Abstract
Background
The current study sought to perform a meta-analysis to compare the preoperative staging of endoscopic ultrasonography (EUS) and multidetector computed tomography (MDCT) in gastric carcinoma.
Methods
Articles published between January 1, 2000, and April 1, 2016, that compared EUS with MDCT were included, and data were presented as 2 × 2 tables. The sensitivities, specificities and summary receiver operating characteristic (ROC) curves for T and N staging were calculated using a bivariate mixed effects model. Data were weighted by generic variance and then pooled by random-effects modeling.
Results
Eight studies comprising 1736 patients were included in this meta-analysis. For T1 staging, the sensitivity value for EUS (82%) was significantly higher than that for MDCT (41%) (relative risk (RR): 2.06, 95% confidence interval (CI) 1.07–3.94; P = 0.030). For lymph node involvement, the sensitivity value for EUS (91%) was also significantly higher than that for MDCT (77%) (RR 1.14, 95% CI 1.05–1.23; P = 0.001). However, the specificity values of both EUS and MDCT were quite low, at 49 and 63%, respectively. No significant differences in T2–4 staging between EUS and MDCT were noted.
Conclusion
This meta-analysis indicates that EUS may be superior to MDCT in preoperative T1 and N staging. Additionally, the low specificity values of EUS and MDCT for N staging merits attention.
Keywords: Gastric carcinoma, Multidetector computed tomography, Endoscopic ultrasonography, Staging, Meta-analysis
Background
On a worldwide scale, gastric cancer is the fourth most common malignancy and the third leading cause of cancer-related death [1]. With early diagnosis, more accurate preoperative staging and standardized curative surgery, gastric cancer patients receive more appropriate and less invasive treatment approaches, thus promoting an increase in overall survival [2–4]. Among these factors, accurate preoperative assessment of tumor invasion depth and lymph node metastasis is a fundamental first step in an optimal therapeutic approach. According to the new National Comprehensive Cancer Network (NCCN) practice guidelines for gastric cancer for clinical Tis or T1a gastric cancer patients, endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) can be considered adequate therapy when the lesion is smaller than 2 cm without associated ulcer formation [5–7]. Results from two large phase III randomized controlled trials demonstrated that locally advanced gastric cancer patients could benefit from neoadjuvant chemotherapy [8, 9]. As a result, for patients who are diagnosed with clinical T2 disease or higher or with positive lymph node involvement, NCCN practice guidelines suggest that perioperative chemotherapy is a preferred treatment strategy.
Regarding the imaging for preoperative staging of gastric cancer, endoscopic ultrasonography (EUS) and multidetector computed tomography (MDCT) are the most commonly used techniques [10], especially in China. However, previous studies have reported conflicting results in preoperative staging between these two modalities [10–16]. Moreover, NCCN practice guidelines for gastric cancer do not recommend specific modalities or workup pathways [7]. Therefore, the purpose of our study was to perform a meta-analysis to compare EUS with MDCT for the preoperative staging of gastric carcinoma.
Methods
Search strategy
The PubMed and Web of Science databases were searched systematically for all relevant articles published between January 1, 2000, and April 1, 2016, that compared EUS with MDCT. The following key words were used in these literature searches: “EUS” OR “endoscopic ultrasound” OR “endosonography” OR “endoscopic ultrasonography” OR “computed tomography” OR “contrast-enhanced computed tomography” OR “multidetector computed tomography” and “stomach cancer” OR “gastric cancer” OR “gastric adenocarcinoma” and “sensitivity” OR “specificity” OR “accuracy” OR “diagnostic”. The reference lists of the articles retrieved were manually reviewed to identify additional relevant references.
Study inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) histologically proven gastric adenocarcinoma in more than 30 patients without any preoperative chemotherapy and/or radiation therapy; (2) histopathologic findings after gastrectomy used as the reference standard; and (3) data sufficient to construct a 2 × 2 contingency table. Studies were excluded based on the following exclusion criteria: (1) animals or ex vivo studies; (2) review articles, meta-analyses, abstracts, case reports, letters, and conference proceedings; and (3) studies published in a language other than English. The most recent or complete article was used if multiple articles described the same population.
Data extraction
Data were extracted and summarized independently by two authors (RC, Nie and S, Chen). Two adjudicating senior authors (SQ, Yuan and YB, Chen) resolved any disagreement. The following data from each included study were extracted: first author, study design, year of publication, and sample size (number of patients).
