Abstract
We report a HER2T798I gatekeeper mutation in a patient with HER2L869R-mutant breast cancer with acquired resistance to neratinib. Laboratory studies suggested that HER2L869R is a neratinib-sensitive, gain-of-function mutation that upon dimerization with mutant HER3E928G, also present in the breast cancer, amplifies HER2 signaling. The patient was treated with neratinib and exhibited a sustained partial response. Upon clinical progression, HER2T798I was detected in plasma tumor cell-free DNA. Structural modeling of this acquired mutation suggested that the increased bulk of isoleucine in HER2T798I reduces neratinib binding. Neratinib blocked HER2-mediated signaling and growth in cells expressing HER2L869R but not HER2L869R/T798I. In contrast, afatinib and the osimertinib metabolite AZ5104 strongly suppressed HER2L869R/T798I-induced signaling and cell growth. Acquisition of HER2T798I upon development of resistance to neratinib in a breast cancer with an initial activating HER2 mutation suggests HER2L869R is a driver mutation. HER2T798I-mediated neratinib resistance may be overcome by other irreversible HER2 inhibitors like afatinib.
Keywords: HER2, neratinib, gatekeeper mutation, breast cancer, precision medicine
Introduction
DNA sequencing efforts have revealed that ERBB2, the gene encoding the HER2 receptor tyrosine kinase (RTK), is mutated a wide variety of cancer types, including 2–3% of primary breast cancers (1–3), with a higher incidence in lobular breast cancers (4). More than 70% of HER2 mutations in breast cancer are found in the absence of HER2 (ERBB2) gene amplification (2). Some of the common HER2 mutations promote HER2 kinase activity and transform breast epithelial cells and other cell types (5–9). Given that irreversible EGFR/HER2 tyrosine kinase inhibitors (TKIs) such as neratinib and afatinib have shown preclinical activity against several HER2 mutants (5,7–9), clinical trials with neratinib (SUMMIT trial; NCT01953926) and afatinib (NCI-MATCH; NCT02465060) focused in patients with HER2-mutant cancers are in progress. However, sustained clinical activity of ATP mimetics in patients with advanced cancer has generally been limited by the acquisition of drug resistance. Mutation of the “gatekeeper” residue within the kinase’s ATP-binding pocket, such as ABLT315I, KITT670I, and EGFRT790M, is a common mechanism of acquired resistance. Here, we report for the first time a case of a HER2 gatekeeper mutation in a patient with non-amplified HER2-mutant breast cancer with acquired resistance to neratinib.
Results
HER2 L869R exhibits a gain-of-function phenotype that is blocked by neratinib
Targeted capture next-generation sequencing (NGS) (10) of DNA from a skin metastasis in a 54 year-old female with ER/PR+, HER2 non-amplified lobular breast carcinoma identified an ERBB2L869R (HER2L869R) somatic mutation (Supplementary Table S1). Prior therapies included chemotherapy, tamoxifen, aromatase inhibitors, everolimus and trastuzumab. The tumor also harbored a truncation mutation in CDH1, ERBB3E928G and amplification of CCND1 and FGF3/4/19. Interrogation of cBioPortal (n>21,000), Project GENIE (n>18,000), COSMIC (n>50,000), Foundation Medicine (n>40,000), and Guardant Health (n>17,000) databases found 16 additional cancers harboring ERBB2L869R and one L869Q mutation, including 12 breast cancers (Supplementary Table S2). Additionally, a recent study reported 4 instances of ERBB2L869R among 413 invasive lobular breast cancers (4).
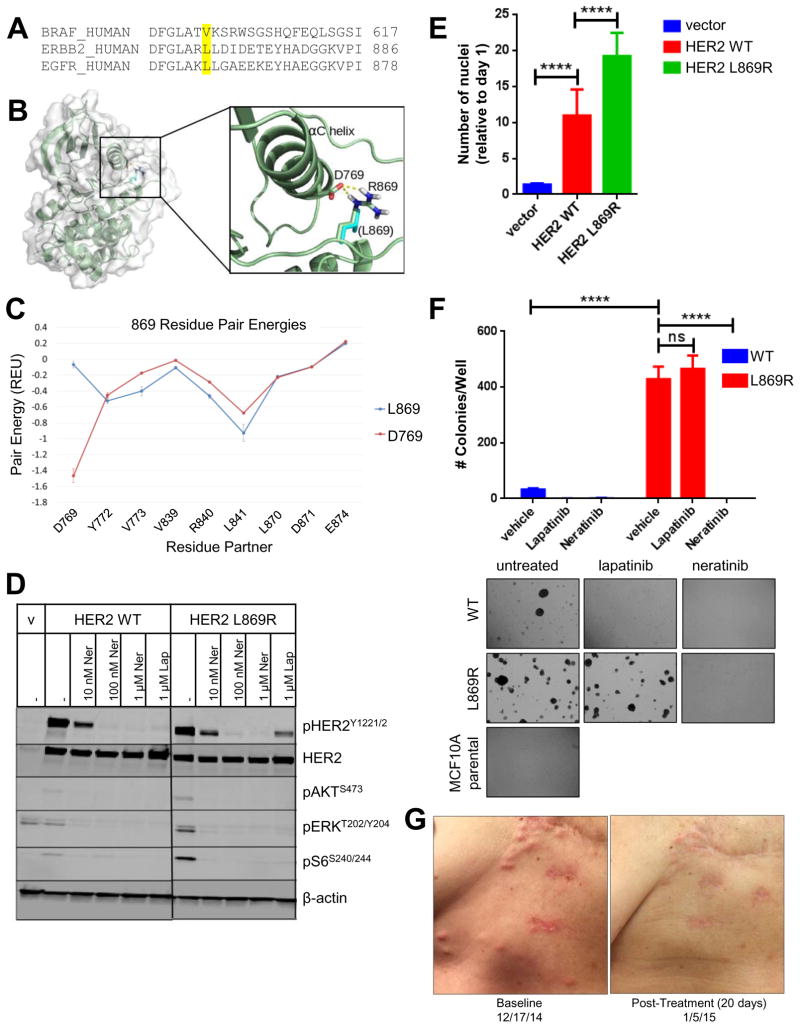
The L869R mutation is located within the activation loop of the HER2 kinase domain. Sequence alignment of the HER2, EGFR, and BRAF kinase domains showed that HER2L869R is homologous to BRAFV600E, a gain-of-function mutation found in >50% of melanomas (11), and EGFRL861R/Q, an activating mutation in non-small cell lung cancer (NSCLC; Fig. 1A) (12). We performed structural modeling of the L869R mutation using Rosetta (13) and examined the residue pair energies involving L869. The mutation resulted in the addition of a strong attractive interaction between R869 and D769 (Fig. 1B,C). This interaction potentially stabilizes the active conformation of the C helix. We also predict that mutating L869 to a polar residue (Arg) disrupts the autoinhibitory contacts between the C helix and the activation loop helix, resulting in destabilization of the inactive conformation of the kinase, similar to EGFRL858R (14).
