Abstract
Background
Staphylococcus aureus is an important pathogen that causes various infections in medical facilities. However, resistance to multiple drugs has made this infection difficult to manage. Thus, new therapeutic strategies are urgently needed to solve this worldwide public health problem. The Streptococcus lactis L16 strain was isolated from the fermented hot chili sauce. To explore whether it can be used as a protective agent against S. aureus infection, we designed a mouse model of S. aureus infection to evaluate the therapeutic potency of S. lactis. Mice were grouped into pre-(P) and post-(T) S. aureus infection groups following oral administration of S. lactis L16. The protection and treatment effects were assessed by examining body weight, internal organ weight, serum cytokines and intestinal secretory IgA alternations.
Result
Oral administration of the S. lactis L16 strain reduced the loss of body weight in mice post-infection and alleviated infection-induced hepatomegaly. In particular, the PL16 group (protection with L16) showed more effective resistance to S. aureus than the TL16 group (treatment with L16). The level of serum cytokine interferon gamma following oral administration of the L16 strain was remarkably increased during infection, as were interleukin-4 levels during convalescence. The probiotic L16 strain induced more sIgA production than S. aureus.
Conclusion
Our data suggest that S. lactis L16 is an effective strain with anti-Staphylococcus activity. By regulating the Th1/Th2 response, S. lactis can effectively reduce lesions from infection, indicating its therapeutic potential in overcoming antibiotic resistance in this mouse infection model that mimics infections observed in humans.
Keywords: Streptococcus lactis, Staphylococcus aureus, Prophylactic effect, Treatment effect, Th1/Th2 response
Background
Staphylococcus aureus is a gram-positive bacterium and a major cause of community- and hospital-acquired bacterial infections [1, 2]. Infection with this bacterium leads to a variety of disease symptoms, such as boils, skin abscesses, endocarditis, and even sepsis. Although S. aureus is initially sensitive to multiple antibiotics, antibiotics abuse can result in the development of multi-drug-resistant strains, such as methicillin-resistant S. aureus (MRSA), vancomycin-intermediate S. aureus, and vancomycin-resistant S. aureus [3, 4], making S. aureus infection difficult to manage.
Additionally, intestinal carriage of MRSA may increase the risk of MRSA infection in children hospitalized in the neonatal intensive care unit [5]. Intestinal colonization of S. aureus facilitates nosocomial skin or nasal infection [6, 7]. Preventing intestinal colonization may become a therapeutic method to protect against S. aureus transmission. Vaccination is an efficient routine used to inhibit intestinal colonization of pathogens. Although promising, S. aureus vaccine development has been impeded due to a lack of sufficiently recapitulated mouse models and a mechanistic understanding of the interaction between S. aureus and the human immune system [8, 9].
Microbiota or bacterial vector based therapies are emerging as potential therapeutic strategies [10–12]. The Streptococcus genus includes human commensal organisms, and specific species have probiotic characteristics [13, 14]. Immunoregulation is one of the important roles of probiotics. Although the precise mechanism of S. aureus immune evasion remains unclear, deregulated T helper cell functions and phagocytic activity are believed to be leading causes of S. aureus infection [9, 15]. Therefore, we selected this strain for an in vivo study. Whether Streptococcus lactis protects against S. aureus in vivo by enhancing the immune response and the immune signalling pathway that can be used to inhibit infection remains unknown. Our previous work isolated the Streptococcus lactis strain L16 from the fermented hot chili sauce, and in vitro experiments indicated that L16 retains antibacterial activity and probiotic characteristics. In this regard, we aimed to characterize the inhibitory efficacy of S. lactis L16 in vivo in a mouse model. Oral administration of S. lactis L16 was used to explore its effectiveness in preventing and treating S. aureus infection. Mouse body weight, internal organ weight, level of sIgA and cytokine changes were assessed to evaluate its prophylactic effect.
