Abstract
Geniposide is an iridoid glycosides purified from the fruit of Gardenia jasminoides Ellis, which is known to have antiinflammatory, anti-oxidative and anti-tumor activities. The present study aimed to investigate the effects of geniposide on experimental rat colitis and to reveal the related mechanisms. Experimental rat colitis was induced by rectal administration of a TNBS solution. The rats were treated with geniposide (25, 50 mg·kg−1·d−1, ig) or with sulfasalazine (SASP, 100 mg·kg−1·d−1, ig) as positive control for 14 consecutive days. A Caco-2 cell monolayer exposed to lipopolysaccharides (LPS) was used as an epithelial barrier dysfunction model. Transepithelial electrical resistance (TER) was measured to evaluate intestinal barrier function. In rats with TNBS-induced colitis, administration of geniposide or SASP significantly increased the TNBS-decreased body weight and ameliorated TNBS-induced experimental colitis and related symptoms. Geniposide or SASP suppressed inflammatory cytokine (TNF-α, IL-1β, and IL-6) release and neutrophil infiltration (myeloperoxidase activity) in the colon. In Caco-2 cells, geniposide (25–100 μg/mL) ameliorated LPS-induced endothelial barrier dysfunction via dose-dependently increasing transepithelial electrical resistance (TER). The results from both in vivo and in vitro studies revealed that geniposide down-regulated NF-κB, COX-2, iNOS and MLCK protein expression, up-regulated the expression of tight junction proteins (occludin and ZO-1), and facilitated AMPK phosphorylation. Both AMPK siRNA transfection and AMPK overexpression abrogated the geniposide-reduced MLCK protein expression, suggesting that geniposide ameliorated barrier dysfunction via AMPK-mediated inhibition of the MLCK pathway. In conclusion, geniposide ameliorated TNBS-induced experimental rat colitis by both reducing inflammation and modulating the disrupted epithelial barrier function via activating the AMPK signaling pathway.
Keywords: geniposide, sulfasalazine, intestinal inflammation, colitis, intestinal barrier function, AMPK signaling pathway, MLCK
Introduction
Intestinal inflammation is related to multiple factors including inflammatory bowel disease (IBD). Although the precise etiology of IBD remains unknown1, it is mainly caused by ulcerative colitis and Crohn's disease2. Crohn's disease causes inflammation that extends through the entire bowel wall, whereas ulcerative colitis affects the colon and/or the large intestine3.
Geniposide (Figure 1), an iridoid glycosides purified from the fruit of Gardenia jasminoides Ellis, is known to have anti-inflammatory, anti-oxidative and anti-tumor effects4,5,6. The anti-inflammatory effects of geniposide have been found to ameliorate arthritis and mastitis7,8. However, whether geniposide can effectively ameliorate intestinal inflammation remains unknown. The present study was designed to investigate the effects of geniposide on intestinal inflammation.
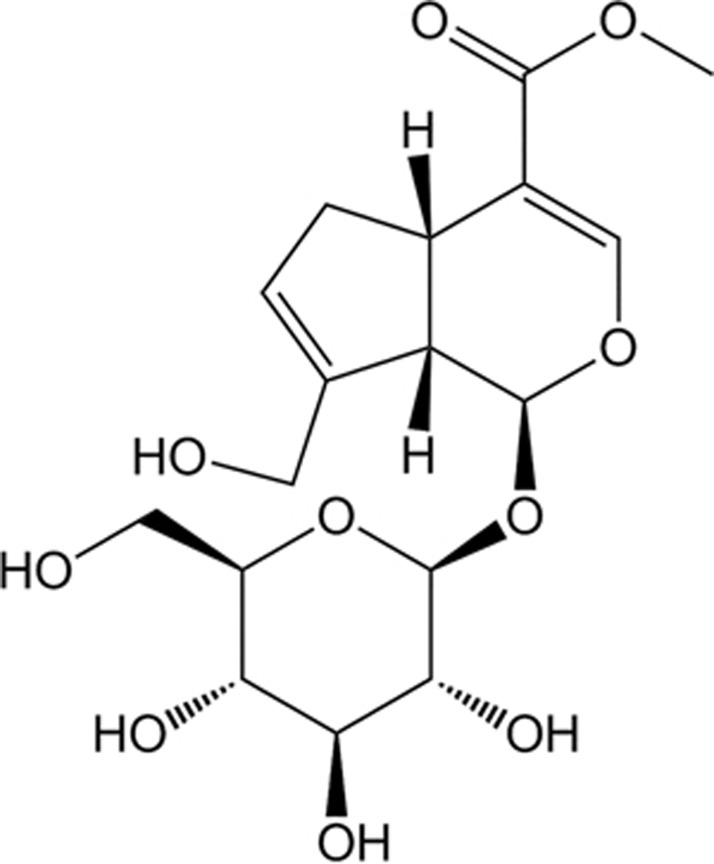
Figure 1.
Chemical structure of geniposide.
To provide valuable information for the potential clinical treatment of bowel inflammation, in the present study, both 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced experimental ulcerative colitis in rats and lipopolysaccharide (LPS)-infected Caco-2 cell monolayers were used as experimental intestinal inflammatory models, and sulfasalazine (SASP) was used as a positive control drug to evaluate and characterize geniposide-induced modulation and reveal the related mechanisms.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats weighing 180–220 g were obtained from the Experimental Animal Center of Dalian Medical University (Certificate of Conformity: No SCXK 2008-0002). The experimental protocol was carried out based on the Declaration of Helsinki and supported by Dalian Medical University Animal Care and Ethics Committee. All rats were housed at a temperature of 22±2 °C, maintained on a 12:12-h light-dark cycle, and provided with food and water ad libitum. Rats were acclimatized for 1 week before the initiation of the study.
Establishment of experimental rat colitis
The rat model of colitis was induced by rectal administration of TNBS according to previously described methods9. After a 24-h period of fasting with ad libitum access to water, a TNBS-ethanol solution (50% v/v) was administered through a catheter into the rat colon at a dose of 100 mg/kg under urethane anesthesia (1.25 g/kg, ip). The vehicle control group was treated with 50% ethanol alone.
Rats were randomly divided into six experimental groups with ten rats in each group: (1) vehicle control group, (2) vehicle + geniposide (H, 50 mg/kg) group, (3) TNBS-treated group, (4) TNBS+SASP (100 mg/kg) group, (5) TNBS+geniposide (L, 25 mg/kg) group, and (6) TNBS+geniposide (H, 50 mg/kg) group. The vehicle and TNBS group were given an equal volume of saline. Agents used in the assay were prepared using saline. Starting 24 h after the initiation of TNBS-induced inflammation, saline, sulfasalazine (100 mg/kg), or geniposide (25, 50 mg/kg) was intragastrically administered once daily in all 6 groups for 14 consecutive days.
