Abstract
Context
Insulin resistance in polycystic ovary syndrome (PCOS) may increase the risk of reactive hypoglycaemia (RH) and decrease glucagon-like peptide-1 (GLP-1) secretion. The possible effects of treatment with oral contraceptives (OCP) and/or metformin on GLP-1 secretion and risk of RH in PCOS is undetermined.
Setting
Outpatient clinic.
Patients and interventions
Randomized, controlled clinical trial. Ninety women with PCOS were randomized to 12-month treatment with OCP (150 mg desogestrel + 30 mg ethinylestradiol), metformin (2 g/day) or metformin + OCP. Five-hour oral glucose tolerance tests (5-h OGTT) measuring fasting and area under the curve (AUC) for GLP-1, glucose, insulin and C-peptide were performed before and after the intervention period. Sixty-five women completed the study and 34 weight-matched healthy women were included as controls.
Main outcome measures
Changes in GLP-1, glucose, insulin and C-peptide during 5-h OGTT.
Results
Fasting GLP-1 levels increased during metformin + OCP vs OCP treatment, whereas AUC GLP-1 levels were unchanged during medical treatment. The prevalence of reactive hypoglycemia increased from 9/65 to 14/65 after intervention (P < 0.01) and was more common after treatment with metformin + OCP (increase from 3/23 to 6/23, P = 0.01). Reactive hypoglycaemia was associated with higher insulin and C-peptide levels during 5-h OGTT, but was unassociated with BMI and AUC GLP-1. GLP-1 levels were comparable in PCOS vs controls. AUC GLP-1 levels were significantly lower in obese vs lean patients and were inversely associated with BMI.
Conclusions
AUC GLP-1 levels were unchanged during treatment. Increased risk of hypoglycemia during metformin + OCP could be associated with increased insulin secretion.
Keywords: PCOS, oral contraceptive, metformin, GLP-1, reactive hypoglycemia
Introduction
Polycystic ovary syndrome (PCOS) is characterized by oligo/anovulation, hyperandrogenaemia and polycystic ovaries (1, 2). Hyperinsulinaemia as a consequence of insulin resistance is present in most patients with PCOS (1). Incretin hormones are released after meal ingestion and account for up to 70% of postprandial insulin secretion (3). Glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide are the most important incretin hormones. GLP-1 secretion is impaired in insulin resistant states such as obesity and type 2 diabetes (3), whereas results in patients with PCOS are conflicting (4, 5, 6).
Reactive hypoglycaemia (RH) is defined as a drop in blood glucose levels 1½–5 h after food consumption without indication of hypoglycaemia due to other causes (7). RH was prevalent in obese and lean patients with PCOS (8, 9) and was associated with insulin resistance and hyperinsulinaemia especially in obese patients (9). RH may contribute to the development of obesity by increasing appetite-evoking hormones such as leptin (10). Increased GLP-1 activity contributed to hypoglycemia after gastric bypass (11), but possible associations between RH and GLP-1 levels in PCOS have not been investigated.
Treatment modalities in PCOS aim at decreasing hyperandrogenism and improving insulin sensitivity along with weight loss (12, 13). Oral contraceptive treatment (OCP) regulates menstrual cycles and SHBG levels are increased, leading to decreased levels of free testosterone (14). OCP may increase body weight (15) and insulin resistance (14, 16), which could be associated with decreased GLP-1 levels. In contrast, animal studies suggested that estradiol and progesterone treatment could increase the effect and secretion of GLP-1 (4, 17). In one recent uncontrolled study, GLP-1 levels were unchanged during a meal tolerance test after 3 months of OCP treatment (ethinyl estradiol 30 µg/drospirenone 3 mg) in 14 lean patients with PCOS (4), but we are not aware of data in obese patients with PCOS. Increased insulin levels during OCP could be associated with increased risk of RH in PCOS, but no study tested this hypothesis.
Treatment with metformin improved insulin sensitivity and ovulatory function in PCOS (14), whereas androgen levels were only slightly decreased (14, 18). We recently reported a significant weight loss of 3.0 kg during 12-month metformin treatment (15). Decreased insulin levels during metformin treatment could be associated with decreased risk of RH and hence decreased appetite (9). Ghrelin levels decreased (19) and GLP-1 levels increased (6) after treatment with metformin in two uncontrolled studies in PCOS, which could have opposite effects on the risk of RH, but we are not aware of controlled studies that investigated the effect of metformin on GLP-1 levels or risk of RH in PCOS.
The primary aim of the present study was to perform 5-h oral glucose tolerance tests (5-h OGTT) and evaluate the possible effects of treatment with OCP and/or metformin on GLP-1 levels and risk of RH in patients with PCOS. Measures of GLP-1 secretion were furthermore compared in women with PCOS and weight-matched controls.
Methods
The methods of the present intervention study have been described recently in a paper regarding the effects of OCP and/or metformin treatment on body composition measures (15). Baseline data in patients and controls were reported in a paper on RH in PCOS (9). Two patients were excluded in (9) due to incomplete data, but were included in the present data as both patients completed study intervention.
Patients
Ninety white women aged 18–39 years with PCOS were included. The patients fulfilled the Rotterdam criteria for PCOS with minimum two of three of the following criteria: (1) Irregular periods during more than a year with a cycle length >35 days; (2) total or free testosterone levels above reference interval (upper limits: total testosterone >1.8 nmol/L, free testosterone >0.035 nmol/L) and/or hirsutism and (3) transvaginal ultrasound with polycystic ovaries. Patients paused OCP for at least three months and metformin for at least one month before evaluation and were not treated with medicine known to affect hormonal or metabolic parameters. Patients randomized to metformin treatment alone consented to use barrier contraception during the study period or had an intrauterine device implanted.
Exclusion criteria
Patients with contraindications for OCP (obesity with BMI ≥35 kg/m2, previous or family history of thrombosis or breast cancer, coagulation defects and heavy smoking) and patients with diabetes (fasting plasma glucose ≥7.0 mmol/L and/or HbA1c ≥48 mmol/mol), elevated liver enzymes, self-reported renal dysfunction, congestive heart disease, depression and eating disorders were excluded. Routine measurements included prolactin, total and free testosterone, TSH, 17-hydroxyprogesterone, liver enzymes, HbA1c, plasma glucose and electrolytes.