Methods
Experimental design
This experiment involved four groups: control (Con), mouse model (Mod), protection with L16 (PL16) and treatment with L16 (TL16) groups. All mice were provided abundant food and water. Mice in the Con group were orally administered phosphate buffer every day, and those in the Mod group were subjected to oral administration of S. aureus daily for 1 week. Mice in the PL16 group were orally administered S. lactis L16 and orally infected with S. aureus. Streptococcus lactis L16 was administered for intervention with post-infection of S. aureus for 1 week in the TL16 group. Schematics of the experimental design are shown in Fig. 1.
Fig. 1.
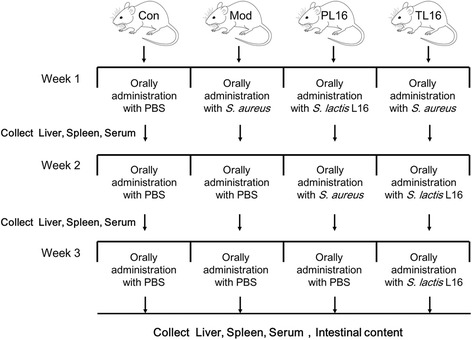
Study design scheme. All of female Kunming mice were fed a normal diet throughout the experimental period. The strains (S. aureus CICC 21600 or Streptococcus lactis L16) were suspended in PBS and administrated into experimental mice. After pre-exposure L16 for 1 week, mice in the PL16 group were then orally administrated with S. aureus for 1 week and with PBS the following week. Mice in the TL16 group were subjected to S. aureus infection for 1 week and treated with L16 the following 2 weeks. In the Mod group, mice were orally infected with S. aureus for 1 week. The Con group remained oral administration of PBS instead of S. aureus or S. lactis L16 for 3 weeks
Mice
Female Kunming mice (age 5 weeks, weight 18–20 g) were obtained from the Changchun Institute of Biological Products Co. Ltd., Changchun, China. All mice were provided a diet and water ad libitum. They were housed in a quiet, ventilated cage under natural light at a temperature of 20 ± 1 °C and a humidity of 50% ± 10%. After 1 week of adaptation to the feeding protocol, mice were randomly divided into four groups (n = 6). All animal handling and experiments were approved by the Academy of Military Medical Sciences Ethics Committee.
Bacterial strains
The strain S. lactis L16 was previously isolated from the fermented hot chili sauce and cultured in de Man, Rogosa, and Sharpe broth (MRS; Qingdao Hope Bio-Technology Co. Ltd., Qingdao, China) at 37 °C for 24 h. Cells were harvested by centrifugation at 7500 rpm for 10 min and then diluted into 109 colony forming units (CFU)/mL with 0.9% sterile saline solution. Staphylococcus aureus (CICC 21600) was obtained from the Laboratory of Food Safety of Jilin Agricultural University, Changchun, China. Cells were cultured with a dilution of 106 CFU/mL in Luria-Bertani (LB) broth at 37 °C for 24 h and stored at 4 °C.
Infection with S. aureus in mice
Based on body weight, pathogenic S. aureus was administered at 107 CFU/kg according to the method described by Kim et al. [16]. Mice were monitored daily. Initial clinical symptoms were sluggishness, loss of appetite, coarse hair, diarrhoea and tremors. In the Mod group, severe congestion and stench of multiple organs were observed after dissection, while mice in the Con group were asymptomatic.
Internal organ weight
Mice in each group were weighed and sacrificed by cervical dislocation. Livers and spleens were collected, and the index was calculated as the percentage of fresh liver or spleen weight to fresh body weight [17] as follows:
Interferon gamma (IFN-γ), interleukin-4 (IL-4) and intestinal sIgA
Blood was collected from the orbital cavity. Mice were anaesthetized with 200 mg/kg amylobarbitone by abdominal injection. Serum was obtained by centrifugation at 3500 rpm for 10 min as described previously [18]. Intestinal lavage from a 1-cm colon sample using PBS was collected and stored at −80 °C [19]. The levels of cytokines (IFN-γ and IL-4) in the serum and sIgA in intestinal fluid (in week 3) were quantified by ELISA [20–24].
Statistical analysis
All data are presented as the mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Cytokine data were analysed and compared between groups by one-way analysis variance followed by Dunnett’s test [25]. A p-value <0.05 was considered to indicate statistical significance.
