEG/MRS study. Hum. Brain Mapp. 2014;35:5040–5051. doi: 10.1002/hbm.22530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Beaumont L, Tremblay S, Poirier J, Lassonde M, Théoret H. Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb. Cortex. 2012;22:112–121. doi: 10.1093/cercor/bhr096. [DOI] [PubMed] [Google Scholar]
- 32.de Beaumont L, et al. Motor system alterations in retired former athletes: the role of aging and concussion history. BMC Neurol. 2013;13:109. doi: 10.1186/1471-2377-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright D, et al. Consolidating behavioral and neurophysiologic findings to explain the influence of contextual interference during motor sequence learning. Psychon. Bull. Rev. 2016;23:1–21. doi: 10.3758/s13423-015-0887-3. [DOI] [PubMed] [Google Scholar]
- 34.Hasan MT, et al. Role of motor cortex NMDA receptors in learning-dependent synaptic plasticity of behaving mice. Nat. Commun. 2013;4:2258. doi: 10.1038/ncomms3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stagg CJ. Magnetic resonance spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. Neuroimage. 2014;86:19–27. doi: 10.1016/j.neuroimage.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- 37.Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn. Psychol. 1987;19:1–32. doi: 10.1016/0010-0285(87)90002-8. [DOI] [Google Scholar]
- 38.Schwarb H, Schumacher EH. Generalized lessons about sequence learning from the study of the serial reaction time task. Adv. Cogn. Psychol. 2012;8:165–178. doi: 10.5709/acp-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abrahamse EL, Noordzij ML. Designing training programs for perceptual-motor skills: practical implications from the serial reaction time task. Eur. Rev. Appl. Psychol. 2011;61:65–76. doi: 10.1016/j.erap.2010.12.001. [DOI] [Google Scholar]
- 40.Tubau E, Hommel B, López-Moliner J. Modes of executive control in sequence learning: from stimulus-based to plan-based control. J. Exp. Psychol. Gen. 2007;136:43–63. doi: 10.1037/0096-3445.136.1.43. [DOI] [PubMed] [Google Scholar]
- 41.Mitter SS, et al. Apolipoprotein E4 influences growth and cognitive responses to micronutrient supplementation in shantytown children from northeast Brazil. Clin. (Sao Paulo) 2012;67:11–18. doi: 10.6061/clinics/2012(01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lima AAM, et al. Zinc, vitamin A, and glutamine supplementation in Brazilian shantytown children at risk for diarrhea results in sex-specific improvements in verbal learning. Clin. (Sao Paulo) 2013;68:351–358. doi: 10.6061/clinics/2013(03)OA11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arwert LI, Deijen JB, Drent ML. Effects of an oral mixture containing glycine, glutamine and niacin on memory, GH and IGF-I secretion in middle-aged and elderly subjects. Nutr. Neurosci. 2003;6:269–275. doi: 10.1080/10284150310001612195. [DOI] [PubMed] [Google Scholar]
- 44.Gleeson M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J. Nutr. 2008;138:2045S–2049S. doi: 10.1093/jn/138.10.2045S. [DOI] [PubMed] [Google Scholar]
- 45.Quetscher C, et al. Striatal GABA-MRS predicts response inhibition performance and its cortical electrophysiological correlates. Brain Struct. Funct. 2015;220:3555–3564. doi: 10.1007/s00429-014-0873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steenbergen L, Sellaro R, Stock A-K, Beste C, Colzato LS. γ-Aminobutyric acid (GABA) administration improves action selection processes: a randomised controlled trial. Sci. Rep. 2015;5:12770. doi: 10.1038/srep12770. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Vaquero JMM, Jiménez L, Lupiáñez J. The problem of reversals in assessing implicit sequence learning with serial reaction time tasks. Exp. Brain Res. 2006;175:97–109. doi: 10.1007/s00221-006-0523-6. [DOI] [PubMed] [Google Scholar]
- 48.Steenbergen L, Sellaro R, Colzato LS. Tryptophan promotes charitable donating. Front. Psychol. 2014;5:1451. doi: 10.3389/fpsyg.2014.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colzato LS, Pratt J, Hommel B. Dopaminergic control of attentional flexibility: inhibition of return is associated with the dopamine transporter (DAT1) Front. Hum. Neurosci. 2010;4:53. doi: 10.3389/fnhum.2010.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colzato LS, et al. Loving-kindness brings loving-kindness: the impact of Buddhism on cognitive self-other integration. Psychon. Bull. Rev. 2012;19:541–545. doi: 10.3758/s13423-012-0241-y. [DOI] [PubMed] [Google Scholar]
- 51.Colzato LS, Hommel B. Effects of estrogen on higher-order cognitive functions in unstressed human females may depend on individual variation in dopamine baseline levels. Front. Neurosci. 2014;8:65. doi: 10.3389/fnins.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Czoty PW, et al. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009;34:548–554. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]
- 53.Jacobs E, Esposito MD. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J. Neurosci. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colzato LS, Jongkees BJ, Sellaro R, van den Wildenberg WPM, Hommel B. Eating to stop: Tyrosine supplementation enhances inhibitory control but not response execution. Neuropsychologia. 2014;62:1–5. doi: 10.1016/j.neuropsychologia.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Steenbergen L, Sellaro R, Hommel B, Colzato LS. Tyrosine promotes cognitive flexibility: evidence from proactive vs. reactive control during task switching performance. Neuropsychologia. 2015;69:50–55. doi: 10.1016/j.neuropsychologia.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Reed J, Johnson P. Assessing implicit learning with indirect tests: determining what is learned about sequence structure. J. Exp. Psychol. Learn. Mem. Cogn. 1994;20:585–594. doi: 10.1037/0278-7393.20.3.585. [DOI] [Google Scholar]
- 57.Willingham DB, Nissen MJ, Bullemer P. On the development of procedural knowledge. J. Exp. Psychol. Learn. Mem. Cogn. 1989;15:1047–1060. doi: 10.1037/0278-7393.15.6.1047. [DOI] [PubMed] [Google Scholar]
- 58.Verwey WB, Abrahamse EL. Distinct modes of executing movement sequences: reacting, associating, and chunking. Acta Psychol. (Amst). 2012;140:274–282. doi: 10.1016/j.actpsy.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Koch I, Hoffmann J. Patterns, chunks, and hierarchies in serial reaction-time tasks. Psychol. Res. 2000;63:22–35. doi: 10.1007/PL00008165. [DOI] [PubMed] [Google Scholar]
- 60.Jiménez L. Taking patterns for chunks: is there any evidence of chunk learning in continuous serial reaction-time tasks? Psychol. Res. 2008;72:387–396. doi: 10.1007/s00426-007-0121-7. [DOI] [PubMed] [Google Scholar]
- 61.Borragán G, Slama H, Destrebecqz A, Peigneux P. Cognitive fatigue facilitates procedural sequence learning. Front. Hum. Neurosci. 2016;10:86. doi: 10.3389/fnhum.2016.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]


