Abstract
Objective
To examine the clinical correlates and survival in patients with anti-fibrillarin antibodies (AFA) in a large international study population consisting of well-characterized systemic sclerosis (SSc) cohorts from Canada, Australia, and the United States of America.
Methods
Baseline clinical data from the prospective cohorts [Canadian Scleroderma Research Group (CSRG), the Australian Scleroderma Cohort Study (ASCS) and the American Genetics versus Environment in Scleroderma Outcome Study (GENISOS)] were investigated. Clinical variables were harmonized and sera were tested for AFA using a commercially available systemic sclerosis profile line immunoassay (LIA), regardless of the immunofluorescence staining pattern. Association of demographic and clinical features with AFA was investigated by logistic or linear regression. Furthermore, a survival analysis was performed by Cox regression analysis.
Results
A total of 1506 SSc patients with complete serological profiles were included in the study. Fifty-two (3.5%) patients had antibodies detected against fibrillarin. Patients of African descent and Native North American ethnicity were more likely to be AFA positive compared to other ethnicities. After adjustment for demographic factors, diffuse involvement and intestinal bacterial overgrowth requiring antibiotics, gastrointestinal reflux disease showed a trend for association with AFA. Furthermore, AFA positivity was associated with shorter survival independently of demographic factors and disease type (HR 1.76, 95% CI 1.11, 2.79, p=0.016).
Conclusion
In this large multinational SSc cohort, AFA was associated with Native American Ethnicity and was an independent predictor of mortality.
Key indexing terms: Systemic sclerosis, Autoimmunity, Anti-fibrillarin antibodies
Introduction
Systemic sclerosis (SSc - scleroderma) is a multisystem rheumatic disease characterized by immune dysregulation, endothelial damage, and fibrosis. It is broadly divided into limited cutaneous and diffuse cutaneous SSc (lcSSc and dcSSc). According to available data, its prevalence can range from 50 to 300 cases per 1 million persons and its incidence from 2.3 to 22.8 cases per 1 million persons per year (1). Women are at higher risk for SSc than men, and a slightly increased susceptibility to SSc among blacks has been reported (2,3).
Most patients with SSc have detectable circulating antibodies against intracellular proteins and their presence is generally associated with different organ system involvement, natural history, and survival among patients with SSc. Anti-fibrillarin (U3-RNP) antibodies (AFA) are a relatively specific biomarker for SSc (4). AFA is directed against a 35-kDa protein component of a nucleolar ribonucleoprotein called fibrillarin (U3RNP). (5) It has been previously reported to occur in 5–8% of patients with SSc. (6–11, 12)
AFA, characterized as a ‘clumpy’ nucleolar indirect immunofluorescence pattern (13), have been detected by a variety of immunoassays including immunoprecipitation of native or in vitro translated proteins, ELISA and line immunoassays (LIA) (12). Despite variances in these immunoassays, there is general consensus that the frequency of AFA differs across ethnic groups; previous studies have indicated a higher prevalence of AFA among African American patients (6,14,15). An association with dcSSc has been reported in a previous study (4). Moreover, male gender and younger age at SSc diagnosis were associated with AFA (6).
Internal organ involvement in association with AFA varies across studies. AFA has been reported to be associated with pulmonary arterial hypertension (PAH), skeletal muscle and gastrointestinal (GI) involvement (4,6,16). Among African American and Japanese patients, AFA was reported to be protective against interstitial lung disease (ILD). (17, 18)
There are conflicting reports on the association of AFA with survival. Some authors have not identified a decreased survival among SSc patients with AFA who are African American or white (4,16,18). On the other hand, in a single center study, Aggarwal et al. reported that the AFA–positive group had reduced cumulative survival from the time of first physician diagnosis of SSc, and this difference was significant after adjustment for age at diagnosis and sex; PAH was the most common cause of death among patients with AFA. (6).
The evaluation of demographic and clinical correlates of AFA has been hampered by its low prevalence. Our objective was to examine the clinical correlates and survival in patients with AFA as determined by LIA (regardless of immunofluorescence staining pattern) in a large international study population consisting of well-characterized SSc patients from Canada, Australia, and the USA.
Patients and Methods
The cohort comprises SSc patients enrolled in the Canadian Scleroderma Research Group (CSRG), the Australian Scleroderma Cohort Study (ASCS) and the American Genetics versus Environment in Scleroderma Outcome Study (GENISOS) cohorts. Ethics committee approval for this study was obtained at the University of Texas Health Science Center at Houston and at all participating CSRG, ASCS, and GENISOS study sites. All patients provided informed written consent to participate in the study.