Study quality
Considering the lack of consensus to assess the quality of non-randomized clinical trials, studies used in this meta-analysis were selected based on data completeness and inclusion criteria.
Statistical analyses
A bivariate mixed effects model was performed to acquire summary estimates of sensitivity and specificity and to fit summary receiver operating characteristic (ROC) curves. We used the relative risk (RR) for the comparison of the sensitivity and specificity of the two modalities. Our studies reported results with 95% confidence intervals (CIs). We used the χ 2 test to assess heterogeneity with the level of significance set at 10%, and the I 2 statistic was used to quantify heterogeneity.
The fixed-effects model was used if there was no significant heterogeneity between studies; otherwise, the random-effects model was performed [17]. We also used funnel plots to screen for publication bias if more than ten studies were included. Egger’s linear regression was used to test the effect of publication bias [18].
Analyses were performed using STATA SE 12.0 for Windows (StataCorp LP, College Station, TX).
Results
Study selection
A total of 5675 articles were identified using our search strategy. After screening their titles and abstracts, 5576 identical studies were excluded. The remaining 99 studies were fully reviewed. Ultimately, eight studies including 1736 cases were included for the final analysis [10–16, 19] (Fig. 1). The characteristics of the included studies are presented in Table 1. Among the eight studies, three were prospective studies [10–12], and five were retrospective studies [13–16, 19]. Agreement between the two reviewers was 97% for study selection.
Fig. 1.
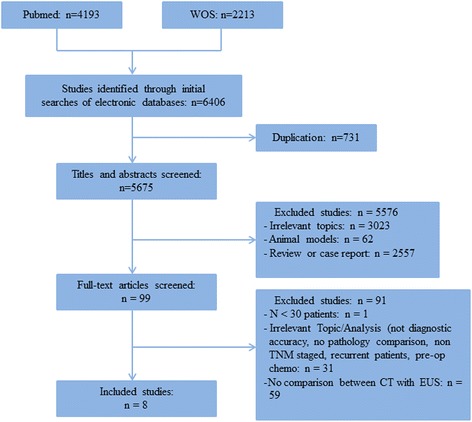
Flow diagram of the studies identified in the meta-analysis. MDCT, multidetector computed tomography; EUS, Endoscopic ultrasonography
Table 1.
Characteristics of included studies
| Study | Study type | No. of patient | Year | EUS MHz | Patient selection | Blinded | Reference test |
|---|---|---|---|---|---|---|---|
| Habermann et al. | R | 51 | 2004 | 7.5/12.0 | No | Yes | Yes |
| Polkowski et al. | P | 88 | 2004 | 7.5/12.0 | No | Yes | Yes |
| Ahn et al. | P | 434 | 2009 | 5/12.0 | Unknown | Yes | Yes |
| SW Hwang et al. | R | 277 | 2010 | 5/7.5/12/20 | No | Unknown | Yes |
| Furukawa et al. | R | 175 | 2011 | 5/7.5/12/20 | No | Yes | Yes |
| Feng et al. | R | 610 | 2013 | 5/7.5/12/20 | No | Yes | Yes |
| Fairweather et al. | R | 49 | 2015 | 5/10 | Unknown | Yes | Yes |
| Giganti et al. | P | 52 | 2016 | 5/10 | No | Yes | Yes |
R retrospective study, P prospective study
Summary estimates of sensitivity and specificity
The overall sensitivity and specificity of EUS and MDCT for gastric cancer patients are presented in Table 2.
Table 2.
Sensitivity and specificity for EUS and MDCT imaging to diagnose T and N staging
| Stage | Sensitivity (%) | Specificity (%) | ||||
|---|---|---|---|---|---|---|
| EUS | MDCT | P value | EUS | MDCT | P value | |
| T1 | 82 (64–92) | 41 (13–77) | 0.030 | 89 (52–98) | 97 (80–100) | 0.228 |
| T2 | 72 (54–85) | 48 (29–68) | 0.056 | 84 (80–88) | 86 (78–92) | 0.629 |
| T3 | 68 (52–80) | 64 (37–84) | 0.749 | 87 (77–93) | 87 (70–95) | 0.455 |
| T4 | 52 (25–78) | 61 (29–86) | 0.613 | 97 (90–99) | 97 (92–99) | 0.731 |
| N | 91 (81–96) | 77 (66–86) | 0.001 | 49 (20–79) | 63 (42–80) | 0.079 |
MDCT multidetector computed tomography, EUS Endoscopic ultrasonography
T1 invasion
The sensitivity value for EUS (82%) was significantly higher than that for MDCT (41%) (RR 2.06, 95% CI 1.07–3.94; P = 0.030), with significant between-study heterogeneity (χ 2 = 329.32, P < 0.001; I 2 = 98.5%). No significant publication bias was observed (P = 0.106). The specificity values for EUS and MDCT were 89 and 97%, respectively, and this difference was not significant (RR 0.95, 95% CI 0.87–1.03; P = 0.228) (Fig. 2).
