Abstract
JS-001 is the first monoclonal antibody (mAb) against programmed cell death protein-1 (PD-1) approved by the China Food and Drug Administration (CFDA) into the clinical trails. To date, however, no pre-clinical pharmacological and pharmacokinetic (PK) data are available. In this study, we investigated the efficacy of JS-001 and conducted a preclinical PK study, including the monitoring of anti-drug antibodies (ADAs). We found that JS-001 specifically bound to PD-1 antigen with an EC50 of 21 nmol/L, and competently blocked the binding of PD-1 antigen to PD-L1 and PD-L2 with IC50 of 3.0 and 3.1 nmol/L, respectively. Furthermore, JS-001 displayed distinct species cross-reactivity: it could bind to the PD-1 antigen on the peripheral blood mononuclear cells (PBMCs) of humans and cynomolgus monkeys, but not to those of mice and woodchucks; the Kd values for the interaction between JS-001 and PD-1 antigens on CD8+ T cells of human and cynomolgus monkey were 2.1 nmol/L and 1.2 nmol/L, respectively. In vitro, treatment with JS-001 (0.01–10 μg/mL) dose-dependently stimulated human T cell proliferation, as well as IFN-γ and TNF-α secretion. In HBsAg-vaccinated cynomolgus monkeys, the expression of PD-1+/CD4+ and PD-1+/CD8+ was significantly elevated, intramuscular injection of JS-001 (1 and 10 mg/kg) resulted in dramatic decreases in PD-1+/CD4+ and PD-1+/CD8+ expression in a dose-dependent manner, which was supported by PD-1 receptor occupancy (RO) results. In the PK study, 18 cynomolgus monkeys treated with single, ascending doses of 1, 10, and 75 mg/kg, and another 6 cynomolgus monkeys received 10 mg/kg successive administration. The plasma clearance of JS-001 followed a linear PK profile with single administration in the 1 and 10 mg/kg groups and a non-linear PK profile in the 75 mg/kg group. In the successive 10 mg/kg administration group, no drug accumulation was observed. But the AUC from the last exposure was lower than that of the first administration, which was probably due to the production of ADAs, as demonstrated in immunogenicity study. These non-clinical data are encouraging and provide a basis for the efficacy and safety of JS-001 in clinical trials.
Keywords: monoclonal antibody, JS-001, programmed cell death 1, T cells, receptor occupancy, pharmacokinetics, anti-drug antibody
Introduction
Targeting programmed cell death protein-1 (PD-1) and its ligand (PD-L) has been an important immunotherapy strategy in facilitating chronic infection and tumor progression through immunoregulatory effect. Three monoclonal antibodies (mAb) against PD-1 and one against PD-L1 have achieved encouraging results when applied in clinical trials of multiple carcinomas, especially melanoma1.
In addition to the more common application for tumors, PD-1 and PD-L1 have demonstrated potential applications for infectious diseases, such as simian immunodeficiency virus (SIV), hepatitis C virus (HCV), and hepatitis B virus (HBV). By blocking the PD-1/PD-L1 pathway, immune regulatory function was restored. In chronic SIV-infected macaque models, PD-1 antibodies reduced plasma viral load and prolonged the survival of SIV-infected macaques2. Chimpanzees with persistent HCV infection were treated with anti-PD-1 antibodies, and control of HCV replication was associated with restoration of intra-hepatic CD4+ and CD8+ T cell immunity3.
JS-001 is the first PD-1 mAb approved by the China Food and Drug Administration (CFDA) in a clinical study. To date, no pre-clinical pharmacological and pharmacokinetic (PK) data are available. In this study, we confirmed the efficacy of a human anti-PD-1 antibody in vitro. Furthermore, in cynomolgus monkeys, in a recombinant hepatitis B vaccine (Engerix B®, GlaxoSmithKline) immunization model, T cell activation and PD-1 expression in CD4+/CD8+ T cells were decreased following treatment with JS-001, increasing receptor occupancy in a dose-dependent manner. The plasma clearance of JS-001 followed a linear PK profile with single administration in the 1 and 10 mg/kg groups and a non-linear PK in the 75 mg/kg group. ADAs were occasionally detected.
Materials and methods
Human PBMC harvest and ethics statement
Human peripheral blood mononuclear cells (PBMCs) were harvested from three healthy volunteers from our institute. The study was performed with the approval of the Ethical Committee of the Beijing Institute of Radiation Medicine. The Ethics Review Board of the Beijing Institute of Radiation Medicine approved the protocol, and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all volunteers.
Antigen binding assay
Different concentrations of JS-001 (starting from 100 mg/mL and using 3-fold dilutions) were incubated with 293F cells transfected with PD-1 plasmids (3×105 cells per reaction) at room temperature (RT) for 30 min. Free JS-001 was washed using PBS containing 1% FBS. Anti-human IgG (Fc gamma-specific) PE (eBioscience, San Diego, CA, USA) was then added. After incubation in the dark at RT for 30 min, the samples were analyzed by Accuri C6 flow cytometer (BD Accuri; Ann Arbor, MI, USA) and FlowJo software (Tree Star, Inc; Ashland, OR, USA).