Rat food intake, body weight, stool consistency (degree of diarrhea), and blood in stool were recorded daily. Rats were sacrificed on d 15, the colon was removed, and the length and weight were measured. The isolated colon was fixed in 4% paraformaldehyde for 24 h and embedded in paraffin. Colon samples were prepared and stained with hematoxylin and eosin (H&E) and then assessed under light microscopy. The pathologic score of the colonic damage was evaluated according to previously described morphological criteria10,11.
Rats were denied access to food but allowed water for 3 h. Then, 150 μL fluorescein isothiocyanate-4 kDa dextran (80 mg/mL, Sigma) was gavaged, and serum was harvested 1 and 3 h later. Serum recovery was measured using a Synergy HT plate reader (BioTek, Winooski, VT, USA) as previously reported12.
LPS-infected Caco-2 cells
Caco-2 cells obtained from the ATCC (Rockville, MD, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) containing 10% fetal bovine serum (FBS, Gibco) were given 1% non-essential amino acids and 1% glutamine. All of the media contained 50 U/mL penicillin and 50 U/mL streptomycin. All cells were maintained at 37 °C in a humidified atmosphere of air and 5% CO2. LPS (1 μg/mL)-stimulated Caco-2 cells were treated with geniposide or an equal volume of vehicle.
Transepithelial electrical resistance (TER) was measured to evaluate the epithelial barrier function in vitro. Decreased TER is a significant indicator of barrier dysfunction13. An epithelial voltohmmeter was used for the measurement of TER of filter-grown Caco-2 intestinal monolayers as previously reported14. TER of Caco-2-plated filters was measured daily. A steady state of TER was achieved after 21 d (always 540±12 Ω·cm2), indicating that the barrier function model was established, and Caco-2-plated filters could be used.
Cell transfection
Caco-2 cells were transfected with small interfering RNA (siRNA) or c-DNA as previously described15. Caco-2 cells were placed into 6-well plates for 24 h. Cells were transfected with specific siRNA (Genepharma, Shanghai, China) or c-DNA targeting AMPK with Lipofectamine 2000 (Invitrogen). After transfection for 48 h, the cells were infected with LPS or LPS+geniposide (50 μg/mL) for an additional 24 h. Then, cells were collected for Western blot analysis.
ELISA assays
The expression levels of pro-inflammatory cytokines and mediators, including tumor necrosis factor-alpha (TNF-α), interleukin-1-beta (IL-1-β), interleukin-6 (IL-6), and myeloperoxidase (MPO), in the rat colon were determined using double-antibody sandwich ELISAs (R&D Systems, USA) according to the manufacturer's instructions.
Western blot analysis
Equal amounts of protein were subjected to Western blot analysis as previously described16. Protein lysates from both rats and cells were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. Membranes were blotted for specific antibodies, including MLCK (Abcam, Cambridge, UK), occludin, ZO-1 (Santa Cruz Biotechnology), NF-κB p65, p-p65, iNOS, COX-2, p-AMPK, and AMPK (CST, Beverly, MA, USA). The blots were developed using an enhanced chemiluminescence method (GE Healthcare). Quantification was performed by densitometric analysis of specific bands on the immunoblots using a Multi Spectral imaging system (UVP, Cambridge, UK).
Reagents
Geniposide (purity >98%) was purchased from Chengdu Must Bio-Technology Co (Chengdu, China). Unless otherwise indicated, all chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA).
Statistical analysis
All data are shown as the mean±standard deviation (SD). One-way analysis of variance (ANOVA) was used where three or more groups of data were compared. All experiments were repeated at least three times, and a P value of less than 0.05 (P<0.05) was considered statistically significant.
Results
Establishment of TNBS-induced experimental colitis in rats
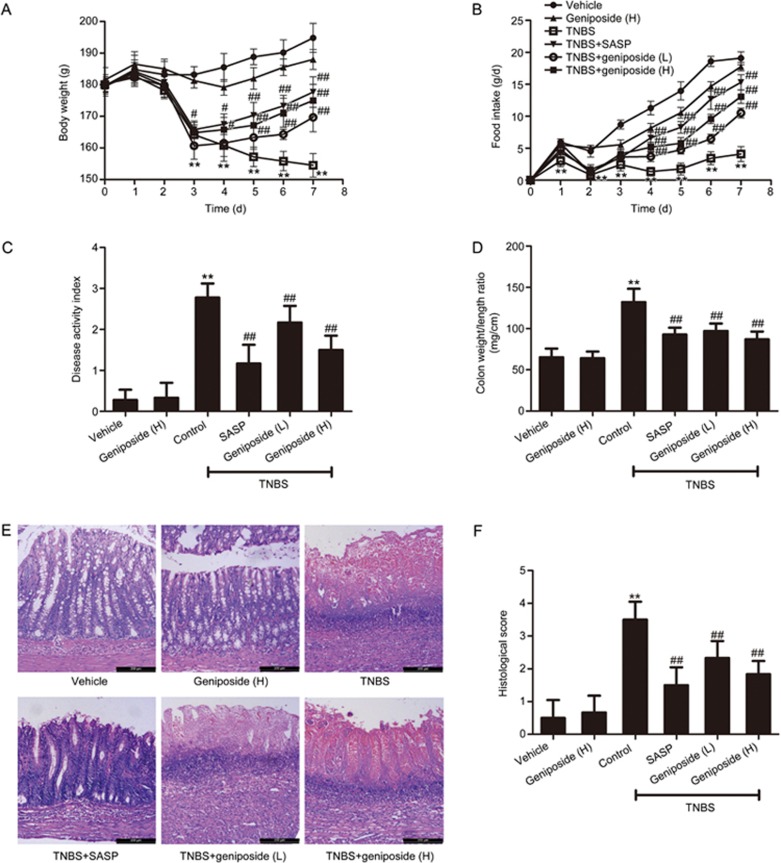
Body weight and food intake of TNBS-treated rats (Figure 2A,2B) were significantly reduced compared with those of the vehicle control group. Macroscopically visible damage, as measured by the disease activity index (DAI) and the colon weight-to-length ratio (Figure 2C,2D), was significantly higher in the rats with TNBS-induced experimental colitis than in the vehicle control. Significant tissue injuries with high microscopic damage scores were found using histological examination (Figure 2E,2F) of resected colons from TNBS-treated rats. Both geniposide (25, 50 mg/kg) and the positive control SASP (100 mg/kg) significantly increased the TNBS-decreased body weight and ameliorated TNBS-induced experimental rat colitis and related symptoms. Although not significant, geniposide decreased the body weight of rats compared with that of the vehicle controls.