Power calculation
Previous short-term studies on metformin and OCP treatment in PCOS using area under the curve (AUC) for insulin during 2-h OGTT as the primary end point included a minimum of 15 patients in each group (20, 21). There was no published data on GLP-1 levels in PCOS by the time of study planning. It was therefore impossible to perform a power calculation on the effect of OCP and/or metformin treatment on GLP-1 levels and 30 patients were arbitrarily included in each treatment arm.
Controls
Thirty-four healthy white women matched to the group of women with PCOS regarding BMI and age were included. All controls had regular menstrual cycles and did not have hirsutism. Controls had paused OCP for at least three months prior to evaluation.
The study was approved by the local ethics committee (Region of Southern Denmark, study ID S-20070020) and by the Danish Medicines Agency, and all subjects gave written informed consent. The trial was registered at www.clinicaltrials.gov, registration number Nbib451568 (intervention study) and Nbib1995773 (controls).
Study protocol
Patients were randomized to 12-month treatment with metformin (1 + 1 g/day), OCP (150 mg desogestrel + 30 mg ethinylestradiol) or combined treatment, metformin + OCP. Before the intervention, patients underwent physical examination including transvaginal ultrasound, fasting blood samples and 5-h OGTT. After 12-month medical treatment, the patients were admitted for repeated examinations similar to the initial evaluation program. Examinations were performed on a random cycle day. Safety tests were performed after 6 and 12 months and included weight, blood pressure, HbA1c, liver enzymes, electrolytes and white blood cell count. Pregnancy tests were performed by the participants each month.
Clinical examination
Clinical examination included Ferriman–Gallwey score, blood pressure, waist-to-hip ratio, height and weight.
Fasting blood samples and 5-h OGTT
A 5-h OGTT was performed after overnight fasting. GLP-1, capillary blood glucose (BG), insulin and C-peptide were measured at baseline (fasting) and at 30, 60, 90, 120, 180, 240 and 300 min after oral ingestion of a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water. Homeostasis model assessment (HOMA-r) was calculated as fasting insulin×fasting BG/22.5. RH was defined as BG <3.3 mmol/L occurring between 120 and 300 min during the 5-h OGTT. Two and 5 h AUC for glucose, insulin and C-peptide during 5-h OGTT was calculated applying the trapezium rule.
Assays
We measured total GLP-1 levels, i.e. the sum of intact GLP-1 and the metabolite generated by dipeptidyl-peptidase 4-mediated degradation, which is the relevant parameter for evaluation of secretion and potential actions of GLP-1 (22). GLP-1 concentrations were measured by radioimmunoassay after extraction of plasma with 70% ethanol (vol/vol, final concentration). The carboxy-terminal GLP-1 immunoreactivity was determined using antiserum 89,390, which has an absolute requirement for the intact amidated carboxy-terminus of GLP-1 7-36-amide and cross-reacts less than 0.01% with carboxy-terminally truncated fragments and at least 89% with GLP-1 9-36-amide, the primary metabolite of dipeptidyl-peptidase 4-mediated degradation. The sum of the two components (total GLP-1 concentration) reflects the rate of secretion of the L-cell. The sensitivity was below 1 pmol/L and the intra-assay coefficient of variation (CV) was below 5% (23). All samples were measured in the same period, using identical reagents and quality controls.
Total testosterone was analyzed using an in-house method based on the extraction of steroids from serum by ether, separation of extracted steroids by liquid chromatography and quantification by radioimmunoassay as previously described (24). In this method, testosterone, dihydrotestosterone and androstendione are extracted before applying radioimmunoassay and overestimation of testosterone levels is hereby avoided. The results of this method correlate closely with the determination of testosterone levels using mass spectrometry (25). SHBG was analyzed by time-resolved immunoassay using an AutoDELFIA commercial kit (Wallac Oy, Turku, Finland). Free Testosterone Index (FTI) was calculated from measurements of total testosterone and SHBG. The intra-assay CV was 8.2 for total testosterone and 5.2% for SHBG. The inter-assay CV was 13.8% for total testosterone and 7.5% for SHBG.
Serum levels of insulin and C-peptide were analyzed by time-resolved fluoroimmunoassay using commercial AutoDELFIA, kits (PerkinElmer Life Sciences). The intra-assay CV was 1.1–5.0% for C-peptide 1.1–5.0% and 2.1–3.7% for insulin. The inter-assay CV was 1.1–3.4% for C-peptide and 3.4–4.0% for insulin.
Ethics
The study was approved by the local ethics committee (Region of Southern Denmark, study ID S-20070020) and by the Danish Medicines Agency and all subjects gave written informed consent.
Statistical analysis
Variables are presented as medians and quartiles. Pre-treatment differences between included patients in the three treatment arms were tested using the Kruskall–Wallis test followed by the Mann–Whitney U test. The Mann–Whitney test was used to compare the differences between patients and controls and between subgroups of patients with PCOS.
Spearman bivariate associations were performed to evaluate associations between AUC GLP-1 and BMI.
Basal differences between patients randomized to the three different interventions and drop-out during follow-up were taken into account by comparing delta (Δ) values of hormonal and metabolic variables between the three intervention groups using the Kruskall–Wallis test followed by the Mann–Whitney U test. Delta values were calculated as post-treatment level minus pre-treatment level of each analyzed variable.
All statistics were performed using SPSS 17.0 (SPSS). P values <0.05 were considered significant.
