Abstract
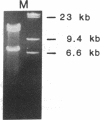
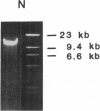
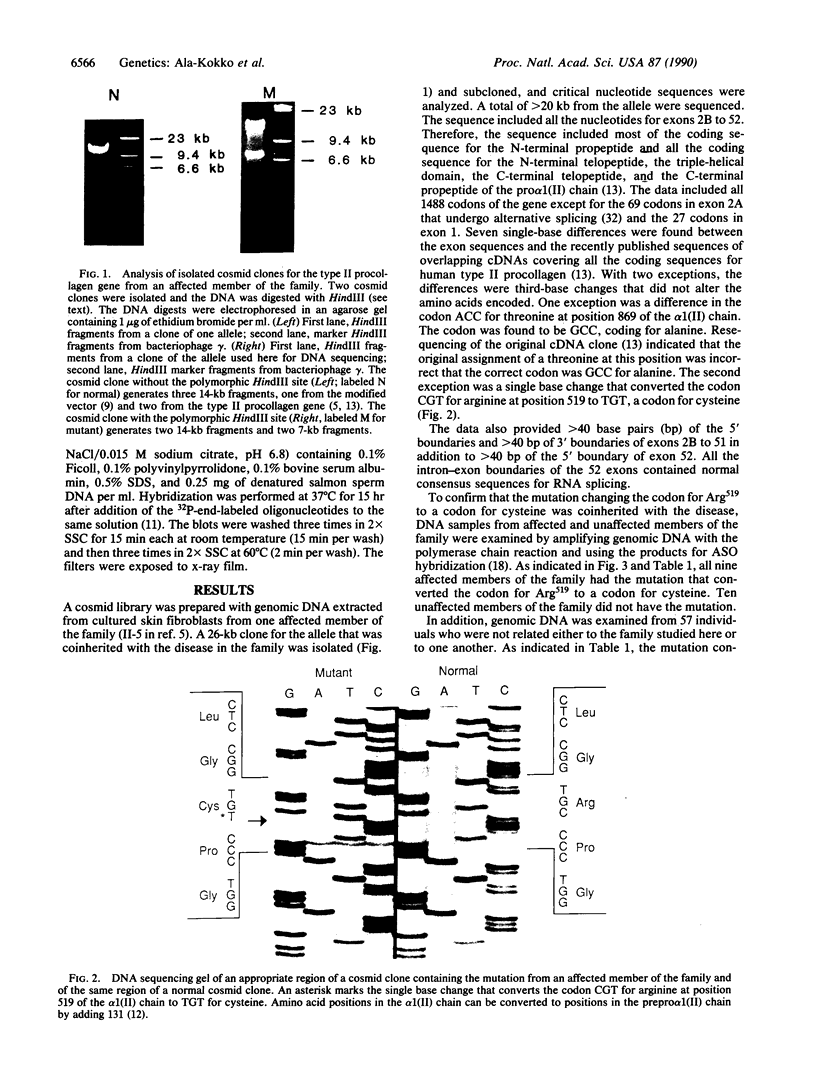
A cosmid clone was isolated that contained an allele for the type II procollagen gene previously shown to be coinherited with primary generalized osteoarthritis in a large family. Affected members of the family had evidence of a mild chondrodysplasia, but they developed progressive osteoarthritic changes in many joints that had no epiphyseal deformities. The clone contained 52 of the 54 exons of the gene. Nucleotide sequencing of greater than 20,000 base pairs from the clone demonstrated that all the coding sequences and all the intron-exon boundaries were normal except for a single base mutation that converted the codon for arginine at position 519 of the alpha 1(II) chain to a codon for cysteine, an amino acid not found in type II collagen from humans or a variety of other species. The mutation was found in all affected members of the family but not in unaffected members or in 57 unrelated individuals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. T., Reginato A. M., Smith C., Jimenez S. A., Prockop D. J. Structure of cDNA clones coding for human type II procollagen. The alpha 1(II) chain is more similar to the alpha 1(I) chain than two other alpha chains of fibrillar collagens. Biochem J. 1989 Sep 1;262(2):521–528. doi: 10.1042/bj2620521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., Bonadio J. F., Cohn D. H., Starman B. J., Wenstrup R. J., Willing M. C. Osteogenesis imperfecta: the molecular basis of clinical heterogeneity. Ann N Y Acad Sci. 1988;543:117–128. doi: 10.1111/j.1749-6632.1988.tb55324.x. [DOI] [PubMed] [Google Scholar]
- Cheah K. S., Stoker N. G., Griffin J. R., Grosveld F. G., Solomon E. Identification and characterization of the human type II collagen gene (COL2A1). Proc Natl Acad Sci U S A. 1985 May;82(9):2555–2559. doi: 10.1073/pnas.82.9.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenberry E. F., Childs B., Sheren S. B., Parry D. A., Craig A. S., Brodsky B. Crystalline fibril structure of type II collagen in lamprey notochord sheath. J Mol Biol. 1984 Jun 25;176(2):261–277. doi: 10.1016/0022-2836(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Furst D. E., Clements P. J., Hillis S., Lachenbruch P. A., Miller B. L., Sterz M. G., Paulus H. E. Immunosuppression with chlorambucil, versus placebo, for scleroderma. Results of a three-year, parallel, randomized, double-blind study. Arthritis Rheum. 1989 May;32(5):584–593. doi: 10.1002/anr.1780320512. [DOI] [PubMed] [Google Scholar]
- KELLGREN J. H., LAWRENCE J. S., BIER F. GENETIC FACTORS IN GENERALIZED OSTEO-ARTHROSIS. Ann Rheum Dis. 1963 Jul;22:237–255. doi: 10.1136/ard.22.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein P. L., Malemud C. J., Pathria M. N., Carter J. R., Sheon R. P., Moskowitz R. W. Early-onset primary osteoarthritis and mild chondrodysplasia. Radiographic and pathologic studies with an analysis of cartilage proteoglycans. Arthritis Rheum. 1990 May;33(5):674–684. doi: 10.1002/art.1780330510. [DOI] [PubMed] [Google Scholar]
- Knowlton R. G., Katzenstein P. L., Moskowitz R. W., Weaver E. J., Malemud C. J., Pathria M. N., Jimenez S. A., Prockop D. J. Genetic linkage of a polymorphism in the type II procollagen gene (COL2A1) to primary osteoarthritis associated with mild chondrodysplasia. N Engl J Med. 1990 Feb 22;322(8):526–530. doi: 10.1056/NEJM199002223220807. [DOI] [PubMed] [Google Scholar]