Fig. 1. HER2L869R exhibits a gain-of-function phenotype that is blocked by neratinib.
(A) The amino acid sequences of human BRAF, ERBB2, and EGFR were aligned using Clustal Omega. BRAF V600, ERBB2 L869, and EGFR L861 residues are highlighted in yellow. (B) The structure of HER2L869R was modeled. The mutation from Leucine (cyan) to Arginine (highlighted in blue) permits favorable charge interaction (dashed yellow lines) with Asp769. (C) Residue pair energies involving residue 869 reveal the addition of a strong attractive (negative) interaction at Asp769 in the HER2L869R model. (D) MCF10A cells stably expressing HER2WT or HER2L869R were treated with vehicle (DMSO), 0.01–1.0 μM neratinib, or 1 μM lapatinib for 4 h in serum-free media. Cell lysates were probed with the indicated antibodies. Scans are all from the same gel/film; the vertical black line indicates an irrelevant lane that was removed from the figure for clarity. (E) Stably transduced MCF10A cells were seeded in 96-well plates in MCF10A starvation media (1% charcoal-stripped serum, no EGF). After 7 days, nuclei were stained with Hoechst and scored using the ImageXpress system. Data points represent the average ± standard deviation (SD) of 4 replicate wells (****, p<0.0001, ANOVA followed by Tukey’s multiple comparisons test). (F) Stably transduced MCF10A cells were plated in 3D Matrigel in presence of the indicated drugs (100 nM). Colonies were grown in media containing 5% charcoal-stripped serum without EGF and insulin. After ~2 weeks, colonies were stained with MTT and counted using the Gelcount system. Data represent the average ± SD of 3 replicates. Representative fields (10X objective) of wells are shown below (****p<0.0001, ANOVA followed by Tukey’s multiple comparisons test). (G) Chest wall skin metastases of patient with invasive lobular breast cancer harboring HER2L869R at baseline and 20 days after starting treatment with neratinib.
Based on these structural data, we hypothesized that HER2L869R would display increased signaling and transforming capacity. To test this, we stably transduced MCF10A breast epithelial cells with lentiviral vectors encoding HER2 wild-type (WT) or HER2L869R. Cells expressing HER2L869R exhibited increased phosphorylation of AKT, ERK, and S6, which were blocked by neratinib (Fig. 1D). Phosphorylation of HER2WT, but not HER2L869R, was blocked by the reversible HER2/EGFR TKI lapatinib, whereas neratinib inhibited phosphorylation of both WT and mutant receptors. Expression of HER2L869R enhanced MCF10A cell proliferation in growth factor-depleted media (Fig. 1E) and colony formation in 3D Matrigel in the absence of EGF and insulin (Fig. 1F) compared to MCF10A/HER2WT cells. Growth of MCF10A/HER2L869R cells was inhibited by neratinib but not by lapatinib, whereas the HER2WT cells were sensitive to both TKIs. With these supporting data, the patient was enrolled in a clinical trial with single agent neratinib (NCT01953926). Upon treatment, the patient exhibited an excellent clinical response, showing near disappearance of multiple skin metastases after 20 days (Fig. 1G), and a 77% reduction in marker lesions by RECIST criteria after 8 weeks.
Since the patient harbored a co-occurring ERBB3E928G mutation, a known activating mutation in HER3 (15), we next asked whether HER2L869R and HER3E928G might cooperate to drive HER2 signaling. Mutations in ERBB2 and ERBB3 often co-occur in cancer. In the AACR Project GENIE dataset (>18,000 sequenced tumors), 8.3% of ERBB2-mutated cancers also harbor mutations in ERBB3, whereas only 2.3% of ERBB2 WT cancers have ERBB3 mutations (q value=1.3×10−10; www.cbioportal.org/genie). ERBB2L869R and ERBB3E928G were found to co-occur in another breast cancer case in the METABRIC data set (16). Structural modeling of the HER2L869R/HER3E928G double-mutant predicted that the HER3 mutation, located at the dimer interface, may enhance heterodimerization of the kinase domains through decreased bulk and electrostatic repulsion (Fig. S1A). Calculating the change in free energy of WT heterodimers compared to mutant heterodimers demonstrated a significant difference in the capacity of the latter to bind to one another (Fig. S1B). Further, co-expression of the HER2L869R and HER3E928G intracellular domains (ICDs) resulted in enhanced transphosphorylation of HER3 and ERK as substrates compared to that induced by expression of either mutant alone (Fig. S1C). Phosphorylation of mutant HER2 and HER3, as well as the elevated downstream signaling induced by expression of both mutants, was blocked by treatment with neratinib (Fig. S1D). These data suggest that these co-occurring mutations in ERBB2 and ERBB3 enhance ERBB signaling output which, in turn, can be blocked by neratinib.
Acquired HER2T798I mediates neratinib resistance
After 5 months on therapy, the patient developed a painful metastasis in the sternum. Addition of the estrogen receptor antagonist fulvestrant to neratinib induced a prompt symptomatic and clinical response. After 10 additional months on the combination, the patient progressed with new skin metastases. Targeted tissue-based NGS analysis of DNA from a new skin metastasis and plasma tumor, cell-free (cf) DNA (Guardant360™) revealed that ERBB2L869R remained (44% allele frequency and 8.7% cfDNA, respectively; Supplementary Table S3). ERBB3E928G remained in the post-treatment biopsy as well. ERBB2T798I was found in plasma (1.3% cfDNA), but not in DNA from the synchronous skin metastasis. Additional single-gene deep sequencing of plasma ERBB2 using two rounds of targeted capture (average >4,000 reads per sample) in an independent plasma sample from that used for the Guardant360 test failed to identify ERBB2T798I in any of the plasma samples obtained at study enrollment or during the first 9 cycles of neratinib, but increased to 1.0% of reads at the time of clinical progression (Fig. 2A and Supplementary Table S4). In contrast, ERBB2L869R was detected in 6.8% of reads in the pre-treatment sample, decreasing considerably during therapy, and rebounding up to 15.2% at progression. These data suggest that ERBB2T798I was acquired during neratinib therapy.