Results
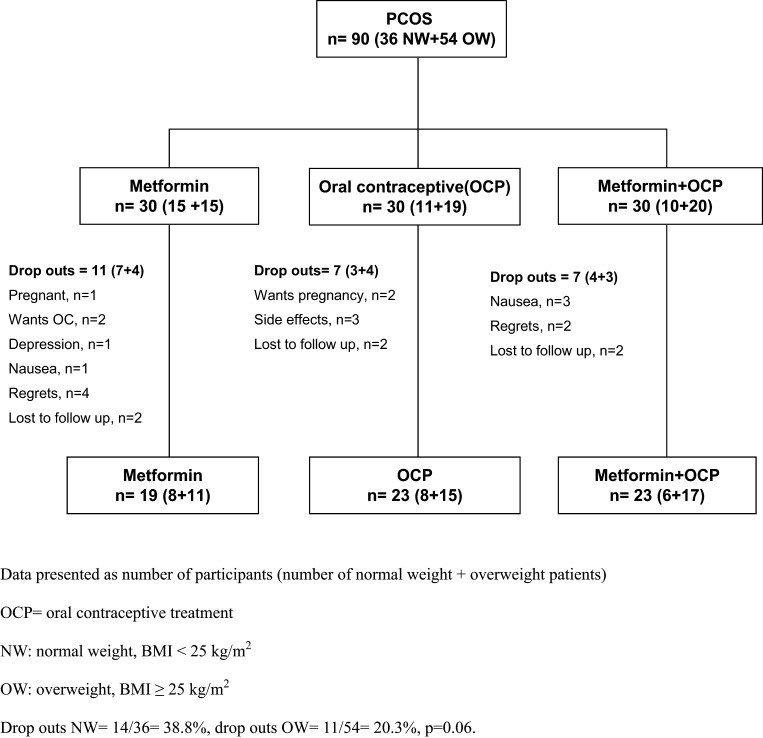
Of the 90 enrolled patients, 65 patients (72%) completed the whole study period (Fig. 1), previously presented in (15). Patients randomized to the three treatment groups were comparable regarding all clinical and biochemical characteristics (Table 1).
Figure 1.
Flow chart of included and excluded subjects and study design.
Table 1.
Baseline clinical and biochemical data in patients with PCOS completing medical intervention (n = 65).
| Metformin (n = 19) | OCP (n = 23) | Metformin + OCP (n = 23) | |
|---|---|---|---|
| Age (years) | 31 (24; 33) | 28 (24; 32) | 30 (24; 31) |
| Weight (kg) | 73.6 (69.2; 83.5) | 86.0 (62.1; 88.8) | 80.2 (70.5; 86.0) |
| BMI (kg/m2) | 25.9 (24.1; 29.6) | 28.0 (22.9; 31.8) | 27.6 (24.3; 31.2) |
| T-testosterone (nmol/L) | 1.96 (1.30; 2.88) | 1.65 (1.39; 2.69) | 1.60 (1.26; 2.32) |
| SHBG (nmol/L) | 44 (32; 62) | 52 (36; 82) | 47 (32; 72) |
| FTI | 0.04 (0.03; 0.05) | 0.03 (0.02; 0.05) | 0.03 (0.02; 0.04) |
| Fasting | |||
| GLP-1 (mmol/L) | 8.5 (6.9; 10.5) | 9.5 (7.5; 11.0) | 8.8 (7.0; 10.6) |
| Insulin (pmol/L) | 51 (34; 71) | 49 (33; 75) | 47 (29; 93) |
| C-peptide (pmol/L) | 611 (467; 906) | 564 (458; 821) | 563 (492; 890) |
| HOMA (mmol/L nmol/L) | 11.7 (7.0; 19.8) | 11.6 (7.8; 17.0) | 10.9 (6.9; 23.5) |
| OGTT | |||
| 2 h AUC GLP-1 (102 mmol/L × h) | 19.8 (16.7; 23.8) | 19.7 (14.6; 22.7) | 17.5 (15.6; 20.3) |
| 5 h AUC GLP-1 (102 mmol/L × h) | 38.4 (32.5; 47.4) | 39.5 (30.9; 43.9) | 37.6 (31.7; 41.9) |
| 2 h AUC BG (102 mmol/L × h) | 9.2 (8.3; 10.0) | 8.5 (8.0; 9.7) | 8.7 (8.3; 9.9) |
| 5 h AUC BG (102 mmol/L × h) | 17.8 (16.4; 19.5) | 17.0 (16.3; 18.7) | 18.5 (16.6; 19.3) |
| 2 h AUC insulin (103 pmol/L × h) | 39.8 (30.1; 54.2) | 44.7 (30.0; 66.8) | 39.8 (27.0; 73.8) |
| 5 h AUC insulin (103 pmol/L × h) | 51.5 (41.3; 77.4) | 56.9 (42.8; 92.8) | 68.0 (41.3; 115.1) |
| 2 h AUC C-peptide (104 pmol/L × h) | 25.8 (22.0; 34.3) | 28.3 (22.2; 33.7) | 26.4 (21.7; 35.4) |
| 5 h AUC C-peptide (104 pmol/L × h) | 46.0 (37.1; 66.6) | 48.0 (39.7; 61.4) | 52.8 (39.4; 61.8) |
Data presented as median (25; 75 quartiles). No significant differences between groups (Kruskall–Wallis test).
FG total, total Ferriman–Gallwey score; FTI, free testosterone index; OCP, oral contraceptive treatment.
Effects of medical intervention
Metformin vs OCP
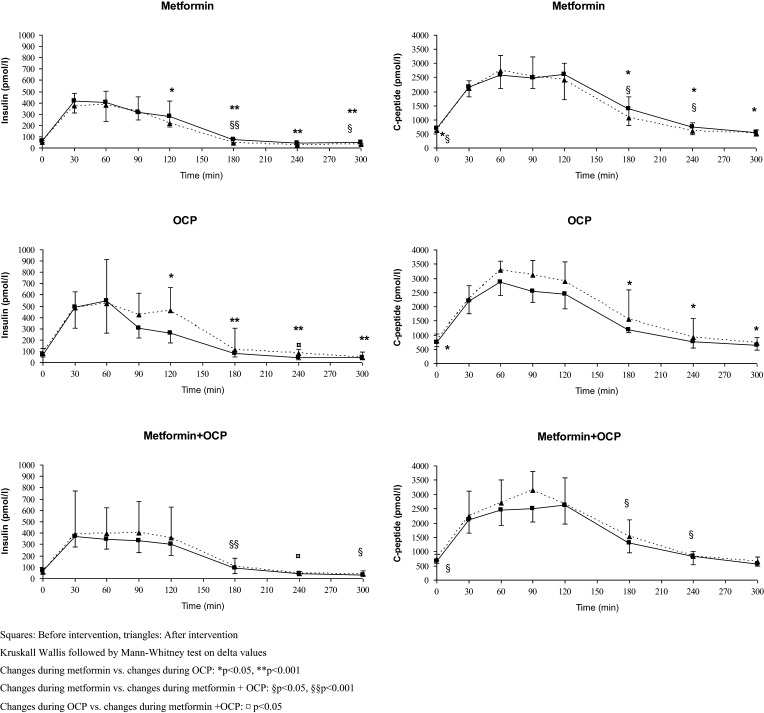
Changes in GLP-1 (fasting, AUC and 5-h OGTT) were comparable during metformin and OCP treatment, whereas treatment with metformin was followed by decreased weight, insulin (time points 120, 180, 240 and 300 min during 5-h OGTT) and C-peptide (fasting and time points 180, 240 and 300 min during 5-h OGTT and 5-h AUC) compared to OCP (Fig. 2 and Table 2).