Selection of study patients and harmonization of clinical variables between the study cohorts has previously been described (19). Briefly, over 98% of the CSRG subjects (20) and all GENISOS subjects meet the 2013 ACR/EULAR classification criteria for SSc (21). Subjects in the ASCS cohort fulfill either the ACR criteria for classification of SSc, or the Leroy and Medsger criteria for SSc (22, 23).
Clinical variables
Patients recruited into these prospective cohorts underwent standardized medical evaluation including medical history, physical examination and laboratory investigations. Demographic information regarding age, sex and ethnicity was collected by subject self-report. Disease duration was recorded as the interval between the onset of the first non-Raynaud phenomenon symptom attributable to SSc and baseline study visit, as determined by the study physician.
Skin involvement was assessed using the modified Rodnan skin score (mRSS). Limited and diffuse cutaneous disease (lcSSc and dcSSc) were defined as previously described (23). A history of inflammatory myositis, calcinosis, telangiectasia, digital tip ulcers or pits, scleroderma renal crisis, malignancy, overlap with Rheumatoid Arthritis (RA) and gastric antral vascular ectasia (GAVE) was recorded by a study physician. To assess gastrointestinal involvement, presence of gastroesophageal reflux disease (GERD), use of antibiotics for bacterial overgrowth, or hyperalimentation was captured by the treating physician. The presence of interstitial lung disease (ILD) was determined using a previously published clinical decision rule (24). Using this algorithm, ILD was considered present if a high resolution computed tomography (HRCT) scan of the lung was interpreted by an experienced radiologist as showing ILD or, in the case where no HRCT was available, if either a chest x-ray was reported as showing either increased interstitial markings (not thought to be due to congestive heart failure) or fibrosis, and/or if a study physician reported the presence of typical “velcro-like crackles” on physical examination. Pulmonary hypertension was defined as an estimated systolic pulmonary artery pressure (sPAP)> 45 mmHg measured using the Doppler flow measurement of the tricuspid regurgitant jet on cardiac echocardiography (an estimate that correlates strongly with right heart catheter studies) (25) for CSRG and GENISOS subjects, or mean pulmonary artery pressure (mPAP) >25 mmHg with a pulmonary capillary wedge pressure (PCWP) <15 mmHg on right heart catheterization for ASCS patients. Forced vital capacity (FVC) as percentage of predicted, hemoglobin and C-reactive protein (CRP) were recorded as continuous variables.
Serology
Autoantibody analyses of the CSRG and GENISOS cohorts were performed in a central laboratory, Mitogen Advanced Diagnostics Laboratory, University of Calgary and the ASCS analyses were performed in Australia using an identical immunoassay kit and protocol. Antibodies against fibrillarin (U3-RNP), and other SSc-related autoantibodies were detected and digitally quantified by the Euroline systemic sclerosis profile LIA (Euroimmun, Lübeck, Germany) according to the manufacturer’s instructions. Patients with positive AFA results by LIA regardless of the immunofluorescence HEP-2 staining pattern were considered as having AFA. The co-occurrence of AFA with other SSc related antibodies as determined by the above LIA was also investigated.
Statistical Analysis
Descriptive statistics were used to compare demographic characteristics of 1,506 SSc patients according to their cohort of origin. First, logistic regression was used to examine the association of demographic variables with AFA (dependent variable) at the baseline visit. Then, logistic or linear regression was used to examine the association of AFA with clinical features (dependent variable) after adjustment for baseline age, gender, and ethnicity.
Cox regression analysis was utilized to investigate the predictive significance of AFA for all-cause mortality after adjusting for age at enrollment, gender, study site, and ethnicity. The starting point of the survival analysis was time of 1st non-Raynaud’s phenomenon symptom. Kaplan Meier analysis and Cox proportional hazard models were used to compare survival between AFA positive versus negative patients. P values < 0.05 were considered statistically significant for the latter analysis. All statistical analyses were performed with Stata version 13.1, StataCorp, College Station, Tx, USA. An adjustment for multiple comparison was not performed for this study.