Figure 2.
Protective effects of geniposide on TNBS-induced colitis in rats. Compared with the TNBS-treated control group, geniposide (L, 25 mg/kg; H, 50 mg/kg) reversed both the decreased body weight (A) and the decreased food intake (B) and decreased the increased disease activity index (C), colon weight/length ratio (D), and histologic injury (E) in TNBS-treated rats. Histologic injury scores of the colon in different groups were quantified (F). All data are expressed as the mean±SD. n=6. **P<0.01 vs vehicle control group. #P<0.05, ##P<0.01 vs TNBS-treated group.
Geniposide-induced amelioration on intestinal inflammation in vivo
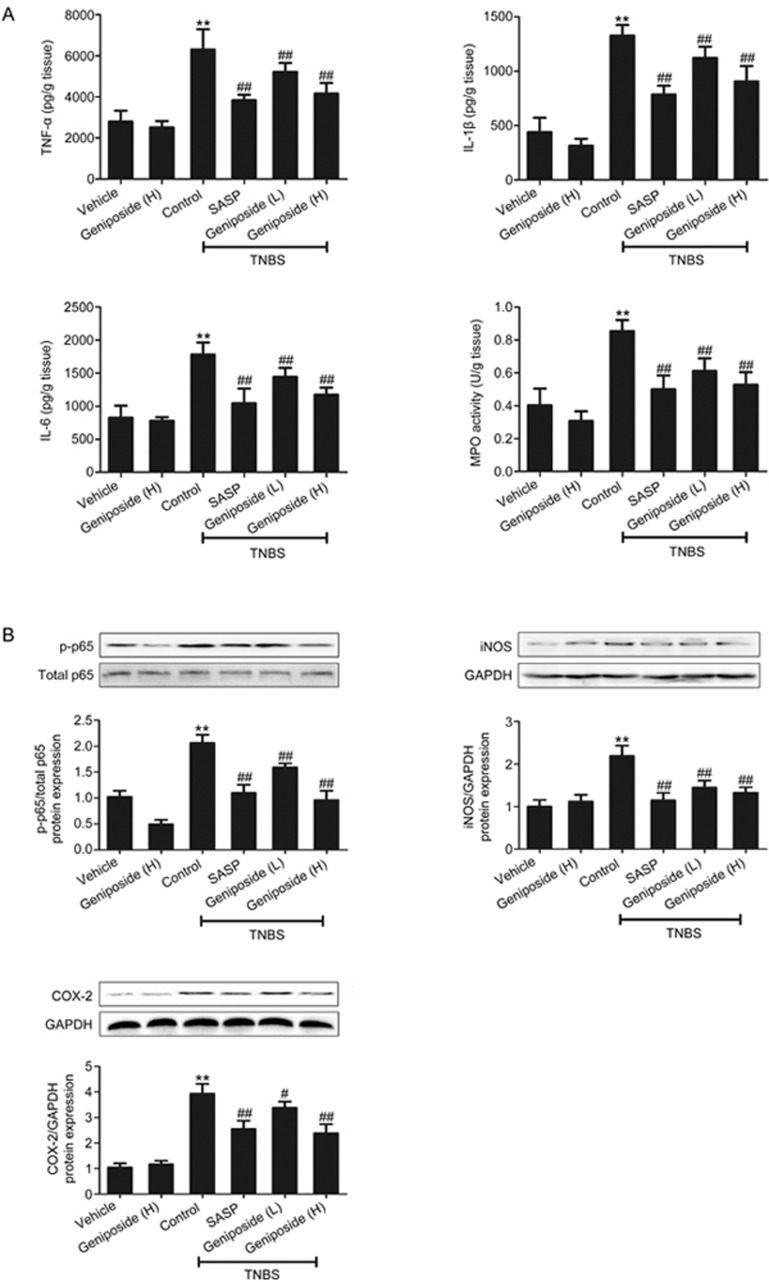
Inflammatory cytokine release and neutrophil infiltration play important roles in the process of inflammation17,18,19. The present study indicated that levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and myeloperoxidase (MPO) activity in the resected colon from TNBS-treated rats were significantly higher than in the vehicle control (Figure 3A). Geniposide (25, 50 mg/kg) significantly decreased both the increased pro-inflammatory cytokines and enhanced MPO activity.
Figure 3.
Geniposide-induced suppression of inflammatory parameters in TNBS-treated rats. (A) Geniposide (L, 25 mg/kg) and geniposide (H, 50 mg/kg) decreased the high expression of tumor necrosis factor alpha (TNF-α), interleukin-1-beta (IL-1β), interleukin-6 (IL-6) and myeloperoxidase (MPO) activity in TNBS-treated rats. Data are expressed as the mean±SD. n=6. (B) Geniposide reduced the increase in nuclear factor kappa-B (NF-κB) p65 phosphorylation, inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) protein expression in TNBS-treated rats. Data are expressed as the mean±SD. n=3. **P<0.01 vs vehicle control group. #P<0.05, ##P<0.01 vs TNBS-treated group.
NF-κB plays an important role in inflammatory processes, initiating transcription of pro-inflammatory cytokine genes20. Inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) are also involved in the process of inflammation21. Our results indicated that the expression levels of NF-κB, COX-2, and iNOS proteins were significantly increased in TNBS-treated rats compared with those in the vehicle control rats (Figure 3B). Both geniposide (25, 50 mg/kg) and the positive control SASP (100 mg/kg) down-regulated the increased expression of NF-κB, COX-2, and iNOS proteins in TNBS-treated rats.