Blocking assay
Different concentrations of JS-001 (starting from 10 mg/mL and using 3-fold dilutions) were mixed with biotin-PD-L1 (1 mg/mL) or PD-L2 (0.5 mg/mL). The mixtures were added to 293F cells transfected with PD-1 plasmids (2×105 cells per reaction) and incubated for 45 min at RT. The unbound PD-1 antibody, PD-L1 and PD-L2 were washed using 1 mL PBS containing 1% FBS. An allophycocyanin-conjugated streptavidin (SA-APC) secondary antibody (Jackson ImmunoResearch Lab, West Grove, PA, USA) was added to a final concentration of 5 mg/mL. The cells were incubated in the dark at 25 °C for 30 min and analyzed by flow cytometry.
T cell proliferation response and IFN-γ, TNF-α determination
PBMCs were isolated and cultured for six days in the presence of IL-4 (WeiKe Biotechnol Co, Ltd, Shanghai, China) and GM-CSF (WeiKe Biotechnol Co, Ltd, Shanghai, China) until immature dendritic cell (imDC) production4. The imDCs were incubated with TNF-α (WeiKe Biotechnol Co, Ltd, Shanghai, China) for three days to obtain mature dendritic cells (mDCs). T cells were isolated from the PBMCs of the same donors using a pan T cell isolation kit (Miltenyi Biotec Technology & Trading Co, Ltd, Shanghai, China) and labeled with 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE) (Invitrogen, CA, USA). The tetanus toxoid (TT, 10 ng/mL, List biological laboratories, Buies Creek, CA, USA), CFSE-T cells, and mDCs were mixed gently. Different concentrations (starting from 10 mg/mL to 10 ng/mL) of PD-1 antibody, Nivolumab (positive control)5, or hIgG4 (negative control) (BD Biosciences, San Diego, CA, USA) were added to the above mixtures and cultured at 7% CO2, 37 °C for 10 d. The TNF-α (R&D Systems Inc, MN, USA) and IFN-γ (R&D Systems Inc, MN, USA) concentrations in the cell culture supernatant were determined using ELISA, and T cells were harvested for flow cytometry (Accuri C6) to determine the CFSE intensity.
Species cross-reactivity
An ELISA assay was used to determine the species cross-reactivity of JS-001 with antigens derived from different species. A 96-well plate was coated with JS-001 from different species (cynomolgus monkey, mouse, woodchuck, and human) at a concentration of 0.5 mg/mL in 100 μL and incubated at 4 °C overnight. After discarding the antigen, 200 μL 2% BSA was added to the wells, which were then incubated for 1 h at 37 °C to block non-specific sites. The wells were emptied, and 100 μL JS-001 was added to a final concentration of 1 μg/mL and incubated for 1 h at 37 °C. Biotin mouse anti-human IgG4 (100 μL) (BD Biosciences Pharmingen, San Diego, CA, USA) was added and incubated at 37 °C for 1 h after five washes. Then, 100 μL peroxidase-conjugated streptavidin (Jackson ImmunoResearch Lab, West Grove, PA, USA) was added and the mixture was incubated at 37 °C for 1 h after five washes. Finally, 100 μL TMB substrate (Sigma, Santa Clara, CA, USA) was added and incubated at 37 °C for 0.5 h. The reactions were stopped by 100 μL 2 mol/mL HCl. The absorption at 450 nm was measured on a tunable microplate reader (Multiskan GO, Thermo).
For further affinity investigations of JS-001 binding to PD-1 molecules in humans and cynomolgus monkeys, PBMCs from humans and cynomolgus monkeys were washed in RPMI-1640 media (Invitrogen, CA, USA) and suspended in cold PBS (1% BSA) (1×106 cell/mL). Approximately 100 μL PBMCs was incubated with human IgG for Fc receptor blocking (Biolegend, San Diego, CA, USA) on ice for 10 min. Different concentrations of JS-001 (starting from 10 μg/mL and using 3-fold dilutions) were then added and incubated on ice for 30 min with gentle mixing. After two washes with 1% BSA in PBS, the antibodies with different fluorescently labeled antibodies, FITC mouse anti-human CD3, PE-Cy7 mouse anti-human CD4 and PE-anti-human IgG4 (BD Biosciences Pharmingen, San Diego, CA, USA) were added. Finally, the cells were suspended in 500 μL PBS (1% BSA) for flow cytometry analysis.
In vivo efficacy evaluation
A total of twelve adult cynomolgus macaques (cynomolgus monkeys) from China that were negative for HBV, HCV, HIV and SIV infection were used as proof-of-concept in the JS-001 activity study. Cynomolgus monkeys were obtained from the Experimental Animal Center at the Beijing Sharing Institute of Biological Resources Co, Ltd. The study was performed with the approval of the Ethical Committee of the Beijing Institute of Radiation Medicine and conducted according to the principles expressed in the Declaration of Helsinki.
Nine cynomolgus macaques were intramuscularly (im) injected with recombinant hepatitis B vaccine (Engerix B®, GlaxoSmithKline, Belgium) at d 28, and then HBsAb and PD-1 expression levels were evaluated within one month. At d 0, HBsAg and JS-001 were simultaneously administered for another 28 d, during which the monkeys underwent pharmacological observation.