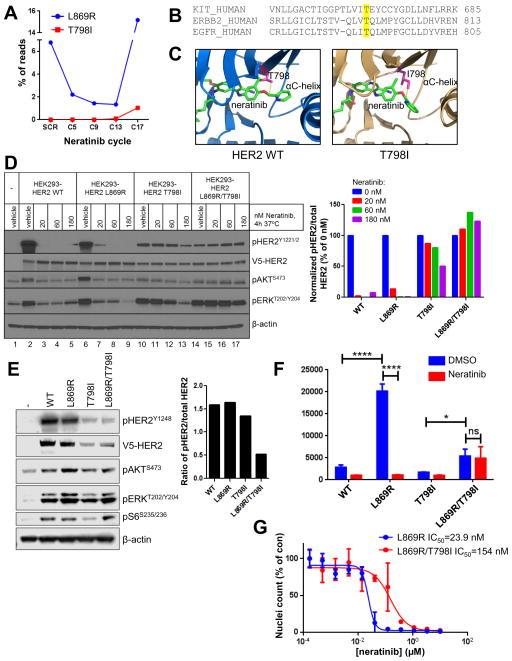
Fig. 2. HER2T798I induces acquired resistance to neratinib.
(A) The patient’s plasma was drawn at the time of clinical trial screening (SCR) and the indicated cycles of neratinib therapy (1 cycle = 28 days). Plasma cfDNA was subjected to ERBB2 targeted capture and sequenced. (B) The amino acid sequences of human KIT, ERBB2, and EGFR were aligned using Clustal Omega. KIT T670, ERBB2 T798, and EGFR T790 gatekeeper residues are highlighted in yellow. (C) The structure of HER2WT and a model of HER2T798I are shown with neratinib bound to the kinase pocket. The threonine (WT) or isoleucine (mutant) residue at position 798 is shown in pink. (D) HEK293 cells were transiently transfected with V5-tagged HER2WT, HER2L869R, HER2T798I or HER2L869R/T798I and treated with the indicated concentrations of neratinib for 4 h in serum-free media. Cell lysates were subjected to immunoblot analyses with the indicated antibodies. The bar graph represents quantification of immunoblot bands using ImageJ software. (E) MCF10A cells stably expressing V5- tagged HER2WT, HER2L869R, HER2T798I or HER2L869R/T798I were cultured in MCF10A growth factor-depleted media. Cell lysates were subjected to immunoblot analyses with the indicated antibodies. The bar graph represents quantification of immunoblot bands using ImageJ software. (F) MCF10A cells from (E) were treated ± 123 nM neratinib in growth factor-depleted media for 6 days. Nuclei were stained with Hoechst and scored using the ImageXpress system. Data represent the average ± SD of 4 replicate wells (*p<0.05, ****p<0.0001, ANOVA followed by Tukey’s multiple comparisons test). (G) MCF10A cells stably expressing HER2L869R (blue) or HER2L869R/T798I (red) were treated with increasing concentrations of neratinib for 6 days. Nuclei were stained with Hoechst and scored using the ImageXpress system. Data represent the average ± SD of 4 replicate wells. IC50 values were calculated using GraphPad Prism.
HER2T798I is homologous to EGFRT790M and imatinib-resistant KITT670I in gastrointestinal stromal tumors (GIST) (Fig. 2B). EGFRT790M drives resistance to first- and second-generation EGFR TKIs in NSCLC by two mechanisms: first, by mediating steric hindrance of ATP-competitive drugs, and second, by increasing the affinity of ATP resulting in enhanced phosphotransfer and kinase activity (17). To determine whether HER2T798I functions in a similar manner, we constructed computational models of HER2WT and HER2T798I bound to neratinib. We found that the increased bulk of the isoleucine at position 798 would result in steric hindrance when neratinib binds (Fig. 2C). The closest approach between non-hydrogen atoms from residue T798 to neratinib is 4.1 Angstrom in HER2WT, whereas this distance is reduced to 3.6 Angstrom in HER2T798I, resulting in a reduced size of the binding pocket. Therefore, the isoleucine substitution at position 798 is expected to reduce neratinib binding.
Next, we asked whether the T798I mutation would block neratinib action. HEK293 cells transfected with HER2WT, HER2L869R, HER2T798I, or HER2L869R/T798I (both mutations in cis) were treated with increasing doses of neratinib for 4 h. Low doses of neratinib (20 nM) blocked pHER2, pAKT and pERK in cells expressing HER2WT or HER2L869R, but not in cells expressing HER2T798I or HER2L869R/T798I treated with up to 180 nM neratinib (Fig. 2D). To confirm these findings, we stably transduced MCF10A cells with WT and mutant HER2. We noted that HER2T798I and HER2T798I/L869R were poorly expressed in HEK293 and MCF10A cells (Fig. 2D,E). Treatment with the proteasome inhibitor MG132 for 24 h restored expression of the T798I mutants (Fig. S2), suggesting that this mutation decreases protein stability. Cells expressing HER2L869R or HER2L869R/T798I, but not HER2T798I alone, displayed enhanced pAKT, pERK and pS6 (Fig. 2E). Furthermore, while HER2L869R and HER2L869R/T798I induced EGF-independent MCF10A cell proliferation, HER2T798I did not (Fig. 2F). Although untreated MCF10A/HER2L869R/T798I cells did not proliferate as fast as cells expressing HER2L869R, they were the only cells that grew in the presence of neratinib. A similar slow growth rate has been reported in EGFR TKI-resistant cell lines and patients’ tumors harboring EGFRT790M (18). MCF10A cells expressing both mutations displayed reduced sensitivity to neratinib (IC50=154 nM) compared to cells expressing HER2L869R (IC50=23.9 nM; Fig. 2G). These results suggest that, like EGFRT790M for gefitinib and erlotinib, HER2T798I confers a growth advantage in the presence of neratinib.
The lack of transforming capacity of HER2T798I alone suggests that it is not a driver oncogene, but an acquired alteration as a result of therapeutic pressure. Consistent with this speculation, HER2T798I is exceedingly rare in tumors from patients not treated with HER2 TKIs (Supplementary Table S2). Out of all of the tumors sequenced in cBioPortal, COSMIC, Foundation Medicine, and Guardant Health databases (over 100,000 samples sequenced in all), HER2T798I was only found in one colorectal cancer (Foundation Medicine) and in one endometrial cancer cell line (CCLE), strongly suggesting that in the patient reported herein, T798I was acquired due to selective pressure of neratinib treatment.