Geniposide-induced amelioration of intestinal barrier dysfunction in vivo
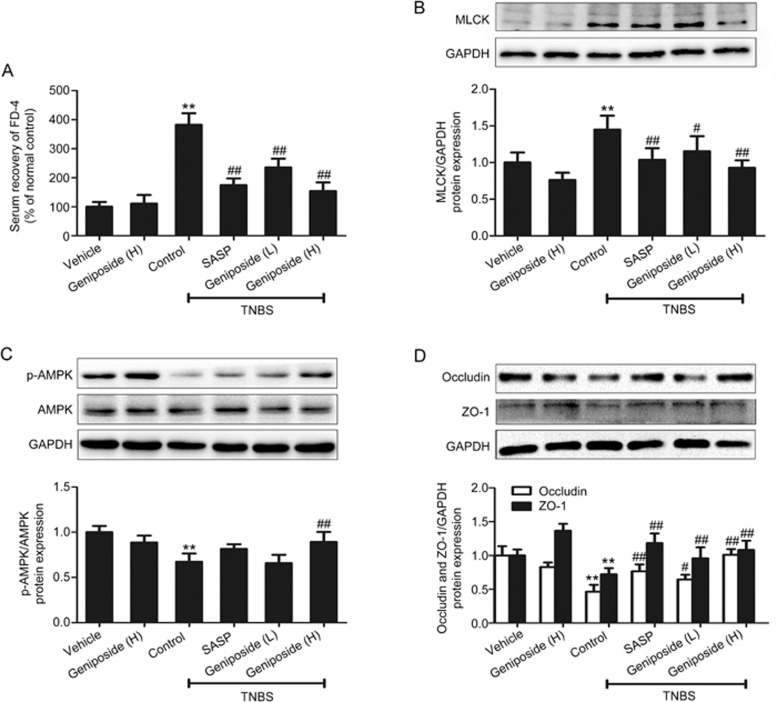
Intestinal barrier dysfunction is found to cause increased intestinal permeability characterized by enhanced serum recovery of FD-422,23. Decreased AMPK phosphorylation, increased MLCK protein expression, and decreased occludin/ZO-1 protein expression (tight junction) are also found to be related to the destruction of intestinal barrier function24,25,26,27. Our results showed that serum FD-4 was significantly higher in TNBS-treated rats than in vehicle control rats. Both geniposide (25, 50 mg/kg) and the positive control SASP (100 mg/kg) reversed the increased serum recovery of FD-4 in TNBS-treated rats (Figure 4A). AMPK phosphorylation was decreased in TNBS-treated rats. Geniposide (25, 50 mg/kg) treatment reversed the decrease in AMPK phosphorylation in TNBS-treated rats (Figure 4B). The protein expression of MLCK was significantly higher in TNBS-treated rats than in vehicle control rats. Geniposide (25, 50 mg/kg) treatment significantly down-regulated the increased protein expression of MLCK in TNBS-treated rats (Figure 4C). Tight junction protein expression (occludin and ZO-1) was decreased in TNBS-treated rats. Geniposide (25, 50 mg/kg) treatment reversed the decrease in the protein expression of occludin and ZO-1 in TNBS-treated rats (Figure 4D).
Figure 4.
Effects of geniposide on intestinal permeability in TNBS-treated rats. (A) Geniposide (L, 25 mg/kg) and geniposide (H, 50 mg/kg) reduced the increase in the serum recovery of FD-4 induced by TNBS. Data are expressed as the mean±SD. n=6. (B) Geniposide reversed the increase in myosin light chain kinase (MLCK) protein level induced by TNBS. (C) Geniposide (25, 50 mg/kg) treatment reversed the decrease in AMP-activated protein kinase (AMPK) protein phosphorylation triggered by TNBS. (D) Geniposide reversed the reduction in the expression of tight junction proteins (occludin and ZO-1) induced by TNBS. Data are expressed as the mean±SD. n=3. **P<0.01 vs vehicle control group. #P<0.05, ##P<0.01 vs TNBS-treated group.
Geniposide-induced amelioration of barrier dysfunction in vitro
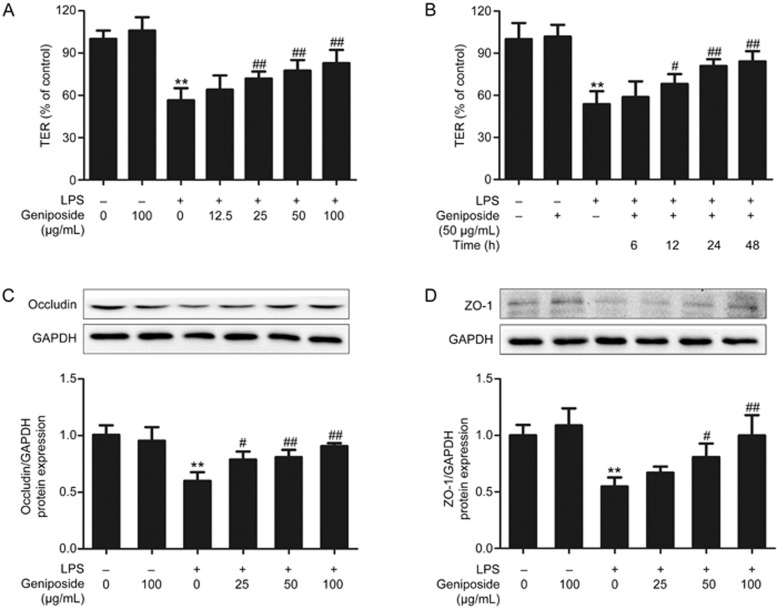
The damage to Caco-2 cells induced by LPS leads to a reduction in TER28. As shown in Figure 5, geniposide at final concentrations of 25 to 100 μg/mL significantly elevated the decreased TER (Figure 5A). With an incubation time of 12 to 48 h, geniposide at a concentration of 50 μg/mL significantly increased LPS-reduced TER (Figure 5B).
Figure 5.
Geniposide-induced amelioration of the impaired barrier function in vitro. (A) Effects of geniposide (0, 12.5, 25, 50, 100 μg/mL) on the decreased transepithelial electrical resistance (TER) induced by LPS. (B) Time-course effect of geniposide (50 μg/mL) on the decreased TER induced by LPS. Data are expressed as the mean±SD. n=6. (C–D) Effects of geniposide (0, 25, 50, 100 μg/mL) on the decreased occludin and ZO-1 protein expression induced by LPS in the Caco-2 monolayer. Data are expressed as the mean±SD. n=3. **P<0.01 vs control group. #P<0.05, ##P<0.01 compared to LPS-infected group.
Destruction of barrier function is found in colitis29. Our results showed that the expression levels of occludin and ZO-1 protein were significantly lower (Figure 5C,5D) in LPS-infected Caco-2 cells than in the control Caco-2 cells. Geniposide (25, 50, 100 μg/mL) significantly elevated the reduced occludin and ZO-1 protein expression in LPS-infected Caco-2 cells.