Sample collection and cynomolgus macaque PBMC isolation
Five milliliters of peripheral blood treated with EDTA were collected from each monkey. PBMCs were isolated using human peripheral blood lymphocyte-separating medium (Hao Yang Biological Manufacture Co, Ltd, Tianjin, China), using Ficoll density gradient centrifugation according to the manufacturer's instructions. The PBMCs were stored at −80 °C until the proliferation and cytotoxic T lymphocyte (CTL) lysis assays of cynomolgus macaque PBMCs were performed.
HBsAb titer determination
An ELISA assay was developed to determine HBsAb levels in monkey serum samples. HBsAb quality control serum (10 ng/mL, National Center for Clinical Laboratory, NCCL) was used as a working standard to make standard curves using a HBsAb ELISA kit (Wantai Biological Pharmacy Enterprise Co, Ltd, Beijing, China). Calibration curves for the ELISA assay appeared as sigmoid curves, and the data were fitted to a 4-parameter logistic model by MicroCal Origin software (Origin Lab).
PD-1 receptor occupancy
For PD-1 receptor occupancy, JS-001 binding to PD-1 molecules on PBMCs was detected by flow cytometry6. Blood samples were collected on d 0, 3, 14, 21 and 28.
Each 80 μL blood sample was divided into two aliquots and diluted with 60 μL PBS (pH 7.0). The blood samples were incubated with JS-001 (10 μg/mL) or purified mouse anti-human IgG4 for 30 min at 4 °C in the dark. The blood samples were centrifuged at 300×g at 25 °C for 10 min and washed twice in PBS (pH 7.0). The samples were incubated with FITC mouse anti-human CD3ɛ, APC mouse anti-human CD95, PE-CyTM7 mouse anti-human CD4 (BD Biosciences, San Diego, CA, USA) and PE mouse anti-human IgG4 (SouthernBiotech, Birmingham, USA) for 30 min at 4 °C in the dark. The remaining erythrocytes were removed with 1 mL RBC lysis buffer for 15 min at 25 °C. PBMCs were washed twice in PBS (pH 7.4), centrifuged at 300×g at 25 °C for 20 min and analyzed by flow cytometry (Guava, Merck Millipore, Germany, guavasoft2.7). PD-1 receptor occupancy=[Percent of fluorescence (Control hIgG4)]/[Percent of fluorescence (PD-1 antibody)].
Pharmacokinetic and ADA study design
Eighteen cynomolgus monkeys (n=6 for each group) were intravenous drip (vd)-treated with three single ascending doses of JS-001 (1, 10 and 75 mg/kg). In addition, six specimens received 10 mg/kg successive administrations on d 0, 7, 14 and 21 in the PK study. Blood samples were collected pre-infusion, and at the indicated time points, until 42 d post infusion (a total of 21 time points).
Forty monkeys were treated with four ascending doses of 0, 10, 30 and 100 mg/kg JS-001 for ADA monitoring. Blood samples were collected pre-infusion and at the indicated time points before and after the first, second, third and fourth infusions.
All samples were stored frozen (−80 °C) until measurement.
Determination of JS-001 concentrations
Serum JS-001 concentrations were measured by a validated ELISA. Briefly, recombinant human PD-1 was coated onto 96-well plates. JS-001 in serum was captured and then combined with goat anti-human IgG (H+L)-HRP conjugated antibodies (R&D Systems Inc). Finally, TMB substrate was added until the reaction was stopped by the addition of 2 mol/L HCl. The absorption at 450 nm was measured on a tunable microplate reader. JS-001 concentrations were calculated by Watson LIMS version 7.3.0.01 (Thermo Scientific Inc). The lower limit of quantification (LLOQ) of the assay was determined to be 19.531 ng/mL. All kinetic parameters were determined independently of a model using a non-compartmental method in WinNonlin.
Analysis of anti-drug antibodies
Anti-drug antibodies in plasma samples were analyzed with a validated bridging ELISA. Briefly, JS-001 was immobilized on the surface of a multi-titer plate as a capture antibody. Samples were added, and antibodies that bound JS-001 were captured and detected via the addition of biotinylated JS-001. Next, a peroxidase-conjugated streptavidin was added. The TMB substrate was finally added until the reaction was stopped by the addition of 2 mol/L HCl. The absorption at 450 nm was measured on a tunable microplate reader. The signal-to-noise ratio (SNR) as a screening cut-off point was 1.35, and the confirmed judgment threshold inhibition rate was 38.1% for free drugs.
Statistical analysis
In vitro pharmacodynamic experiments, including T cell proliferation response, IFN-γ and TNF-α secretion and in vivo receptor occupancy results, were analyzed by one-way ANOVA for each time-point or JS-001 concentration. Pharmacokinetic parameters were calculated and statistically analyzed using the WinNonlin software program (version 5.2.1, Pharsight corporation, Mountain View, CA, USA). Non-parametric Spearman correlation coefficients, rho (ρ), were calculated between the HBsAb levels to PD-1 expression on CD4+ or CD8+ T cells score for the whole sample of n=12 subjects7. Differences with P<0.05 were considered significant.
