Abstract
Pancreatic adenocarcinoma (PDAC) is the fourth most common cause of cancer death in the United States. PDAC is difficult to manage effectively, with a five-year survival rate of only 5%. PDAC is largely driven by activating KRAS mutations, and as such, cannot be directly targeted with therapeutic agents that affect the activated protein. Instead, inhibition of downstream signaling and other targets will be necessary to effectively manage PDAC. Here, we describe a tiered single-agent and combination compound screen to identify targeted agents that impair growth of a panel of PDAC cell lines. Several of the combinations identified from the screen were further validated for efficacy and mechanism. Combination of the bromodomain inhibitor JQ1 and the neddylation inhibitor MLN4294 altered production of reactive oxygen species in PDAC cells, ultimately leading to defects in the DNA damage response. Dual bromodomain/neddylation blockade inhibited in vivo growth of PDAC cell line xenografts. Overall, this work revealed novel combinatorial regimens, including JQ1 plus MLN4294, which show promise for the treatment of RAS-driven PDAC.
Keywords: Pancreatic adenocarcinoma, combination drug screening, bromodomain inhibitors, neddylation inhibitors, reactive oxygen species
Introduction
Pancreatic cancers are the tenth most prevalent cancer in the US, but disproportionately rank as fourth for cancer deaths (1). 90% of these cancers develop from malignant transformation of pancreatic duct cells resulting in pancreatic ductal adenocarcinoma (PDAC), an aggressively metastasizing exocrine pancreatic cancer. PDAC frequently presents with advanced disease at the time of diagnosis and is associated with a five-year survival rate of only 5% (2,3). Strikingly, it is suggested that pancreatic tumors will be the second most common cause of cancer death in the US by 2030 (4).
Approximately 90% of PDAC harbor mutations in the oncogene KRAS (4-6). These mutations are common in the precursor lesion pancreatic intraepithelial neoplasia and promote pancreatic carcinogenesis in genetically-engineered mouse models (4,7-9). The full extent by which co-driver mutations cooperate with mutant KRAS to promote human PDAC is unclear. The prevalence of KRAS drivers contributes to the difficulty in managing PDAC: RAS proteins are largely “undruggable” owing to their structural properties, and multiplicity of signaling pathways activated by oncogenic KRAS limits the impact of targeting single pathways. Even for other solid tumors with targetable driver mutations, such as BRAF-driven melanoma and EGFR-driven lung adenocarcinoma, durability of treatment response is limited by rapid development of treatment-selected resistance. Some of these issues may be overcome with identification of appropriate combination therapies (10,11). Drug combinations may counteract KRAS signaling by simultaneously interdicting multiple parallel RAS output pathways. Drug combinations may also prevent resistance by countering adaptive epigenetic and signaling changes that restore setpoints perturbed by treatment intervention, and that promote cell survival. Hence, recent efforts have focused on finding combination regimens to slow the development of resistance during treatment and to increase therapeutic response rate (4,6,12).
Conventional chemotherapeutics are used in combination for a variety of tumors, including the FOLFIRINOX (leucovorin, 5FU, irinotecan and oxaliplatin) regimen for PDAC (13). Combination strategies will be critical for better management of PDAC as well as other tumors. In diffuse large B-cell lymphoma, activation of AKT and BTK signaling followed monotherapy treatment with BTK inhibitor ibrutinib and PI3Kα/δ inhibitor copanlisib led to striking improvement in efficacy (14). Cross-resistance to BRAF and MEK inhibitors in melanoma can be overcome with PI3K pathway targeting (15). Reactivation of MAPK signaling, a common consequence of BRAF or MEK inhibition, can be alleviated through upstream inhibition of receptor tyrosine kinases (11). Combined CDK and MEK inhibition triggers both cell cycle arrest and pro-apoptotic pathways in NRAS mutant melanomas (16). Together, these studies illustrate the impact of combination small-molecule regimens in managing cancers that are notorious for therapeutic resistance.
Combination targeted small molecules or biologics have improved benefit for many tumor types, but have not been found effective or approved for treatment of PDAC (17). Furthermore, the “combination space” of therapeutic partners needs more systematic exploration; combination efficacy cannot be readily predicted from single agent efficacy. Compound screening allows for robust empirical and agnostic testing of a myriad of possible therapeutic combinations. We and others have used combinatorial screening to uncover drug combinations active on BRAF inhibitor-resistant and NRAS-driven melanomas, KRAS-driven lung adenocarcinomas, and B-cell lymphomas (18,19). We hypothesize that combinatorial screening will identify novel and effective therapeutic approaches for PDAC that may work by concurrent targeting of multiple signaling pathways activated by KRAS, by forestalling resistance through epigenetic and kinome rewiring that promote resistance, and by promoting apoptosis in the face of stress induced by therapeutic interventions.
Here we present results from a tiered screen beginning with single agent triage of 129 curated agents, most either US FDA-approved or in advanced preclinical and clinical development, followed by a combinatorial screen of forty agents to identify most effective drug combinations that control growth of PDAC cells. The results identify several novel combinations that reduce cell proliferation and promote apoptosis. Agents that contribute most often to effective combinations include SN-38, the active metabolite of irinotecan (topoisomerase I inhibitor), PF-431396 (FAK, PYK2 inhibitor), MLN4924 (neddylation activating enzyme inhibitor), JQ1 (BET family inhibitor), Foretinib (MET, VEGFR2 inhibitor), and GSK1120212 (MEK inhibitor). Of note, the bromodomain inhibitor JQ1 and neddylation inhibitor MLN4924 combine superadditively to halt growth and inhibit viability and colony formation in a broad range of PDAC cell lines, and control human PDAC grown as mouse xenografts. Effectiveness of this combination occurs in part through the induction of reactive oxygen species (ROS) that promote increased DNA damage, leading to apoptosis. This combination and other combinations identified from this screening series should be suitable for further clinical exploration of the PDAC therapeutic arsenal.