Results
Detection of weight change associated with S. aureus infection
Body weight was monitored every 2 days. Body weight changes were shown in Fig. 2. Compared with the Con group, infection with S. aureus led to a loss of weight. Mice in both the PL16 and TL16 groups maintained a stable weight for at least 3 weeks. Although a slight decrease in mean weight was observed in the PL16 group compared with the TL16 group, the mean weight of both groups was approximately 25 g, with no significant difference.
Fig. 2.
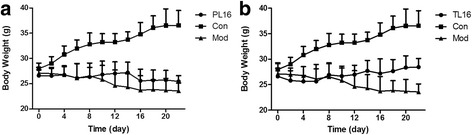
Changes in body weight. a Protection group (PL16): Oral administration of L16 for 1 week (week 1) and continued feeding with S. aureus the following week (week 2). Normal feeding during the last week (week 3). b Treatment group (TL16): Oral administration of S. aureus for 1 week and continued feeding with L16 the following 2 weeks. The control group (Con): Oral administration of PBS instead of S. aureus or S. lactis L16 for 3 weeks. All groups were fed a normal diet throughout the experimental period. Data are presented as the mean ± SD (n = 2)
Detection of mouse internal organ weight
Mice in the PL16 group were orally administered S. lactis L16 for 1 week and fed S. aureus the following week. Mice were fed a normal diet the last week. Mice in the TL16 group were fed S. aureus for 1 week and fed S. lactis L16 the following 2 weeks. The control group (Con) was fed a normal diet throughout the experimental period. Data are presented as the mean ± SD (n = 4).
Internal organ weight data are shown in Table 1. Mice who received oral administration of S. lactis (PL16 group) and were infected with S. aureus exhibited a similar index to the Con group. The liver index of mice in the TL16 group was 18% higher than that of the Con group (p < 0.05) post-infection with S. aureus. After intervention therapy for 1 week, the index decreased and was 8% higher than that of the PL16 group.
Table 1.
The ratios of organs to body weight during infection
| Week 1 | Week 2 | Week 3 | ||||
|---|---|---|---|---|---|---|
| Liver | Spleen | Liver | Spleen | Liver | Spleen | |
| Con | 48.15 ± 0.17 | 4.61 ± 0.99 | 48.61 ± 1.99 | 4.55 ± 1.12 | 47.87 ± 5.65 | 4.62 ± 0.41 |
| PL16 | 48.26 ± 0.18 | 3.54 ± 1.54 | 48.49 ± 1.88 | 3.44 ± 1.02 | 42.91 ± 5.32 | 3.61 ± 0.88 |
| TL16 | 54.35 ± 2.80 | 4.75 ± 0.86 | 52.65 ± 1.05 | 4.98 ± 1.50 | 46.56 ± 9.01 | 5.65 ± 0.81 |
| Mod | 58.18 ± 1.14** | 4.67 ± 1.78 | 56.18 ± 5.37** | 4.76 ± 1.83 | 51.77 ± 4.70 | 5.06 ± 0.97 |
The data is presented by ratio of organ to body weight by mean ± SD. (**, p < 0.05).
Groups are matched with experimental design in Method section. Con: the control group with orally administration of PBS. PL16: the protection group with administration of L16 strain before Staphylococcus aureus infection. TL16: the treatment group with administration of L16 strain after Staphylococcus aureus infection. Mod: murine model group with Staphylococcus aureus infection daily for 1 week.
Detection of serum IFN-γ and IL-4
The serum levels of IFN-γ and IL-4 over the 3-week experimental period are shown in Fig. 3. The expression of IFN-γ was significantly increased (p < 0.001) during infection in both the PL16 (Fig. 2a) and TL16 (Fig. 2b) groups, while IL-4 remained stable. During convalescence, IFN-γ significantly increased (p < 0.001) and IL-4 decreased (p < 0.001). Further analysis revealed that the ratio of IFN-γ to IL-4 significantly decreased during infection and during the recovery phase.
Fig. 3.