Results
Biological function of JS-001 in vitro
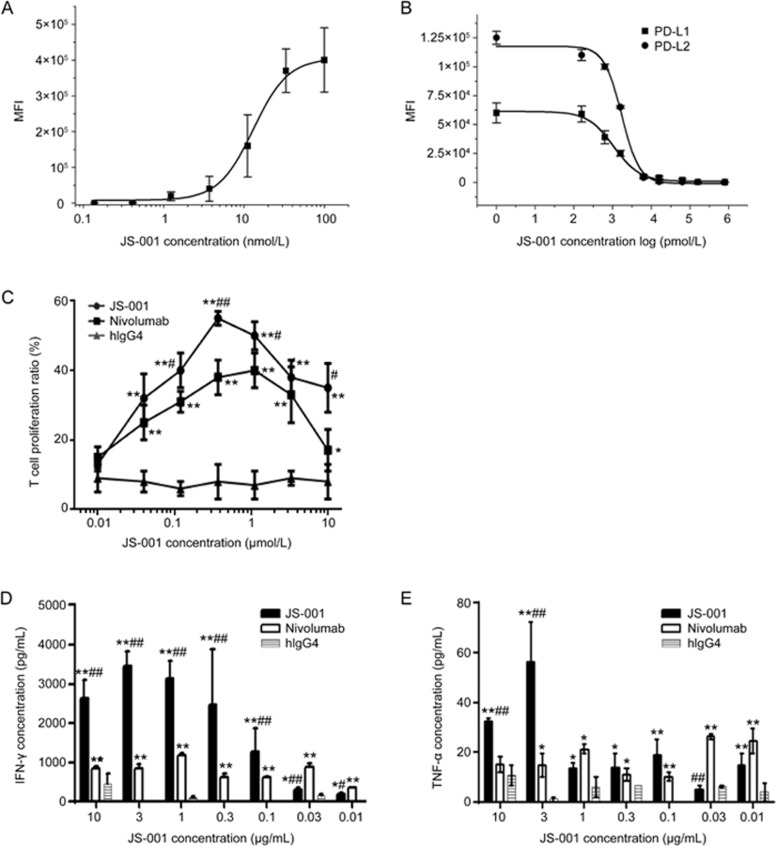
JS-001 was evaluated for its ability to specifically bind the PD-1 molecule and to subsequently block PD-1/PD-L1 and PD-1/PD-L2 interactions. Representative curves of FCA-determined binding and blocking are shown in Figure 1A and 1B. The effective concentration (EC50) of JS-001 was 21 nmol/L (Figure 1A). JS-001 could competently inhibit the binding of PD-1 antigen to PD-L1 and PD-L2. The inhibitory concentration (IC50) was 3.0 nmol/L and 3.1 nmol/L for PD-1/PD-L1 and PD-1/PD-L2, respectively (Figure 1B). Therefore, JS-001 was able to specifically bind PD-1 antigen and efficiently block the PD-1/PD-L1 and PD-1/PD-L2 interactions.
Figure 1.
In vitro activity of JS-001. (A) In vitroFCA experiments demonstrate that JS-001 specifically binds to the PD-1 antigen and (B) efficiently blocks the PD-1/PD-L1 and PD-1/PD-L2 interactions. Apparent affinity was determined using median fluorescence intensity (MFI). (C) The T cell proliferation response was performed using flow cytometry to determine the CFSE intensity. *P<0.05, **P<0.01 vs hIgG4. #P<0.05, ##P<0.01 vs Nivolumab. (D) IFN-γ and (E) TNF-α levels were determined using ELISA. Nivolumab, positive control; hIgG4, negative control. *P<0.05, **P<0.01 vs hIgG4. #P<0.05, ##P<0.01 vs Nivolumab. Data are shown as the mean±SD from 3 independently analyzed experiments.
The T cell proliferation response showed that JS-001 and the positive control, Nivolumab, both promoted T cell proliferation, as well as IFN-γ and TNF-α secretion, at dosages higher than that of the negative control, hIgG4. JS-001 was more effective in the range of 0.1–3 μg/mL, whereas Nivolumab showed higher efficacy at doses of 0.01 and 0.03 μg/mL (Figure 1C–1E).
Species cross-reactivity
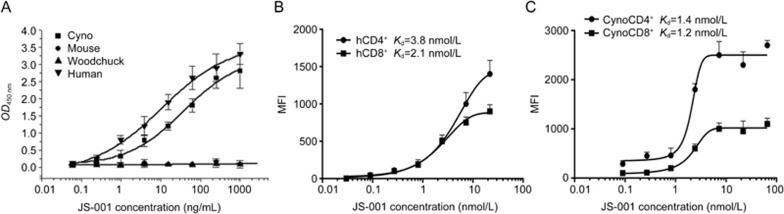
The species reactivity of JS-001 showed that it could bind to the PD-1 antigen on the PBMCs of humans and cynomolgus monkeys, but not to those of mice and woodchucks (no reactivity). The EC50 values of JS-001 with humans (h) and cynomolgus monkeys (cyno) were 11 ng/mL and 38 ng/mL, respectively (Figure 2A). Furthermore, the affinities of JS-001 and PD-1 on human and cynomolgus monkey PBMCs were evaluated. The Kd values for the interaction between JS-001 and human and cynomolgus monkey CD4+ T cells were 3.8 nmol/L and 1.4 nmol/L, respectively. In addition, the Kd values for the interaction between JS-001 and CD8+ T cells of human and cynomolgus monkey were 2.1 nmol/L and 1.2 nmol/L, respectively (Figure 2B, 2C).