Geniposide-induced amelioration of inflammation in vitro
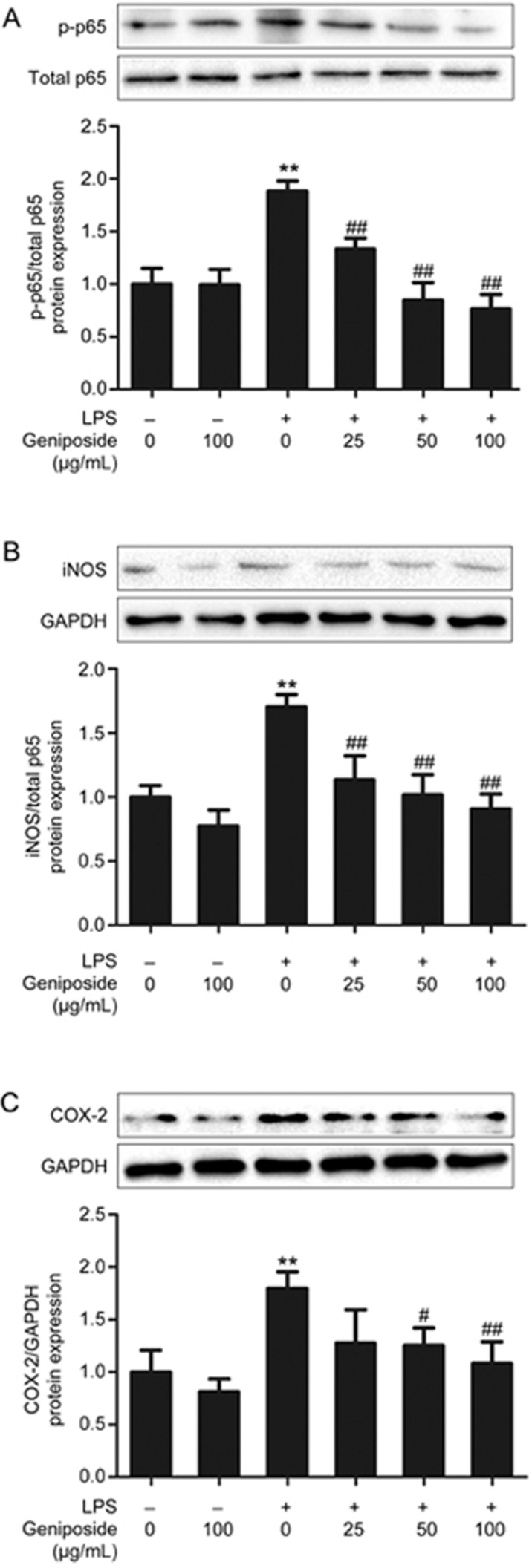
The results showed that the expression levels of NF-κB, COX-2, and iNOS proteins were significantly higher in LPS-infected Caco-2 cells (Figure 6A–6C) than in control Caco-2 cells. Geniposide (25, 50, 100 μg/mL) significantly down-regulated NF-κB, COX-2, and iNOS protein expression in LPS-infected Caco-2 cells.
Figure 6.
Geniposide-induced suppression of inflammation in vitro. (A) Effects of geniposide (0, 25, 50, 100 μg/mL) on the increased nuclear factor kappa-B (NF-κB) p65 phosphorylation inducible nitric oxide synthase (iNOS) (B) and cyclooxygenase 2 (COX-2) protein expression (C) induced by LPS in the Caco-2 monolayer. All data are expressed as the mean±SD. n=3. **P<0.01 vs control group. #P<0.05, ##P<0.01 vs LPS-infected group.
Geniposide-induced modulation of the AMPK/MLCK pathway
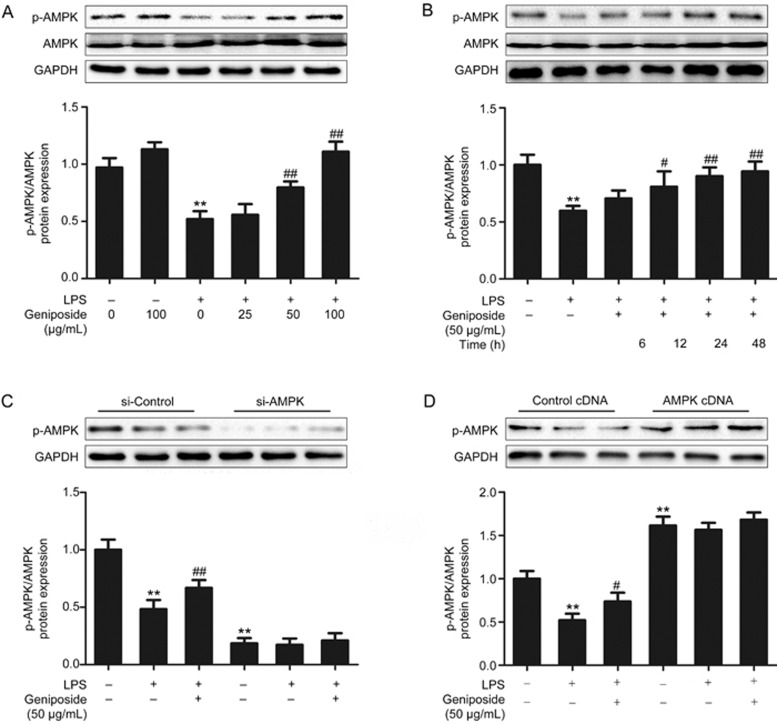
Decreased AMPK phosphorylation is found in LPS-infected Caco-2 cells30. At doses of 50–100 μg/mL (Figure 7A) and with incubation times of 12–48 h (Figure 7B), geniposide significantly enhanced the decreased AMPK phosphorylation in LPS-infected Caco-2 cells in a dose- and time-dependent manner. Both siRNA-inhibited and cDNA-facilitated endogenous expression of AMPK in Caco-2 cells were used to further characterize the role of geniposide in the modulation of AMPK. The results indicated that geniposide-mediated AMPK up-regulation was significantly abrogated following AMPK siRNA transfection (Figure 7C), and geniposide could not further up-regulate cDNA-facilitated AMPK expression (Figure 7D).
Figure 7.
Effects of geniposide on the decreased AMPK phosphorylation induced by LPS in vitro. (A) Effects of geniposide (0, 25, 50, 100 μg/mL) on the decreased AMPK phosphorylation induced by LPS in the Caco-2 monolayer. (B) Time-course effects of geniposide (50 μg/mL) on the decreased AMPK phosphorylation by LPS in the Caco-2 monolayer. (C) siRNA-induced knockdown of AMPK prevented the facilitative effects of geniposide on AMPK phosphorylation. (D) cDNA-induced overexpression of AMPK prevented the facilitative effects of geniposide on AMPK phosphorylation. All data are expressed as the mean±SD. n=3. **P<0.01 vs control group. #P<0.05, ##P<0.01 vs LPS- infected group.