Figure 2.
Insulin and C-peptide levels before and after intervention.
Table 2.
Median changes in clinical and biochemical variables during medical intervention.
| Metformin (n = 19) | OCP (n = 23) | Metformin + OCP (n = 23) | |
|---|---|---|---|
| Weight (kg)** | −3.0 (−10.3; 0.6)¤§ | 1.2 (−0.8; 3.0) | −1.9 (−4.9; 0.1)¤ |
| BMI (kg/m2)** | −1.0 (−3.7; 0.2)¤§ | 0.4 (−0.4; 1.2) | −0.8 (−1.8; 0.03)¤ |
| T-testosterone (nmol/L) | −0.35 (−0.97; −0.06) | −0.36 (−1.17; −0.04) | −0.42 (−1.19; 0.01) |
| SHBG (nmol/L)** | 9 (−2.19)¤¤§§ | 138 (89; 162) | 106 (59; 175) |
| FTI** | −0.007¤§ (−0.019; 0.0036) | −0.023 (−0.034; −0.008) | −0.019 (−0.029; −0.014) |
| Fasting | |||
| GLP-1 (mmol/L)* | 2.5 (0.3; 3.8) | 1.3 (−1.0; 2.4) | 3.0 (1.4; 4.6)¤ |
| Insulin (pmol/L)* | 2 (−22; 16) | 9 (−6; 46) | −8 (−18; 6)¤ |
| C-peptide (pmol/L)* | −86 (−237; 78)¤§ | 95 (−73; 247) | −97 (−289; 43)¤ |
| HOMA (mmol/L nmol/L) | −0.2 (−5.5; 4.1) | 1.7 (−1.1; 10.4) | −0.6 (−5.1; 1.8) |
| OGTT | |||
| 2 h AUC GLP-1 (102 mmol/L × h) | 0.4 (−2.9; 3.4) | −2.6 (−1.7; 10.9) | 1.7 (−3.1; 8.9) |
| 5 h AUC GLP-1 (102 mmol/L × h) | 3.2 (−4.1; 7.0) | 0.3 (−0.4; 1.0) | −0.2 (−1.8; 2.3) |
| 2 h AUC insulin (103 pmol/L × h) | 0.5 (−13.0; 7.2) | 6.5 (−5.3; 23.0) | −0.3 (−9.0; 12.3) |
| 5 h AUC insulin (103 pmol/L × h) | 1.0 (−20.2; 8.7) | 13.1 (−4.4; 54.9) | 3.6 (−11.5; 21.4) |
| 2 h AUC C-peptide (104 pmol/L × h) | 1.6 (−42.3; 53.8) | 2.6 (−3.2; 7.1) | 0.8 (−1.5; 10.0) |
| 5 h AUC C-peptide (104 pmol/L × h)* | 0.8 (−10.8; 5.4)¤ | 8.7 (−3.1; 18.6) | 4.4 (−1.6; 10.4) |
Data presented as median (25; 75% quartiles). The median changes in BG (fasting, 2 h and 5 h AUC) were comparable in the three intervention groups (data not shown).
P < 0.05, **P < 0.001, Kruskall–Wallis test between groups; ¤P < 0.05 vs OCP, Kruskall–Wallis test followed by Mann Whitney; §P < 0.05, §§P < 0.001 vs metformin + OCP, Kruskall–Wallis test followed by Mann Whitney.
FTI, free testosterone index; OCP, oral contraceptive treatment.
Metformin + OCP vs OCP
Treatment with metformin+OCP was associated with increased fasting GLP-1, whereas changes in AUC GLP-1 were comparable. Weight, insulin (fasting and time point 240 min during 5-h OGTT) and fasting C-peptide decreased during metformin + OCP compared to OCP.
Metformin vs metformin + OCP
Changes in GLP-1 (fasting, AUC and during 5-h OGTT) were comparable during metformin vs metformin + OCP. Metformin treatment was followed by decreased weight, BMI, insulin (time points 180 and 300 min during 5-h OGTT) and C-peptide (fasting and at time points 180 and 240 min during 5-h OGTT) compared to changes during treatment with metformin + OCP.
RH during medical intervention
The prevalence of RH increased significantly from 9/65 to 14/65 after study intervention (P = 0.01). The prevalence of RH during metformin + OCP increased significantly (from 3/23 to 6/23, P = 0.01) and was unchanged during metformin (from 1/19 to 3/19, P = 0.16) and during OCP (before and after 5/23).
Patients with RH after study intervention were characterized by higher 2-h AUC insulin (61.3 (39.6; 80.4) vs 40.0 (28.3; 60.9) × 103 pmol/L × h) and 5-h AUC insulin (85.4 (61.4; 106.7) vs 59.3 (42.6; 97.8) × 103 pmol/L × h) at 12 months compared to patients without RH, whereas fasting GLP-1 levels (9.0 (7.0; 11.0) vs 11.3 (9.3; 13.3) mmol/L) were lower. 2-h and 5-h AUC GLP-1, age and BMI were comparable in patients with and without RH (data not shown). In patients with BMI ≥25 kg/m2, the prevalence of RH increased from 7/43 to 10/43 (P = 0.04) and in normal-weight patients, the prevalence of RH increased from 2/22 to 4/22 (P = 0.03) during medical intervention.
