Abstract
To address the need for efficient and biocompatible delivery systems for systemic siRNA delivery, we developed 1,2-Dioleoyl-sn-Glycero-3-Phosphatidylcholine (DOPC) nanoliposomal EphA2-targeted therapeutic (EPHARNA). Here, we performed safety studies of EPHARNA in murine and primate models. Single dosing of EPHARNA was tested at 5 concentrations in mice (N=15 per group) and groups were sacrificed on days 1, 14, and 28 for evaluation of clinical pathology and organ toxicity. Multiple dosing of EPHARNA was tested in mice and Rhesus macaques twice weekly at two dose levels in each model. Possible effects on hematologic parameters, serum chemistry, coagulation, and organ toxicity were assessed. Following single dose EPHARNA administration to mice, no gross pathological or dose-related microscopic findings were observed in either the acute (24 hrs) or recovery (14 and 28 days) phases. The no-observed-adverse-effect level (NOAEL) for EPHARNA is considered > 225 μg/kg when administered as a single injection intravenously in CD-1 mice. With twice weekly injection, EPHARNA appeared to stimulate a mild to moderate inflammatory response in a dose-related fashion. There appeared to be a mild hemolytic reaction in the female mice. In Rhesus macaques, minimal to moderate infiltration of mononuclear cells was found in some organs including the GI tract, heart, and kidney. No differences attributed to EPHARNA were observed. These results demonstrate that EPHARNA is well tolerated at all doses tested. These data, combined with previously published in vivo validation studies, have led to an ongoing first-in-human Phase I clinical trial (NCT01591356).
Keywords: EphA2, RNAi, toxicology
INTRODUCTION
The use of short interfering RNA (siRNA) as a method of gene silencing has rapidly become a powerful tool in protein function delineation, gene discovery, and drug development (1–3). The promise of specific RNA degradation has also generated much excitement as a possible therapeutic modality, but in vivo siRNA delivery has proven difficult (4, 5). Delivery methods that are effective for other nucleic acids are not necessarily effective for siRNAs in experimental models (6). In vivo gene silencing with siRNA has been reported using both viral vector delivery and high-pressure, high-volume intravenous injection of synthetic siRNAs, but these approaches have limited, if any, clinical use (7–10). SiRNA delivery has been effective by injections directly into the tumor, or into the thecal, vitreal, nasal, or joint cavities (11–14). The only study to show in vivo uptake and associated target downregulation after normal systemic dosing required chemical modulation of the siRNA that may have unknown toxicities, and may affect siRNA activity and longevity (15). Recently, an angiogenesis targeted liposomal siRNA, Atu027, was reported to be tolerated in humans in a Phase I clinical trial (16).
Several liposomal constructs have been developed for systemic delivery of siRNA (17). Charged nanoliposomes can result in significant toxicity, therefore, neutral liposomes are desired (18–20).1,2-Dioleoyl-sn-Glycero-3-Phosphatidylcholine (DOPC) is one of the few lipid constructs developed for nucleic acid delivery to cells that is neutral. It has been found to deliver inhibitory nucleic acids with ten-fold greater efficiency compared to cationic liposomes (15), and is therefore an attractive construct for further investigation.
EphA2 is a member of the largest subfamily of receptor tyrosine kinases, with 14 receptors and 8 ligands currently known. This receptor was initially studied in the nervous system, where it plays important roles in neuronal development and in embryogenesis (21–23). In adult animals, EphA2 is expressed primarily in epithelial cells. Although its primary functions are not completely understood, tumor-based models suggest roles in proliferation, survival, migration, invasion, and angiogenesis (24, 25). EphA2 is overexpressed in human cancers including breast, prostate, lung, ovarian, endometrial, and pancreatic, and is associated with adverse outcomes (26–32). We previously described the development of liposome-incorporated EphA2 siRNA (EPHARNA) and demonstrated its efficacy in orthotopic in-vivo models (15). Based on these data, EPHARNA represents a novel method for therapeutically delivering siRNA and is a potential therapeutic for a range of human cancers. Here, we report preclinical toxicological assessments of EPHARNA in two mammalian species, mouse and Rhesus monkeys.
MATERIALS AND METHODS
EPHARNA Production and Handling
The siRNA was incorporated into a specific liposome, 18:1 PC (cis) 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) to enhance intracellular delivery. DOPC (Avanti Polar Lipids, Inc., Alabaster, AL) was dissolved in tertiary butanol, and the lyophilized siRNA was added to the DOPC solution. A trace amount of the detergent, Tween-20, was added to enhance the incorporation of siRNA into liposomes. Excess tertiary butanol (< 10 ml) was added to the mixture. The mixture was frozen in a dry ice/acetone bath and lyophilized overnight. SiRNA was supplied in sterile vials as a white powder by the manufacturer (Alnylam, Cambridge MA) and stored at −15 to −25°C until the time of drug formulation. Following reconstitution of the lyophilized powder, the test article was stored at room temperature and used within 6 hours.
Single dose toxicology in mice
Following an IACUC approved protocol, ninety (90) mice of each sex (15/sex/dose group) were assigned to five dose groups and a vehicle control group and administered EPHARNA intravenously via the tail vein at doses of 0 (empty liposomes), 75, 112.5, 150, 187.5, and 225 μg/kg as a single dose on Study Day 0. Groups of animals were sacrificed on Study Days 1 (24 hrs), 14 and 28 for evaluation of clinical pathology and organ toxicity. One female in the lowest dose group died before drug administration and was excluded from the analysis. Blood was collected from 5 mice/sex/group at days 1, 14, 28, and prior to sacrifice of any animal in a moribund condition. Five mice of each gender from each group were necropsied on days 1, 14, and 28. Tissues were collected from mice which were found dead or accidentally killed (tissue integrity permitting), sacrificed in extremis or sacrificed by design, and fixed in 10% neutral buffered formalin.