Figure 2.
Species cross-reactivity of JS-001 and its affinity to PD-1 molecules in humans and cynomolgus monkeys. (A) An ELISA assay was used to determine the species cross-reactivity of JS-001 with antigens derived from different species. FCA analysis confirmed the affinity of JS-001 and PD-1 molecules to human and cynomolgus monkey PBMCs. (B) JS-001 binds to the PD-1 molecules on human PBMCs and (C) cynomolgus monkey PBMCs (CD4+ and CD8+ T cells). Data are given as the mean±SD from 3 independently analyzed experiments.
In vivo efficacy evaluation of JS-001
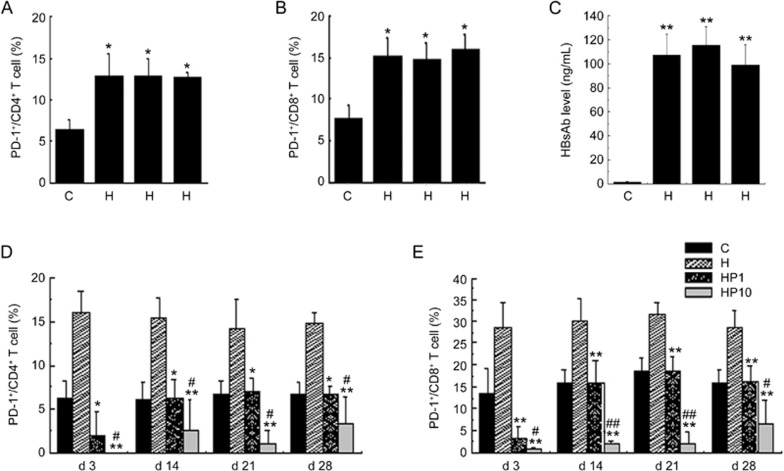
To evaluate the probable efficacy of JS-001 in vivo, we immunized the cynomolgus monkeys with a HBsAg vaccine to increase the PD-1 expression level. We compared PD-1 expression on CD4+ and CD8+ T cells in PBMCs between HBsAg-immunized and non-immunized cynomolgus macaques. As shown in Figure 3A and 3B, the percentages of PD-1/CD4+ and PD-1/CD8+ T cells in blood from the HBsAg-vaccination group were higher those of the healthy controls (HBsAg-) (P<0.05); up-regulation of HBsAb in serum was also observed (Figure 3C). Non-parametric Spearman correlation analysis indicated that the Spearman correlation coefficient for HBsAb levels to PD-1 expression on CD4+ T cells was 0.644 and that of PD-1 expression on CD8+ T cells was 0.706 (0.587 and 0.727 for P=0.05 and P=0.01). In conclusion, the up-regulation of PD-1 and HBsAb expression was correlated with the HBsAg vaccination, which demonstrated the feasibility of the JS-001 efficacy evaluation.
Figure 3.
JS-001 decreased the expression of PD-1+/CD4+ and PD-1+/CD8+T cells in an HBsAg-immunized primate model. HBsAg vaccination was performed at d 0 and the HBsAb and PD-1 expression levels were evaluated. At d 14 post-HBsAg vaccination, the PD-1 expressions on CD4+ (A) and CD8+ (B) cells were observed in the control and HBsAg immunization groups using FCA. (C) HBsAb determination at d 14 post-HBsAg vaccination. *P<0.05, **P<0.01 vs C (n=3 for control and n=9 for HBsAg immunization, 3 per HBsAg dose group). Data are shown as the mean±SD from 3 independently analyzed monkeys. At d 28, the HBsAg and PD-1 antibodies were simultaneously administered for another 28 d of observation. The nine monkeys immunized with the HBsAg vaccine were randomly divided into three groups, H, HP1, and HP10, and the indexes for the preparation of H, HP1, and HP10 group were then tested. H: HBsAg vaccination only; HP1: HBsAg vaccination+JS-001 (1 mg/kg); HP10: HBsAg vaccination+JS-001 (10 mg/kg). PBMCs were harvested at d 3, 14, 21 and 28 post-HBsAg vaccination and PD-1 antibody blockade. (D) PD-1-expressing CD4+ and (E) PD-1-expressing CD8+ cell in the four groups were determined by flow cytometry. Data are shown as the mean±SD from 3 independently analyzed monkeys. *P<0.05, **P<0.01 vs H. #P<0.05, ##P<0.01 vs HP1.