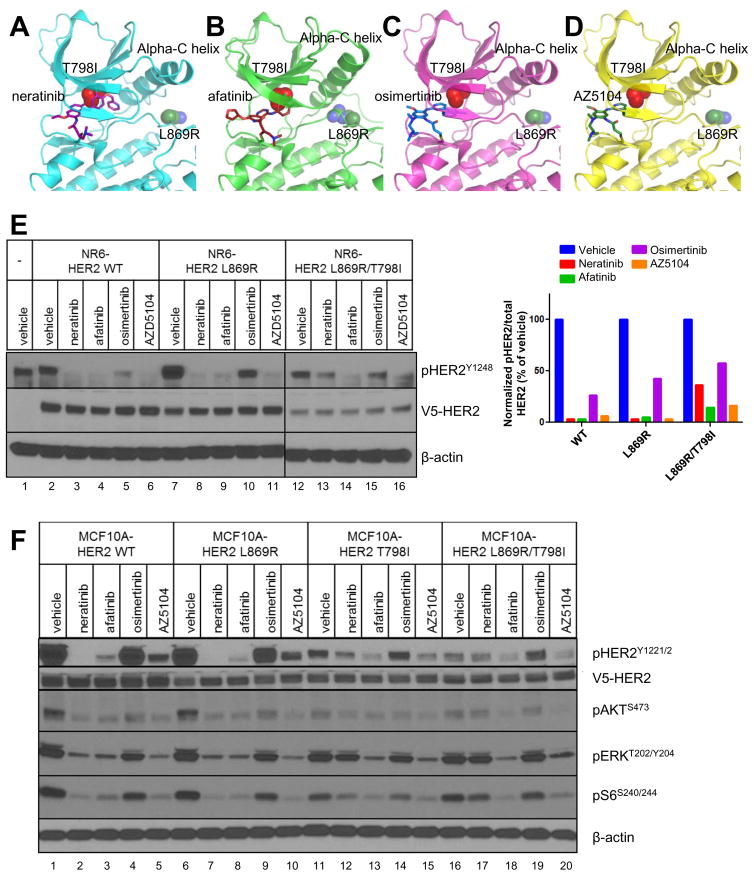
We next examined a panel of other irreversible EGFR/HER2 TKIs for their ability to block HER2L869R/T798I. These included afatinib, a covalent EGFR/HER2 inhibitor, the EGFR inhibitor osimertinib (AZD9291), which exhibits selectivity against mutant EGFR (including T790M) but does not block WT HER2 (19), and AZ5104, an osimertinib metabolite that inhibits WT HER2 and EGFR (20). We performed computational modeling of HER2L869R/T798I bound to neratinib, afatinib, osimertinib, and AZ5104 (Fig. 3A–D). These small molecules are expected to bind HER2 using the same mechanism and position by which they bind EGFR. By analogy with EGFR, the HER2 kinase is predicted to adopt distinct conformations when bound by each inhibitor. Afatinib and neratinib have covalent binding modes that project deeply into the substrate binding pocket of the HER2 kinase (Fig. 3A,B). The sterically larger side chain of HER2T798I decreases the available space and decreases the polar character of the binding pocket. This is predicted to affect neratinib binding, which, by being the largest of these small molecules, extends the deepest into the pocket. While afatinib is predicted to make slight contact with T798I, it does not insert as far into the tunnel as neratinib does. Osimertinib and AZ5104 are predicted to bind much less deeply on the lip of the pocket (Fig. 3C,D). Based on these studies, HER2T798I is predicted to disrupt neratinib binding, but is not expected to significantly affect the binding of afatinib, osimertinib, or AZ5104.
Fig. 3. Afatinib and AZ5104 block HER2L869R/T798I signaling.
(A–D) Computational modeling of the HER2 kinase domain in complex with (A) neratinib, (B) afatinib, (C) osimertinib, and (D) AZ5104 was performed. The N-terminal lobe and part of the C-terminal lobe of the tyrosine kinase domain (TKD) is shown in ribbon style. Each inhibitor is represented as sticks bound in the substrate-binding pocket. The T798I mutation is shown as red spheres deep in the pocket. The L869R mutation is shown as blue and green spheres on the far side of the alpha-C helix. (E) NR6 cells stably expressing V5-tagged HER2WT, HER2L869R, HER2T798I or HER2L869R/T798I (LR/TI) were treated with the indicated drugs at 100 nM for 4 h in serum-free media. Cell lysates were subjected to immunoblot analyses with the indicated antibodies. Scans are all from the same gel/film; the vertical black line indicates an irrelevant lane that was removed from the figure for clarity. The bar graph represents quantification of immunoblot bands using ImageJ software. (F) Stably transduced MCF10A cells were treated with the indicated drugs at 100 nM for 4 h in EGF- and serum-free media. Cell lysates were subjected to immunoblot analyses with the indicated antibodies as described in Methods.
We next tested the ability of the panel of inhibitors to block mutant HER2 in stably transduced NR6 mouse fibroblasts, which lack endogenous EGFR (21), and MCF10A cells. In both cell types, neratinib more efficiently blocked pHER2 in cells expressing HER2WT or HER2L869R compared to cells expressing HER2L869R/T798I (Fig. 3E, F). Treatment with afatinib and AZ5104 blocked phosphorylation of HER2WT as well as both HER2 mutants. In contrast, osimertinib failed to inhibit HER2WT, HER2L869R or HER2T798I. Inhibition of pAKT, pERK, and pS6 with all small molecules mirrored that of pHER2 in MCF10A cells (Fig. 3F).