Patients with PCOS vs controls and obese vs lean patients with PCOS
GLP-1
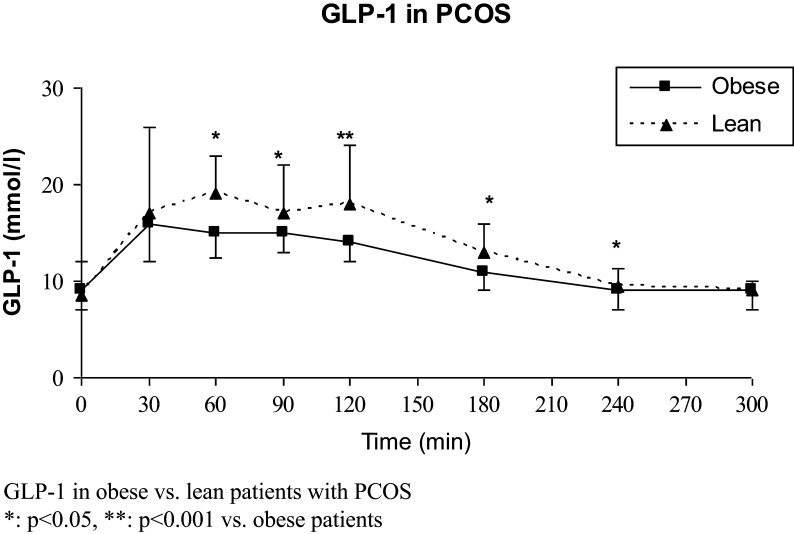
GLP-1 levels (fasting and during 5 h OGTT) were comparable in patients vs controls (Table 3), in lean patients vs lean controls, and in obese patients vs obese controls (data not shown). GLP-1 levels were higher in lean vs obese patients (Fig. 3 and Table 4) and in lean vs obese controls (data not shown). Two-hour and 5-h AUC GLP-1 levels were inversely associated with BMI in patients with PCOS (r = −0.31 and r = −0.34, respectively, P < 0.004), but these associations were non-significant in controls (Tables 3 and 4).
Table 3.
Baseline clinical and biochemical data in patients and controls.
| PCOS (n = 90) | Controls (n = 34) | |
|---|---|---|
| Age (years) | 28 (24–32) | 26 (22–32) |
| Weight (kg) | 73.7 (67.1; 85.3) | 71.9 (67.6; 82.1) |
| BMI (kg/m2) | 26.8 (23.3–30.8) | 25.1 (22.6–27.4) |
| Fasting | ||
| GLP-1 (mmol/L) | 9.0 (7.5; 10.9) | 8.3 (7.0; 10.6) |
| Insulin (pmol/L) | 71 (50–95)* | 55 (48–68) |
| C-peptide (pmol/L) | 707 (591–930)* | 631 (561–727) |
| HOMA (mmol/L nmol/L) | 16.1 (11.7; 21.0) | 14.2 (11.4; 16.5) |
| OGTT | ||
| 2 h AUC GLP-1 (102 mmol/L*h) | 18.0 (15.4; 22.7) | 18.1 (16.1; 23.7) |
| 5 h AUC GLP-1 (102 mmol/L*h) | 38.6 (31.7; 44.4) | 37.0 (34.5; 46.6) |
| 2 h AUC BG (102 mmol/L*h) | 8.9 (8.3; 9.8) | 8.6 (8.3; 9.3) |
| 5 h AUC BG (102 mmol/L*h) | 17.7 (16.5; 19.1) | 18.5 (17.6; 19.0) |
| 2 h AUC insulin (103 pmol/L*h) | 41.3 (30.7; 66.0)* | 34.1 (22.9; 51.2) |
| 5 h AUC insulin (103 pmol/L*h) | 60.8 (44.7; 89.9)* | 48.3 (33.3; 70.6) |
| 2 h AUC C-peptide (104 pmol/L*h) | 27.2 (22.7; 34.5)* | 25.1 (20.4; 27.3) |
| 5 h AUC C-peptide (104 pmol/L*h) | 50.4 (39.7; 63.2)** | 42.6 (37.9; 47.2) |
Data presented as median (25; 75 quartiles).
P < 0.05, **P < 0.001 vs controls.
Figure 3.
GLP-1 levels in obese and lean patients with PCOS.
Table 4.
Baseline clinical and biochemical data in patients and controls.
| PCOS (n = 90) | Controls (n = 34) | |
|---|---|---|
| Age (years) | 28 (24–32) | 26 (22–32) |
| Weight (kg) | 73.7 (67.1; 85.3) | 71.9 (67.6; 82.1) |
| BMI (kg/m2) | 26.8 (23.3–30.8) | 25.1 (22.6–27.4) |
| Fasting | ||
| GLP-1 (mmol/L) | 9.0 (7.5; 10.9) | 8.3 (7.0; 10.6) |
| Insulin (pmol/L) | 71 (50–95)* | 55 (48–68) |
| C-peptide (pmol/L) | 707 (591–930)* | 631 (561–727) |
| HOMA (mmol/L nmol/L) | 16.1 (11.7; 21.0) | 14.2 (11.4; 16.5) |
| OGTT | ||
| 2 h AUC GLP-1 (102 mmol/L*h) | 18.0 (15.4; 22.7) | 18.1 (16.1; 23.7) |
| 5 h AUC GLP-1 (102 mmol/L*h) | 38.6 (31.7; 44.4) | 37.0 (34.5; 46.6) |
| 2 h AUC BG (102 mmol/L*h) | 8.9 (8.3; 9.8) | 8.6 (8.3; 9.3) |
| 5 h AUC BG (102 mmol/L*h) | 17.7 (16.5; 19.1) | 18.5 (17.6; 19.0) |
| 2 h AUC insulin (103 pmol/L*h) | 41.3 (30.7; 66.0)* | 34.1 (22.9; 51.2) |
| 5 h AUC insulin (103 pmol/L*h) | 60.8 (44.7; 89.9)* | 48.3 (33.3; 70.6) |
| 2 h AUC C-peptide (104 pmol/L*h) | 27.2 (22.7; 34.5)* | 25.1 (20.4; 27.3) |
| 5 h AUC C-peptide (104 pmol/L*h) | 50.4 (39.7; 63.2)** | 42.6 (37.9; 47.2) |
Data presented as median (25; 75 quartiles).
P < 0.05, **P < 0.001 vs controls.
RH
RH was found in 15/90 (16.7%) patients and 0 controls (9). Patients with RH had significantly higher fasting GLP-1 8.0 (6.0; 10.0) mmol/L vs patients without RH 7.0 (9.5; 11.0) mmol/L, P < 0.04. GLP-1 levels during 5-h OGTT were comparable in patients with RH vs patients without RH. As recently presented, the presence of RH was independent of BMI (9), data not shown.