Multiple dose toxicology in mice
Mice were dosed with 0.9% USP NaCl, or EPHARNA at theoretical dose concentrations of 0.08 and 0.16 mg/ml at a volume based on the animal’s body weight and rounded to the nearest 0.01 ml. Ten animals, five of each gender, were included in each group in accordance with GLP and IACUC regulations for toxicology studies and consistent with previously published toxicology reports (33, 34). Dose materials were administered twice weekly for 4 consecutive weeks by intravenous (IV) injection into the tail vein using a 0.5 ml syringe with attached 27 gauge, ½ inch needle. Body weights of all animals were recorded on Days 1, 3, 8, 10, 15, 17, 22, 24, and 25. All animals were observed twice daily for moribund state and mortality. On each dose administration day (Study Days 1, 3, 8, 10, 15, 17, 22, and 24), all mice were observed for clinical signs of toxicity (cage side observations) at one hour post-dose administration. Mice were bled by cardiac puncture for Clinical Pathology on Day 25 and sacrificed for necropsy and organ histopathological staining on day 25, with the exception of one group 1 male that was sacrificed at day 22 due to paralysis.
Mouse Clinical Pathology
Serum samples collected in serum microtainer tubes for clinical chemistry analysis were prepared from each animal sacrificed. Whole blood samples were collected into K2-EDTA-treated tubes and prepared from each animal sacrificed. Erythrocyte count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelet count, total leukocyte count, differential leukocyte count, nucleated red blood cell count, blood urea nitrogen, creatinine, serum aspartate aminotransferase, serum alanine aminotransferase, lactic dehydrogenase, alkaline phosphatase, total bilirubin, total protein, albumin, and triglycerides.
Mouse Sacrifice and Necropsy
An extensive necropsy was performed on all study animals. All mice were anesthetized by CO2 asphyxiation, weighed, and bled by cardiac puncture. Necropsies were performed under the direction and supervision of an anatomic pathologist. Tissues/organs were collected and fixed in 10% neutral buffered formalin (10% NBF). The lungs were inflated with fixative prior to immersion in fixative. The eyes, pituitary, Harderian glands, and testes were fixed in Davidson’s solution. All gross lesions observed at necropsy were also collected and preserved in 10% NBF. The following organs were harvested: Liver, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, submandibular salivary gland, pancreas, gallbladder, rectum, kidneys, bladder, lungs, trachea, heart, aorta, thyroid, parathyroid glands, adrenal glands, pituitary gland, spleen, thymus, sub-iliac lymph nodes, mesenteric lymph node, bone marrow (femur), brain (including cerebellum and medulla/pons), spinal cord, sciatic nerve, tongue, thigh muscle, distal femur and proximal tibia, epididymis, testes, seminal vesicles, ovaries, vagina, mammary glands, skin (right flank), eyes, and Harderian gland.
Organ weights were collected on all animals at the time of necropsy. The brain, heart, liver, kidneys, spleen, testes, and thymus were weighed. Extraneous tissues and fat were carefully removed from all organs prior to weighing.
Mouse Histopathology
All gross lesions and tissues were collected at the time of necropsy and embedded in paraffin, sectioned to 3–5 microns and fixed on slides. All sections were stained with hematoxylin and eosin (H&E). Designated tissues, as referenced in the necropsy table list above, from all animals were evaluated microscopically and recorded into the Pathology Module of ProvantisTM, versions 8.1.0.1 and 9.2.5. Remaining fixed tissues will be held until data are analyzed and final disposition is retained in the archives at the Keeling Center for Comparative Medicine and Research (KCCMR) in accordance with 21 CFR § 58.195.
In situ hybridization
The formalin-fixed paraffin embedded tissue sections were dewaxed in xylenes, and rehydrated through an ethanol dilution series. Tissue sections were then loaded onto Ventana Discovery Ultra for in situ hybridization analysis. The tissue slides were hybridized with the double-DIG labeled mercury LNA microRNA probe (Exiqon) for 2 hrs (sequence TGACATGCCGATCTACAT for siRNA targeted probe). The digoxigenins can then be detected with a polyclonal anti-DIG antibody and Alkaline Phosphatase conjugated second antibody (Ventana) using NBT-BCIP as the substrate.
Toxicology in Macaca mulatta
Three-year-old rhesus macaques (Macaca mulatta) were randomized by weight into a control group (0 mg/kg, n=2), low dose group (0.5 mg/kg, n=4) and high dose group (0.75mg/kg, n=4). Each group contained an equal number of males and females. The control DOPC was dosed at 0.75 mg/m2. The test and control articles were administered by intravenous infusion through a cephalic vein. They were reconstituted in USP 0.9% normal saline for injection and administered over 30–60 seconds through a 22 gauge indwelling catheter. Doses were given weekly for four weeks. One animal developed signs consistent with an infusion reaction, so this animal was dosed with a slow intravenous infusion over 15–30 minutes beginning on day 8 and continuing with each infusion throughout the remainder of the dosing period.
A physical examination, bone marrow aspiration and clinical pathologic evaluation (complete blood count, serum chemistry, coagulation, and urinalysis) were performed on each animal four days prior to dosing. Physical examination and clinical pathologic evaluation were repeated immediately prior to each dose administration. Animals were observed continuously for at least one hour following injection.
Primate Care and Housing
The care and use of laboratory animals used in this study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (ILAR/NRC, 1996), the U. S. Department of Agriculture through the Animal Welfare Act (7 USC 2131), 1985, and the Animal Welfare Standards incorporated in 9 CFR Part 3, 1991. Monkeys were single housed in Harford 4.3 squeeze back stainless steel cages. Daily disinfection of these cages was accomplished utilizing water under pressure and a phenolic compound (Wexcide 128). Every two weeks the monkeys were removed from their cages, and the cages were sanitized utilizing water under pressure and a bleach phenolic mixture. The test system was maintained in an environmentally controlled space. Temperature and humidity readings in the animal room(s) were recorded by the Johnson Controls, Inc. Metasys System for Extended Architecture (MSEA) v2.2, and reviewed and documented daily by the MSEA Data Reviewers. Each animal received daily enrichment to include treats and manipulata. Administration of the enrichment procedures and acceptance by each animal was documented and maintained with the study records.