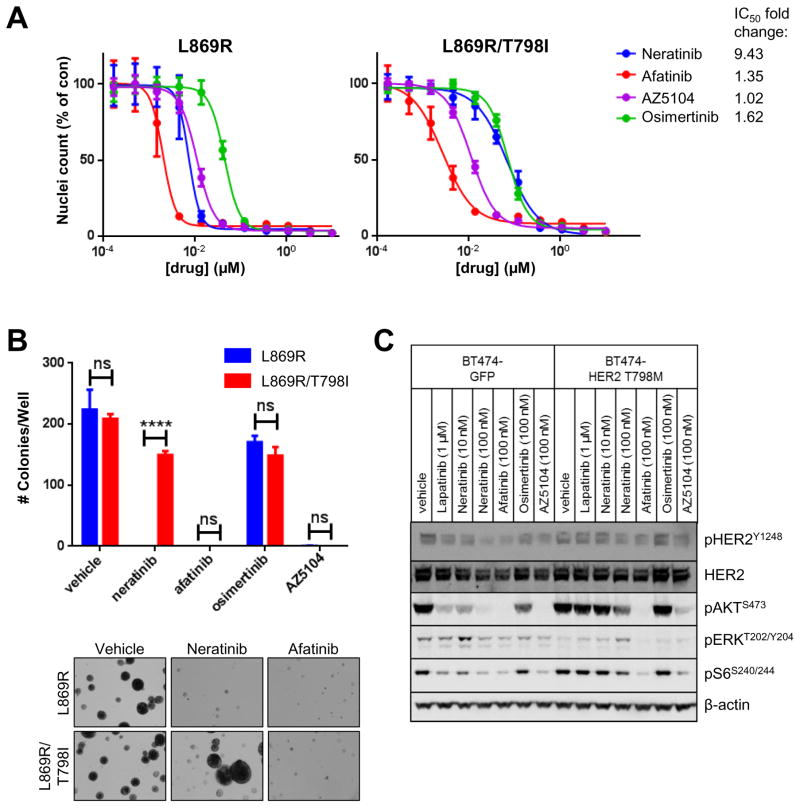
MCF10A/HER2L869R and MCF10A/HER2L869R/T798I were highly sensitive to afatinib and AZ5104 in growth factor-depleted media, whereas higher doses of osimertinib were required to block the growth of both cell types (Fig. 4A). Neratinib and AZ5104 showed similar IC50 values in HER2L869R-expressing cells, whereas neratinib was less effective against HER2L869R/T798I-expressing cells. In 3D Matrigel, 100 nM of neratinib, afatinib, or AZ5104 completely blocked acini formation by MCF10A/HER2L869R cells, whereas 100 nM of osimertinib only slightly suppressed acini growth (Fig. 4B). Both neratinib and osimertinib failed to suppress growth of MCF10A/HER2L869R/T798I cells in 3D Matrigel, whereas this was completely blocked by afatinib and AZ5104, suggesting that the latter two inhibitors are able to overcome HER2T798I-mediated drug resistance.
Fig. 4. Afatinib and AZ5104 block HER2L869R/T798I-induced growth.
(A) MCF10A cells stably expressing HER2L869R and HER2L869R/T798I were treated with increasing concentrations of neratinib, afatinib, osimertinib, or AZ5104. After 5 days, nuclei were stained with Hoechst and scored using the ImageXpress system. Data represent the average ± SD of 4 replicate wells. The fold change in IC50 values of MCF10A L869R/T798I cells relative to L869R cells is shown. (B) Stably transduced MCF10A cells were plated in 3D Matrigel in presence of the indicated drugs (100 nM). After 9 days, colonies were stained with MTT and counted using the Gelcount system. Data represent the average ± SD of 3 replicates. Representative fields (10X objective) of wells treated with vehicle (DMSO), 100 nM neratinib, and 100 nM afatinib are shown (****p<0.0001, ANOVA followed by Tukey’s multiple comparisons test). (C) BT474GFP (control) and BT474HER2-T798M were treated with the indicated drugs for 4 h in serum-free media. Cells lysates were tested in immunoblot anlayses using the indicated antibodies.
Recent reports have proposed the acquisition of HER2 mutations in patients with WT HER2 amplification treated with anti-HER2 therapies (22). In addition, neratinib has shown clinical activity and is being used in patients with WT HER2 amplification (23). Thus, we tested if a HER2 gatekeeper mutation would confer resistance to neratinib when present in a background of WT HER2 amplification. We used HER2-amplified BT474 cells stably expressing HER2T798M, which we previously reported to be lapatinib-resistant (24). BT474GFP control cells and BT474HER2-T798M cells were treated with vehicle (DMSO), lapatinib, neratinib, afatinib, osimertinib or AZ5104. Lapatinib failed to suppress pHER2, pAKT, pERK and pS6 in HER2T798M-expressing cells (Fig. 4C). Treatment with neratinib inhibited pHER2, pAKT and pS6 in BT474GFP cells but not in BT474HER2-T798M cells. Consistent with the findings in MCF10A cells, afatinib and AZ5104, but not osimertinib, blocked pAKT, pERK, and pS6 in both BT474GFP and BT474HER2-T798M cells. Since only ~3% of the ERBB2 alleles in the BT474HER2-T798M cells harbor the mutation (24), these data suggest that just a few HER2T798M alleles can confer resistance to neratinib, but not afatinib, in cells with WT HER2 gene amplification.
Discussion
We report herein the identification of a HER2T798I gatekeeper mutation in a patient with HER2-mutant, non-amplified breast cancer with acquired resistance to neratinib. Structural modeling showed that the T798I mutation results in a steric clash with neratinib, which would reduce drug binding. HER2T798I directly promoted resistance to neratinib in lentivirally-transduced cell lines. In contrast to neratinib, afatinib and the metabolite of osimertinib, AZ5104, blocked HER2T798I-induced signaling and cell growth.
While the initial neratinib-sensitizing HER2L869R mutation induced constitutive phosphorylation of AKT, ERK, and S6, and displayed gain-of-function activity when expressed in breast epithelial cells (Fig. 1), we failed to observe increased phosphorylation of this mutant compared to WT HER2 (Figs. 1D; 2D,E). We speculate that the L869R mutation likely removes autoinhibitory interactions, thus placing the kinase in a better position to interact with other ERBB receptors and adaptor proteins/downstream substrates (25,26). Notably, the HER2mutant cancer also harbored a known activating HER3E928G mutation (15). We speculate these co-mutations resulted in increased dependence on the ERBB pathway and contributed to the tumor’s initial sensitivity to neratinib. Consistent with this speculation, preliminary results from the SUMMIT trial show that among 17 patients who exhibited clinical benefit from neratinib, 2 patients harbored ERBB3 missense mutations, whereas none of the 25 patients who did not benefit harbored ERBB3 alterations in their cancer (27).
Our findings parallel the identification of the EGFRT790M gatekeeper mutation in NSCLC resistant to EGFR inhibitors. We note that for EGFR, 2 nucleotides would need to be mutated to change the threonine codon at position 790 to an isoleucine [ACG (Thr) > ATA, ATC, or ATT (Ile)], whereas only one nucleotide change is needed for the T790M mutation (ACG>ATG). The opposite is true for ERBB2 [ACA (Thr) > ATA (Ile) vs ACA > ATG (Met)]. Thus, it is easier for the tumor to mutate ERBB2 codon 798 to an isoleucine rather than a methionine.