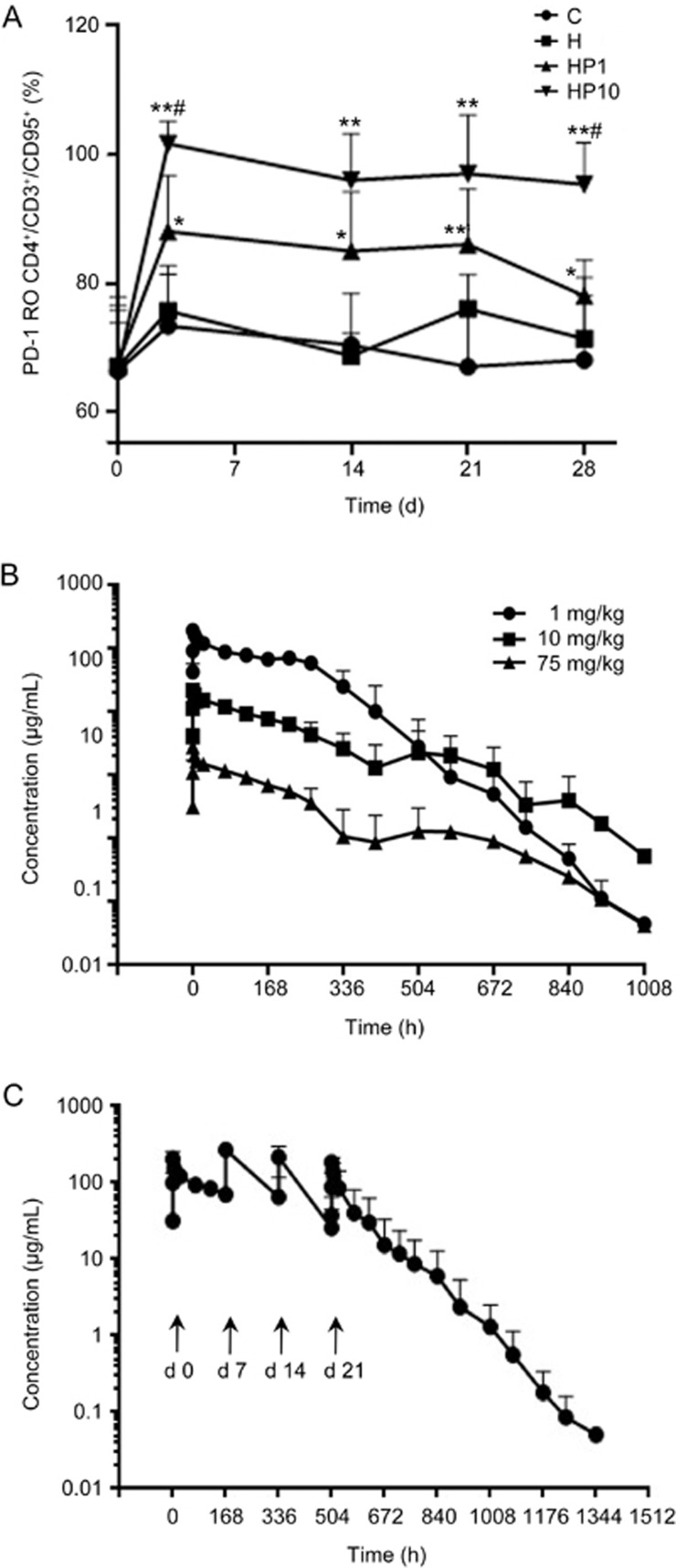
Next, we treated HBsAg-immunized cynomolgus monkeys with JS-001 twice at 14-day intervals. Compared to HBsAg immunization alone, JS-001 dramatically inhibited the elevated expression of PD-1/CD4+ and PD-1/CD8+ in a dose-dependent manner. The phenomenon lasted throughout the 28 d experimental period (Figure 3D, 3E). PD-1 receptor occupancy (RO) results appeared to be dose-independent, such that 1 mg/kg and 10 mg/kg dosing led to high RO percentages of ∼90% (range, 85% to 94%) and ∼100% (range, 95% to 112%), respectively, on d 3. A plateau in occupancy was observed from d 3 to d 28 in the 10 mg/kg group. In the 1 mg/kg group, a decrease in the RO was observed at d 28 (Figure 4A). At d 28, the RO percentages for 1 mg/kg and 10 mg/kg were 72%–83% (n=3) and 89%–102% (n=3), respectively.
Figure 4.
PD-1 receptor occupancy and PK profiles. (A) PD-1 RO experiment was performed by FCA; a detailed description is available in Materials and Methods. Long-term PD-1 occupancy analysis in the four groups. *P<0.05, **P<0.01 vs H. #P<0.05 vs HP1. Data are shown as the mean±SD from 3 independently analyzed monkeys. (B) Drug concentration time curves of cynomolgus macaques after a single vd administration of JS-001 at low, mid, and high dosages (mean±SD, n=6). (C) Drug concentration-time curves of cynomolgus macaques after successive vd administrations of 10 mg/kg JS-001 (mean±SD, n=6).
Pharmacokinetics profile and low immunogenicity of JS-001
The lower limit of quantification (LLOQ) of the assay was determined to be 19.531 ng/mL. The PK profile of JS-001, plotted by serum concentration, is shown in Figure 4B for the low (1 mg/kg), medium (10 mg/kg) and high dose (75 mg/kg) treatments. The Cmax and AUC values were significantly different among the three groups (P<0.01). The ratios of Cmax and AUC(0–t) in the three groups were 1:7.8:68.3 and 1:11.3:98.2, respectively. The effective t1/2_7 d and t1/2_14 dshowed a significant difference between the low and medium dose groups (P<0.05). There was no significant difference in terminal t1/2 (P>0.05) among the three groups, either with CLsor Vd(P>0.05). All detailed PK parameters are listed in Table 1. Taken together, JS-001 showed a linear PK profile within the dose range of 1–10 mg/kg and non-linear PK behavior at high dosages (75 mg/kg).