Fig. 2.
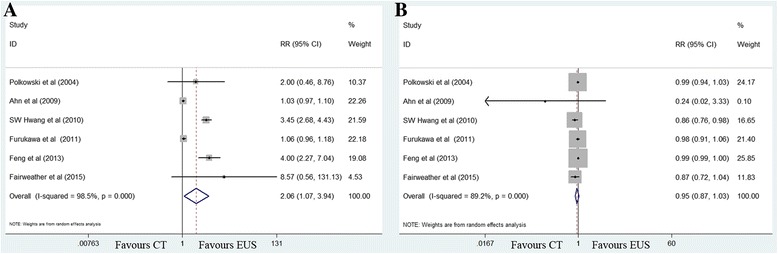
Forest plots of sensitivity (a) and specificity (b) for T1 staging. MDCT, multidetector computed tomography; EUS, Endoscopic ultrasonography. RR, relative risk; CI, confidence interval
T2 invasion
The sensitivity value for EUS (72%) was marginally higher than that for MDCT (48%) (RR 1.48, 95% CI 0.99–2.20; P = 0.056), with significant between-study heterogeneity (χ 2 = 19.41, P = 0.002; I 2 = 74.2%). The specificity values for EUS (84%) and MDCT (86%) were similar (RR 0.98, 95% CI 0.92–1.06; P = 0.629) (Fig. 3).
Fig. 3.
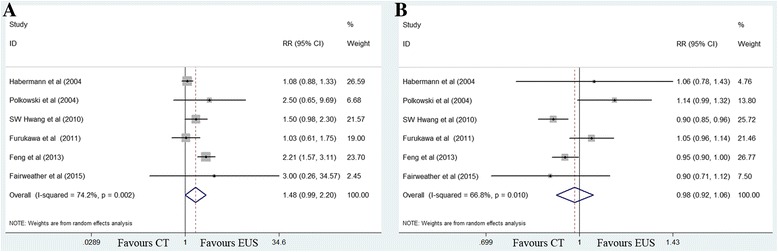
Forest plots of sensitivity (a) and specificity (b) for T2 staging. MDCT, multidetector computed tomography; EUS, Endoscopic ultrasonography. RR, relative risk; CI, confidence interval
T3 invasion
No difference in sensitivity for EUS (68%) and MDCT (64%) was noted (RR 1.05, 95% CI 0.77–1.43; P = 0.749). EUS and MDCT imaging exhibited similar specificity estimates of 87% (Fig. 4).
Fig. 4.
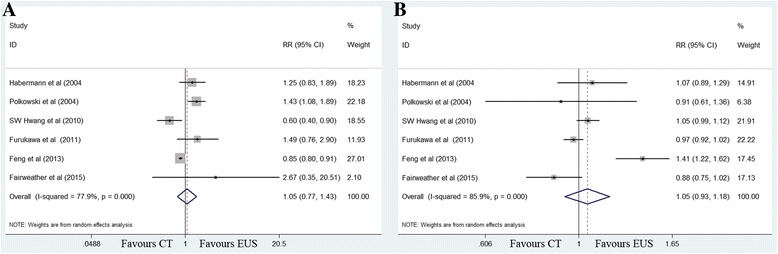
Forest plots of sensitivity (a) and specificity (b) for T3 staging. MDCT, multidetector computed tomography; EUS, Endoscopic ultrasonography. RR, relative risk; CI, confidence interval
T4 invasion
Sensitivity estimates between the two imaging modalities were comparable: 52% for EUS and 61% for MDCT. Specificity values were also comparable: 97% for both EUS and MDCT (Fig. 5).
Fig. 5.