RESULTS
Study population, disease, and autoantibody characteristics
As shown in Table 1, a total of 1506 SSc patients with complete serological profiles from the cohorts was included in the study, 714 from the CSRG, 493 from the ASCS and 299 from the GENISOS group. The mean age (± SD) of patients at enrollment was 55 (12.8) years, and the majority of patients were female (1292 or 86%) and of white ethnicity (1249 or 83%). The distribution of other ethnicities was 5% of African descent, 6% Latino, 3% Asian, 2.6% Native North American and 1.6% Australian Aboriginal. At enrollment, 588 (or 40%) SSc patients were diagnosed with diffuse cutaneous involvement. The mean disease duration (±SD), defined as the interval between the onset of the first non-Raynaud disease manifestation and baseline study visit, was 9.5 (9.2) years for the overall cohort.
Table 1.
Demographic characteristics of the SSc patients in the three cohorts at the baseline visit.
| Demographic characteristics | CSRG | ASCS | GENISOS | Total |
|---|---|---|---|---|
| (n=714) | (n=493) | (n=299) | n=1506 | |
| Female gender, n (%) | 615(86.1) | 429(87) | 248 (82.9) | 1292(85.8) |
| Ethnicity, n (%) | ||||
| White | 644(90.2) | 464(94.1) | 141(47.2) | 1249(82.9) |
| African descent | 10(1.4) | 0 | 61(20.4) | 71(4.7) |
| Latino | 3(0.4) | 1(0.2) | 86(28.8) | 90(6) |
| Asian | 17(2.4) | 20(4.1) | 10(3.3) | 47(3.1) |
| Native North American | 38(5.3) | 0 | 1(0.3) | 39(2.6) |
| Australian Aboriginal | 0 | 8(1.6) | 0 | 8(1.6) |
| Other | 2(0.3) | 0 | 0 | 2(0.1) |
| Diffuse involvement, n(%) | 286(40.1) | 127(26.7) | 175(58.5) | 588(39.5) |
| Age at enrollment, years, mean (±SD) | 55.6(12.1) | 57.8(12.4) | 48.7(12.8) | 54.9(12.8) |
| Disease duration, years, mean (±SD) | 10.7(9.0) | 11.9(10.0) | 2.5(1.6) | 9.5(9.2) |
| Anti-Scl 70, n (%) | 128(17.9) | 93(18.9) | 45(15) | 266(17.7) |
| Anti-Centromere B, n (%) | 248(34.7) | 42(14.1) | 200(40.8) | 490(32.6) |
| Anti-RNA polymerase III, n (%) | 126 (17.6) | 62 (12.6) | 41 (13.7) | 229 (15.2) |
| Th/To, n (%) | 10 (1.4) | 9 (1.8) | 5 (1.7) | 24 (1.6) |
Of the 1506 SSc patients, 52 (3.5%) were AFA positive, 31 (4.%) from the CSRG, 6 (1.2%) from the ASCS and 15 (5%) from the GENISOS cohorts, while the comparison group (AFA negative group) comprised 1454 patients. Among 52 AFA positive patients, anti-centromere B, anti-Scl-70, anti-RNA polymerase III, and Th/To antibodies were present in 9, 6, 11, and 1 cases, respectively while 27 with AFA patients (51.7%)did not have neither of the aforementioned antibodies.
The mean (±SD) age at enrollment was 51.7 (±15.5) years for AFA positive patients and 55.1 (±12.7) years for AFA negative participants [p= 0.14 (95% CI-1.09 to 7.72)]. The mean disease duration (± SD) was 9.9 (±8.9) and 9.5 (±9.2) years for AFA positive and AFA negative patients, respectively, with no significant difference between the two groups (p=0.72).
Demographic correlates of anti-fibrillarin antibodies
In logistic regression analysis, patients of Native North American ethnicity were more likely to be AFA positive compared to other ethnicities (o OR 3.85, 95% CI 1.30–11.41, p=0.01, respectively; Table 2). Consistent with previously published data, patients of African descent showed a trend for higher frequency of AFA (p=0.06). Of the 8 Australian Aboriginal patients none had AFA. Noteworthy, 10% (4/39) of patients of Native North American descent had AFA positivity. Supplemental Table 1 shows the frequency of AFA among investigated ethnic groups.
Table 2.