Materials and Methods
Cell culture
Cell lines were obtained directly from the American Type Culture Collection (ATCC; Manassas, VA) and cultured in 5% CO2. ATCC uses STR testing to authenticate cell lines. Cell lines were grown in media recommended by ATCC supplemented with 1% penicillin/streptomycin (Gibco) and fetal bovine or horse sera as recommended by ATCC.
Single agent dose-response analysis
High throughput screens were performed at the Yale Center for Molecular Discovery. Single-agent and combination-agent screens were performed essentially as described in (18). For single-agent screening, eleven pancreatic carcinoma cell lines were screened against 140 agents that met quality control standards (PANC-1, PL45, MiaPaCa-2) or 131 agents (the other eight lines tested). Dose response experiments were conducted either in sixteen, 2-fold dilutions or five 8-fold dilutions for overall dose range of 1 nM to 10 μM. Forty agents were chosen for combination compound screening. Single-agent screens were performed in technical duplicate or triplicate and incubated for three days after drug addition. CellTiter-Glo reagent (Pierce) was added on day three to measure total ATP accumulation as a surrogate for cell proliferation. For combinatorial screening, a total of 22 master plates encompassing 40 agents at three three doses each (7503 drug-dose combinations) were produced by robotic hit-picking to distribute single agents, combinations, negative vehicle controls, and positive controls for growth inhibition (10 μM staurosporine). Four pancreatic adenocarcinoma lines that represent a range of overall drug sensitivity (MiaPaCa-2, PANC-1, PL45, and SU-86-86; Supplementary Fig. S3A) were evaluated in triplicate after three days incubation in drugs by CellTiter-Glo assays for effect relative to staurosporine positive control. The analytical pipeline for CellTiter-Glo assays is as described in (18), except that dose-response curves were analyzed differently after quality control and background subtraction. Four parameter log-logistic curves were fit with the R drc library. AUC was calculated with numeric integration, using the base R package, from the EC10 to the highest concentration used. This AUC value was then divided by the concentration range (maximal drug concentration - EC10) to obtain average area in order to compensate for the fact that different drugs were used at different concentration ranges. These “AUC average” values were used for further analyses. The R gplots package heatmap.2 function was used for heatmaps in Figs. 2B and 2C, and Supplementary Figs S2 and S5. Fig. 3 plots were produced using R circlize (20) and R ColorBrewer packages.
Fig. 2. Single agent analysis of PDAC drug sensitivity.
A. Scatter plot of AUC by cell line for each agent. Agents are ordered according to mean AUC across all cell lines, with AUC plotted for each cell line as marked in legend. Each agent is denoted (X axis) by a number indicating rank order of AUC mean from high to low. Agent names and corresponding AUC mean values keyed to rank order are listed in Supplementary Table S2. Solid line, mean AUC for each agent across cell lines plotted with R standard graphics. Dashed lines, 95% confidence interval for mean AUC. B. Heatmap of hierarchical clustering of the subset of agents with greatest heterogeneity after normalization to a uniform mean AUC (Supplementary Fig. S2). The heatmap is colored green to black to red with ascending normalized AUC as shown in the legend. C. Heatmap of AUC for each target class, with clustering by cell line and by target.
Fig. 3. Response to single agents and drug combinations.
A. Sensitivities (AUC) of individual cell lines to single agents, ordered by categories of drug targets. Links connect agents for which the linked cell line(s) have normalized AUC value of 40 or greater. B. Highest-ranked drug combinations. Links mark drug combinations with six or more drug/dose observations surviving data filtering as described in the text and listed in Table 1. Link widths increase with number of drug-dose combinations.
PCA
Fig. 4A. Drug response was evaluated by average AUC, with drug response scored using a cutoff of AUC = 50. Then, a matrix was constructed with cell lines versus drug categories with the values being the ratio of responders to non-responders in each category for each cell line. PCA was performed on this matrix. Fig. 4B. Direct and indirect associations inferred between genes and drug sensitivity or resistance were manually curated from the published research literature and compiled in a database. Genes were categorized by grouping them according to the drug categories to which their associated drugs belong. Some genes that are associated may fall into more than one category.
Fig. 4. Principal component analysis (PCA) of single agent data.

A. PCA of single-agent drug responses (AUC) across cell lines. The proportion of drugs in each category with AUC > 50 was considered as the overall effectiveness of the drug category. The arrows show the “directions” of drug effectiveness with the individual cell lines being projected on this space. Cell lines that map away from the origin and in the direction opposite to the arrows are inferred to show lack of sensitivity. The circle denotes the 95% confidence region. B. PCA of transcription profiles of the genes encoding targets of the drugs in each category. Expression levels for each gene in each cell line were transformed to tertiles among all pancreatic cancer cell lines in the CCLE database (21). Genes in the highest tertile were considered to be up-regulated and called as “up”, and those in lowest tertile were called as “down”. PCA compared the proportion of targets in each drug category that were called as “up” or “down” for each cell line.
Log2 expression values from the publicly available CCLE expression profiling data (21) were transformed to tertiles for each cell line relative to all pancreatic cancer cell lines in the CCLE data set. A matrix was constructed with cell lines versus drug-associated genes by category, up- and down-regulated. The values are the ratio of drug associated genes in the first tertile for up-regulated and the third tertile for down-regulated. PCA was performed on this matrix.
Proliferation assays
Cells were seeded in 96-well, clear-bottom cell culture plates at 1500 cells/well. Agents were added the next day. Three days later, cells were assayed with Cell Titer-Glo to measure ATP accumulation. Combination indices were calculated with CompuSyn software (ComboSyn).
Clonogenicity assays
Colony formation assays were performed as described (22).
Measurement of mitochondrial superoxide
Su.86.86 cells were seeded at ∼60% confluence and allowed to attach overnight. The next day vehicle, JQ1, MLN4924 and trolox were added as indicated. Approximately 36 hours later cells were detached from the substrate using 0.25% trypsin, washed once in pre-warmed complete medium and then re-suspended in complete medium. Each treatment was split into two tubes, one receiving MitoTracker Green FM (ThermoFisher Scientific) (50nM) and the other MitoSOX Red (ThermoFisher Scientific) (200nM). The cells were then incubated for an additional 30 min. at 37°C. Cells were next washed once with PBS and the overall level of staining assessed by flow cytometry.