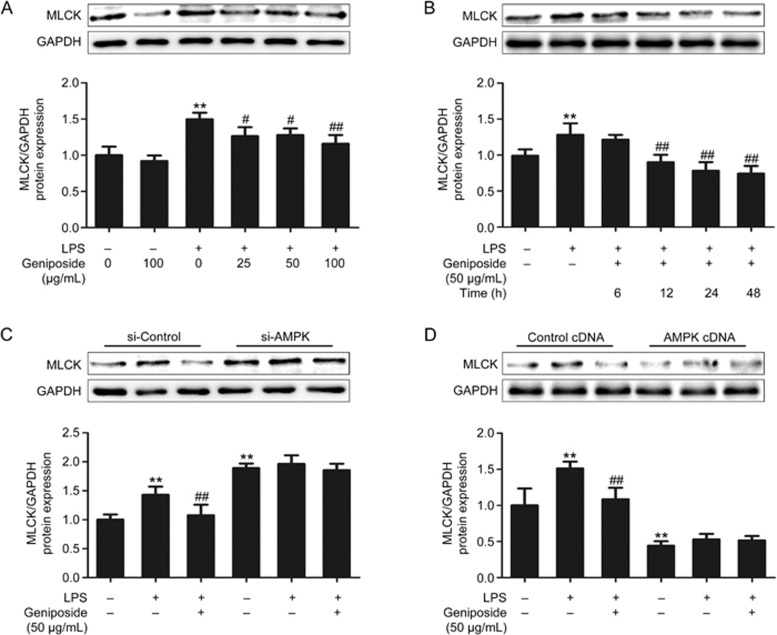
Our results indicated that MLCK expression was significantly increased in LPS-infected Caco-2 cells. At doses of 25–100 μg/mL (Figure 8A) and with incubation times of 12–48 h (Figure 8B), geniposide exerted dose- and time-dependent inhibitory effects on the increase in MLCK protein expression in LPS-infected Caco-2 cells. To assess whether MLCK is involved in the AMPK signaling pathway in geniposide-induced modulation, the effects of geniposide on the status of MLCK protein expression following AMPK siRNA and cDNA treatment of Caco-2 cells were measured. As shown in Figure 8C,8D, knockdown of AMPK significantly increased the expression of MLCK compared with the control siRNA in LPS-infected Caco-2 cells, and geniposide-mediated down-regulation of MLCK in LPS-infected Caco-2 cells was abolished by AMPK siRNA transfection. Overexpression of AMPK decreased MLCK protein expression compared with the control cDNA in Caco-2 cells, and geniposide did not further decrease the down-regulated MLCK induced by AMPK overexpression.
Figure 8.
Modulation of the AMPK/MLCK pathway by geniposide in the LPS-induced barrier dysfunction model. (A) Effects of geniposide (0, 25, 50, 100 μg/mL) on the increased MLCK protein expression induced by LPS in the Caco-2 monolayer. (B) Time-course effects of geniposide (50 μg/mL) on the increased MLCK protein expression induced by LPS in the Caco-2 monolayer. (C) siRNA-induced knockdown of AMPK prevented the inhibitory effects of geniposide on MLCK protein expression. (D) cDNA-induced overexpression of AMPK prevented the inhibitory effects of geniposide on MLCK protein expression. All data are expressed as the mean±SD. n=3. **P<0.01 vs control group. #P<0.05, ##P<0.01 vs LPS-infected group.
Discussion
Geniposide, a traditional Chinese medicine from the fruit of Gardenia jasminoides Ellis, has been found to possess antidiarrheal, hepatoprotective, anti-inflammatory, and anti-endotoxin activities31,32,33. Recent studies have shown that geniposide is an efficient anti-inflammatory agent in experimental arthritis and mastitis7,34. The present study was carried out to reveal the characteristics of geniposide in the protection against and amelioration of experimental rat intestinal inflammation and the underlying mechanisms.
In the present study, geniposide (25, 50 mg/kg) ameliorated TNBS-induced experimental rat colitis and related symptoms. Geniposide significantly increased the TNBS-decreased body weight compared with that of the TNBS controls, demonstrating its ameliorative effects; however, geniposide was found to decrease the body weight of rats compared with that of vehicle controls without statistical significance (Figure 2). This phenomenon can be explained as follows. Geniposide is one of the active ingredients in traditional Chinese medicine used to fight obesity35. For instance, geniposide has been used for the amelioration of spontaneously obese type 2 diabetic mice and has been shown to suppress body weight and visceral fat accumulation36.
Inflammatory cytokine release and neutrophil infiltration play an important role in colitis; MPO can indirectly reflect the vitality of neutrophil infiltration37. NF-κB, an important regulatory factor of inflammation, regulates the expression of genes that are involved in the production of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-638. A pro-inflammatory cytokine inhibitor such as infliximab, an anti-TNF-α antibody, is used in treatment of IBD patients39. iNOS and COX-2 are also involved in the process of inflammation, regulating the expression of inflammatory mediators NO and PGE240,41. The above-mentioned evidence indicates that increased expression of inflammatory mediators is found in intestinal inflammation, suggesting that down-regulation of those inflammatory mediators would be beneficial in the amelioration of intestinal inflammation. The present study indicated that geniposide treatment not only improved the symptoms of TNBS-treated rats, including reversing the decreased body weight and food intake and the amelioration of histological injury, but also reduced MPO activity and down-regulated pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 and protein expression of NF-κB, iNOS and COX-2, indicating the potential mechanisms involved in geniposide-induced amelioration of TNBS-induced experimental colitis.
Damaged intestinal epithelial barrier function is correlated with triggering or worsening intestinal inflammatory disorder42. Both maintenance and disruption of epithelial barrier function are related to intestinal inflammation. For instance, intestinal barrier dysfunction is found to result in increased intestinal permeability characterized by enhanced serum recovery of FD-422,23. AMPK activity has a specific role in epithelial barrier function43. LPS-induced endothelial hyperpermeability occurs in parallel with a decrease in AMPK activity. Activation of AMPK by 5-aminoimidazole-4-carboxamide-1-d-ribofuranoside, a potent AMPK activator, attenuates LPS-induced endothelial hyperpermeability in vitro30. Increased MLCK protein expression and decreased occludin/ZO-1 protein expression (tight junction) are also found to induce the destruction of intestinal barrier function25,26,27. All of these studies indicate that the amelioration of the disruption of epithelial barrier function, characterized by restoration of these parameters back to normal, is therapeutically beneficial.