Association of demographic variables with anti-fibrillarin antibodies.
| OR | 95% CI | p-value | |
|---|---|---|---|
| Female gender | 0.78 | 0.38, 1.6 | 0.53 |
| Ethnicity | |||
| White | 1 | 1 | 1 |
| African descent | 2.55 | 0.97, 6.72 | 0.06 |
| Latino | 1.98 | 0.76, 5.18 | 0.16 |
| Asian | 1.5 | 0.35, 6.41 | 0.58 |
| Native North American | 3.85 | 1.30, 11.41 | 0.01 |
| Australian Aboriginal | N/A | N/A | N/A |
| Age at enrollment, years | 0.98 | 0.96, 1.00 | 0.07 |
Clinical correlates of anti-fibrillarin antibodies
There were more AFA-positive patients classified as having dcSSc (56%) than lcSSc (44%), although there were some differences according to ethnicity (within the African descent subgroup, all five patients had dcSSc and within the Native North American ethnicity subgroup, three of the four patients had dcSSc, whereas only 50% of white patients had dcSSc). In multivariable regression analysis after adjustment for age, gender, and ethnicity (Table 3), dcSSc (p=0.057), bacterial overgrowth requiring antibiotics (p=0.053), and gastroesophageal reflux disease (p=0.076) showed a trend for association with AFA positivity. No association between the other clinical variables, including myositis or digital ulcers and AFA positivity was demonstrated.
Table 3.
Association of anti-fibrillarin antibodies with clinical features of SSc*.
| Clinical Characteristic | b or OR | 95% CI | P-value |
|---|---|---|---|
| Diffuse | 1.8 | 0.98, 3.28 | 0.057 |
| Myositis | 1.34 | 0.57, 3.13 | 0.504 |
| Calcinosis | 1.45 | 0.79, 2.66 | 0.234 |
| Telangiectasia | 1.47 | 0.73, 2.99 | 0.284 |
| Digital tip ulcers/pits | 0.95 | 0.52, 1.74 | 0.875 |
| mRSS | 1.93 | −0.91, 4.76 | 0.183 |
| SRC | 0.44 | 0.06, 3.26 | 0.418 |
| ILD | 1.41 | 0.79, 2.50 | 0.248 |
| FVC% predicted | −3.72 | −9.83, 2.39 | 0.233 |
| Pulmonary hypertension | 1.94 | 0.83, 4.54 | 0.126 |
| Malignancy | 1.82 | 0.74, 4.50 | 0.194 |
| Overlap with RA | 1.46 | 0.34, 6.31 | 0.61 |
| GERD | 2.55 | 0.91, 7.20 | 0.076 |
| Bacterial overgrowth requiring antibiotics | 2.44 | 0.99, 6.02 | 0.053 |
| Hyperalimentation | 2.82 | 0.76, 10.40 | 0.12 |
| GAVE | 1.04 | 0.11, 10.08 | 0.97 |
| Hemoglobin, mg/dl | −0.17 | −0.57, 0.22 | 0.385 |
| CRP, mg/L | −2.42 | −8.81, 3.97 | 0.458 |
| Disease duration, years, mean (±SD) | 1.11 | −1.31, 3.54 | 0.368 |
| DLCO corrected | −1.47 | −8.30, 5.36 | 0.67 |
Adjusted for age at enrollment, gender, and ethnicity.
SSc: Systemic sclerosis; mRSS: modified Rodnan skin score; SRC: Scleroderma renal crisis; ILD: interstitial lung disease; FVC: forced vital capacity; RA: rheumatoid arthritis; GERD: gastro-esophageal reflux disease; GAVE: gastric antral vascular ectasia; CRP: C-reactive protein.
AFA is associated with decreased survival
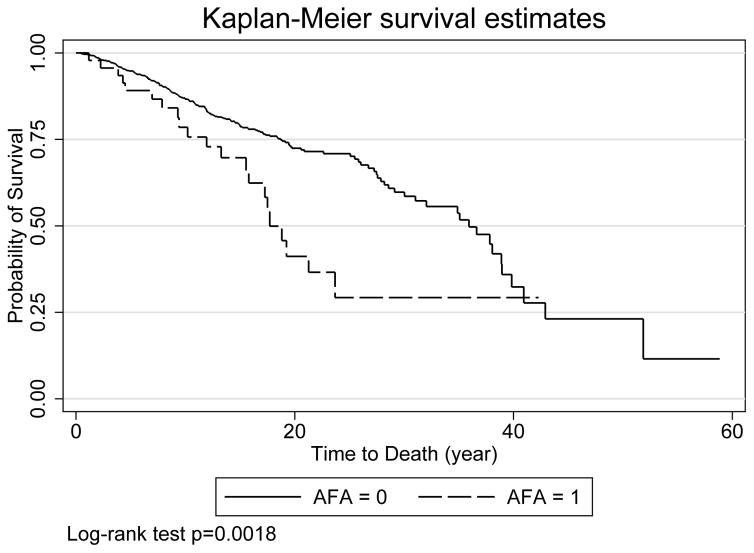
In unadjusted survival analysis, AFA-positive patients had decreased survival compared to AFA-negative patients (HR 2.02, p=0.002, Figure 1). In the multivariable model, AFA positivity was still a significant predictor of mortality even after adjusting for gender, ethnicity, age at enrollment and site (HR 1.88, 95% CI 1.19, 2.97, p=0.007, Table 4).