Measurement of DNA damage
Su.86.86 cells were seeded at ∼60% confluence and allowed to attach overnight. The next day vehicle, JQ1, MLN4924 and trolox were added as indicated. Approximately 70 hours later etoposide (100μM) was added to an untreated well as control and allowed to incubate at 37°C for 2 hours. Following this period cells were detached from the substrate by trypsinization, washed, and the amount of total and γ-H2A.X was determined by flow cytometry using the FlowCellect DNA Damage Histone H2A.x Dual Detection kit (Millipore) according to the manufacturer's recommendations. The percentage of cells staining positive for γ-H2A.X was determined by gating for fluorescence greater than cells stained for total H2A.x alone.
Measurement of Apoptosis
Su.86.86 cells were seeded at ∼60% confluence and allowed to attach overnight. The next day vehicle, JQ1, MLN4924 and trolox were added as indicated. Approximately 70 hours later cells were detached from the substrate by trypsinization, washed, and the amount propidium iodide (PI) and annexin V (AV) staining were determined by flow cytometry using the FITC Annexin V Apoptosis detection kit (BD Pharmingen) according to the manufacturer's recommendations. Positivity for AV or PI was determined by gating for fluorescence greater than cells stained for PI or AV alone, respectively.
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at Yale University and conformed to the legal mandates and federal guidelines for the care and maintenance of laboratory animals. Female J:NU nude mice were obtained from Jackson Laboratory and used when five to six weeks old. MiaPaCa-2 cells were subcutaneously implanted in the dorsal flank of nude mice. When the size of the xenograft (n = 3-5 per group) reached approximately 80-120 mm3, the mice were randomized and intraperitoneally treated with JQ1 (50 mg/kg, daily) and/or MLN4924 (25 mg/kg, every other day) for 14 days and showed significant synergistic effect. Control mice were treated with vehicle (5% Polyethylene glycol in PBS). The volume of tumors was calculated in Excel (Microsoft) using the formula v= length (mm) × width (mm)2.
Results
Single agent screening of pancreatic adenocarcinoma cell lines
We first developed a custom panel of 129 agents with: i. broad coverage of the target landscape, including standard-of-care agents (genotoxins, antimetabolites, and cytotoxic drugs); ii. signaling targets including RAS-activated pathways; iii. pro-apoptotic agents; iv. cell cycle-active agents; and v. agents that may be preferentially active under hypoxia or conditions of metabolic stress (Supplementary Table S1). The panel included agents of special interest for PDAC and lung adenocarcinoma that were analyzed in parallel. Where possible, we chose US FDA-approved agents or agents in advanced therapeutic development in order to facilitate rapid clinical translation.
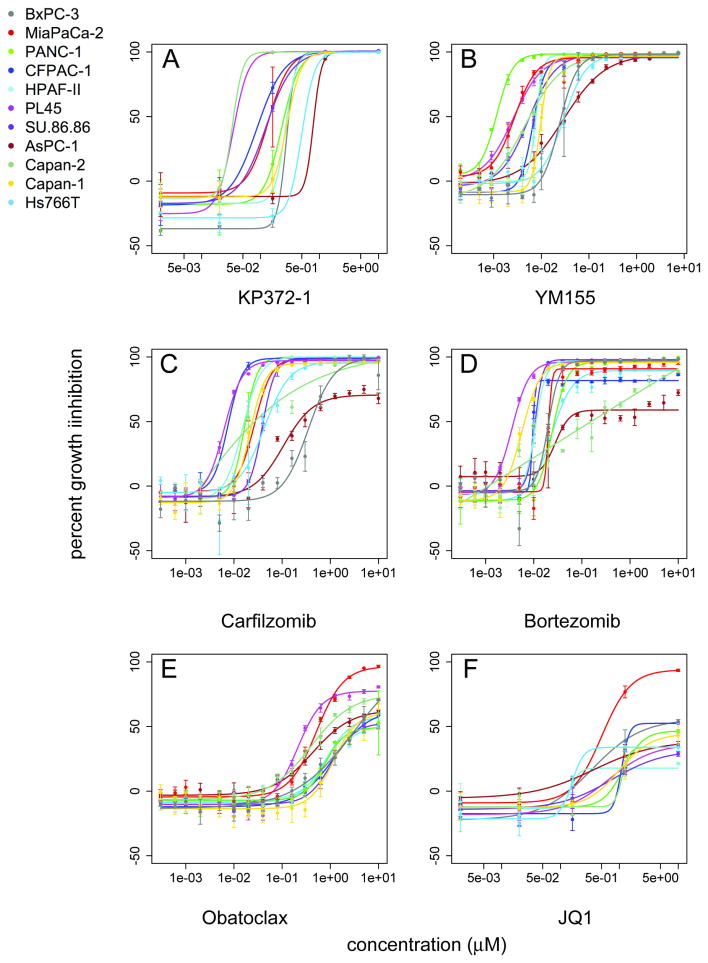
In initial experiments, eleven pancreatic cell lines were evaluated in three day CellTiter-Glo ATP assays in multi-point dose-response experiments (Fig. 1, Supplementary Tables S1 and S2). Cell lines displayed a range of sensitivities to the agents for maximal effect or for potency or for both (for example, compare Fig. 1B, YM155 to Fig. 1D, bortezomib). The responses were quantified using average area under the curve (AUC) calculations in order to capture differences in both potency and maximal effect as a single metric. Substantial responses to at least half of the agents were observed in all cell lines (Fig. 2A, Supplementary Fig. S1). Responses of individual cell lines generally tracked with the mean AUC of all the cell lines, marked with a solid line in Fig. 2A (agent numbers in X axis correspond to AUC rank listed in Supplementary Table S2.) Agents yielding highest mean AUC across cell lines are PDK1 inhibitor KP372-1 (Fig. 1A), followed by survivin inhibitor YM155 (Fig. 1B), proteasome inhibitors carfilzomib (Fig. 1C) and bortezomib (Fig. 1D), transcription inhibitor dactinomycin, sodium-potassium ATPase inhibitor digoxin, triptolide, topoisomerase inhibitor SN-38, and IKK-2 inhibitor IMD-0354 (Supplementary Table S2).