Our results indicated that the mechanisms involved in geniposide-ameliorated intestinal epithelial barrier dysfunction in TNBS-treated rats had the following characteristics. Geniposide reversed the increase in the serum recovery of FD-4, enhanced AMPK phosphorylation, inhibited the increase in protein expression of MLCK, and reversed the decrease in the protein expression of occludin and ZO-1. Consistent with the above results obtained from the in vivo experiments, geniposide also significantly reversed the decrease in TER in LPS-infected Caco-2 cells.
Geniposide-induced modulation of epithelial barrier dysfunction was confirmed by in vitro assays. Geniposide significantly up-regulated the decreased p-AMPK (Figure 7A,7B) and significantly down-regulated the increased MLCK protein expression (Figure 8A,8B) in LPS-infected Caco-2 cells. As AMPK activation has been found to inhibit MLCK44,45, these results suggest that geniposide attenuates LPS-induced intestinal barrier dysfunction by AMPK up-regulation and activation (phosphorylation). It should be noted that following AMPK siRNA transfection, geniposide neither up-regulated AMPK protein expression (Figure 7C) nor down-regulated the increased MLCK protein expression (Figure 8C). Similarly, following cloning of MLCK cDNA, geniposide neither further up-regulated the increased AMPK protein expression (Figure 7D) nor further down-regulated the MLCK protein expression (Figure 8D) in LPS-infected Caco-2 cells, showing that geniposide-induced modulation is characterized by reversing the disrupted epithelial function back to normal.
The present study indicated that geniposide treatment ameliorated TNBS-induced experimental colitis in vivo and attenuated LPS-induced barrier dysfunction in vitro by reducing pro-inflammatory cytokine release and restoring impaired intestinal barrier function. Geniposide down-regulates the protein expression of MLCK by activating AMPK phosphorylation. Our results suggest that geniposide has potential clinical implications for alleviating intestinal inflammatory disorders.
Author contribution
Bin XU, Yan-li LI, Chang-chun YU, and Meng-qiao LIAN performed the experiments; Bin XU, Yan-li LI, Ming XU, Chuan-xun LI, and Ze-yao TANG analyzed the data; Bin XU wrote the paper; Yuan LIN guided the research.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No 30772601).
References
- Yadav V, Varum F, Bravo R, Furrer E, Bojic D, Basit AW. Inflammatory bowel disease: exploring gut pathophysiology for novel therapeutic targets. Transl Res 2016; 176: 38–68. [DOI] [PubMed] [Google Scholar]
- Li C, Xi Y, Li S, Zhao Q, Cheng W, Wang Z, et al. Berberine ameliorates TNBS induced colitis by inhibiting inflammatory responses and Th1/Th17 differentiation. Mol Immunol 2015; 67: 444–54. [DOI] [PubMed] [Google Scholar]
- Murad HA, Abdallah HM, Ali SS. Mentha longifolia protects against acetic-acid induced colitis in rats. J Ethnopharmacol 2016; 190: 354–61. [DOI] [PubMed] [Google Scholar]
- Shi Q, Cao J, Fang L, Zhao H, Liu Z, Ran J, et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kappaB, MAPK and AP-1 signaling pathways in macrophages. Int Immunopharmacol 2014; 20: 298–306. [DOI] [PubMed] [Google Scholar]
- Lv C, Liu X, Liu H, Chen T, Zhang W. Geniposide attenuates mitochondrial dysfunction and memory deficits in APP/PS1 transgenic mice. Curr Alzheimer Res 2014; 11: 580–7. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Lai CC, Lai CH, Sue SC, Lin CW, Hung CH, et al. Inhibition of enterovirus 71 infections and viral IRES activity by Fructus gardeniae and geniposide. Eur J Med Chem 2013; 62: 206–13. [DOI] [PubMed] [Google Scholar]
- Song X, Zhang W, Wang T, Jiang H, Zhang Z, Fu Y, et al. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation 2014; 37: 1588–98. [DOI] [PubMed] [Google Scholar]
- Li R, Cai L, Tang WJ, Lei C, Hu CM, Yu F. Apoptotic effect of geniposide on fibroblast-Like synoviocytes in rats with adjuvant-induced arthritis via inhibiting ERK signal pathway in vitro. Inflammation 2016; 39: 30–8. [DOI] [PubMed] [Google Scholar]
- Yue Y, Wu S, Li Z, Li J, Li X, Xiang J, et al. Wild jujube polysaccharides protect against experimental inflammatory bowel disease by enabling enhanced intestinal barrier function. Food Funct 2015; 6: 2568–77. [DOI] [PubMed] [Google Scholar]
- Yang M, Lin HB, Gong S, Chen PY, Geng LL, Zeng YM, et al. Effect of Astragalus polysaccharides on expression of TNF-alpha, IL-1beta and NFATc4 in a rat model of experimental colitis. Cytokine 2014; 70: 81–6. [DOI] [PubMed] [Google Scholar]
- Zhu T, Liu TJ, Shi YY, Zhao Q. Vitamin D/VDR signaling pathway ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis by inhibiting intestinal epithelial apoptosis. Int J Mol Med 2015; 35: 1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 2013; 145: 407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian MW, Bao S, Knoell DL, Fahy RJ, Shao G, Crouser ED. Intestinal epithelium is more susceptible to cytopathic injury and altered permeability than the lung epithelium in the context of acute sepsis. Int J Exp Pathol 2011; 92: 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KW, Kuo CY. 6-Gingerol modulates proinflammatory responses in dextran sodium sulfate (DSS)-treated Caco-2 cells and experimental colitis in mice through adenosine monophosphate-activated protein kinase (AMPK) activation. Food Funct 2015; 6: 3334–41. [DOI] [PubMed] [Google Scholar]
- Guo L, Zheng X, Liu J, Yin Z. Geniposide suppresses hepatic glucose production via AMPK in HepG2 cells. Biol Pharm Bull 2016; 39: 484–91. [DOI] [PubMed] [Google Scholar]
- Alam R, Schultz CR, Golembieski WA, Poisson LM, Rempel SA. PTEN suppresses SPARC-induced pMAPKAPK2 and inhibits SPARC-induced Ser78 HSP27 phosphorylation in glioma. Neuro Oncol 2013; 15: 451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YS, Li YY, Wang LH, Kang Y, Zhang J, Liu ZQ, et al. Tanshinone IIA attenuates chronic pancreatitis-induced pain in rats via downregulation of HMGB1 and TRL4 expression in the spinal cord. Pain Physician 2015; 18: E615– 28. [PubMed] [Google Scholar]