Primate Diet
Animals were fed Hi Fiber Primate Purina diet in an amount equal to 4–6% of their body weight divided into two daily doses. In addition, they were fed either a fresh fruit or vegetable early in the afternoon. Specialty foods, such as seeds, peanuts, raisins, yogurt, cereals, frozen juice cups and peanut butter, were distributed as enrichment daily. Food consumption was measured qualitatively at the time clinical observations were noted. Water potable for human consumption was provided ad libitum through lix-it devices. Based on yearly inspection and evaluation, no contaminations were identified that could have affected the results of the study.
Primate Feed Consumption
Qualitative feed consumption was monitored twice daily by the Animal Technician or the Clinical Veterinarian. Consumption was reported as Good (ate 1/2 to all of the biscuits), Fair (ate 1/4 to ½ of the biscuits), or Poor (ate less than 1/4 of the biscuits).
Primate Clinical Pathology
Blood was collected from the femoral vein and urine was collected as a voided sample or by cystocentesis under sedation for clinical pathology and urine analysis on Days −4 (for baseline comparison), 1 (prior to treatment), 8, 15, 22, 31, and prior to necropsy. The animals were fasted overnight prior to blood collection. Anesthesia was required for obtaining all clinical pathology specimens
Hematology Parameters
Approximately 2–5 mls of blood was collected into an EDTA (lavender top) tube by venipuncture using the femoral vein. The samples were transferred to the KCCMR Clinical Pathology Laboratory for processing and kept at ambient temperature until analyzed. A complete blood count was performed on a Cell-Dyn® 3500R (Abbott Diagnostics) and included the following: Erythrocyte count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelet, total leukocyte, and nucleated red blood cell counts.
Serum Chemistry Parameters
Approximately 3–5 mls of whole blood was collected into a serum (red top) tube by venipuncture using the femoral vein. The samples were transferred to the KCCMR Clinical Pathology Laboratory for processing. A serum chemistry panel was performed on an Olympus AU400e® Chemistry Immuno Analyzer. The panel included serum aspartate aminotransferase, serum alanine aminotransferase, gamma-glutamyl transpeptidases, alkaline phosphatase, lactate dehydrogenase, creatine Kinase, total bilirubin, total protein, albumin, globulin, blood urea nitrogen, creatinine, glucose, calcium, phosphorus, sodium, potassium, chloride, CO2, iron, TIBC, iron saturation
Coagulation Parameters
Approximately 1.8 mls of blood was collected into a 3.2% sodium citrate (blue top) tube by venipuncture from the femoral vein. Samples were processed within 2 hours and analyzed immediately or frozen at −70°C for batch analysis. All samples were performed on the ACL 7000 Coagulation Analyzer (Beckman Coulter). Activated partial thromboplastin time, fibrinogen, and prothrombin time were tested.
Urine Collection and Urinalysis
One to 6 mls (typically 2–3 ml) of urine were collected as a voided sample or by cystocentesis under sedation. A large majority of the samples were collected as voided samples. Urinalysis included specific gravity, microscopic sediment analysis, and urine dipstick (glucose, ketones, bilirubin, pH, blood, protein, urobilinogen, nitrite and leukocytes).
Bone Marrow Aspiration and Evaluation
A bone marrow aspirate was obtained from the proximal aspect of the femur on each animal once during the pre-dose phase (Days −4) and immediately prior to euthanasia at the end of the recovery period. Preparation and staining of the bone marrow aspirates as well as cytologic evaluation of were performed by a veterinary clinical pathologist.
Necropsy
An extensive necropsy was performed on all monkeys. On Days 44– 45 (males) and 43–44 (females) all animals were sacrificed by a barbiturate overdose and weighed to the nearest tenth of a kilogram. Necropsies were performed under the direction and supervision of an anatomic pathologist. The necropsy included a thorough and systematic examination and dissection of the animal viscera and carcass.
Tissue Collection and Preservation
Tissues were collected from all monkeys. Organs were carefully dissected and trimmed to remove fat and other contiguous tissue and then weighed to the nearest gram or tenth of a gram immediately to minimize the effects of drying on organ weight. All tissues were routinely fixed in 10% neutral buffered formalin with the exception of the eyes, which were fixed with Davidson’s solution. The lungs were inflated with 10% neutral buffered formalin prior to immersion in fixative. Tissues collected included adrenal glands, aorta, mammary gland (when present in regular abdominal skin section), bone, femoral head with articular surface, pancreas, brain, cecum, parathyroid gland (when present in regular thyroid gland section), colon, pituitary gland, duodenum, prostate gland, epididymides, salivary gland, esophagus, eyes, sciatic nerve, skeletal muscle, gall bladder, skin (ventral abdomen and injection sites, gonads, spinal cord, gross lesions, spleen, heart, stomach (cardiac, fundic, and pyloric), Ileum, thymus, jejunum, thyroid glands (with parathyroid glands), kidneys, tongue, lips, tonsils, liver, trachea, lungs, urinary bladder, lymph nodes (bronchial, mandibular, mesenteric, injection site), uterus (in females).
Histopathology
After immersion fixation, tissues were routinely processed, embedded in paraffin, sectioned at 3–5 um, and placed on glass slides. All tissue sections were stained with hematoxylin and eosin (HE). In addition, some selections were stained with a Prussian blue stain for iron, a Steiner stain for certain microorganisms (e.g. Helicobacter pylori, Legionella pneumophila, spirochetes and some fungi), and a Von Koss stain for calcium deposits. Slides were evaluated by light microscopy for the presence or absence of lesions. Lesions were scored based on a severity scale of minimal, mild, moderate, and marked; the scale correlated with estimates of lesion distribution and extent of tissue involvement (minimal = 2– 10%; mild = >10–20%, moderate = >20–50%, marked = >50%).