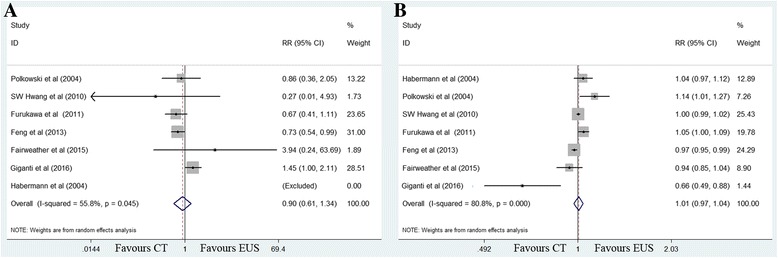
Forest plots of sensitivity (a) and specificity (b) for T4 staging. MDCT, multidetector computed tomography; EUS, Endoscopic ultrasonography. RR, relative risk; CI, confidence interval
Lymph node involvement
The sensitivity value for EUS (91%) was significantly higher than that for MDCT (77%) (RR 1.14, 95% CI 1.05–1.23; P = 0.001), with moderate between-study heterogeneity (χ 2 = 12.68, P = 0.048; I 2 = 52.7%). No significant publication bias was observed (P = 0.073). Specificity was comparable between modalities, with values of 49% for EUS and 63% for MDCT (RR 0.80, 95% CI 0.62–1.03; P = 0.079) (Fig. 6).
Fig. 6.
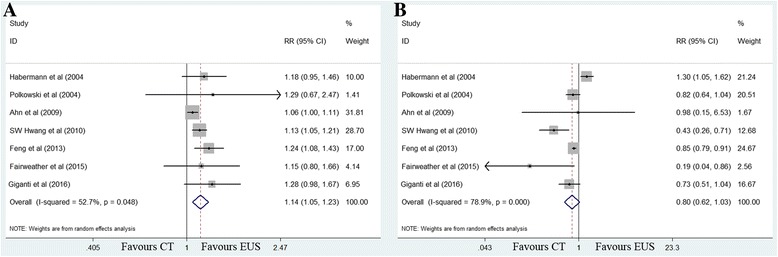
Forest plots of sensitivity (a) and specificity (b) for N staging. MDCT, multidetector computed tomography; EUS, Endoscopic ultrasonography. RR, relative risk; CI, confidence interval
Summary ROC curves
Summary ROC curves of T1 invasion and lymph node involvement are presented in Figs. 7 and 8. A summary ROC curve located near the upper left corner with an increased area under the curve (AUC) indicates a better diagnostic modality. Summary ROC curves for T1 invasion using EUS were located closer to the upper left corner than those using MDCT, indicating the better diagnostic performance of EUS (Fig. 7). In addition, summary ROC curves demonstrated the better diagnostic performance of EUS than that of MDCT for lymph node involvement (Fig. 8).
Fig. 7.
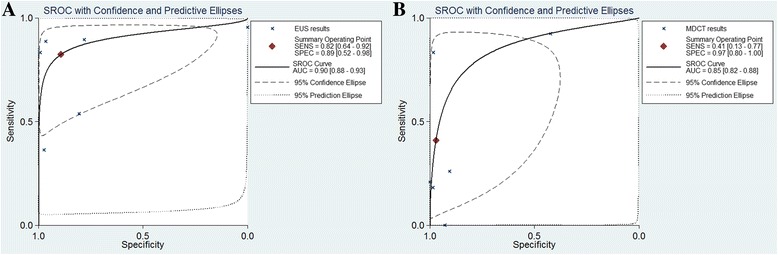
Summary ROC curves for T1 staging with EUS (a) and MDCT (b). MDCT, multidetector computed tomography; EUS, Endoscopic ultrasonography
Fig. 8.
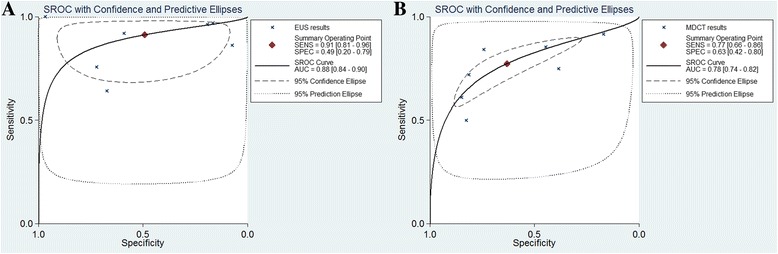
Summary ROC curves for lymph node involvement with EUS (a) and MDCT (b). MDCT, multidetector computed tomography; EUS, Endoscopic ultrasonography
Discussion
Accurate preoperative evaluation for T and N staging of gastric cancer relies on precise imaging. Among various imaging modalities, EUS and computed tomography (CT) are the most common and valuable tools for the preoperative evaluation of gastric cancer. EUS was first introduced in clinical practice in the 1980s and exhibits high accuracy in detecting the depth of gastric cancer [20, 21]. Moreover, EUS shows greater accuracy than incremental CT for both the T and N staging assessment. However, MDCT exhibits a remarkably improved resolution and an accuracy and diagnostic performance similar to EUS [22–24]. Previous studies have reported conflicting results in preoperative staging between EUS and MDCT [10–16, 19]. This meta-analysis of three prospective studies and five retrospective studies including 1736 patients comparing the sensitivity and specificity of EUS and MDCT demonstrated that EUS was superior to MDCT in preoperative T1 and N staging. No significant differences in T2–4 staging were noted between EUS and MDCT.