Fig. 1. Single-agent screening of pancreatic adenocarcinoma cell lines.
Dose response curves were generated from CellTiter-Glo growth inhibition experiments following 72 hours of incubation with test agents. Drug concentration (μM) is plotted against percent growth inhibition relative to positive control for maximal growth inhibition. A. KP372-1, B. YM155, C. carfilzomib, D. bortezomib, E. obatoclax, and F. JQ1.
Sensitivity of cell lines to single agents
There was a two-fold difference in overall drug sensitivity of cell lines (mean AUC across all agents tested), ranging from the most sensitive line MiaPaCa-2 (mean AUC, 30.1) to the least sensitive line CFPAC.1 (mean AUC, 14) (Supplementary Fig. S3, left panel). Hierarchical clustering of mean AUC suggested a remarkably similar pattern of responses to single agents across cell lines (Supplementary Fig. S1), which was even more uniform after normalization of AUC values to a common mean AUC across agents (Supplementary Fig. S2). (BxPC-3 was omitted from Fig. 2B and from most other analyses as marked, since this line was analyzed against a smaller number of agents [Supplementary Fig. S3, left panel]. A heat map including BxPC-3 is shown in Supplementary Fig. S4.) Agents eliciting heterogeneous responses, as shown in Supplementary Fig. S2, were re-clustered (Fig. 2B) to enhance visualization of patterns of drug responses across cell line subsets. This revealed some differences, but they were modest in comparison to our similar analyses of melanoma cells (18).
Agents that were effective across cell lines included GDC-0941, vandetanib, dasatinib, EHT-1864, dovitinib, rapamycin, I-BET, JQ1, Gemcitabine, BMS-754807, simvastatin, trifluorothymidine, lovastatin, atorvastatin, and CYT-387. Two lines that were most resistant overall (CFPAC-1 and SU-86-86), show more sensitivity to atorvastatin (HMGCoA reductase inhibitor), MLN4924 (neddylation inhibitor), ABT-263 (BCL-2 family), MK-1775 (WEE1), and Stattic (STAT3) than other lines. By contrast, these lines are more resistant to inhibitors affecting single pathway arms modulated by or contributing to RAS activity including AKT inhibitors MK-2206 and GSK690693; p38 inhibitor CMPD-1and farnesyl transferase inhibitor tipifarnib (Fig. 2B).
Functional categories of targets for single agents
We next evaluated sensitivity to single agents (AUC) in the context of drug target category denoted in Supplementary Table S1 (Fig. 2C, 3A, Supplementary Fig. S4, Supplementary Table S3). To facilitate comparison of drug response patterns across cell lines, data were analyzed after normalization of mean AUC for each cell line. By target category, cells were most sensitive to drugs affecting protein stability and localization (neddylation inhibitor MLN4924, which indirectly affects activity of E3 ubiquitin ligases and targeting to proteasomes; proteasome inhibitors; eEF-2 kinase inhibitor NH125), and agents that promote apoptosis (BCL-2 inhibitors ABT-263 and obatoclax; and survivin inhibitor YM155). A third high-ranking category is composed of “immune signaling” agents (including NF-κB inhibitors Bay-11-7082 and Triptolide, IKK inhibitor IMD 0354, JAK inhibitor CYT-387, STAT inhibitor Stattic, and chemokine receptor inhibitor BX513). Agents belonging to HIV, metabolism, WNT/HH signaling and “other signaling” categories were generally least effective. Some inhibitor categories including “Cell cycle”, “DNA/RNA”, “PI3K” “MAPK” “WNT,HH signaling” and “stress” varied in efficacy, and were more heterogeneous among cell lines, as each category included some effective and some ineffectual agents. (Fig. 2C, Supplementary Fig. S5). Notably, resistant cell lines SU-86-86 and CFPAC-1 were comparatively resistant to agents in MAPK and WNT/HH signaling categories.
Principle Component Analysis (PCA)(Fig. 4)
Functional associations were also investigated by principal component analysis (Fig. 4A). Specifically, drugs in “metabolism” and “epigenetic” categories were connected with similar activity patterns as single agents across cell lines and they were particularly effective against MiaPaCa-2 cells. Drugs in the “immune”, “DNA/RNA”, “stress”, “TK”, “RTK” and “MAPK” categories were similarly active and particularly effective against Capan.2, HPAF.II and PL45 cells (Fig. 4A). Single agent drug sensitivity was also compared to transcription profiles of these cell lines available through the Cancer Cell Line Encyclopedia, CCLE, (21). PCA of the expression patterns of the genes that encode for proteins targeted by drugs in the different categories suggest that sensitivity of MiaPaCa-2 cells to “epigenetic” drugs and of Capan-2 and PL45 cells to “immune”, “DNA/RNA” and “RTK” drugs correspond to low relative expression of the corresponding targets (Fig. 4B).
Comparison to other single agent drug response collections
Other groups have published single-agent screens of PDAC cell lines, including the CCLE, and the Genomics of Drug Sensitivity of Cancer (GDSC) (21,23,24). Where these datasets overlap, they produce partly inconsistent results for a variety of reasons (25,26), and independent analyses provide important information on the variation in outcomes of such assays. We compared results of CCLE, GDSC, and our dataset (current data, denoted “Yale”) to evaluate their relatedness (Supplementary Fig. S6). Cell lines were compared individually, as the overlap in drugs tested varied by cell line. Overall, there was substantial correlation in pairwise combinations of the three sets, with differences among P values partly driven by varying overlap in drugs tested (and hence number of comparisons) among the pairwise combinations.