- Knowlton R. G., Weaver E. J., Struyk A. F., Knobloch W. H., King R. A., Norris K., Shamban A., Uitto J., Jimenez S. A., Prockop D. J. Genetic linkage analysis of hereditary arthro-ophthalmopathy (Stickler syndrome) and the type II procollagen gene. Am J Hum Genet. 1989 Nov;45(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Lee B., Vissing H., Ramirez F., Rogers D., Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989 May 26;244(4907):978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- MORTON N. E. Sequential tests for the detection of linkage. Am J Hum Genet. 1955 Sep;7(3):277–318. [PMC free article] [PubMed] [Google Scholar]
- Palotie A., Väisänen P., Ott J., Ryhänen L., Elima K., Vikkula M., Cheah K., Vuorio E., Peltonen L. Predisposition to familial osteoarthrosis linked to type II collagen gene. Lancet. 1989 Apr 29;1(8644):924–927. doi: 10.1016/s0140-6736(89)92507-5. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Constantinou C. D., Dombrowski K. E., Hojima Y., Kadler K. E., Kuivaniemi H., Tromp G., Vogel B. E. Type I procollagen: the gene-protein system that harbors most of the mutations causing osteogenesis imperfecta and probably more common heritable disorders of connective tissue. Am J Med Genet. 1989 Sep;34(1):60–67. doi: 10.1002/ajmg.1320340112. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Ryan M. C., Sandell L. J. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J Biol Chem. 1990 Jun 25;265(18):10334–10339. [PubMed] [Google Scholar]
- STECHER R. M., HERSH A. H., HAUSER H. Heberden's nodes; the family history and radiographic appearance of a large family. Am J Hum Genet. 1953 Mar;5(1):46–60. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi F. O., Benson-Chanda V., de Wet W. J., Sobel M. E., Tsipouras P., Ramirez F. Isolation and partial characterization of the entire human pro alpha 1(II) collagen gene. Nucleic Acids Res. 1985 Apr 11;13(7):2207–2225. doi: 10.1093/nar/13.7.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann B., Nicholls A., Pope F. M. Clinical variability of osteogenesis imperfecta reflecting molecular heterogeneity: cysteine substitutions in the alpha 1(I) collagen chain producing lethal and mild forms. J Biol Chem. 1986 Jul 5;261(19):8958–8964. [PubMed] [Google Scholar]
- Studencki A. B., Wallace R. B. Allele-specific hybridization using oligonucleotide probes of very high specific activity: discrimination of the human beta A- and beta S-globin genes. DNA. 1984;3(1):7–15. doi: 10.1089/dna.1.1984.3.7. [DOI] [PubMed] [Google Scholar]
- Su M. W., Benson-Chanda V., Vissing H., Ramirez F. Organization of the exons coding for pro alpha 1(II) collagen N-propeptide confirms a distinct evolutionary history of this domain of the fibrillar collagen genes. Genomics. 1989 Apr;4(3):438–441. doi: 10.1016/0888-7543(89)90353-4. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A., Gugler E., Gitzelmann R., Steinmann B. Ehlers-Danlos syndrome type IV: a multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J Biol Chem. 1988 May 5;263(13):6226–6232. [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Shikata H., Prockop D. J. A single base mutation that substitutes serine for glycine 790 of the alpha 1 (III) chain of type III procollagen exposes an arginine and causes Ehlers-Danlos syndrome IV. J Biol Chem. 1989 Jan 25;264(3):1349–1352. [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Stolle C., Pope F. M., Prockop D. J. Single base mutation in the type III procollagen gene that converts the codon for glycine 883 to aspartate in a mild variant of Ehlers-Danlos syndrome IV. J Biol Chem. 1989 Nov 15;264(32):19313–19317. [PubMed] [Google Scholar]
- Vikkula M., Peltonen L. Structural analyses of the polymorphic area in type II collagen gene. FEBS Lett. 1989 Jul 3;250(2):171–174. doi: 10.1016/0014-5793(89)80713-6. [DOI] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]