Sample Size Determinations
The mouse studies were performed after seeking guidance from the FDA and were conducted under current FDA guidelines. There were ten mice per group, five of each gender to ensure a balance between suitable numbers of animals per sex per group. This sample size is consistent with similarly published toxicology studies (2, 3) and has been standard for our group and has previously passed FDA scrutiny. The sample size was chosen after both consulting with the FDA and the IACUC, with a goal of balancing the statistical justification for the study as well as to ensure the smallest possible number of animals for this bridge study, which was required before testing EPHARNA in human subjects.
RESULTS AND DISCUSSION
EPHARNA can be given safely as single dose in mice
Following an initial guidance call with the FDA, we first performed toxicological studies of EPHARNA in mice. There is approximately 65% homology between mouse and human EphA2. Therefore, the mouse experiments were formulation-based studies to assess possible toxicity with DOPC-siRNA and not target specific to assess for systemic effects from EphA2 inhibition.
A single dose of EPHARNA was given to mice and they were assessed for toxicity at 24 hours and 28 days (Figure 1). No test article-related morbidity or mortality was observed in the study. No test article-related adverse clinical signs were observed. Group average body weight gain of treated animals was similar to control in all groups over the course of the study. In female animals, no reduction in food consumption was observed compared to control. In male animals, by study day 21 and 28 during the recovery period, total average food consumption was reduced to 83 and 85% of control, but no clear dose-related trend in severity was observed.
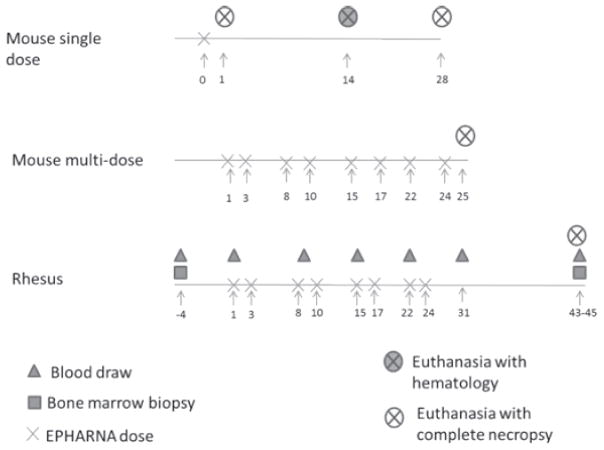
Figure 1. Schematic of Dosing Experiments.
Single dose effects were assessed by administering a dose of EPHARNA and groups of mice underwent necropsy at days +1, +14, or day +28 from EPHARNA dosing. To assess continuous dosing of EPHARNA, groups of mice were given EPHARNA twice weekly and underwent necropsy after 4 weeks of treatment. Non-human primates were given EPHARNA twice weekly for 9 administrations and underwent necropsy 43–45 days after the initial dosing.
No toxicologically-important test article-related alterations in group average hematology or clinical chemistry parameters were observed. Also, no gross pathological or dose-related microscopic findings were observed in either the acute (24 hours) or recovery (28 days) phases of this study. Slight increases in relative spleen weights were observed at 24 hrs post-dose in female treated groups compared to control. The increases were not dose-related, and lacked morphologic correlates, and therefore, were considered not toxicologically important. Under the conditions of this study, the no-observed-adverse-effect level (NOAEL) for EPHARNA is considered > 225 μg/kg when administered as a single injection intravenously in CD-1 male and female mice.
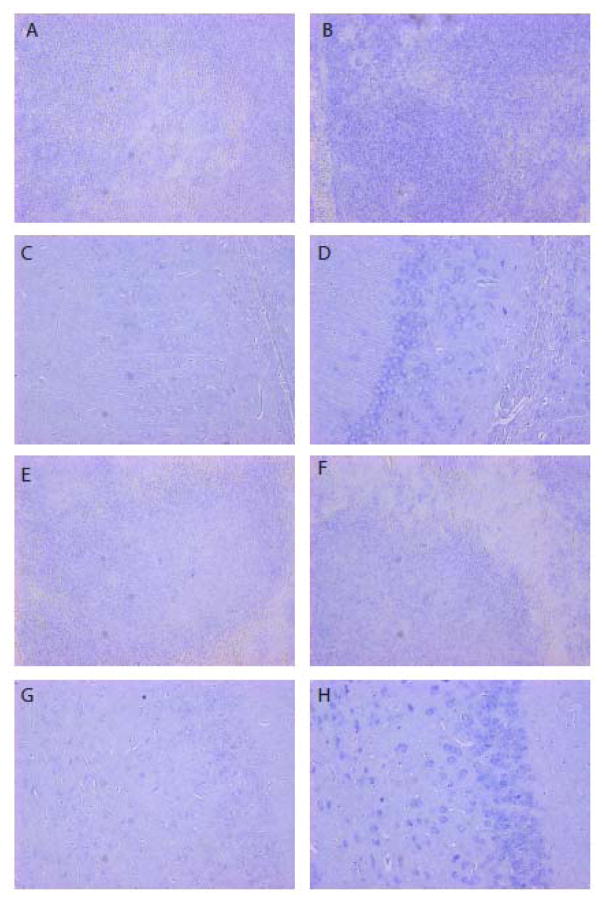
In situ staining for the EphA2 targeted siRNA on FFPE from mice taken 24 hours after EPHARNA infusion revealed delivery of EPHARNA to the spleen. Low level of EphA2 siRNA was detected in the brains of the mice treated with the highest test particle dose. However, some of this staining was felt to be non-specific as there was also a low level of positive staining in the control DOPC treated mouse brain (Figure 2).
Figure 2. In situ hybridization for anti-EphA2 siRNA in mouse spleen and brain.