Figure 1.
Kaplan Meier curve to compare survival in the AFA positive and negative patients.
Table 4.
Predictive significance of AFA for mortality in the multivariable model
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Anti-fibrillarin positivity | 1.88 | 1.19, 2.97 | 0.007 |
| Female gender | 0.49 | 0.35, 0.68 | <0.001 |
| Ethnicity | |||
| White | 1 | 1, 1 | 1 |
| African descent | 2.05 | 1.25, 3.36 | 0.005 |
| Latino | 1.34 | 0.81, 2.22 | 0.26 |
| Asian | 1.15 | 0.54, 2.47 | 0.717 |
| Native North American | 1.04 | 0.50, 2.16 | 0.924 |
| Australian Aboriginal* | 15.78 | 2.15, 115.58 | 0.007 |
| Age at enrollment, years | 1.02 | 1.01, 1.04 | <0.001 |
| Site | |||
| GENISOS | 2.6 | 1.78, 3.8 | <0.001 |
| ASCS | 1.27 | 0.60, 2.66 | 0.534 |
AFA: Anti-fibrillarin antibodies.
No AFA positive patients in this ethnic group
After adjusting for differences in baseline demographic characteristics, site and diffuse disease subtype, AFA-positive patients still had decreased survival compared to AFA-negative patients (HR 1.76, 95% CI 1.11, 2.79, p=0.016).
Among the 21 deaths occurring in AFA group, the cause of death information was only available for 8 patients. The cause of death in these 8 patients was as follows: 3 non-SSc related, 2 combined severe ILD and PAH, 1 PAH, and 1 scleroderma gut involvement.
DISCUSSION
The overall prevalence of AFA in this large tri-nation SSc cohort was 3.5%, relatively lower than other cohorts where prevalence has been reported to be 5–8% (6–11); however, if separated by cohorts, the prevalence of AFA was 5% for GENISOS. It is possible that this can be attributed to the sensitivity of the LIA. In this study, we confirm the previously reported association of AFA with African descent and report for the first time an association of these antibodies with Native North American ethnicity. We also observed a strong association between AFA and decreased survival which was independent of demographic factors, site and disease type. AFA also showed a trend for association with SSc GI manifestations.
Prior studies have indicated a higher prevalence of AFA among African American patients (6,14,15), but little was known to date regarding the prevalence of AFA among Native North American patients. The relatively high prevalence of AFA among the latter is a novel observation. This finding also contributes to the higher frequency of AFA in the CSRG compared to the ASCS cohort, despite the fact that these two cohorts have similar clinical characteristics (e.g. disease duration).
The association of internal organ manifestations in previous studies has been summarized in Table 5. Overall, AFA have been more consistently associated with dcSSc, pulmonary hypertension and GI tract involvement. In agreement with previous studies, (4, 16), we observed that the majority of AFA positive patients had dcSSc. AFA was also associated with SSc GI manifestations (in particular bacterial overgrowth requiring antibiotics and gastrointestinal reflux disease).
Table 5.
Clinical correlates of AFA in previous publications.
| First Author (year of publication). (Reference) | Sample size | Race | Clinical association |
|---|---|---|---|
| Reimer, et al (1988). (26) | 646 SSc (22 AFA) | NA | Lung, heart and GI involvement |
| Okano, et al (1992). (11) | 416 SSc (24 AFA) | White – African Americans | Myositis, GI involvement and pulmonary arterial hypertension |
| Jacobsen, et al (1998). (27) | 230 SSc (8 AFA) | Mostly White | No clinical correlations were found for AFA within this group of patients |
| Tormey, et al (2001). (4) | 1026 SSc (42 AFA) | White - Afro-Caribbean | dcSSc, isolated pulmonary hypertension and myositis |
| Aggarwal, et al (2009). (6) | 2579 SSc (108 AFA) | White - African Americans | Skeletal muscle involvement and PAH |
| Sharif, et al (2011). (18) | 278 SSc (52 AFA) | All African American | Digital ulcers and lower GI involvement |
| Steen, et al (2012). (16) | 3148 SSc (114 AFA) | White-African American | PAH and GI involvement |
| Nihtyanova, et al (2014). (28) | 398 SSc (23 AFA) | Mostly White | Pulmonary hypertension |
| Otero, et al (2016) | 1544 SSc (52 AFA) | White -African descent – Native North American and Australians | dcSSc, GI involvement and mortality |
AFA: Anti-fibrillarin antibodies.