Combinatorial screen
Use of single targeted agents for treatment of RAS-driven cancers, including nearly all PDAC, has been largely unproductive, prompting interest in combination therapies. Armed with information from the single agent screen that identified most effective agents and revealed drug dose sensitivities, we selected a panel of forty agents for combinatorial screening. These included conventional agents and agents targeting a broad range of cancer-associated targets (Supplementary Table S1). Criteria for selection included effect in single agent analyses, maximal coverage of target range, and likelihood of success of the agent or related agents in therapeutic development. These agents were screened at three doses each in all pairwise combinations, and in parallel as single agents at the same concentrations. We prefer to use agents at concentrations approximating GI10, GI25, and GI50 values based on single agent assays, but, with intrinsic differences in drug sensitivity across cell lines (Supplementary Fig. S3, left), choices for each agent dose-point were made manually, taking into consideration the range of dose responses for each agent over multiple cell lines.
After preliminary data processing for background subtraction and quality control (Methods), additional analysis was performed to identify drug-concentration combinations that were informative (i.e. substantial inhibition of cell growth) and super-additive. Data were filtered to include only drug combinations where the single agent response was between 15% and 80%; the combination yielded at least 50% of maximal growth inhibition; the combination yielded a 10% or more increase in growth inhibition over a prediction metric (Bliss additive sum) calculated from the single agent responses at the same concentrations; and where the replicate data points show less than 15% standard deviation. This stringent filtration series reduced the number of observations surviving quality control by 94% (PL45 and SU.86.86) and 97% (MiaPaCa-2 and PANC-1). Filtered drug combinations were then rank-ordered according to the highest number of positively interacting drug-dose combinations across the four cell lines tested (Table 1).
Table 1. Top drug combinations, in rank order.
| combination | number observationsa | agent 1 targetb | agent 2 targetb | Rankc |
|---|---|---|---|---|
| Flavopiridol & JQ1 | 11 | CDK | Bromodomain | 1 |
| Flavopiridol & PF-431396 | 10 | CDK | FAK/PYK2 | 2 |
| Gemcitabine & JQ1 | 10 | nucleoside analog | Bromodomain | 2 |
| GSK1120212 & PF-431396 | 9 | MEK1 & 2 | FAK/PYK2 | 3 |
| JQ1 & MLN-4924 | 9 | Bromodomain | Neddylation | 3 |
| SB225002 & YM155 | 9 | CXCR2 | Survivin | 3 |
| Foretinib & GDC-0941 | 8 | MET & AXL &… | PI3K | 4 |
| GDC-0941 & GSK1120212 | 8 | PI3K | MEK1 & 2 | 4 |
| JQ1 & SN-38 | 8 | Bromodomain | Topoisomerase | 4 |
| MLN-4924 & PF-431396 | 8 | Neddylation | FAK/PYK2 | 4 |
| AZD-8055 & SN-38 | 7 | mTOR-specific | Topoisomerase | 5 |
| BI-2536 & JQ1 | 7 | Polo-like kinase | Bromodomain | 5 |
| Dasatinib & Foretinib | 7 | Abl & Src | MET & AXL &… | 5 |
| Flavopiridol & GSK1120212 | 7 | CDK | MEK1 & 2 | 5 |
| Foretinib & GSK1120212 | 7 | MET & AXL &… | MEK1 & 2 | 5 |
| GDC-0941 & PF-431396 | 7 | PI3K | FAK/PYK2 | 5 |
| MLN-4924 & SN-38 | 7 | Neddylation | Topoisomerase | 5 |
| SB225002 & SN-38 | 7 | CXCR2 | Topoisomerase | 5 |
| SN-38 & Simvastatin | 7 | Topoisomerase | HMGCoA Reductase | 5 |
| AZD-7762 & SN-38 | 6 | Chk1/2 | Topoisomerase | 6 |
| AZD-8055 & Foretinib | 6 | mTOR-specific | MET & AXL &… | 6 |
| AZD-8055 & MK-1775 | 6 | mTOR-specific | p53/ wee1 | 6 |
| BI-2536 & BRD-7389 | 6 | Polo-like kinase | p90 RS6K | 6 |
| BI-2536 & Dasatinib | 6 | Polo-like kinase | Abl & Src | 6 |
| BYL-719 & Flavopiridol | 6 | PI3Kalpha | CDK | 6 |
| Dasatinib & GSK1120212 | 6 | Abl & Src | MEK1 & 2 | 6 |
| Dasatinib & PF-431396 | 6 | Abl & Src | FAK/PYK2 | 6 |
| Flavopiridol & Simvastatin | 6 | CDK | HMGCoA Reductase | 6 |
| Foretinib & MK-1775 | 6 | MET & AXL &… | p53/ wee1 | 6 |
| Foretinib & SB225002 | 6 | MET & AXL &… | CXCR2 | 6 |
| Foretinib & Vorinostat | 6 | MET & AXL &… | HDAC | 6 |
| GDC-0941 & SN-38 | 6 | PI3K | Topoisomerase | 6 |
| Gemcitabine & MLN-4924 | 6 | nucleoside analog | Neddylation | 6 |
| GSK1120212 & MK-2206 | 6 | MEK1 & 2 | AKT1-3 | 6 |
| JQ1 & MLN-8237 | 6 | Bromodomain | Aurora Kinase | 6 |
| JQ1 & Pemetrexed | 6 | Bromodomain | folate agent | 6 |
| JQ1 & PF-431396 | 6 | Bromodomain | FAK/PYK2 | 6 |
| MK-1775 & SN-38 | 6 | p53/ wee1 | Topoisomerase | 6 |
| MLN-4924 & MLN-8237 | 6 | Neddylation | Aurora Kinase | 6 |
| MLN-4924 & Oligomycin | 6 | Neddylation | OxPhos | 6 |
| MLN-4924 & Pemetrexed | 6 | Neddylation | folate agent | 6 |
| MLN-8237 & SN-38 | 6 | Aurora Kinase | Topoisomerase | 6 |
| Paclitaxel & SB225002 | 6 | microtubule | CXCR2 | 6 |
| Paclitaxel & SN-38 | 6 | microtubule | Topoisomerase | 6 |
| Simvastatin & YM155 | 6 | HMGCoA Reductase | Survivin | 6 |
| SN-38 & YM155 | 6 | Topoisomerase | Survivin | 6 |
Combinations with six or more observations meeting filtering and thresholding criteria described in the text are listed.