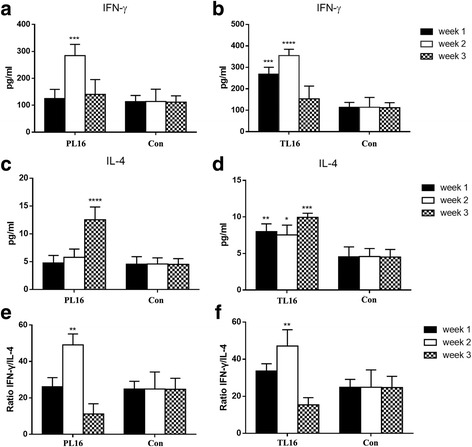
Concentration and ratio of serum cytokines in mice. Concentration of serum IFN-γ (a) and IL-4 (c) in the PL16 group. Concentration of serum IFN-γ (b) and IL-4 (d) in the TL16 group. Ratio of IFN-γ to IL-4 in the PL16 (e) and TL16 (f) groups. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 compared with Con. L16: S. lactis, Con: control group. Data are presented as the mean ± SD
Detection of sIgA in the colon content
The level of sIgA in the colon content was shown in Fig. 4. The secretion of sIgA following L16 administration was 2-fold higher in the PL16 and TL16 groups than in the Con group (p < 0.05). Intestinal sIgA secretion was modestly higher in protection than in treatment, which was consistent with clinical symptoms [26]. However, oral administration of L16 elevated intestinal sIgA in both the PL16 and TL16 groups to a greater extent than in the Con and Mod groups.
Fig. 4.
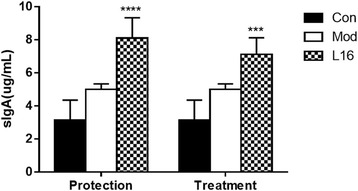
Final concentration of sIgA in intestinal fluid. ELISA was used to detect sIgA in intestinal fluid. Con: control group, Mod: mice infected with S. aureus without S. lactis administration. L16: S. lactis. *p < 0.05 compared with Con
Discussion
Staphylococcus aureus is a major source of community- and hospital-acquired bacterial infections [27]. It is also a leading cause of morbidity and mortality in medical facilities. It frequently colonizes human skin and mucosa and conditionally causes a wide range of infections, leading to various clinical symptoms, including boils, impetigo, arthritis and sepsis. Staphylococcus aureus, an important opportunistic pathogen, causes a variety of infectious diseases [28, 29]. It has become prevalent because of the use of drugs, such as antibiotics, and the development of MRSA. Several new therapies are under investigation to replace antibiotics therapy [30]. Thus, a novel vaccine or therapy is urgently needed to solve this worldwide public health problem [31]. A microbiome-based therapeutic strategy, which may play a therapeutic role in a variety of infectious diseases, has received growing recognition [32, 33]. Microbiota transplantation has been applied for several years to antagonize infectious diseases, such as recurrent Clostridium difficile [34]. However, whether one or several species-specific strains play a vital role against S. aureus infection that can be developed into a novel treatment therapy remains unclear.
It is important to note that S. lactis inhibits the growth of S. aureus in vitro [35]. An independent study also showed that an enhanced Th1 response was beneficial to attenuate infection [36]. To our knowledge, the present study is the first to demonstrate that S. lactis, an isolate from the fermented food, exhibits inhibitory activity against S. aureus by oral administration in a murine infection model. Our results show that feeding S. lactis can reduce weight loss induced by S. aureus infection in both the PL16 and TL16 groups. The internal organ index was introduced to present liver and spleen pathological characteristics and severity. Reduced severity of pathology was shown in the PL16 group, which indicates that S. lactis L16 may have a prophylactic effect on S. aureus infection.
We also found that in contrast to serum IL-4, serum IFN-γ increased in the PL16 and TL16 groups during infection and returned to normal during convalescence, consistent with previous observations [37]. Serum IFN-γ represents a cytokine expressed from Th1, an indicator of cellular immunity [38, 39]. Elevated IFN-γ may promote cytotoxic lymphocyte proliferation to eliminate invasive pathogens. After removal, the Th2 response increases to continue the clearance of the surviving pathogen and establishes a memory immune response [40, 41]. Finally, we also evaluated the concentration of sIgA in intestinal fluid. We conclude that L16 causes higher secretion of sIgA in the intestinal tract. In mice, secretory IgA would initially be beneficial to protect the intestine from S. aureus invasion [42, 43]. An enhanced Th1 response and increased secretion of sIgA illustrate the protective roles of L16.