(A) Spleen, EPHARNA treated mouse, control probe; (B) Spleen, EPHARNA treated mouse, anti-siRNA probe; (C) CNS, EPHARNA treated mouse, control probe; (D) CNS, EPHARNA treated mouse, anti-siRNA probe; (E) Spleen, Control DOPC treated mouse, control probe (F) Spleen, control DOPC treated mouse, anti-siRNA probe; (G) CNS, control DOPC treated mouse, control probe; (H) CNS, control DOPC treated mouse, anti-siRNA probe
EPHARNA can be safely administered to mice with twice weekly dosing
General tolerance
All mice (5 mice/sex/group), with the exception of one control group male that was euthanized due to paralysis on day 22, were sacrificed on day 25 of study. The test article appeared to have no effect on clinical presentation at any time during the study. No significant differences in absolute body weights were noted across the study days for either male or female animals.
Hematologic Effects
At the doses and frequency utilized here (0, 0.3, and 0.6 mg/kg), EPHARNA appears to stimulate a mild to moderate inflammatory response in a dose-related fashion in subjects of both sexes (Table 1). This was characterized primarily by a significant continual increase in monocyte numbers between the treatment groups. Lymphocytes were also similarly significantly elevated in a dose-related fashion in males, and while there was a similar trend in females, these changes did not reach statistical significance. Additionally, a trend toward elevated eosinophil numbers in male subjects and elevated neutrophil numbers in female subjects was also identified to occur in a dose-related manner though neither change was statistically significant.
Table 1.
Hematologic parameters after twice weekly EPHARNA administration in mice. EPHARNA appeared to cause a mild, dose dependent inflammatory response.
| EPHARNA dose group | ||||||
|---|---|---|---|---|---|---|
| 0 mg/kg | 0.3 mg/kg | 0.6 mg/kg | ||||
| Male (n=4) | Female (n=5) | Male (n=5) | Female (n=5) | Male (n=4) | Female (n=5) | |
| WBC (SD) | 5.720 (1.220) | 7.382 (1.759) | 5.840 (1.208) | 8.332 (1.018) | 8.734 (1.656) | 8.636 (2.840) |
| Absolute Neutrophil | 0.888 (0.253) | 0.908 (0.205) | 0.854 (0.148) | 1.170 (0.290) | 1.076 (0.212) | 1.200 (0.401) |
| Absolute Monocyte | 0.175 (0.083) | 0.282 (0.217) | 0.290 (0.135) | 0.774 (0.240) | 0.524 (0.271) | 0.840 (0.215) |
| Absolute Lymphocyte | 4.535 (1.274) | 6.036 (1.906) | 4.552 (1.041) | 6.092 (0.768) | 6.958 (1.500) | 6.302 (2.558) |
| Absolute Eosinophil | 0.116 (0.111) | 0.154 (0.127) | 0.146 (0.036) | 0.296 (0.231) | 0.178 (0.269) | 0.278 (0.216) |
| RBC | 9.67 (0.261) | 9.35 (0.664) | 9.52 (0.289) | 9.19 (0.493) | 9.70 (0.440) | 9.12 (0.210) |
| Hgb | 15.1 (0.46) | 14.9 (1.03) | 15.44 (0.61) | 14.76 (0.85) | 15.30 (0.58) | 14.30 (0.71) |
| HCT | 52.0 (1.30) | 50.2 (2.96) | 52.1 (1.15) | 49.4 (2.70) | 51.1 (1.70) | 46.92 (1.94) |
| MFCV | 53.85 (0.97) | 53.74 (0.94) | 54.70 (1.15) | 53.78 (1.09) | 52.72 (0.55) | 51.46 (0.40) |
| MCHC | 15.63 (0.26) | 15.98 (0.49) | 16.18 (0.33) | 16.08 (0.45) | 15.76 (0.55) | 15.66 (0.58) |
As albumin is a negative acute phase protein, this analyte often decreases in the serum as part of the inflammatory response. Consistent with an inflammatory dose-related response to EPHARNA, a trend toward decreased albumin/globulin ratios in a dose-related fashion was noted in both sexes and a similar dose-related decrease was also identified in albumin for the female subjects. While male subjects did not demonstrate a classical dose-related pattern of distribution in their group means for albumin, the mean albumin value of the control group was significantly higher than the mean values of the treatment groups.
In both male and female subjects, there was a significant dose-related increase in mean corpuscular hemoglobin concentration (MCHC) identified between control and 0.6 mg/kg that suggest the test article may induce hemolysis in a dose-responsive manner. The degree of hemolysis observed in these serum samples continually increased between groups. For the male subjects, two control males had mild (1+) hemolysis, two 0.3 mg/kg males had mild-moderate (2+) hemolysis, and one 0.6 mg/kg male had 2+ and a second had marked (4+) hemolysis. In the female subjects, no control animals were noted to have hemolysis while one in each treatment group had 1+. With regard to other erythrocyte parameters, the mean corpuscular volume (MCV) for the 0.6 mg/kg group was significantly decreased below the 0.3 mg/kg group MCV values in male subjects and the mean MCV value for the 0.6 mg/kg group was significantly decreased below both control and 0.3 mg/kg group MCV values in female subjects. Finally, in the female subjects alone, the red blood cell (RBC), hematocrit (HCT) and hemoglobin (HGB) all demonstrated a dose-related decrease between the control and 0.6 mg/kg groups. Notably, however, in these animals the patterns of change to RBC, HCT and HGB were not statistically significant and each analyte remained within the calculated reference intervals used for this study. Collectively, these data obtained from female subjects, in particular, could suggest that the test article is associated with a mild, non-regenerative loss of erythrocytes that is occurring through hemolysis. These changes may be clinically insignificant as even in the 0.6 mg/kg group female subjects the RBC, HCT and HGB values remained within the reference intervals. One possible mechanism for the observed hemolysis is that EPHARNA may stimulate a mild local inflammatory reaction (see below), which can be associated with anemia (35).