The GI tract has been reported to be the most frequently involved internal organ in SSc, affecting more than 90% of patients, with the esophagus being the most frequent GI organ involved (29). The proposed pathophysiology of GI involvement in SSc includes vasculopathy, neural dysfunction, smooth muscle atrophy, and tissue fibrosis (30). Small intestinal bacterial overgrowth has been reported to affect 33% to 43% of the patients with SSc (31) and is considered to be the main cause of malabsorption. Malabsorption is a poor prognostic factor, with a 50% mortality rate at 8.5 years (32,33). Furthermore, gastroesophageal disease has been suggested to be a risk factor for the development of ILD (34). Although the high prevalence of GI tract involvement in SSc is well recognized, and the association with AFA positivity has been described, there are no studies investigating the pathogenic role (if any) of AFA in GI involvement in SSc.
There was a strong association between AFA and decreased survival in the present study, which was independent of demographic factors, site and disease type. The observed associations with pulmonary hypertension and GI manifestations might potentially explain this finding. PAH was the most common cause of death among AFA-positive patients in a single center study by Aggarwal et al (6). Severe GI tract involvement has also been associated with disease related mortality (35).
The main strength of our study is its large sample size and the inclusion of patients from three countries from a wide geographic area. Furthermore, all the antibody determinations were performed using the same platform and protocol. Nevertheless, this study has some limitations. Causes of death was not available for the patients in the GENISOS cohort. Therefore, our analysis focused on all-cause mortality. Furthermore, right heart catheterization results were not available from patients with pulmonary hypertension in the CSRG and GENISOS cohorts. Nevertheless, the SPAP cut-off of >45 mmHg on cardiac echo used in this study has been reported to correlate strongly with right heart catheter studies (25). Another limitation of the present study is that we did not validate the LIA results in comparison to the immunoprecipitation assays, because our goal was to investigate the demographic and clinical correlates of AFA by LIA, which is a widely used method that has received CE (Conformité Européenne) approval for clincial use. A previous study has shown that the performance of LIA is comparable to the gold standard method (36), immunoprecipitation of radiolabeled proteins (37), although in that study, only samples were investigated that had a positive nucleolar immunofluorescence pattern and lacked the other SSc specific antibodies.
In summary, AFA was strongly associated with bacterial overgrowth requiring antibiotics and an independent predictor of mortality in this large multinational SSc cohort. Furthermore, we confirmed the association of AFA with African descent and reported for the first time an association with the North American ethnicity. The association of AFA with GI manifestations and decreased survival supports the value of this antibody as a clinically useful biomarker.
Supplementary Material
Acknowledgments
Grant Support: The CSRG received funds and/or gifts in kind from the Canadian Institutes of Health Research (CIHR) (grant #FRN 83518), the Scleroderma Society of Canada and its provincial Chapters, Scleroderma Society of Ontario, Sclérodermie Québec, Cure Scleroderma Foundation, INOVA Diagnostics Inc. (San Diego, CA, USA), Euroimmun (Lubeck, Germany), Fonds de la recherche en santé du Québec (FRSQ), the Canadian Arthritis Network (CAN), and the Lady Davis Institute of Medical Research of the Jewish General Hospital, Montreal, QC. The CSRG has also received educational grants from Pfizer and Actelion pharmaceuticals. Dr. Hudson is funded by the Fonds de la recherche en santé du Québec. Dr. Nikpour holds an NHMRC research fellowship (APP1071735). ASCS receives research support from Actelion, Pfizer and GSK, and is also supported by Scleroderma Australia. Dr. Assassi is funded by the NIH/NIAMS K23AR061436. Dr. Mayes is funded by NIH/NIAMS AR055258. The GENISOS cohort receives funding from DoDW81XWH-13-1-0452. The funding sources had no role in the design of the study, analysis of the data, preparation of the manuscript and decision to submit for publication.
We thank Dr. Suman Murthy for the assistance in obtaining the 2013 ACR/EULAR classification information on ASCS patients.