With the increase in global aging, the proportion of gastric cancer patients older than 70 years is increasing, resulting in an increase in morbidity and mortality after curative gastrectomy. This trend has led to the need for less invasive treatment options, such as EMR and ESD, for early gastric cancer (EGC). Isomoto et al. demonstrated that 5-year overall and disease-specific survival rates after ESD reached 97.1 and 100%, respectively, indicating the excellent prognosis of ESD for EGC [6]. However, the standard imaging modality for clinical assessment of EGC remains debatable. Whether EUS is more accurate than MDCT in diagnosing EGC remains unknown [12, 15, 25]. This meta-analysis demonstrated that the sensitivity of EUS was significantly higher than that of MDCT for EGC, indicating that EUS was a preferred imaging modality in diagnosing EGC. For example, the EUS result was more reliable if a gastric cancer patient was preoperatively staged as T1 using EUS but T2 using MDCT. The reasons why EUS was superior to MDCT for T1 staging are as follows: (1) most EGC cases were easily treated by MDCT because EGC appeared to have small size without obvious enhancement of mucosa; and (2) the integrality of the low-density zone that corresponded to the submucosa was applied to distinguish T1 and T2. However, MDCT could easily distort the results in some cases due to edema or fatty deposition [26].
Feng et al. revealed that MDCT showed higher sensitivity than EUS with regard to lymph node metastasis [15], whereas other studies suggested comparable results in N staging [10, 11, 13, 19]. The pooled results of this meta-analysis demonstrated that EUS was superior to MDCT in preoperative N staging, with a higher sensitivity and better diagnostic performance. Our study revealed that EUS was more sensitive to recognize positive lymph node involvement and thus apply perioperative chemotherapy, which can reduce the tumor stage and significantly improved progression-free and overall survival [8, 9].
Notably, the pooled specificity values of EUS and MDCT were both quite low (49% for EUS and 63% for MDCT, respectively), indicating that the pooled value might not be reliable, even if EUS or MDCT suggested negative lymph node involvement. It is possible that in some cases, pathological positive lymph nodes may appear to be small without obvious enhancement. Zhao et al. also demonstrated that the rate of lymph node metastasis of EGC is quite high (25.27%), and EMR or ESD should be cautiously used in high-risk EGC patients [27]. Therefore, more accurate imaging modalities to predict the negative lymph node for EGC are needed, and further studies are required to test whether the combination of MDCT and EUS could improve the specificity of N staging.
There are also some limitations in the present meta-analysis. First, the main limitation is that most of the included studies were retrospective, with the exception of three prospective studies. Second, significant between-study heterogeneity was noted in this meta-analysis. Finally, the incorporation of various classification systems may have influenced the results of this meta-analysis. However, we applied strict criteria to include high-quality studies, and the use of random-effects modeling could adequately address the heterogeneity. In addition, T staging of all editions is similar; therefore, the comparison of pooled data was not influenced. Given that N staging varies in different editions, the present meta-analysis compared only the preoperative identification of N0 versus N+ disease of EUS and MDCT to make the included studies comparable.
Conclusions
This meta-analysis indicates that EUS may be superior to MDCT in preoperative T1 and N staging. Additionally, the low specificity of both EUS and MDCT for N staging merits further attention.
Acknowledgements
The authors thank Miss. Minglan Shi for her encouragement support.
Funding
This work was supported in part by a grant from National Natural Science Foundation of China (81302144) and the Guangdong Science and Technology Department (No2012B061700087).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Authors’ contributions
RCN independently searched references and took charge of data statistics and drafted the manuscript. RCN and SC searched references and extracted the parameters from each study. SQY, XJC, LPX, YMC, BYZ, XWS, ZWZ and YBC participated in the manuscript revision. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 3.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 4.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 5.Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88–92. doi: 10.1007/s10120-005-0357-0. [DOI] [PubMed] [Google Scholar]
- 6.Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–6. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Gastric Cancer, Version 1. 2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 29 May 2017.