In summary, S. lactis L16 is a probiotic with potent anti-Staphylococcus activity in vivo. It promotes IFN-γ and IL-4 secretion and stimulates the Th1 response at the early phase of disease development by enhancing the phagocytosis of pathogens, thus preventing further harm from infection, which is synergistically supported by the upregulation of sIgA. These results imply the therapeutic anti-infection potential of L16 for future human clinical applications.
Conclusion
This study shows S. lactis L16, an isolated from the fermented hot chili sauce, owns a potentially immunoregulatory role on protecting mice against pathogenic S. aureus. Besides, the changes of cytokines and sIgA seems to be one of important factors to improve clinical symptons in a murine model. Therefore, this probiotic strain L16 may be used as a potential and promising therapeutic drug to prevent or treat S. aureus.infection in the gastro-Intestinal tract.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China [grant number 31401475] and the Fund Project of Jilin Provincial Department of Education [2015 (No. 196)].
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Authors’ contributions
MPW and SJG prepared the animal model, performed the statistical analysis and drafted the manuscript. SWD, YLZ, and FJR participated in animal feeding and preparation of the animal model. MPW, RRP and YD performed the immunoassays. CL, DYR, and NYJ helped design the study, performed the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
All animal handling and experiments were approved by the Academy of Military Medical Sciences Ethics Committee [No. SYXK 2009–045].
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- IFN-γ
Interferon-gamma
- IL-4
Interleukin-4
- MRSA
Methicillin-resistant S. aureus
- sIgA
Secretory immunoglobulin A
- Th1/2
Type 1/2 helper cell
Contributor Information
Maopeng Wang, Email: wangmaopenga@126.com.
Shengjie Gong, Email: 1550042107@qq.com.
Shouwen Du, Email: du_shouwen@126.com.
Yilong Zhu, Email: yilong_zhu1988@126.com.
Fengjun Rong, Email: 838284150@qq.com.
Rongrong Pan, Email: 2281078335@qq.com.
Yang Di, Email: 492272246@qq.com.
Chang Li, Email: lichang78@163.com.
Dayong Ren, Email: rdy79@163.com.
Ningyi Jin, Email: ningyik@126.com.
References
- 1.Masalha M, Borovok I, Schreiber R, Aharonowitz Y, Cohen G. Analysis of transcription of the Staphylococcus aureus aerobic class Ib and anaerobic class III ribonucleotide reductase genes in response to oxygen. J Bacteriol. 2001;183(24):7260–7272. doi: 10.1128/JB.183.24.7260-7272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Torok ME, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11(3):208–222. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 3.Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect. 2014;20(7):605–623. doi: 10.1111/1469-0691.12705. [DOI] [PubMed] [Google Scholar]
- 4.Lowy FD. Staphylococcus aureus Infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 5.Nakao A, Ito T, Han X, Lu YJ, Hisata K, Tsujiwaki A, et al. Intestinal carriage of methicillin-resistant Staphylococcus aureus in nasal MRSA carriers hospitalized in the neonatal intensive care unit. Antimicrobial Resistance Infect Control. 2014;3(1):14. [DOI] [PMC free article] [PubMed]
- 6.Bhalla A, Aron DC, Donskey CJ. Staphylococcus aureus Intestinal colonization is associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect Dis. 2007;7(1):1–7. doi: 10.1186/1471-2334-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acton DS, Plat-Sinnige MJ, van Wamel W, de Groot N, van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis. 2009;28(2):115–127. doi: 10.1007/s10096-008-0602-7. [DOI] [PubMed] [Google Scholar]
- 8.Salgado-Pabon W, Schlievert PM. Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol. 2014;12(8):585–591. doi: 10.1038/nrmicro3308. [DOI] [PubMed] [Google Scholar]