EGFRT790M is reported to promote resistance by simultaneously increasing ATP affinity and decreasing drug binding (28). While our data suggest that the HER2T798I mutation could affect neratinib binding through steric interactions, it could similarly affect ATP binding and kinase activity. Although the change in distance (0.5 Angstrom) from residue 798 to neratinib could theoretically be accommodated by conformational changes, the structural evidence suggests that replacing a polar amino acid (Thr) with a hydrophobic residue (Ile) would decrease ATP affinity. The wild-type Thr side chain contains an -OH group that faces the ATP binding site. In the AMP-PNP-bound crystal structure of EGFR (2GS7.pdb), that -OH group is within 3.4 Angstrom of the N6 of AMP-PNP. Replacing the Thr with Ile would remove that favorable interaction and is expected to decrease ATP affinity. These structural assessments are consistent with our cell-based findings that T798I-expressing cells do not show increased HER2 phosphorylation, even when corrected for expression levels (Fig. 2D–E).
HER2T798I and EGFRT790M also differ in that the former is exceedingly rare in untreated tumors (Supplementary Table S2) whereas EGFRT790M also occurs in germline DNA and can promote lung cancer formation (29), suggesting that EGFRT790M itself is oncogenic. This is also consistent with the notion that HER2T798I alone is not oncogenic, but requires another activating mutation in cis (e.g., L869R) to promote HER2 signaling and oncogenic growth (Fig. 2).
We previously reported that a HER2T798M gatekeeper mutation increased HER2 autophosphorylation and association of HER3 with the p85 regulatory subunit of PI3K (24). In the current study, HER2T798I alone did not appear to enhance HER2 signaling or HER2-induced proliferation more than WT HER2 (Fig. 2E,F). This discrepancy may be due to differences in experimental conditions (i.e., serum starvation), differences between the Met and Ile residues, or lower expression of the mutant receptor compared to WT, which we observed in multiple cell lines expressing T798I alone or in cis with L869R (Figs. 2E, 3E). We speculate that the decreased expression of the mutant may be due to decreased protein stability (Supplementary Fig. S2). Despite this decreased expression, MCF10A cells expressing HER2T798I/L869R displayed increased phosphorylation of HER2 signaling targets and EGF-independent proliferation compared to MCF10A/HER2WT cells, as well as robust growth in the presence of neratinib (Figs. 2E–G, 4B), altogether suggesting that even low levels of HER2T798I can promote neratinib resistance.
We are unable to determine whether ERBB2L869R and ERBB2T798I occur in cis in the patient’s plasma, since these two mutations are 213 bp apart, longer than the length of cfDNA fragments shed from tumor cells. In NSCLC, EGFRT790M is usually found on the same allele as the initial TKI-sensitizing EGFR mutation (30), suggesting that the two ERBB2 mutations may also occur in cis. Additionally, the allele frequency of ERBB2T798I in plasma tumor cell free DNA in the patient progressing on neratinib was lower than the frequency of ERBB2L869R (Fig. 2A and Supplementary Table S3) consistent with HER2L869R being the initial driver mutation, and HER2T798I representing an acquired subclonal drug-resistant mutation. A similar relationship is typically seen with somatic EGFRT790M in the plasma of patients progressing on EGFR inhibitors compared to the level of the original drug-sensitive EGFR mutation (31). We also note that HER2T798I was not found in a new skin metastasis synchronous with the progression on neratinib, suggesting spatially heterogeneous mechanisms of drug resistance. This finding is consistent with other reports where plasma may serve as a repository of different acquired drug-resistant mutations found in some but not all metastatic sites, whereas a tissue biopsy of a single lesion may produce a less complete picture, as suggested by studies with drug-resistant NSCLC expressing EGFRT790M. For example, a subset of patients with EGFR TKI-resistant NSCLC with EGFRT790M detected in plasma but not in a tumor biopsy still responded to osimertinib (32).
PIK3CAM1043I, an activating mutation in the p110α catalytic subunit of PI3K (33), was found at 0.1% frequency in the same plasma sample where HER2T798I was first detected (Supplementary Table S3). PIK3CA mutations are associated with resistance to anti-HER2 therapy in HER2-overexpressing breast cancers (34). Whether PIK3CAM1043I contributes to a multifactorial resistance to neratinib is also possible but beyond the scope of this report. While afatinib and neratinib are both irreversible covalent EGFR/HER2 TKIs, we found that afatinib, but not neratinib, was able to block HER2L869R/T798I activity. We speculate that because neratinib is larger than afatinib, the former is more likely to be affected by a steric clash with the bulkier isoleucine residue in HER2T798I (Fig. 3A,B). Treatment with low doses of afatinib (10 nM), easily achievable in patients (35), completely blocked growth of MCF10A/HER2L869R/T798I cells, while treatment with neratinib at clinically achievable concentrations (36) failed to do so (Fig. 4A,B). We also observed moderate activity of the osimertinib metabolite AZ5104 (Fig. 4). However, this drug is not being developed independently of osimertinib, and only ~10% of osimertinib is metabolized into AZ5104 in humans (20).
Immediately following progression on neratinib, the patient was treated with capecitabine chemotherapy. The patient responded well and remains in a partial response ~one year later. We repeated next gen sequencing of her plasma tumor DNA after ~6 months on capecitabine; ERBB2L869R cfDNA dropped to 0.4%, while ERBB2T798I and CCND1 amplification were no longer detectable, consistent with the decrease in tumor burden and the patient’s clinical response. If the patient progresses on capecitabine and the ERBB2 mutations are once again detectable, there will be strong consideration for treatment with afatinib at this time. As more patients with HER2-mutant cancers are treated with HER2 TKIs such as neratinib, we expect that acquired HER2T798I may be observed more frequently. We propose afatinib is active against HER2T798I and is an alternative worthy of clinical investigation in cancers harboring the HER2 gatekeeper mutation. Finally, this report supports the development of HER2T798I-selective inhibitors that would spare the toxicity associated with therapeutic inhibition of WT ERBB receptors.
Methods
ERBB2 Single-Gene Targeted Capture
Extraction of cell free DNA from plasma was performed using a fully automated QIAGEN platform, QIAsymphony SP, and QIAsymphony DSP Virus/Pathogen Midi Kit following centrifugation. Sequence libraries were prepared according to the KAPA Hyper protocol (Kapa Biosystems) with the ligation of Illumina sequence adaptors followed by PCR amplification and clean-up. Barcoded libraries were hybridized with DNA probes targeting all coding exons of ERBB2 (Integrated DNA Technologies) in two successive captures, using a protocol modified from the NimbleGen SeqCap Target Enrichment system. The first capture was incubated at 55°C for 16 h followed by post-capture washes and 16 cycles of PCR amplification. The second capture was incubated at 65°C for 4 h followed by post-capture washes and 3–5 cycles of PCR amplification. Captured libraries were sequenced on an Illumina HiSeq as paired-end 100 bp reads.