Footnotes
Declaration of interest
ASCS has received grant support from Scleroderma Australia and Arthritis Australia. Dr Nikpour holds a University of Melbourne Faculty of Medicine Dentistry and Health Science David Bickart Clinician Researcher Fellowship and is a recipient of an NHMRC Fellowship (APP1071735).
ASCS has received financial support from Actelion Australia, Bayer, CSL Biotherapies, GlaxoSmithKline Australia and Pfizer.
MJF has been a member of the Scientific Advisory Committee for Inova Diagnostics (San Diego, CA) and has received gifts in kind from Euroimmun GmbH (Luebeck, Germany) and honoraria from BioRad (Hercules, CA, USA).
References
- 1.Chifflot H, Fautzi B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37:223–35. doi: 10.1016/j.semarthrit.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–55. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 3.Reveille JD. Ethnicity and race in systemic sclerosis: how it affects susceptibility, severity, antibody genetics, and clinical manifestations. Curr Rheumatol Rep. 2003;5:160–7. doi: 10.1007/s11926-003-0045-1. [DOI] [PubMed] [Google Scholar]
- 4.Tormey VJ, Bunn CC, Denton CP, Black CM. Anti-fibrillarin antibodies in systemic sclerosis. Rheumatology. 2001;40:1157–62. doi: 10.1093/rheumatology/40.10.1157. [DOI] [PubMed] [Google Scholar]
- 5.Ochs RL, Lischwe MA, Spohn WH, Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54:123–33. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal R, Lucas M, Fertig N, Oddis CV, Medsger TA., Jr Anti–U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum. 2009;60:1112–8. doi: 10.1002/art.24409. [DOI] [PubMed] [Google Scholar]
- 7.Kipnis RJ, Craft J, Hardin JA. The analysis of antinuclear and antinucleolar autoantibodies of scleroderma by radioimmunoprecipitation assays. Arthritis Rheum. 1990;33:1431–7. doi: 10.1002/art.1780330917. [DOI] [PubMed] [Google Scholar]
- 8.Kuwana M, Kaburaki J, Okano Y, Tojo T, Homma M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum. 1994;37:75–83. doi: 10.1002/art.1780370111. [DOI] [PubMed] [Google Scholar]
- 9.Reimer G, Steen VD, Penning CA, Medsger TA, Jr, Tan EM. Correlates between autoantibodies to nucleolar antigens and clinical features in patients with systemic sclerosis (scleroderma) Arthritis Rheum. 1988;31:525–32. doi: 10.1002/art.1780310409. [DOI] [PubMed] [Google Scholar]
- 10.Arnett FC, Reveille JD, Goldstein R, Pollard KM, Leaird K, Smith EA, et al. Autoantibodies to fibrillarin in systemic sclerosis (scleroderma): an immunogenetic, serologic, and clinical analysis. Arthritis Rheum. 1996;39:1151–60. doi: 10.1002/art.1780390712. [DOI] [PubMed] [Google Scholar]
- 11.Okano Y, Steen VD, Medsger TA., Jr Autoantibody to U3 nucleolar ribonucleoprotein (fibrillarin) in patients with systemic sclerosis. Arthritis Rheum. 1992;35:95–100. doi: 10.1002/art.1780350114. [DOI] [PubMed] [Google Scholar]
- 12.Mehra S, Walker J, Patterson K, Fritzler MJ. Autoantibodies in systemic sclerosis. Autoimmun Rev. 2013;12:340–54. doi: 10.1016/j.autrev.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Reimer G, Steen VD, Penning CA, Medsger TA, Jr, Tan EM. Correlates between autoantibodies to nucleolar antigens and clinical features in patients with systemic sclerosis (scleroderma) Arthritis Rheum. 1988;31:525–32. doi: 10.1002/art.1780310409. [DOI] [PubMed] [Google Scholar]
- 14.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Reveille JD, Fischbach M, McNearney T, Friedman AW, Aguilar MB, Lisse J, et al. Systemic sclerosis in 3 US ethnic groups: a comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum. 2001;30:332–46. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 16.Steen V, Domsic RT, Lucas M, Fertig N, Medsger TA., Jr A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum. 2012;64:2986–94. doi: 10.1002/art.34482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimagi E, Tochimoto A, Kawaguchi Y, Satoh T, Kuwana M, Takagi K, et al. Characteristics of patients with early systemic sclerosis and severe gastrointestinal tract involvement. J Rheumatol. 2007;34:2050–5. [PubMed] [Google Scholar]