Insulin and C-peptide
Women with PCOS had significantly higher levels of insulin (fasting, 2- and 5-h AUC) and C-peptide (fasting, 2- and 5-h AUC) vs controls (Table 3). Obese patients with PCOS had significantly higher levels of insulin (fasting, 2- and 5-h AUC), C-peptide (fasting, 2- and 5-h AUC) and HOMA-IR compared to lean patients (Table 4).
Discussion
To our knowledge, this is the first long-term randomized controlled clinical trial examining the effect of OCP and/or metformin treatment on GLP-1 secretion and risk of RH in PCOS. Fasting GLP-1 levels and the risk of RH increased in patients treated with metformin + OCP, whereas changes in AUC GLP-1 levels were comparable between the medical intervention groups despite significant changes in insulin sensitivity, body weight and testosterone levels. Increased risk of RH after medical intervention was associated with increased insulin levels during 5-h OGTT and was not predicted by AUC GLP-1 or BMI.
Few previous studies evaluated GLP-1 secretion during medical intervention in women with PCOS. In agreement with our study, a recent uncontrolled study reported unchanged GLP-1 levels during a standardized meal test following 3 months treatment with a 4th generation OCP (30 µg ethinyl estradiol/3 mg drospirenone) in 14 lean patients with PCOS (4). Limited data are available on possible interactions between sex hormones and GLP-1. Treatment with GLP-1 increased LH secretion and induced puberty in female rats (17, 26). Increased LH in PCOS (27) could therefore be associated with higher GLP-1 levels. We found no association between testosterone and GLP-1 levels and GLP-1 levels were unchanged despite normalized testosterone levels during treatment with OCP, which did not support an association between testosterone and GLP-1 secretion. In support of this hypothesis, total testosterone levels were unchanged during treatment with GLP-1 despite significant weight loss in patients with PCOS (28, 29).
We found that metformin treatment was associated with unchanged GLP-1 secretion, whereas insulin levels decreased during 5-h OGTT and the median weight loss was 3.0 kg (15). In contrast, Svendsen et al. reported increased GLP-1 secretion during 2-h OGTT after 8-month treatment with metformin in 22 lean and obese women with PCOS (6). Measures of insulin resistance were unchanged and no reduction in BMI was reported (6). In vitro and in vivo studies in patients with type 2 diabetes and obese non-diabetic subjects, supported that treatment with metformin could increase GLP-1 secretion directly by stimulating the GLP-1 producing cells and by decreasing soluble dipeptidyl peptidase-4 activity and thereby inhibit GLP-1 degradation (30, 31). We did therefore expect increased GLP-1 levels during metformin treatment. The median changes in fasting, 2-h and 5-h AUC GLP-1 levels during metformin treatment were positive, but the present long-term study design did not allow the inclusion of a placebo group. It is possible that metformin treatment could have short-term effects on GLP-1 secretion that does not persist during long-term treatment, but more studies are needed to test this hypothesis.
We tested the hypothesis that medical treatment in PCOS could change the risk of RH and that the risk of RH could be mediated by changes in GLP-1 and/or insulin secretion. The prevalence of RH increased in women treated with metformin + OCP, but OCP treatment and metformin treatment alone did not affect the risk of RH. Women with RH after study intervention had higher insulin secretion, whereas glucose stimulated GLP-1 secretion and BMI was comparable in patients with and without RH. Increased risk of RH was seen in both lean and obese individuals. These findings supported that increased insulin secretion were the primary mediator of increased risk of RH. The power of the present study did not allow us to test the possibility that the mechanism for RH could differ between lean and obese subgroups of patients (9). We are not aware of previous studies that investigated the effect of treatment with metformin and/or OCP on the risk of RH in PCOS. The present study did not support our hypothesis that weight gain during treatment with OCP was associated with increased risk of RH (32). However, insulin resistance was deteriorated during treatment with OCP as fasting and glucose-stimulated insulin levels increased. It is possible that the effects of OCP treatment on insulin and C-peptide levels were too modest to affect the risk of RH. In meta-analyses, treatment with OCP was associated with unchanged fasting insulin in PCOS (16), but fasting insulin is only a rough measure of insulin resistance (33) and different generation OCPs could have divergent effects on metabolic risk factors (34). Increased ghrelin levels during OCP treatment could be a marker of increased appetite, but previous data on ghrelin secretion during treatment with OCP in patients with PCOS were conflicting (35, 36). More data are therefore needed on the possible effects of different generation OCPs on metabolic risk including RH, appetite regulation and insulin resistance in PCOS.
We found that GLP-1 secretion was comparable in patients and controls both at fasting and during 5-h OGTT and GLP-1 levels were also comparable in obese and lean subgroups of patients and weight-matched controls. Results from recent studies on GLP-1 secretion in PCOS were conflicting. The majority of studies found no significant differences in GLP-1 levels between patients with PCOS and weight-matched controls (5, 6, 37), whereas lower GLP-1 levels in lean patients with PCOS vs weight-matched controls were reported in two studies (4, 38). Our findings are in agreement with the study by Svendsen et al. in 40 patients with PCOS (6), reporting comparable GLP-1 levels between patients with PCOS and controls overall and in lean and obese subgroups during 2-h OGTT, whereas less than 25 patients were included in the remaining studies (4, 5, 37, 38). Furthermore, we used up-to-date methods for the measurement of GLP-1 (22) in contrast to previous studies (4, 5, 37, 38). Comparable measures of insulin resistance between patients and controls (4, 5, 6, 38) could have affected study outcomes in previous studies. Our findings of significantly higher measures of insulin resistance in PCOS vs controls further supported that BMI is the most important predictor of GLP-1 secretion in PCOS and that PCOS itself is not associated with changed GLP-1 secretion.
The strength of the present study was the randomized controlled design. A recent publication of similar sample size reported significant changes in GLP-1 secretion during metformin treatment (6). Furthermore, up-to-date methods were applied for the measurement of GLP-1 (22). This supported that the present study had power to detect changes in GLP-1 secretion during medical treatment. A study limitation was the inclusion of relatively lean women and that the long intervention period did not allow for the inclusion of a placebo group. Normal-weight patients had high drop-out rates especially in the two treatment arms including metformin, which could lead to type 1 error, thus lack of power. P values were, however, close to one, which support our conclusions. The pathogenesis of insulin resistance in PCOS is multifactorial and insulin resistance is difficult to assess by mathematical indices (39). Furthermore, there are intra-individual variations in glucose levels, which could lead to misclassification of RH (40). Our findings regarding risk of RH during medical treatment in PCOS therefore need to be reproduced in future studies.