Table 1. PK parameters of JS-001 after single vd administrations of 1, 10, and 75 mg/kg in cynomolgus macaques (mean±SD, n=6).
| PK parameters | Units | Group 2 1 mg/kg | Group 3 10 mg/kg | Group 4 75 mg/kg |
|---|---|---|---|---|
| t1/2_7 d | h | 133.91±19.50 | 168.87±50.96 | 193.79±27.5* |
| t1/2_14 d | h | 64.48±41.70 | 114.16±42.82 | 156.32±53.99* |
| t1/2a | h | 35.13±16.14 | 36.82±4.75 | 34.96±5.74 |
| Tmax | h | 0.5±0 | 0.5±0 | 0.5±0 |
| Cmax | μg/mL | 27.70±12.29 | 216.11±34.52** | 1891.72±270.16**,## |
| AUC(0–t) | h·μg·mL−1 | 2744.55±834.19 | 31075.53±9849.79** | 269472.4±50585.96**,## |
| AUC(0–inf) | h·μg·mL−1 | 2749.51±833.39 | 31096.05±9849.95** | 269488.17±50574.79**,## |
| AUC(t–inf)% | % | 0.20±0.26 | 0.07±0.06 | 0.01±0.01# |
| Vd | mL/kg | 18.22±3.77 | 17.94±3.63 | 14.28±2.54 |
| CLs | mL·h−1·kg−1 | 0.39±0.12 | 0.35±0.09 | 0.29±0.05 |
| MRT | h | 121.91±38.84 | 147.5±62.6 | 149.25±24.68 |
note: t1/2_7 d and t1/2_14 d, effective half-time, which was calculated by terminal phase data harvested from the first 7 or 14 d;
a t1/2: terminal t1/2, calculated by total terminal phase data;
*P<0.05,
**P<0.01 vs Group 2;
#P<0.05,
##P<0.01 vs Group 3.
In the 10 mg/kg successive administration group (Figure 4C), no drug accumulation was observed. The final AUC exposure was lower than that of the first administration (Table 2), which was partly due to the production of ADAs at the last administration (21 d). In accordance with this observation, positive ADA results were observed in some samples in immunogenicity assays. The 10% of each group was positive 28 d after the first administration. Our data indicate that low immunogenicity of JS-001 was observed in cynomolgus monkeys.
Table 2. PK parameters of JS-001 after successive administrations of 10 mg/kg in cynomolgus macaques (mean±SD, n=6).
| PK parameters | Units | First | Group 1 vd JS-001 10 mg/kg Last (0–168 h) | Last (0–inf) |
|---|---|---|---|---|
| t1/2 | h | 175±11.82 | 39.8±25.06** | 37.6±25.83 |
| Tmax | h | 1.75±3.06 | 0.5±0 | 0.5±0 |
| Cmax | μg/mL | 205.82±51.71 | 185.6±44.21 | 185.6±44.21 |
| AUC(0–t) | h·μg·mL−1 | 16513.89±1212.45 | 8310.92±6404.33* | 9794.67±8406.78 |
| AUC(0–inf) | h·μg·mL−1 | 34408.03±3689.04 | 9558.99±8170.37** | 9816.77±8386.49 |
| AUC(t–inf)% | % | 51.85±1.92 | 7.80±7.78** | 0.92±2.20 |
| Vd | mL/kg | 73.72±4.79 | 73.24±23.95 | 67.74±25.87 |
| CLs | mL·h−1·kg−1 | 0.29±0.03 | 2.07±1.71* | 2.05±1.72 |
| MRT | h | 252.9±13.75 | 52.83±35.25** | 55.9±39.66 |
| ARa | NA | NA | 0.50±0.37 | NA |
a AR: accumulation rate, last drug administration AUC(0–168 h)/ first drug administration AUC(0–168 h);
*P<0.05,
**P<0.01 vs Group 1 first administration.
Discussion
Here, we reported the in vitro and in vivo efficacy, PK and immunogenicity of JS-001, which is the first anti-PD-1 mAb approved for clinical study in China. The promising in vitro results confirmed the efficacy of JS-001 in vivo. According to species cross-reactivity experiments, we determined that JS-001 effectively targeted the PD-1 of primate species. We utilized HBsAg antigen immunization to activate T cells and elevate PD-1 expression. We successfully observed the expected indices in our primate models. A non-parametric Spearman correlation analysis was performed, which confirmed a moderate to high-correlation relationship (P<0.05). We observed decreases in PD-1+/CD4+ and PD-1+/CD8+ after blockade with 1 or 10 mg/kg JS-001 treatment. It has been reported that the proportion of CD8+ T cells expressing PD-1 and the levels of PD-1 on virus-specific T cells are strongly correlated with viral load in the plasma. The basis of effective antiviral treatment is that it decreases PD-1 expression in CD8+ T cells, which makes it possible to rescue exhausted T cells by blocking the PD-1/PD-L1 interaction8,9. The up-regulation of PD-L1 on PBMCs was observed, and its expression was positively correlated with levels of PD-1 in circulating CD4+ and CD8+ T cells. Using this proof-of-concept study in cynomolgus monkeys, we confirmed the PD-1 blocking activity of JS-001.