- 9.Brown AF, Murphy AG, Lalor SJ, Leech JM, O'Keeffe KM, Mac Aogain M, et al. Memory Th1 tells are protective in invasive Staphylococcus aureus infection. PLoS Pathog. 2015;11(11):e1005226. doi: 10.1371/journal.ppat.1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young VB. Therapeutic manipulation of the microbiota: past, present, and considerations for the future. Clin Microbiol Infect. 2016;22(11):905–909. doi: 10.1016/j.cmi.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marotz CA, Zarrinpar A. Treating obesity and metabolic syndrome with fecal microbiota transplantation. Yale J. Biol. Med. 2016;89(3):383–388. [PMC free article] [PubMed] [Google Scholar]
- 12.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 13.Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, et al. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun. 2008;76(9):4163–4175. doi: 10.1128/IAI.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swain MR, Anandharaj M, Ray RC, Parveen Rani R. Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnol Res Int. 2014;2014:250424. doi: 10.1155/2014/250424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litjens NH, Huisman M, van den Dorpel M, Betjes MG. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J Am Soc Nephrol. 2008;19(8):1483–1490. doi: 10.1681/ASN.2007090971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HK, Missiakas D, Schneewind O. Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods. 2014;410:88–99. doi: 10.1016/j.jim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webster SH, Liljegren EJ, Zimmer DJ. Organ; body weight ratios for liver, kidneys and spleen of laboratory animals; albino rat. Am J Anat. 1947;81(3):477–513. doi: 10.1002/aja.1000810307. [DOI] [PubMed] [Google Scholar]
- 18.Ren D, Li C, Qin Y, Yin R, Du S, Liu H, et al. Evaluation of immunomodulatory activity of two potential probiotic lactobacillus strains by in vivo tests. Anaerobe. 2015;35(Pt B):22–7. [DOI] [PubMed]
- 19.Wan MLY, Turner PC, Allen KJ, El-Nezami H. Lactobacillus rhamnosus GG modulates intestinal mucosal barrier and inflammation in mice following combined dietary exposure to deoxynivalenol and zearalenone. J Funct Foods. 2016;22:34–43. doi: 10.1016/j.jff.2016.01.014. [DOI] [Google Scholar]
- 20.Biedermann T, Rocken M, Carballido JM. TH1 and TH2 lymphocyte development and regulation of TH cell-mediated immune responses of the skin. J. Investig. Dermatol. Symp. Proc. 2004;9(1):5–14. doi: 10.1111/j.1087-0024.2004.00829.x. [DOI] [PubMed] [Google Scholar]
- 21.Nan CL, Lei ZL, Zhao ZJ, Shi LH, Ouyang YC, Song XF, et al. Increased Th1/Th2 (IFN-gamma/IL-4) cytokine mRNA ratio of rat embryos in the pregnant mouse uterus. J. Reprod. Dev. 2007;53(2):219–228. doi: 10.1262/jrd.18073. [DOI] [PubMed] [Google Scholar]
- 22.Torres KC, Dutra WO, Gollob KJ. Endogenous IL-4 and IFN-gamma are essential for expression of Th2, but not Th1 cytokine message during the early differentiation of human CD4+ T helper cells. Hum Immunol. 2004;65(11):1328–1335. doi: 10.1016/j.humimm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Kamogawa Y, Minasi LA, Carding SR, Bottomly K, Flavell RA. The relationship of IL-4- and IFN gamma-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 1993;75(5):985–995. doi: 10.1016/0092-8674(93)90542-X. [DOI] [PubMed] [Google Scholar]