In male subjects, there was a significant dose-related increase in creatine kinase (CK) between control and treatment groups that suggest the test article may induce a mild dose-related pathologic change within the musculature of these animals. Supportive of this suggestion is the observation that the mean value of alanine aminotransferase (ALT) in the high dose male subjects was significantly elevated over those of control and 0.3 mg/kg and also the observation that aspartate aminotransferase (AST) in male subjects exhibited a dose-effect response-like pattern of distribution (Table 2). However, in light of the facts that ALT does not exhibit a classical dose-effect response-like pattern of distribution and the AST pattern of distribution is not statistically significant, it is also possible that this finding may be spurious. No similar trends in CK, ALT or AST were identified in the female subjects of this study. In the female subjects, there was a significant continual increase in mean glucose values between groups. This was not seen in males.
Table 2.
EPHARNA had no significant liver toxicity when administered twice weekly in mice.
| EPHARNA dose group | ||||||
|---|---|---|---|---|---|---|
| 0 mg/kg | 0.3 mg/kg | 0.6 mg/kg | ||||
| Male (n=4) | Female (n=5) | Male (n=5) | Female (n=5) | Male (n=4) | Female (n=5) | |
| AST (SD) | 43.8 (4.6) | 66.8 (21.7) | 61.2 (29.2) | 60.8 (29.2) | 71.8 (20.1) | 57.2 (20.7) |
| ALT | 33.5 (2.9) | 27.6 (7.1) | 29.2 (4.0) | 30.2 (12.9) | 20.1 (7.2) | 27.2 (6.5) |
| Total Bilirubin | 0.258 (0.021) | 0.189 (0.029) | 0.270 (0.070) | 0.218 (0.042) | 0.258 (0.089) | 0.224 (0.071) |
| Albumin | 3.28 (0.1) | 33.22 (0.08) | 3.06 (0.05) | 3.18 (0.18) | 3.08 (0.05) | 3.06 (0.21) |
| Globulin | 2.68 (0.19) | 2.10 (0.19) | 2.64 (0.21) | 2.20 (0.19) | 2.75 (0.17) | 2.24 (0.11) |
Anatomic Pathology
Anatomic pathology evaluation identified test article related lesions at the injection site, draining lymph node for the injection site, and spleen. Injection site lesions occurred in both 0.3 and 0.6 mg/kg group mice and included inflammation and associated lesions that were generally minimal to moderate in severity with moderate lesions more common in 0.6 mg/kg mice. The draining lymph nodes of two 0.6 mg/kg mice had neutrophilic infiltrates or extramedullary hematopoiesis (EMH). Dose dependent, minimal to mild EMH was identified in the spleen of several 0.3 and 0.6 mg/kg group mice. This is consistent with a possible mild inflammatory reaction related to the infusion. A possible test article related lesion (EMH) was present in the liver and lung of 0.3 and 0.6 mg/kg group mice. No changes were seen in brain in any of the treatment groups. One mouse each in the 0.3 and 0.6 mg/kg dose groups had mild neutrophilic and moderate lymphohistiocytic inflammation in the esophagus, which was not felt to be due to the test particle after review by the pathologist. No other adverse effects could be attributed to the administration of the test article.
Statistical analysis of organ weights was performed relative to terminal body weight. In male mice, the liver weight and ratio of liver to body weight in the 0.3 mg/kg group were significantly smaller than those in the 0.6 mg/kg group, however, the changes were not linear with regard to dose and there was no corresponding histologic lesion. The spleen weight and ratio of spleen to body weight for control mice were significantly lower than EPHARNA treated mice. The latter changes were linear and are considered test article related. The histologic finding of increased extramedullary hematopoiesis in some EPHARNA treated mice may partially explain the differences in spleen measurements between groups. In female mice, the kidney weight and ratio of kidney to body weight for the 0.3 mg/kg group was significantly lower than the 0.6 mg/kg group; however, the changes were not linear with regard to dose and there was no corresponding histologic lesion. The spleen weight and ratio of spleen to body weight for control mice were significantly lower than those for 0.6 mg/kg mice. Spleen measurements for 0.3 mg/kg mice were intermediate, but not statistically different from either control or 0.6 mg/kg mice. The latter changes are considered EPHARNA related. Again, the histologic finding of increased EMH in all 0.6 mg/kg treated mice may partially explain the differences in spleen measurements.
Multi-dosing of EPHARNA is well tolerated in non-human primates
Rhesus monkeys were chosen as a second model due to the near 100% homology between Rhesus and human EphA2. This model allowed us to evaluate for systemic toxicity related to both the DOPC formulation as well as EphA2 silencing.
General Tolerance
In general, all animals tolerated EPHARNA administration well and no abnormal observations were noted as the result of a test article effect. One low dose group (0.5 mg/kg) female was diagnosed with vaginitis and treated by the clinical veterinarian for three days. This was not considered a result of EPHARNA treatment. One control group female had a mild to moderate reaction that was noted approximately one hour following the initial drug administration. The reaction consisted of cutaneous swelling of the face and neck, erythema around the lips and chin, hypersalivation, and rubbing of the face. These clinical signs generally returned to normal within three hours of dose administration. To lessen the reaction, the animal was premedicated with diphenhydramine, dexamethasone and atropine prior to each subsequent injection. During scheduled anesthesia for blood collection (without dose administration) near the end of the study, another animal experienced the same constellation of clinical signs. Thus, it was concluded that the drug reaction occurred as a result of ketamine anesthesia and was not related to administration of EPHARNA. Body weights were stable for both males and females. Overall food consumption was acceptable for singly-housed rhesus macaques throughout the duration of the study.