The dosages used in pharmacokinetics studies need to be taken into consideration with respect to the comprehensive effects of the effective doses, including the minimum effective dose, as well as the emerging toxic dose. For the currently available anti-PD-1/PD-L1 antibody drug on the market, the normal dosage used in clinical trials is 0.3–10 mg/kg10,11. At dosages greater than 10 mg/kg, dosage increase does not correlate with increased response. JS-001 toxicity was observed in monkeys at a dosage range of 30–100 mg/kg. In this study, the JS-001 dosages used in monkeys were 1, 10, and 75 mg/kg, which correspond to 0.3, 3, and 25 mg/kg in humans. We discovered that the optimal dose is 10 mg/kg in monkeys, which is consistent with a human dose of 2–3 mg/kg for Nivolumab or Pembrolizumab10,12. In addition, in JS-001 clinical I trials for non-small cell lung cancer (NSCLC), melanoma, breast cancer and renal cell carcinoma, optimal doses of 3 mg/kg were also reported.
RO is an important index for PD-1 mAb evaluation. Few previous reports have examined the RO of PD-1 mAb, with the exception of one anti-PD-1 mAb, MDX-1106 (also named BMS-936558), which has been studied in clinical trials6,10. In that study, the effect of the PD-1 receptor on circulating CD3+ T cells was determined using FCA. We improved this FCA method for RO determination, focusing on PD-1+/CD3+/CD4+/CD95+ instead of PD-1+/CD3+. CD95+ to represent activated T cell markers13, including memory T cell (CD28+ CD95+) and effector T cell (CD28−CD95+) markers. CD28 and CD95 represent one method for T cell classification, whereas CD45RA and CD45RO represent a different method. Ideally, if eight-color FCA were available, CD45RA and CD45RO would also have been included. However, only four-color FCA was available in this study, and three channels were already occupied with CD3, CD4 and IgG4. With only channel left, CD95 was chosen to detect activated (non-naive) T cells.
For efficacy, the administration of MDX-1106 at doses of 0.1 mg/kg to 10.0 mg/kg every 2 weeks resulted in a change in the median PD-1 receptor occupancy by the anti–PD-1 antibody of 64% to 70%, depending on the dosage. The background RO ranges of the patients and monkeys pre-administration were not available. The RO results suggest that the effect of JS-001 on PD-1 was dose-dependent and could reach ∼100% compared to MDX-1106. The consistently high levels of PD-1 occupancy observed in PBLs of JS-001 indicate its potentially better efficacy. Administration periods in clinic trials need to be considered to avoid adverse effects.
In successive vd administrations of 10 mg/kg JS-001, the exposure level after the last infusion was lower than that following the first infusion. Similarly, in the later administration periods, the clearance of JS-001 in the 75 mg/kg group became more rapid than that of the 10 mg/kg group. The presence of ADAs may cause this phenomenon. Regardless, JS-001 showed relatively low immunogenicity in pre-clinical trials.
In conclusion, this study is the first report on the efficacy, PK and immunogenicity of JS-001 in cynomolgus monkeys. The non-clinical data are encouraging and could provide a basis for its efficacy and safety evaluation in clinic trials.
Author contribution
Ruo-xian DENG, Hui FENG, Bo CHEN, and Hai-feng SONG designed the research; Jie FU, Fang WANG, Li-hou DONG, Jing ZHANG, Cheng-lian DENG, Jing ZHANG, Li-bo ZHANG, and Hai WU performed the research; Jie FU, Hui Feng, Bo CHEN, Li-hou DONG, Xue-li WANG, and Xin-yao XIE analyzed the data; Jie FU, Fang WANG, and Hai-feng SONG wrote the paper.
Acknowledgments
This study was supported by National Thirteen Five Major Special Fund “Non-clinical and Clinical PK, PD, and Immunogenicity Evaluation of Biosimilar” (2015ZX09501008-006) and “Core Technology-based Non-clinical Evaluation of Biological macromolecular drugs” (2015ZX09501007-002). We thank Dr Qing-qing WANG and Zhe WANG for language assistance during the preparation of this manuscript.
References
- McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med 2013; 2: 662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009; 458: 206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, et al. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1). Proc Natl Acad Sci U S A 2013; 110: 15001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C, Thieme S, Bandoła J, Laugsch M, Anastassiadis K, Brenner S. Generation of inducible immortalized dendritic cells with proper immune function in vitro and in vivo. PLoS One 2013; 8: e62621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015; 16: 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust L, D'journo XB. The use of correlation functions in thoracic surgery research. J Thorac Dis 2015; 7: E11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade inchronic hepadnaviral infection. PLoS Pathog 2014; 10: e1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 2006; 203: 2281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li F, Jiang F, Lv X, Zhang R, Lu A, et al. A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway & translational blockade of immune checkpoints. Int J Mol Sci 2016; 17: 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen M, Janssen O. Pro- and anti-apoptotic CD95 signaling in T cells. Cell Commun Signal 2011; 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]