In conclusion, the present study supported that GLP-1 levels were unchanged during medical intervention with metformin and/or OCP and increased risk of RH could be associated with increased insulin levels during medical intervention with metformin + OCP. GLP-1 secretion was predicted by BMI and not by PCOS-status.
Declaration of interest
None of the authors have any conflict of interest that could be perceived as prejudicing the impartiality of the research reported. Sandoz sponsored tablets, but were otherwise not involved in the projects’ economy, planning or writing of article.
Funding
Financial grants for the studies were supported by Institute of Clinical Research, Odense University Hospital, Kolding Hospital, A P Møller’s Foundation, The Novo Nordisk Foundation, The Danish Medical Association. Oral contraceptive pills and metformin tablets were sponsored by Sandoz.
Author contribution statement
D G and H M performed experiments; D G and M A conceived and planned the study; J J H contributed reagents and performed analyses; D G drafted the manuscripts; H M, J J H and M A helped writing manuscript and constructive criticism.
Acknowledgements
The authors thank Jeannette Fogh Lindegaard, Mette Brøchner Hansen, Anne Mette Hangaard, Susanne Møller Pedersen, Geraldine Rasmussen, Thon Kowall Andersen and Lene Bruus Albæk for excellent technical assistance.
References
- 1.Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecological Endocrinology 2010. 4 281–296. ( 10.3109/09513590903247873) [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility 2004. 1 19–25. ( 10.1093/humrep/deh098) [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ. Enteroendocrine secretion of gut hormones in diabetes, obesity and after bariatric surgery. Current Opinion in Pharmacology 2013. 6 983–988. ( 10.1016/j.coph.2013.09.014) [DOI] [PubMed] [Google Scholar]
- 4.Aydin K, Arusoglu G, Koksal G, Cinar N, Aksoy DY, Yildiz BO. Fasting and post-prandial glucagon like peptide 1 and oral contraception in polycystic ovary syndrome. Clinical Endocrinology 2014. 4 588–592. ( 10.1111/cen.12468) [DOI] [PubMed] [Google Scholar]
- 5.Pontikis C, Yavropoulou MP, Toulis KA, Kotsa K, Kazakos K, Papazisi A, Gotzamani-Psarakou A, Yovos JG. The incretin effect and secretion in obese and lean women with polycystic ovary syndrome: a pilot study. Journal of Women’s Health 2011. 6 971–976. ( 10.1089/jwh.2010.2272) [DOI] [PubMed] [Google Scholar]
- 6.Svendsen PF, Nilas L, Madsbad S, Holst JJ. Incretin hormone secretion in women with polycystic ovary syndrome: roles of obesity, insulin sensitivity, and treatment with metformin. Metabolism 2009. 5 586–593. ( 10.1016/j.metabol.2008.11.009) [DOI] [PubMed] [Google Scholar]
- 7.Brun JF, Fedou C, Mercier J. Postprandial reactive hypoglycemia. Diabetes and Metabolism 2000. 5 337–351. [PubMed] [Google Scholar]
- 8.Altuntas Y, Bilir M, Ucak S, Gundogdu S. Reactive hypoglycemia in lean young women with PCOS and correlations with insulin sensitivity and with beta cell function. European Journal of Obstetrics and Gynecology and Reproductive Biology 2005. 2 198–205. ( 10.1016/j.ejogrb.2004.07.038) [DOI] [PubMed] [Google Scholar]
- 9.Mumm H, Altinok ML, Henriksen JE, Ravn P, Glintborg D, Andersen M. Prevalence and possible mechanisms of reactive hypoglycemia in polycystic ovary syndrome. Human Reproduction 2016. 31 1105–1112. ( 10.1093/humrep/dew046) [DOI] [PubMed] [Google Scholar]
- 10.Schmitz O, Fisker S, Orskov L, Hove KY, Nyholm B, Moller N. Effects of hyperinsulinaemia and hypoglycaemia on circulating leptin levels in healthy lean males. Diabetes and Metabolism 1997. 1 80–83. [PubMed] [Google Scholar]
- 11.Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014. 3 669–680. ( 10.1053/j.gastro.2013.11.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glintborg D. Endocrine and metabolic characteristics in polycystic ovary syndrome. Danish Medical Journal 2016. 63 B5232. [PubMed] [Google Scholar]
- 13.Diamanti-Kandarakis E, Kouli C, Tsianateli T, Bergiele A. Therapeutic effects of metformin on insulin resistance and hyperandrogenism in polycystic ovary syndrome. European Journal of Endocrinology 1998. 3 269–274. ( 10.1530/eje.0.1380269) [DOI] [PubMed] [Google Scholar]
- 14.Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2007. 1 CD005552 ( 10.1002/14651858.cd005552.pub2) [DOI] [PubMed] [Google Scholar]
- 15.Glintborg D, Altinok ML, Mumm H, Hermann AP, Ravn P, Andersen M. Body composition is improved during 12 months treatment with metformin alone or combined with oral contraceptives compared to treatment with oral contraceptives in polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2014. 99 2584–2591. ( 10.1210/jc.2014-1135) [DOI] [PubMed] [Google Scholar]
- 16.Halperin IJ, Kumar SS, Stroup DF, Laredo SE. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with polycystic ovary syndrome: a systematic review and meta-analysis of observational studies. Human Reproduction 2011. 1 191–201. ( 10.1093/humrep/deq301) [DOI] [PubMed] [Google Scholar]
- 17.Asarian L, Abegg K, Geary N, Schiesser M, Lutz TA, Bueter M. Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology 2012. 2 325–327. ( 10.1053/j.gastro.2012.05.008) [DOI] [PubMed] [Google Scholar]
- 18.Palomba S, Falbo A, Zullo F, Orio F., Jr Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocrine Reviews 2009. 1 1–50. ( 10.1210/er.2008-0030) [DOI] [PubMed] [Google Scholar]
- 19.Schofl C, Horn R, Schill T, Schlosser HW, Muller MJ, Brabant G. Circulating ghrelin levels in patients with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2002. 10 4607–4610. ( 10.1210/jc.2002-020505) [DOI] [PubMed] [Google Scholar]