Nominal “Targets” listed for each agent were curated manually from the research literature, may not apply to all drug doses, and are not all-inclusive.
Combinations with an identical number of observations meeting criteria have identical ranks and are ordered alphabetically.
All but one of the agents tested contributed to one or more combinations meeting filtering criteria, but the frequency distribution varied considerably by agent (Supplementary Table S4). Agents that most frequently combined with other agents to pass through the filter series were the pan-CDK inhibitor Flavopiridol (5.3% of combinations), topoisomerase inhibitor SN-38 (5%), FAK/PYK inhibitor PF-431396 (4.5%), neddylation inhibitor MLN4924 (4.5%), bromodomain inhibitor JQ1 (4.3%), multi-targeted receptor kinase inhibitor Foretinib (4%), and MEK inhibitor GSK1120212/Trametinib (4%). Together, these agents comprise 17% of the agents tested, but contributed to 31% of the effective combinations. Agents categorized as modulators of cell cycle (18.4%), DNA and RNA metabolism (17.9%), and the PI3K pathway (13%) were the most common of the filtered data set, and together account for close to 50% of combinations.
A subset of combinations was chosen for further analysis based on the above ranking, their novelty, and the potential of the agents or in-class analogs for clinical translation. The first group includes agents that are US FDA-approved, or have the same target class as agents in clinical trials: simvastatin plus vorinostat (both US FDA-approved), simvastatin plus SN-38 (active metabolite of FDA-approved irinotecan), and foretinib plus PI3K inhibitor GDC-0941. A second group includes two combinations that are not represented in clinical trials: the RSKp90 inhibitor BRD-7389 plus vorinostat, and the IKK inhibitor IMD-0354 plus flavopiridol. Finally, we evaluated three combinations with JQ1, a bromodomain inhibitor: gemcitabine, which has been approved by the US FDA for treatment of PDAC, piperlongumine, which induces reactive oxygen species (ROS), and neddylation inhibitor MLN4924.
Synergy analysis
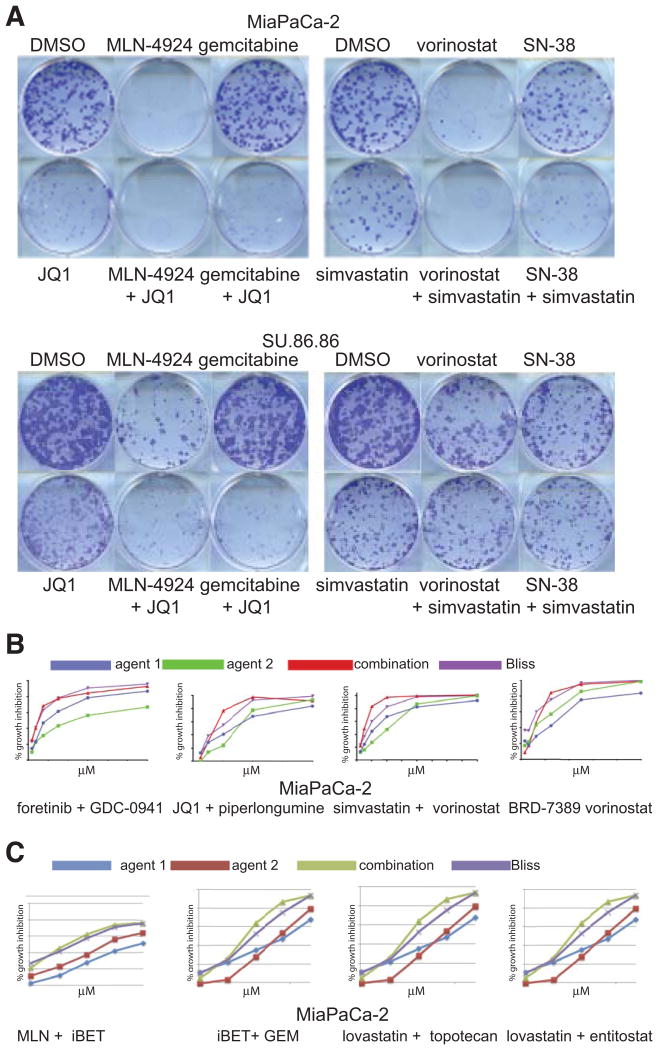
In clonogenicity assays, combinations of JQ1 plus MLN4924, JQ1 plus gemcitabine, vorinostat plus simvastatin, and SN-38 plus simvastatin all showed substantially greater effect combined than when used alone (Fig. 5A). To test whether combination effects applied to target class rather than idiosyncratic qualities of a particular agent, we evaluated effects of agents with similar targets using Cell-Titer Glo ATP-based assays (Fig. 5C). Bromodomain inhibitor i-BET762, (JQ1 biosimilar), worked additively with MLN4924, as well as with gemcitabine. Likewise, lovastatin performed similarly to simvastatin, topotecan to SN-38, and entitostat to vorinostat. Similar results were obtained with SU.86.86, which were more resistant.
Fig. 5. Impact of selected drug combinations on cell growth and clonogenicity.
A. Clonogenic colony formation assays for MiaPaCa-2 and SU.86.86 cells incubated with DMSO vehicle control, single agents, or drug combinations as marked. B. CellTiter-Glo dose responses for single agents and drug combinations. Combination index values calculated from these and replicate experiments are listed in Supplementary Table S5. C. Target class analysis. Dose-response CellTiter-Glo ATP accumulation curves of drug combinations that affect the same target classes as some of the high-ranked drug combinations.
The small number of data points evaluated in combinatorial screening is inadequate for true synergy analysis, so we performed manual isobologram analyses of some candidate combinations (Fig. 5B). We found that candidate combinations matched or exceeded additive phenotypes suggested in screens (Bliss additivity model) (Fig. 5B). At the concentrations tested, Chou-Talalay analyses yielded combination indices (CI) generally indicative of additivity for simvastatin plus vorinostat, and JQ1 plus piperlongumine, or of antagonism for flavopiridol plus IMD-0354. Foretinib plus GDC-0941 was synergistic in MiaPaCa-2 and SU.86.86 cells, whereas BRD-7389 plus vorinostat were antagonistic in MiaPaCa-2 cells and synergistic in SU.86.86 cells (Supplementary Table S5).