Computational modeling
Structural modeling of inhibitor-bound HER2WT, HER2L869R/HER3E928G and HER2L869R/T798I was performed using Rosetta. Detailed procedures are available in Supplementary Methods.
Cell lines and inhibitors
MCF10A breast epithelial cells (ATCC® CRL-10317™; purchased in 2012) and HEK293 human embryonic kidney cells (ATCC® CRL-1573™; purchased in 2006) were from ATCC. Cell lines were authenticated by ATCC prior to purchase by the short tandem repeat (STR) method. 293FT cells were purchased from Invitrogen (Cat. No. R70007). NR6 cells have been previously described (21), as have BT474GFP and BT474HER2T798M (24). ERBB2T798M was verified by sequencing cDNA using primers for ERBB2. Other than routinely checking cell morphology for consistency with published images, no other authentication was performed.
293FT, HEK293, and NR6 cells were maintained in DMEM supplemented with 10% FBS and 1X Antibiotic-Antimycotic (Gibco). BT474 cells were maintained in IMEM supplemented with 10% FBS, 1X Antibiotic-Antimycotic, and 100 μg/ml G418. MCF10A cells were maintained in MCF10A complete media (DMEM/F12 supplemented with 5% horse serum, 20 ng/ml EGF, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, 0.1 μg/ml cholera toxin, and 1X antibiotic/antimycotic). For experiments under growth factor-depleted conditions, MCF10A cells were grown in DMEM/F12 supplemented with 1% charcoal/dextran-stripped serum (CSS), 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, 0.1 μg/ml cholera toxin, and 1X Antibiotic-Antimycotic. Cell lines were routinely evaluated for mycoplasma contamination. All experiments were completed less than 2 months after thawing early-passage cells.
The following inhibitors were used: MG132 (Selleck Chemicals), lapatinib (LC Laboratories), neratinib (PUMA Biotechnology), afatinib (Selleck Chemicals), and osimertinib and AZ5104 (AstraZeneca Pharmaceuticals).
Immunoblot analysis
Cells were washed with PBS and lysed on ice in RIPA lysis buffer plus protease and phosphatase inhibitors. Protein concentration was measured using the BCA protein assay reagent (Pierce). Lysates were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). Immunoreactive bands were detected by enhanced chemiluminescence following incubation with horseradish peroxidase-conjugated secondary antibodies (Promega). Detailed information on antibodies is available in Supplementary Methods. Immunoblot bands were quantified from inverted images using ImageJ software.
Cell growth assays
MCF10A cells were seeded in black clear-bottom 96-well plates (Greiner Bio-One) at a density of 1,000 cells per well in growth factor-depleted media. The next day, media was replaced with 100 μl media containing increasing amounts of inhibitor (0.17 nM–10 μM in 3-fold dilutions). After 5–6 days, nuclei were stained with 10 μg/ml Hoechst 33342 (Thermo Fisher Scientific) at 37°C for 20 min. Fluorescent nuclei were counted using the ImageXpress Micro XL automated microscope imager (Molecular Devices).
For three-dimensional (3D) growth assays, cells were seeded on growth factor–reduced Matrigel (BD Biosciences) in 48-well plates following published protocols (37). Inhibitors were added to the medium at the time of cell seeding. Fresh media and inhibitors were replenished every 3 days. Following 9–14 days, colonies were stained with 5 mg/ml MTT for 20 min. Plates were scanned and colonies measuring ≥100 μm were counted using GelCount software (Oxford Optronix). Colonies were photographed using an Olympus DP10 camera mounted in an inverted microscope.
Patient studies
Informed consent was obtained from the patient described in this study. The clinical trial (NCT01953926) was conducted in accordance with the Declaration of Helsinki and approved by an institutional review board.
Statistical analysis
All experiments were performed using at least three technical replicates and at least two independent times. P values were generated by ANOVA followed by Tukey’s multiple comparisons test unless otherwise indicated. Data are presented as mean ± SD. IC50 values were generated through GraphPad Prism (version 6.0).
Detailed descriptions of NGS, multiple sequence alignment, determination of mutation frequencies, transient transfections, and generation of stable cell lines are available in Supplementary Methods.
Supplementary Material
Statement of Significance.
We found an acquired HER2 gatekeeper mutation in a patient with HER2-mutant breast cancer upon clinical progression on neratinib. We speculate that HER2T798I may arise as a secondary mutation following response to effective HER2 TKIs in other cancers with HER2 activating mutations. This resistance may be overcome by other irreversible HER2 TKIs such as afatinib.
Acknowledgments
Financial Support: This work was supported by NIH/NCI K12 Award CA090625 (ABH); NCI R01 grant CA080195 (CLA); ACS Clinical Research Professorship Award CRP-07-234-06-COUN (CLA); a research grant from Puma Biotechnology; NIH Breast Cancer Specialized Program of Research Excellence (SPORE) grant P50 CA098131; and Vanderbilt-Ingram Cancer Center Support Grant P30 CA68485.
We would like to acknowledge the American Association for Cancer Research and its support in the development of the AACR Project GENIE registry, as well as members of the consortium for their commitment to data sharing. Interpretations are the responsibility of study authors. In addition, we offer our sincere gratitude to the patient for her contribution to this study.
Footnotes
Disclosure of potential conflicts of interest: R.N. and R.L. are employees of Guardant Health. J.H. and V.M. are employees of Foundation Medicine. R.E.C. and A.S.L. are employees of Puma Biotechnology. D.C. is an employee of AstraZeneca. Other authors declare no conflicts of interest.