Hematologic Effects
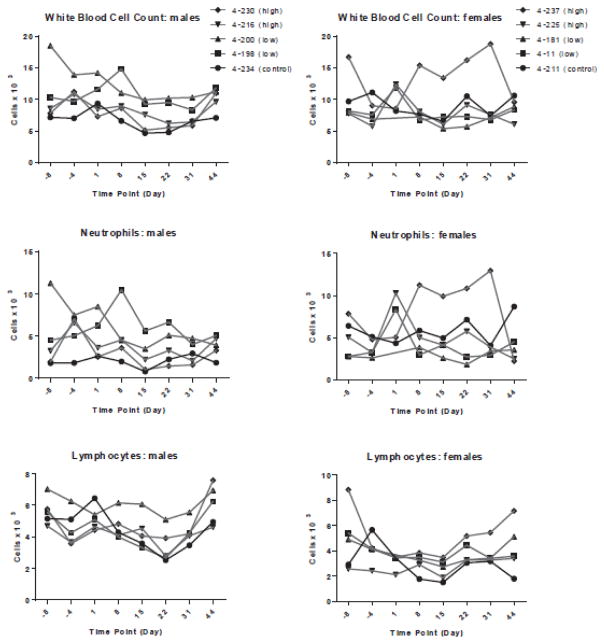
One female in the high dose group (0.75 mg/kg) had a borderline to mild leukocytosis characterized by a neutrophilia during the dosing interval. The mild serological inflammatory response seen in mice was not seen in macaques (Figure 3). This was likely related to a stress or physiologic response to anesthesia and frequent manipulation. There was an initial mild drop in the RBC count, hemoglobin and hematocrit in all animals immediately prior to administration of the first dose likely reflecting the frequent bleeding and bone marrow aspiration prior to dosing (Supp. Figure 1s).
Figure 3.
Immunologic parameters in non-human primates did not appear affected by EPHARNA administration.
Sporadic increases in creatine kinase (CK) and lactate dehydrogenase (LDH) were attributed to ketamine anesthesia (Supp. Figure 2s). Infrequent elevations in potassium, phosphorus and total bilirubin were attributed to sample handling (Supp. Figure 3s). Slight to mild variations in BUN, total protein, albumin, globulin, total CO2, iron and iron saturation occurred occasionally and were considered incidental (Supp. Figure 4s). There were no effects attributed to the test article in urinalysis (Supp. Table 1s) or coagulation parameters (Supp. Figure 5s).
Anatomic Pathology
All monkeys received a complete necropsy. No gross lesions attributable to EPHARNA were noted. No alterations in organ weights were attributed to the test article. Minimal to moderate infiltrates of mononuclear cells were found in multiple organs in all 10 animals. The organs that mostly commonly contained these infiltrates included the gall bladder, liver, heart, kidney, thyroid, esophagus, stomach, duodenum, ileum, cecum, colon, tongue and injection sites. The severity of the infiltrates varied between individuals and anatomical locations. Acute inflammation of the tonsillary mucosa was observed in all animals with the exception of one animal from the high-dose group. Although these infiltrates are not related to EPHARNA, the significance of the infiltrates to the general health is not known. Eight sections of heart muscle per animal were examined. Nine of 10 animals (including one control) were confirmed to have minimal myocardial infiltrations of mononuclear cells. This lesion is commonly described for the rhesus macaque. It was considered incidental and not related to EPHARNA.
One female control animal and one female in the high dose group had ovarian mineralization. The lesions in the treated female were moderate compared to minimal for the control female. Mineralization of the ovary is a common finding in the rhesus macaque, and was considered most likely an incidental lesion in this study. However, given the low number of animals in each group, the possibility that the test article increased the severity of the mineralization in the treated female cannot be entirely excluded.
Although significant lesions were identified in the gastrointestinal tract and the heart, and to a lesser extent the ovaries, they were not considered to be related to the test article. A minimal multifocal infiltration of mononuclear cells was present in the submucosa of the esophagus of two monkeys dosed at 0.5 mg/kg, which was not attributed to the test particle and was not seen in any of the monkeys dosed at 0.75 mg/kg. No changes were seen in brain or salivary gland in any of the treatment groups. Lesions in other tissues were considered incidental and/or of equivocal significance. No significant bone marrow alterations were attributed to EPHARNA. There was an overall increased percentage of lymphocytes that is likely normal for this colony of SPF macaques. A mild increase in the degree of erythroid immaturity in the control female and one female in the high dose group was attributed to erythroid stimulation following blood loss associated with the menstrual cycle.
CONCLUSIONS
These results demonstrate that all animals tolerated EPHARNA at all doses tested without significant complications. Body weights showed minimal to no variation throughout the course of the study. Clinical pathologic analysis, gross examination, and histopathologic evaluation did not reveal findings that could be specifically attributed to EPHARNA. There was a suggestion of a dose related inflammatory response in mice, but this was not replicated in Rhesus macaques. Immune infiltration was seen on histopathologic exam in macaques in many tissues, but this did not seem to be clinically significant and was not attributed to the study drug as it was seen in all groups. It is possible that the baseline immune infiltration in macaques masked a slight inflammatory response to EPHARNA in these animals. We have previously demonstrated the efficacy of EPHARNA using in vivo mouse models (15). This study demonstrates that EPHARNA is safe in multiple mammalian species including non-human primates at dose levels that elicited a therapeutic effect without significant toxicity. A first in human Phase I clinical trial of EPHARNA is currently underway (NCT01591356).
Supplementary Material
Acknowledgments
Financial Support:
M.J. Wagner is supported by NCI-DHHS-NIH T32 Training Grant (T32CA009666). A.K. Sood is supported by NCI P50 CA083639, P50 CA098258, U54 CA151668, UH3 TR000943, CPRIT (RP110595 and RP120214), a Ovarian Cancer Research Fund Program Project Development Grant, the Frank McGraw Memorial Chair in Cancer Research, the RGK Foundation, the Judi A. Rees Ovarian Cancer Research Fund, the Meyer and Ida Gordon Foundation, the American Cancer Society Research Professor Award, the Ann Rife Cox Chair in Gynecology, and the Blanton-Davis Ovarian Cancer Research Program.
Footnotes
Conflicts of Interest: Anil K. Sood and Gabriel Lopez-Berestein are co-inventors on a patent related to this work. Kirstin Barnhart is currently an employee at Abbvie Inc. The other authors declare no potential conflicts of interest.