- 18.Sharif R, Fritzler MJ, Mayes MD, Gonzalez EB, McNearney TA, Draeger H, et al. Anti-fibrillarin antibody in African American patients with systemic sclerosis: immunogenetics, clinical features, and survival analysis. J Rheumatol. 2011;38:1622–30. doi: 10.3899/jrheum.110071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wodkowski M, Hudson M, Proudman S, Walker J, Stevens W, Nikpour M, et al. Clinical correlates of monospecific anti-PM75 and anti-PM100 antibodies in an international cohort of 1574 systemic sclerosis subjects. Autoimmunity. 2015;48:542–51. doi: 10.3109/08916934.2015.1077231. [DOI] [PubMed] [Google Scholar]
- 20.Alhajeri H, Hudson M, Fritzler M, Pope J, Tatibouet S, Markland J, et al. 2013 American College of Rheumatology/European League against rheumatism classification criteria for systemic sclerosis outperform the 1980 criteria: data from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken) 2015;67:582–7. doi: 10.1002/acr.22451. [DOI] [PubMed] [Google Scholar]
- 21.Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of Rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–55. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 22.Nikpour M, Hissaria P, Byron J, Sahhar J, Micallef M, Paspaliaris W, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Res Ther. 2011;13:R211. doi: 10.1186/ar3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 24.Steele R, Hudson M, Lo E, Baron M Canadian Scleroderma Research Group. Clinical decision rule to predict the presence of interstitial lung disease in systemic sclerosis. Arthritis Care Res (Hoboken) 2012;64:519–24. doi: 10.1002/acr.21583. [DOI] [PubMed] [Google Scholar]
- 25.Hsu VM, Moreyra AE, Wilson AC, Shinnar M, Shindler DM, Wilson JE, et al. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol. 2008;35:458–65. [PubMed] [Google Scholar]
- 26.Reimer G, Steen VD, Penning CA, Medsger TA, Jr, Tan EM. Correlates between autoantibodies to nucleolar antigens and clinical features in patients with systemic sclerosis (scleroderma) Arthritis Rheum. 1988;31:525–32. doi: 10.1002/art.1780310409. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen S, Halberg P, Ullman S, Van Venrooij WJ, Høier-Madsen M, Wiik A, et al. Clinical features and serum antinuclear antibodies in 230 Danish patients with systemic sclerosis. Br J Rheumatol. 1998;37:39–45. doi: 10.1093/rheumatology/37.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Nihtyanova SI, Schreiber BE, Ong VH, Rosenberg D, Moinzadeh P, Coghlan JG, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 2014;66:1625–35. doi: 10.1002/art.38390. [DOI] [PubMed] [Google Scholar]
- 29.Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii36–9. doi: 10.1093/rheumatology/ken485. [DOI] [PubMed] [Google Scholar]
- 30.Sjogren RW. Gastrointestinal features of scleroderma. Curr Opin Rheumatol. 1996;8:569–75. doi: 10.1097/00002281-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Gyger G, Baron M. Gastrointestinal manifestations of scleroderma: recent progress in evaluation, pathogenesis, and management. Curr Rheumatol Rep. 2012;14:22–9. doi: 10.1007/s11926-011-0217-3. [DOI] [PubMed] [Google Scholar]
- 32.Sjogren RW. Gastrointestinal motility disorders in scleroderma. Arthritis Rheum. 1994;37:1265–82. doi: 10.1002/art.1780370902. [DOI] [PubMed] [Google Scholar]
- 33.Jaovisidha K, Csuka ME, Almagro UA, Soergel KH. Severe gastrointestinal involvement in systemic sclerosis: report of five cases and review of the literature. Semin Arthritis Rheum. 2005;34:689–702. doi: 10.1016/j.semarthrit.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 34.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann-Vold AM, Molberg O, Midtvedt O, Garen T, Gran JT. Survival and causes of death in an unselected and complete cohort of Norwegian patients with systemic sclerosis. J Rheumatol. 2013;40:1127–33. doi: 10.3899/jrheum.121390. [DOI] [PubMed] [Google Scholar]
- 36.Satoh M, Tanaka S, Chan EK. The uses and misuses of multiplex autoantibody assays in systemic autoimmune rheumatic diseases. Front Immunol. 2015;21:181. doi: 10.3389/fimmu.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson LK, Jaskowski TD, Mayes MD, Tebo AE. Detection of anti-U3-RNP/fibrillarin IgG antibodies by line immunoblot assay has comparable clinical significance to immunoprecipitation testing in systemic sclerosis. Immunol Res. 2016;64:483–8. doi: 10.1007/s12026-015-8710-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.