JQ1 and MLN4924
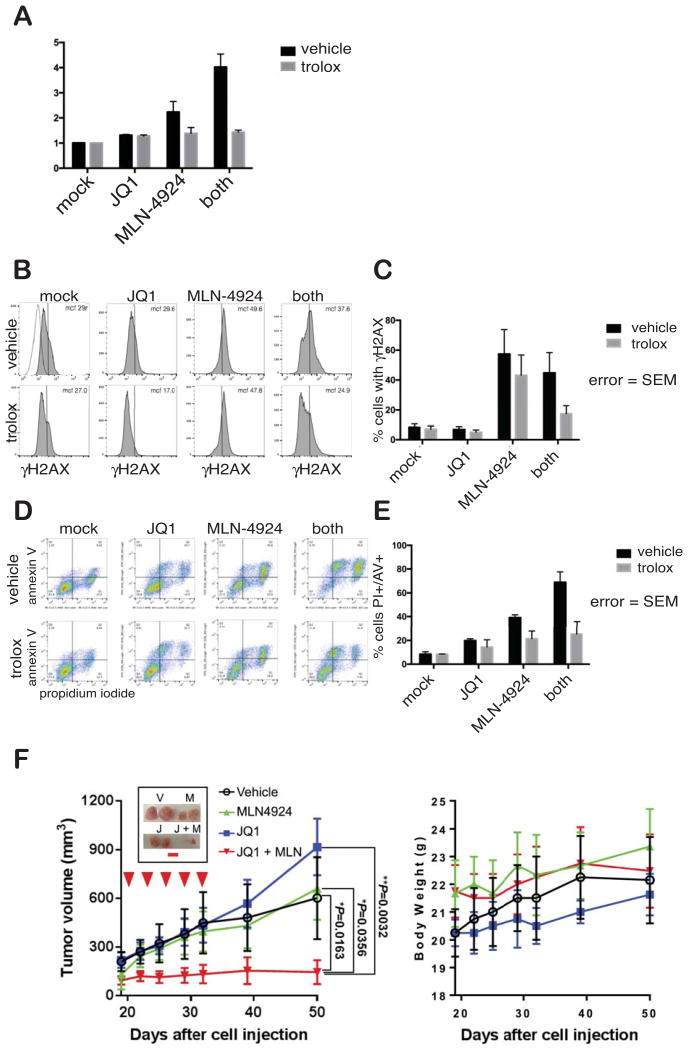
We chose to follow up on JQ1 plus MLN4924 as it was one of the higher ranked combinations; because bromodomain inhibitors and MLN4924 are moving through clinical trials; and, finally, because both bromodomains and neddylation are vanguard targets for new categories of therapeutic agents. Importantly, combination of JQ1 and MLN4924 suppressed growth of MiaPaCa-2 mouse xenografts under conditions where the single agents were ineffectual (Fig. 6F, left). This reinforces the potential for substantial improvement over single-agent responses with this combination regimen. Furthermore, the combination was tolerated without loss of weight, indicating a lack of general toxicity (Fig. 6F, right).
Fig. 6. MLN4924 alone and in combination with JQ1 modulates oxidative stress of Su.86.86 cells to promote DNA damage and apoptosis.
A. Reactive oxygen species levels detected with MitoSOX Red mitochondrial superoxide indicator (Molecular Probes) in Su.86.86 cells. B. DNA damage determined by flow cytometry of cells immunostained for γ-H2A.X after incubation withy JQ1 and/or MLN4924 in the absence or presence of anti-oxidant Trolox. C. Quantification of panel B. D. Annexin V/propidium iodide flow cytometry of Su.86.86 cells after approximately 70 hours of treatment with indicated compounds. E. Quantification of panel D. F. MiaPaCa-2 xenografts are sensitive to dual JQ1 and MLN4294 treatment. Inset shows representative tumors post-treatment, with red scale bar representing one cm. Left Panel. Tumor volumes were calculated based on caliper measurements. Right Panel. Body weight was measured to determine the gross toxicity. Statistical analysis was done by one-way ANOVA.
JQ1 or MLN4924 used alone modestly induced apoptosis, evaluated by flow cytometric analysis of Annexin V/Propidium Iodide levels (Fig. 6D, 6E), but apoptosis was significantly heightened by their combination. MLN4924 increased formation of phospho-epitope γ-H2AX, which marks sites of DNA damage and is essential for the DNA damage response. In contrast, JQ1slightly reduced γ-H2AX when used alone in combination with MLN4924 (Fig. 6B,6C). A recent report described enhanced ROS production induced by MLN4924 treatment of gastric adenocarcinoma cells (27), so we next determined the impact of JQ1 and MLN4924 on ROS (Fig. 6A). While JQ1 only modestly increased ROS levels, MLN4924 strongly induced ROS. Strikingly, the JQ1/MLN4924 combination induced the highest level of ROS. Importantly, these effects were largely reversed by incubation of cells with the anti-oxidant Trolox (Fig. 6A). Incubation with Trolox also significantly reduced phospho-γ-H2AX and increased survival in the presence of MLN4924 alone or in combination with JQ1. We conclude that the MLN4924/JQ1 combination promotes apoptosis through a mechanism involving DNA damage mediated by ROS and through other means augmented by co-treatment with bromodomain inhibitor JQ1.