References
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998 Feb 19;391(6669):806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001 May 24;411(6836):494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 3.Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004 Sep 16;431(7006):371–8. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- 4.Wu SY, Lopez-Berestein G, Calin GA, Sood AK. RNAi therapies: drugging the undruggable. Science translational medicine. 2014 Jun 11;6(240):240ps7. doi: 10.1126/scitranslmed.3008362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nature reviews Cancer. 2011 Jan;11(1):59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassani Z, Lemkine GF, Erbacher P, Palmier K, Alfama G, Giovannangeli C, et al. Lipid-mediated siRNA delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. The journal of gene medicine. 2005 Feb;7(2):198–207. doi: 10.1002/jgm.659. [DOI] [PubMed] [Google Scholar]
- 7.Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer cell. 2002 Sep;2(3):243–7. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 8.Yang G, Thompson JA, Fang B, Liu J. Silencing of H-ras gene expression by retrovirus-mediated siRNA decreases transformation efficiency and tumorgrowth in a model of human ovarian cancer. Oncogene. 2003 Aug 28;22(36):5694–701. doi: 10.1038/sj.onc.1206858. [DOI] [PubMed] [Google Scholar]
- 9.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nature biotechnology. 2002 Oct;20(10):1006–10. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 10.Devroe E, Silver PA. Therapeutic potential of retroviral RNAi vectors. Expert opinion on biological therapy. 2004 Mar;4(3):319–27. doi: 10.1517/14712598.4.3.319. [DOI] [PubMed] [Google Scholar]
- 11.Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, et al. siRNA relieves chronic neuropathic pain. Nucleic acids research. 2004;32(5):e49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiffelers RM, Xu J, Storm G, Woodle MC, Scaria PV. Effects of treatment with small interfering RNA on joint inflammation in mice with collagen-induced arthritis. Arthritis and rheumatism. 2005 Apr;52(4):1314–8. doi: 10.1002/art.20975. [DOI] [PubMed] [Google Scholar]
- 13.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nature medicine. 2005 Jan;11(1):50–5. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 14.Tolentino MJ, Brucker AJ, Fosnot J, Ying GS, Wu IH, Malik G, et al. Intravitreal injection of vascular endothelial growth factor small interfering RNA inhibits growth and leakage in a nonhuman primate, laser-induced model of choroidal neovascularization. Retina. 2004 Aug;24(4):660. doi: 10.1097/00006982-200408000-00039. [DOI] [PubMed] [Google Scholar]
- 15.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer research. 2005 Aug 1;65(15):6910–8. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 16.Schultheis B, Strumberg D, Santel A, Vank C, Gebhardt F, Keil O, et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Dec 20;32(36):4141–8. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 17.Buyens K, De Smedt SC, Braeckmans K, Demeester J, Peeters L, van Grunsven LA, et al. Liposome based systems for systemic siRNA delivery: stability in blood sets the requirements for optimal carrier design. Journal of controlled release : official journal of the Controlled Release Society. 2012 Mar 28;158(3):362–70. doi: 10.1016/j.jconrel.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Li J, He F, Wilson A, Pitt B, Li S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochemical and biophysical research communications. 2005 May 13;330(3):755–9. doi: 10.1016/j.bbrc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 19.Omidi Y, Barar J, Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Current drug delivery. 2005 Oct;2(4):429–41. doi: 10.2174/156720105774370249. [DOI] [PubMed] [Google Scholar]
- 20.Dokka S, Toledo D, Shi X, Castranova V, Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharmaceutical research. 2000 May;17(5):521–5. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg RA, Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Molecular and cellular biology. 1990 Dec;10(12):6316–24. doi: 10.1128/mcb.10.12.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annual review of neuroscience. 1998;21:309–45. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 23.Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996 Jul;17(1):9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 24.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000 Nov 20;19(49):5614–9. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 25.Lamorte L, Park M. The receptor tyrosine kinases: role in cancer progression. Surgical oncology clinics of North America. 2001 Apr;10(2):271–88. viii. [PubMed] [Google Scholar]
- 26.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer research. 2001 Mar 1;61(5):2301–6. [PubMed] [Google Scholar]
- 27.Kinch MS, Moore MB, Harpole DH., Jr Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003 Feb;9(2):613–8. [PubMed] [Google Scholar]
- 28.Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004 Aug 1;10(15):5145–50. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 29.Kamat AA, Coffey D, Merritt WM, Nugent E, Urbauer D, Lin YG, et al. EphA2 overexpression is associated with lack of hormone receptor expression and poor outcome in endometrial cancer. Cancer. 2009 Jun 15;115(12):2684–92. doi: 10.1002/cncr.24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merritt WM, Kamat AA, Hwang JY, Bottsford-Miller J, Lu C, Lin YG, et al. Clinical and biological impact of EphA2 overexpression and angiogenesis in endometrial cancer. Cancer biology & therapy. 2010 Dec 15;10(12):1306–14. doi: 10.4161/cbt.10.12.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mudali SV, Fu B, Lakkur SS, Luo M, Embuscado EE, Iacobuzio-Donahue CA. Patterns of EphA2 protein expression in primary and metastatic pancreatic carcinoma and correlation with genetic status. Clinical & experimental metastasis. 2006;23(7–8):357–65. doi: 10.1007/s10585-006-9045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin YG, Han LY, Kamat AA, Merritt WM, Landen CN, Deavers MT, et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007 Jan 15;109(2):332–40. doi: 10.1002/cncr.22415. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Lee J, Kim S. A Study on the Single-dose Oral Toxicity of Super Key in Sprague-Dawley Rats. Journal of pharmacopuncture. 2015 Sep;18(3):63–7. doi: 10.3831/KPI.2015.18.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selby MJ, Engelhardt JJ, Johnston RJ, Lu LS, Han M, Thudium K, et al. Preclinical Development of Ipilimumab and Nivolumab Combination Immunotherapy: Mouse Tumor Models, In Vitro Functional Studies, and Cynomolgus Macaque Toxicology. PloS one. 2016;11(9):e0161779. doi: 10.1371/journal.pone.0161779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurado RL. Iron, infections, and anemia of inflammation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997 Oct;25(4):888–95. doi: 10.1086/515549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.