Authors’ Contributions
Conception and design: A.B. Hanker, M. Red Brewer, J.H. Sheehan, G. Sliwoski, J. Meiler, C.L. Arteaga
Development of methodology: A.B. Hanker, M. Red Brewer, J.H. Sheehan, G. Sliwoski, J. Meiler, C.L. Arteaga
Acquisition of data: A.B. Hanker, M. Red Brewer, J.H. Sheehan, J.P. Koch, G. Sliwoski, M.F. Berger
Analysis and interpretation of data: A.B. Hanker, M. Red Brewer, J.H. Sheehan, J.P. Koch, G. Sliwoski, R. Lanman, R. Nagy, M.F. Berger, D.M. Hyman, J. He, V. Miller, J. Meiler, C.L. Arteaga
Acquisition of clinical samples and/or collection/interrogation of patient data: R. Lanman, R. Nagy, M. Berger, D.M. Hyman, D.B. Solit, J. He, V. Miller, R.E. Cutler, Jr., A.S. Lalani, C.L. Arteaga
Writing, review, and/or revision of manuscript: A.B. Hanker, M. Red Brewer, J.H. Sheehan, G. Sliwoski, R. Lanman, D.M. Hyman, C.M. Lovly, J. Meiler, C.L. Arteaga
Provided reagents: D. Cross and C.M. Lovly
Participated in discussions: D.M. Hyman, R.E. Cutler, Jr., A.S. Lalani, D. Cross, C.M. Lovly
Study supervision: J. Meiler and C.L. Arteaga
References
- 1.Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross JS, Gay LM, Wang K, Ali SM, Chumsri S, Elvin JA, et al. Nonamplification ERBB2 genomic alterations in 5605 cases of recurrent and metastatic breast cancer: An emerging opportunity for anti-HER2 targeted therapies. Cancer. 2016;122(17):2654–62. doi: 10.1002/cncr.30102. [DOI] [PubMed] [Google Scholar]
- 3.Chmielecki J, Ross JS, Wang K, Frampton GM, Palmer GA, Ali SM, et al. Oncogenic alterations in ERBB2/HER2 represent potential therapeutic targets across tumors from diverse anatomic sites of origin. Oncologist. 2015;20(1):7–12. doi: 10.1634/theoncologist.2014-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmedt C, Zoppoli G, Gundem G, Pruneri G, Larsimont D, Fornili M, et al. Genomic Characterization of Primary Invasive Lobular Breast Cancer. J Clin Oncol. 2016;34(16):1872–81. doi: 10.1200/JCO.2015.64.0334. [DOI] [PubMed] [Google Scholar]
- 5.Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer discovery. 2013;3(2):224–37. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10(1):25–38. doi: 10.1016/j.ccr.2006.05.023. S1535-6108(06)00179-6 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Minami Y, Shimamura T, Shah K, LaFramboise T, Glatt KA, Liniker E, et al. The major lung cancer-derived mutants of ERBB2 are oncogenic and are associated with sensitivity to the irreversible EGFR/ERBB2 inhibitor HKI-272. Oncogene. 2007;26(34):5023–7. doi: 10.1038/sj.onc.1210292. [DOI] [PubMed] [Google Scholar]
- 8.Greulich H, Kaplan B, Mertins P, Chen TH, Tanaka KE, Yun CH, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012;109(36):14476–81. doi: 10.1073/pnas.1203201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavuri SM, Jain N, Galimi F, Cottino F, Leto SM, Migliardi G, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer discovery. 2015;5(8):832–41. doi: 10.1158/2159-8290.CD-14-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature biotechnology. 2013;31(11):1023–31. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong DJ, Ribas A. Targeted Therapy for Melanoma. Cancer Treat Res. 2016;167:251–62. doi: 10.1007/978-3-319-22539-5_10. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci. 2016;107(9):1179–86. doi: 10.1111/cas.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leaver-Fay A, Tyka M, Lewis SM, Lange OF, Thompson J, Jacak R, et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 2011;487:545–74. doi: 10.1016/B978-0-12-381270-4.00019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11(3):217–27. doi: 10.1016/j.ccr.2006.12.017. S1535-6108(07)00028-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell. 2013;23(5):603–17. doi: 10.1016/j.ccr.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eck MJ, Yun CH. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer. Biochim Biophys Acta. 2010;1804(3):559–66. doi: 10.1016/j.bbapap.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berruti A, Brizzi MP, Generali D, Ardine M, Dogliotti L, Bruzzi P, et al. Presurgical systemic treatment of nonmetastatic breast cancer: facts and open questions. Oncologist. 2008;13(11):1137–48. doi: 10.1634/theoncologist.2008-0162. theoncologist.2008-0162 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer discovery. 2014;4(9):1046–61. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruss RM, Herschman HR. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977;74(9):3918–21. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trowe T, Boukouvala S, Calkins K, Cutler RE, Jr, Fong R, Funke R, et al. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res. 2008;14(8):2465–75. doi: 10.1158/1078-0432.CCR-07-4367. 14/8/2465 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367–77. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 24.Rexer BN, Ghosh R, Narasanna A, Estrada MV, Chakrabarty A, Song Y, et al. Human breast cancer cells harboring a gatekeeper T798M mutation in HER2 overexpress EGFR ligands and are sensitive to dual inhibition of EGFR and HER2. Clin Cancer Res. 2013;19(19):5390–401. doi: 10.1158/1078-0432.CCR-13-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125(6):1137–49. doi: 10.1016/j.cell.2006.05.013. S0092-8674(06)00584-8 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Red Brewer M, Yun CH, Lai D, Lemmon MA, Eck MJ, Pao W. Mechanism for activation of mutated epidermal growth factor receptors in lung cancer. Proc Natl Acad Sci U S A. 2013;110(38):E3595–604. doi: 10.1073/pnas.1220050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyman DM, Piha-Paul SA, Saura C, Arteaga C, Mayer I, Shapiro GI, et al. Neratinib + fulvestrant in ERBB2-mutant, HER2 non-amplified, estrogen receptor-positive, metastatic breast cancer: preliminary analysis form the phase II SUMMIT trial. Thirty-Ninth Annual CTRC-AACR San Antonio Breast Cancer Symposium; San Antonio, TX, USA. December 6–10, 2016. [Google Scholar]
- 28.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105(6):2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell DW, Gore I, Okimoto RA, Godin-Heymann N, Sordella R, Mulloy R, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37(12):1315–6. doi: 10.1038/ng1671. ng1671 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng D, Ye X, Zhang MZ, Sun Y, Wang JY, Ni J, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Scientific reports. 2016;6:20913. doi: 10.1038/srep20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34(28):3375–82. doi: 10.1200/JCO.2016.66.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gymnopoulos M, Elsliger MA, Vogt PK. Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci U S A. 2007;104(13):5569–74. doi: 10.1073/pnas.0701005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25(3):282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wind S, Schmid M, Erhardt J, Goeldner RG, Stopfer P. Pharmacokinetics of afatinib, a selective irreversible ErbB family blocker, in patients with advanced solid tumours. Clinical pharmacokinetics. 2013;52(12):1101–9. doi: 10.1007/s40262-013-0091-4. [DOI] [PubMed] [Google Scholar]
- 36.Wong KK, Fracasso PM, Bukowski RM, Lynch TJ, Munster PN, Shapiro GI, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15(7):2552–8. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 37.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30(3):256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.