- 24.Bodhankar S, Sun X, Woolard MD, Simecka JW. Interferon gamma and interleukin 4 have contrasting effects on immunopathology and the development of protective adaptive immunity against mycoplasma respiratory disease. J. Infect. Dis. 2010;202(1):39–51. doi: 10.1086/653121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed DM, Foldes G, Gatheral T, Paschalaki KE, Lendvai Z, Bagyura Z, et al. Pathogen sensing pathways in human embryonic stem cell derived-endothelial cells: role of NOD1 receptors. PLoS One. 2014;9(4):e91119. doi: 10.1371/journal.pone.0091119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selle M, Hertlein T, Oesterreich B, Klemm T, Kloppot P, Müller E, et al. Global antibody response to Staphylococcus aureus live-cell vaccination. Sci. Rep. 2016;6:24754. doi: 10.1038/srep24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46(4):1147–1152. doi: 10.1128/AAC.46.4.1147-1152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merghni A, Ben Nejma M, Dallel I, Tobji S, Ben Amor A, Janel S, et al. High potential of adhesion to biotic and abiotic surfaces by opportunistic Staphylococcus aureus strains isolated from orthodontic appliances. Microb Pathog. 2016;91:61–67. doi: 10.1016/j.micpath.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Korea CG, Balsamo G, Pezzicoli A, Merakou C, Tavarini S, Bagnoli F, et al. Changing frequencies of opportunistic infections in an HIV cohort 1995–1997: the role of highly active antiretroviral therapy (HAART): C Murphy, S hood, J bond, E Wilkins. Department of Infectious Diseases, North Manchester general hospital, Manchester M. Infect. Immun. 2014;82(10):4144–4153. doi: 10.1128/IAI.01576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen HK, Trachsel J, Looft T, Casey TA. Finding alternatives to antibiotics. Ann N Y Acad Sci. 2014;1323:91–100. doi: 10.1111/nyas.12468. [DOI] [PubMed] [Google Scholar]
- 31.Dietert RR, Silbergeld EK. Biomarkers for the 21st century: listening to the microbiome. Toxicol Sci. 2015;144(2):208–216. doi: 10.1093/toxsci/kfv013. [DOI] [PubMed] [Google Scholar]
- 32.Narrowe AB, Albuthi-Lantz M, Smith EP, Bower KJ, Roane TM, Vajda AM, et al. Perturbation and restoration of the fathead minnow gut microbiome after low-level triclosan exposure. Microbiome. 2015;3(1):1–18. doi: 10.1186/s40168-015-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, et al. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity. 2016;44(3):647–658. doi: 10.1016/j.immuni.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez E, Lee CH, Petrof EO. A practical method for preparation of fecal microbiota transplantation. Methods Mol Biol. 2016;1476:259–267. doi: 10.1007/978-1-4939-6361-4_19. [DOI] [PubMed] [Google Scholar]
- 35.Haines WC, Harmon LG. Effect of variations in conditions of incubation upon inhibition of Staphylococcus aureus by Pediococcus cerevisiae and Streptococcus lactis. Appl Microbiol. 1973;25(2):169–172. doi: 10.1128/am.25.2.169-172.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillen C, McInnes IB, Vaughan DM, Kommajosyula S, Van Berkel PH, Leung BP, et al. Enhanced Th1 response to Staphylococcus aureus infection in human lactoferrin-transgenic mice. J Immunol. 2002;168(8):3950–3957. doi: 10.4049/jimmunol.168.8.3950. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki S, Nishikawa S, Miura T, Mizuki M, Yamada K, Madarame H, et al. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect Immun. 2000;68(5):2424–2430. doi: 10.1128/IAI.68.5.2424-2430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1998;17(6):733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 39.Luo Y, Chen X, O'Donnell MA. Role of Th1 and Th2 cytokines in BCG-induced IFN-γ production: cytokine promotion and simulation of BCG effect. Cytokine. 2003;21(1):17–26. doi: 10.1016/S1043-4666(02)00490-8. [DOI] [PubMed] [Google Scholar]
- 40.Vohr HW. T helper 2 (Th2) response. Enc Immunotoxicol. 2016. doi:10.1007/978-3-642-54596-2_201437.
- 41.Shirtliff M, Harro J, Leid J. Multivalent vaccine protection from Staphylococcus aureus infection. 2016. [Google Scholar]
- 42.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin N Am. 2009;23(1):17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rooijakkers SH, van Kessel KP, van Strijp JA. Staphylococcal innate immune evasion. Trends Microbiol. 2005;13(12):596–601. doi: 10.1016/j.tim.2005.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.