- 20.Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Martikainen HK, Tapanainen JS. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: a randomized study. Journal of Clinical Endocrinology and Metabolism 2000. 9 3161–3168. ( 10.1210/jc.85.9.3161) [DOI] [PubMed] [Google Scholar]
- 21.Morin-Papunen L, Vauhkonen I, Koivunen R, Ruokonen A, Martikainen H, Tapanainen JS. Metformin versus ethinyl estradiol-cyproterone acetate in the treatment of nonobese women with polycystic ovary syndrome: a randomized study. Journal of Clinical Endocrinology and Metabolism 2003. 1 148–156. ( 10.1210/jc.2002-020997) [DOI] [PubMed] [Google Scholar]
- 22.Kuhre RE, Wewer Albrechtsen NJ, Hartmann B, Deacon CF, Holst JJ. Measurement of the incretin hormones: glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. Journal of Diabetes and its Complications 2015. 29 445–450. ( 10.1016/j.jdiacomp.2014.12.006) [DOI] [PubMed] [Google Scholar]
- 23.Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994. 4 535–539. ( 10.2337/diab.43.4.535) [DOI] [PubMed] [Google Scholar]
- 24.Lykkesfeldt G, Bennett P, Lykkesfeldt AE, Micic S, Moller S, Svenstrup B. Abnormal androgen and oestrogen metabolism in men with steroid sulphatase deficiency and recessive X-linked ichthyosis. Clinical Endocrinology 1985. 4 385–393. ( 10.1111/j.1365-2265.1985.tb01096.x) [DOI] [PubMed] [Google Scholar]
- 25.Nielsen TL, Hagen C, Wraae K, Brixen K, Petersen PH, Haug E, Larsen R, Andersen M. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. Journal of Clinical Endocrinology and Metabolism 2007. 7 2696–2705. ( 10.1210/jc.2006-1847) [DOI] [PubMed] [Google Scholar]
- 26.Outeirino-Iglesias V, Romani-Perez M, Gonzalez-Matias LC, Vigo E, Mallo F. GLP-1 increases preovulatory LH source and the number of mature follicles, as well as synchronizing the onset of puberty in female rats. Endocrinology 2015. 11 4226–4237. ( 10.1210/en.2014-1978) [DOI] [PubMed] [Google Scholar]
- 27.Morales AJ, Laughlin GA, Butzow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. Journal of Clinical Endocrinology and Metabolism 1996. 8 2854–2864. ( 10.1210/jc.81.8.2854) [DOI] [PubMed] [Google Scholar]
- 28.Kahal H, Abouda G, Rigby AS, Coady AM, Kilpatrick ES, Atkin SL. Glucagon-like peptide-1 analogue, liraglutide, improves liver fibrosis markers in obese women with polycystic ovary syndrome and nonalcoholic fatty liver disease. Clinical Endocrinology 2014. 4 523–528. ( 10.1111/cen.12369) [DOI] [PubMed] [Google Scholar]
- 29.Nylander M, Frossing S, Kistorp C, Faber J, Skouby SO. Liraglutide in polycystic ovary syndrome: a randomized trial, investigating effects on thrombogenic potential. Endocrine Connections 2017. 2 89–99. ( 10.1530/EC-16-0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Messeri G, Rotella CM. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care 2001. 3 489–494. ( 10.2337/diacare.24.3.489) [DOI] [PubMed] [Google Scholar]
- 31.Kappe C, Patrone C, Holst JJ, Zhang Q, Sjoholm A. Metformin protects against lipoapoptosis and enhances GLP-1 secretion from GLP-1-producing cells. Journal of Gastroenterology 2013. 3 322–332. ( 10.1007/s00535-012-0637-5) [DOI] [PubMed] [Google Scholar]
- 32.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013. 6116 172–177. ( 10.1126/science.1230721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocrine Reviews 2012. 6 981–1030. ( 10.1210/er.2011-1034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kriplani A, Periyasamy AJ, Agarwal N, Kulshrestha V, Kumar A, Ammini AC. Effect of oral contraceptive containing ethinyl estradiol combined with drospirenone vs. desogestrel on clinical and biochemical parameters in patients with polycystic ovary syndrome. Contraception 2010. 2 139–146. ( 10.1016/j.contraception.2010.02.009) [DOI] [PubMed] [Google Scholar]
- 35.Arusoglu G, Koksal G, Cinar N, Tapan S, Aksoy DY, Yildiz BO. Basal and meal-stimulated ghrelin, PYY, CCK levels and satiety in lean women with polycystic ovary syndrome: effect of low-dose oral contraceptive. Journal of Clinical Endocrinology and Metabolism 2013. 11 4475–4482. ( 10.1210/jc.2013-1526) [DOI] [PubMed] [Google Scholar]
- 36.Sagsoz N, Orbak Z, Noyan V, Yucel A, Ucar B, Yildiz L. The effects of oral contraceptives including low-dose estrogen and drospirenone on the concentration of leptin and ghrelin in polycystic ovary syndrome. Fertility and Sterility 2009. 2 660–666. ( 10.1016/j.fertnstert.2008.07.008) [DOI] [PubMed] [Google Scholar]
- 37.Gama R, Norris F, Wright J, Morgan L, Hampton S, Watkins S, Marks V. The entero-insular axis in polycystic ovarian syndrome. Annals of Clinical Biochemistry 1996. 33 190–195. ( 10.1177/000456329603300303) [DOI] [PubMed] [Google Scholar]
- 38.Vrbikova J, Hill M, Bendlova B, Grimmichova T, Dvorakova K, Vondra K, Pacini G. Incretin levels in polycystic ovary syndrome. European Journal of Endocrinology 2008. 2 121–127. ( 10.1530/EJE-08-0097) [DOI] [PubMed] [Google Scholar]
- 39.Diamanti-Kandarakis E, Kouli C, Alexandraki K, Spina G. Failure of mathematical indices to accurately assess insulin resistance in lean, overweight, or obese women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2004. 3 1273–1276. ( 10.1210/jc.2003-031205) [DOI] [PubMed] [Google Scholar]
- 40.Schousboe K, Henriksen JE, Kyvik KO, Sorensen TI, Hyltoft PP. Reproducibility of S-insulin and B-glucose responses in two identical oral glucose tolerance tests. Scandinavian Journal of Clinical and Laboratory Investigation 2002. 8 623–630. ( 10.1080/003655102764654358) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a