- 8.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 9.Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 10.Giganti F, Orsenigo E, Arcidiacono PG, Nicoletti R, Albarello L, et al. Preoperative locoregional staging of gastric cancer: is there a place for magnetic resonance imaging? Prospective comparison with EUS and multidetector computed tomography. Gastric Cancer. 2016;19:216–25. doi: 10.1007/s10120-015-0468-1. [DOI] [PubMed] [Google Scholar]
- 11.Polkowski M, Palucki J, Wronska E, Szawlowski A, Nasierowska-Guttmejer A, et al. Endosonography versus helical computed tomography for locoregional staging of gastric cancer. Endoscopy. 2004;36:617–23. doi: 10.1055/s-2004-814522. [DOI] [PubMed] [Google Scholar]
- 12.Ahn HS, Lee HJ, Yoo MW, Kim SG, Im JP, et al. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J Surg Oncol. 2009;99:20–7. doi: 10.1002/jso.21170. [DOI] [PubMed] [Google Scholar]
- 13.Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010;25:512–8. doi: 10.1111/j.1440-1746.2009.06106.x. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa K, Miyahara R, Itoh A, Ohmiya N, Hirooka Y, et al. Diagnosis of the invasion depth of gastric cancer using MDCT with virtual gastroscopy: comparison with staging with endoscopic ultrasound. AJR Am J Roentgenol. 2011;197:867–75. doi: 10.2214/AJR.10.5872. [DOI] [PubMed] [Google Scholar]
- 15.Feng XY, Wang W, Luo GY, Wu J, Zhou ZW, et al. Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer - results of a single institution study of 610 Chinese patients. PLoS One. 2013;8:e78846. doi: 10.1371/journal.pone.0078846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairweather M, Jajoo K, Sainani N, Bertagnolli MM, Wang J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol. 2015;111:1016–20. doi: 10.1002/jso.23919. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J, Sally G. Cochrane handbook for systematic reviews of interventions. New York: Cochrane Collaboration, John Wiley and Sons; 2008. [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habermann CR, Weiss F, Riecken R, Honarpisheh H, Bohnacker S, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004;230:465–71. doi: 10.1148/radiol.2302020828. [DOI] [PubMed] [Google Scholar]
- 20.Shimoyama S, Yasuda H, Hashimoto M, Tatsutomi Y, Aoki F, et al. Accuracy of linear-array EUS for preoperative staging of gastric cardia cancer. Gastrointest Endosc. 2004;60:50–5. doi: 10.1016/S0016-5107(04)01312-4. [DOI] [PubMed] [Google Scholar]
- 21.Xi WD, Zhao C, Ren GS. Endoscopic ultrasonography in preoperative staging of gastric cancer: determination of tumor invasion depth, nodal involvement and surgical resectability. World J Gastroenterol. 2003;9:254–7. doi: 10.3748/wjg.v9.i2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JW, Shin SS, Heo SH, Choi YD, Lim HS, et al. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur Radiol. 2012;22:654–62. doi: 10.1007/s00330-011-2283-3. [DOI] [PubMed] [Google Scholar]
- 23.Yu T, Wang X, Zhao Z, Liu F, Liu X, et al. Prediction of T stage in gastric carcinoma by enhanced CT and oral contrast-enhanced ultrasonography. World J Surg Oncol. 2015;13:184. doi: 10.1186/s12957-015-0577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barros RH, Penachim TJ, Martins DL, Andreollo NA, Caserta NM. Multidetector computed tomography in the preoperative staging of gastric adenocarcinoma. Radiol Bras. 2015;48:74–80. doi: 10.1590/0100-3984.2014.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei Q, Wang L, Pan J, Ling T, Lv Y, et al. Endoscopic ultrasonography for staging depth of invasion in early gastric cancer: A meta-analysis. J Gastroenterol Hepatol. 2015;30:1566–73. doi: 10.1111/jgh.13014. [DOI] [PubMed] [Google Scholar]
- 26.Fukuya T, Honda H, Kaneko K, Kuroiwa T, Yoshimitsu K, et al. Efficacy of helical CT in T-staging of gastric cancer. J Comput Assist Tomogr. 1997;21:73–81. doi: 10.1097/00004728-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Zhao BW, Chen YM, Jiang SS, Chen YB, Zhou ZW, et al. Lymph node metastasis, a unique independent prognostic factor in early gastric cancer. PLoS One. 2015;10:e0129531. doi: 10.1371/journal.pone.0129531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