- Li J, Zhong W, Wang W, Hu S, Yuan J, Zhang B, et al. Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-kappaB activation. PLoS One 2014; 9: e87810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol 2008; 84: 1213–21. [DOI] [PubMed] [Google Scholar]
- Joh EH, Kim DH. Kalopanaxsaponin A ameliorates experimental colitis in mice by inhibiting IRAK-1 activation in the NF-kappaB and MAPK pathways. Br J Pharmacol 2011; 162: 1731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh EH, Lee IA, Jung IH, Kim DH. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation–the key step of inflammation. Biochem Pharmacol 2011; 82: 278–86. [DOI] [PubMed] [Google Scholar]
- Michielan A, D'Inca R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm 2015; 2015: 628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terc J, Hansen A, Alston L, Hirota SA. Pregnane X receptor agonists enhance intestinal epithelial wound healing and repair of the intestinal barrier following the induction of experimental colitis. Eur J Pharm Sci 2014; 55: 12–9. [DOI] [PubMed] [Google Scholar]
- Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan Q, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol 2010; 80: 1708–17. [DOI] [PubMed] [Google Scholar]
- Du J, Chen Y, Shi Y, Liu T, Cao Y, Tang Y, et al. 1,25-Dihydroxyvitamin D protects intestinal epithelial barrier by regulating the myosin light chain kinase signaling pathway. Inflamm Bowel Dis 2015; 21: 2495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Wang J, Chu H, Chen D, Guo H. Salvianolic acid B restored impaired barrier function via downregulation of MLCK by microRNA-1 in rat colitis model. Front Pharmacol 2016; 7: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Sheng B, Zhang Z, Pu A, Yin J, Wang Q, et al. Aryl hydrocarbon receptor activation in intestinal obstruction ameliorates intestinal barrier dysfunction via suppression of MLCK-MLC phosphorylation pathway. Shock 2016; 46: 319–28. [DOI] [PubMed] [Google Scholar]
- Yang T, Wang L, Sun R, Chen H, Zhang H, Yu Y, et al. Hydrogen-rich medium ameliorates lipopolysaccharide-induced barrier dysfunction via rhoa-mdia1 signaling in Caco-2 cells. Shock 2016; 45: 228–37. [DOI] [PubMed] [Google Scholar]
- Meckel K, Li YC, Lim J, Kocherginsky M, Weber C, Almoghrabi A, et al. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am J Clin Nutr 2016; 104: 113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol 2013; 182: 1021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Tao W, Zhang H, Xue W, Tang J, Wu R, et al. Two standardized fractions of Gardenia jasminoides Ellis with rapid antidepressant effects are differentially associated with BDNF up-regulation in the hippocampus. J Ethnopharmacol 2016; 187: 66–73. [DOI] [PubMed] [Google Scholar]
- Song X, Guo M, Wang T, Wang W, Cao Y, Zhang N. Geniposide inhibited lipopolysaccharide-induced apoptosis by modulating TLR4 and apoptosis-related factors in mouse mammary glands. Life Sci 2014; 119: 9–17. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ding Y, Zhong X, Guo Q, Wang H, Gao J, et al. Geniposide acutely stimulates insulin secretion in pancreatic beta-cells by regulating GLP-1 receptor/cAMP signaling and ion channels. Mol Cell Endocrinol 2016; 430: 89–96. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu H, Xu GB, Dai MM, Hu SL, Sun LL, et al. Determination of geniposide in adjuvant arthritis rat plasma by ultra-high performance liquid chromatography tandem mass spectrometry method and its application to oral bioavailability and plasma protein binding ability studies. J Pharm Biomed Anal 2015; 108: 122–8. [DOI] [PubMed] [Google Scholar]
- Zhang WL, Zhu L, Jiang JG. Active ingredients from natural botanicals in the treatment of obesity. Obes Rev 2014; 15: 957–67. [DOI] [PubMed] [Google Scholar]
- Kojima K, Shimada T, Nagareda Y, Watanabe M, Ishizaki J, Sai Y, et al. Preventive effect of geniposide on metabolic disease status in spontaneously obese type 2 diabetic mice and free fatty acid-treated HepG2 cells. Biol Pharm Bull 2011; 34: 1613–8. [DOI] [PubMed] [Google Scholar]
- Daneshmand A, Mohammadi H, Rahimian R, Habibollahi P, Fakhfouri G, Talab SS, et al. Chronic lithium administration ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats; potential role for adenosine triphosphate sensitive potassium channels. J Gastroenterol Hepatol 2011; 26: 1174–81. [DOI] [PubMed] [Google Scholar]
- Quaglio AE, Castilho AC, Di Stasi LC. Experimental evidence of heparanase, Hsp70 and NF-kappaB gene expression on the response of anti-inflammatory drugs in TNBS-induced colonic inflammation. Life Sci 2015; 141: 179–87. [DOI] [PubMed] [Google Scholar]
- Wen J, Teng B, Yang P, Chen X, Li C, Jing Y, et al. The potential mechanism of Bawei Xileisan in the treatment of dextran sulfate sodium-induced ulcerative colitis in mice. J Ethnopharmacol 2016; 188: 31–8. [DOI] [PubMed] [Google Scholar]
- Zwolinska-Wcislo M, Brzozowski T, Ptak-Belowska A, Targosz A, Urbanczyk K, Kwiecien S, et al. Nitric oxide-releasing aspirin but not conventional aspirin improves healing of experimental colitis. World J Gastroenterol 2011; 17: 4076–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, You C, Dong K, Sun J, You H, Dong Y, et al. Ameliorative effects of 3,4-oxo-isopropylidene-shikimic acid on experimental colitis and their mechanisms in rats. Int Immunopharmacol 2013; 15: 524–31. [DOI] [PubMed] [Google Scholar]
- Novak EA, Mollen KP. Mitochondrial dysfunction in inflammatory bowel disease. Front Cell Dev Biol 2015; 3: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Jin X, Chen Y, Song Z, Jiang X, Hu F, et al. Polyphenol-rich propolis extracts strengthen intestinal barrier function by activating AMPK and ERK signaling. Nutrients 2016; 8. pii: E272. doi: 10.3390/nu 8050272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Choi HC. Acetylcholine-induced AMP-activated protein kinase activation attenuates vasoconstriction through an LKB1-dependent mechanism in rat aorta. Vascul Pharmacol 2013; 59: 96–102. [DOI] [PubMed] [Google Scholar]
- Sung JY, Choi HC. Metformin-induced AMP-activated protein kinase activation regulates phenylephrine-mediated contraction of rat aorta. Biochem Biophys Res Commun 2012; 421: 599–604. [DOI] [PubMed] [Google Scholar]