Discussion
We report here a tiered single agent and combinatorial compound screen designed to identify novel treatment approaches for PDAC. We identified forty-six candidate drug combinations. In contrast to our findings with melanoma (18), and lung adenocarcinoma, the pattern of drug responsiveness across the cell line panel was remarkably consistent. Effective single agent inhibitors most commonly targeted processes of protein localization, degradation, and post-translational modification, as well as apoptosis, and immune cell response signaling. Inhibition of protein and lipid kinases downstream of KRAS in the MAPK and PI3K pathways yielded intermediate and variable responses. Agents that were most frequently represented in effective drug pairs included flavopiridol, JQ1, MLN4924, SN-38, PF-431396, foretinib, and trametinib. Pairwise combination of these seven agents reduced clonogenic colony formation and were often super-additive. The combination of JQ1 plus MLN4924 promotes cell death by inducing DNA damage enhanced by the accumulation of ROS.
GDSC and CCLE data are partly inconsistent with each other, and with our data as well. Causes for inter-study variability in such datasets have been discussed exhaustively (25,26,28). They can begin with differences in the cell lines tested, arising from phenotypic drift of independent cell cultures, and include technical differences in mechanics of the screens in liquid and cell handling and variations in data analysis. In contrast to GDSC and CCLE (which used FBS-supplemented RPMI, DMEM, or DMEM/F12), we used cell culture media recommended by American Type Culture Collection, which may reduce cell stress and also affect responses to specific growth inhibitors. The current study, and the CCLE assayed cell accumulation with CellTiter-Glo, which measures ATP levels, which generally tracks with cell number and size, and is affected by metabolic changes. In contrast, the GDSC used SYTO 60, a nucleic acid stain, which is more variable than Cell TiterGlo (28). We extended analysis of some combinations with clonogenic and apoptosis assays to confirm that that reduced aggregate ATP was associated with changes in cell number and viability. These differences reinforce the value of comparing drug screening hits from different sources (26).
Most PDAC tumors are driven by activating mutations in KRAS. PDAC is intrinsically resistant to the majority of chemotherapy and targeted therapeutic agents. KRAS activating mutations have proven difficult to attack in the clinic because of the challenging pharmacologic properties of RAS proteins. The multiplicity of signaling outputs of activated KRAS also reduce impact of targeted monotherapies, which are unable to substantially quench all possible “escape routes” following downstream pathway blockade. This leads to both ab initio and acquired resistance to small molecule monotherapies. Therefore, combination small molecule therapies, perhaps in further combination with other treatment modalities such as immune checkpoint inhibition, will be needed to effectively manage and treat PDAC patients. Thus far, the approach of targeting multiple KRAS signaling outputs has been defeated by toxicities, so we have used empirical screening to identify other possibilities.
A few high-ranked combinations have been reported by others. One study revealed that proteasome inhibitors in combination with gemcitabine impede growth of PDAC models more effectively than single agents alone (29). Precursor PDAC lesions rely on proteasomal activity as an initiating factor in Kras-mutant mouse models of PDAC (30). Indirect inhibition of E3 ubiquitin ligases with NEDD8-activating enzyme inhibitor MLN4924 radiosensitizes PDAC cells (31). Hence PDAC may rely on tight regulation of proteostasis, perhaps in the context of active KRAS.
Modulating apoptotic proteins with anti-BCL2 family agents or SMAC mimetics inhibits survival of PDAC cells. Combination of MEK and BCL-2 family antagonists such as navitoclax augments growth inhibition of PDAC cells over each individual agent (32), but toxicity concerns make clinical implementation of this combination challenging. The combination of gemcitabine with SMAC mimetic JP1201 inhibits PDAC growth and may prove to be more tolerable, as suggested in pre-clinical PDAC xenografts and transgenic mouse models (33). Several of our top combinations affected immune/inflammatory signaling pathways. Immune signaling is an important component of PDAC development (34-36) and apoptosis modulators work in conjunction with immune response signaling pathways, such as JAK/STAT3/NF-κB axis control of BCL-2 expression (37). STAT3 promotes PDAC with mutant KRAS in PDAC mouse models (38). Nonetheless, immune-related transcriptional regulators are difficult to target using small molecules, and their inhibition may result in undesired effects on immune cell function. Indirect inhibition of STAT3 and NF-κB signaling with the natural product nexrutine partially inhibits growth of PDAC cells and induces apoptosis (39). The involvement of proteasomes in activation of NF-κB through destruction of IκB may contribute to the sensitivity of PDAC to proteasome inhibition.
MEK, PI3K, CDK, neddylation, and bromodomain inhibition were over-represented in our ranking of combinations with the greatest growth inhibitory effects on PDAC cell lines. MEK, PI3K, and CDK inhibitors have been found effective in pre-clinical models of RAS mutant tumors (40,41). The combination of multi-receptor tyrosine kinase (RTK) foretinib with the pan-PI3K inhibitor GDC-0941 synergistically inhibited growth of both MIAPaCa-2 and SU.86.86 cells. Foretinib inhibits the activity of several Type III and V RTKs plus other receptors including c-MET and AXL (42). The interplay between these two signaling nodes drives targeted therapy resistance in other cancer types, including breast carcinoma and melanoma (22,43,44). Furthermore, PI3K pathway activation limits the effectiveness of dual MAPK and RTK blockade in PDAC cells which illustrates the importance of these pathways in maintaining PDAC (10). Additional study on the combination of foretinib and GDC-0941 (or like agents) is warranted based on our findings.
We show for the first time that the combination of the JQ1 bromodomain inhibitor and MLN4924 neddylation inhibitor is highly effective for PDAC in both in vitro and in vivo studies. Agents with these target classes are now in clinical trials, and it can be anticipated that treatment-selected resistance will become an issue for agents with each of these target classes. Hence, it is noteworthy that we identified a series of effective combination partners both for JQ1 and for MLN4924. JQ1 combined well with gemcitabine, BI-2536, MLN-8237, pemetrexed, and PF-431396. MLN4924 combination partners included PF-431396, SN-38, MLN-8237, oligomycin, and pemetrexed. Of note, gemcitabine, SN-38 (as irinotecan), and pemetrexed are all FDA-approved, too.
Bromodomain inhibitors simultaneously affect a broad range of transcriptional targets, and a number of mechanisms may explain their impact on carcinoma cells. Bromodomain inhibitors can suppress MYC-driven tumors by disrupting bromodomain binding to the super-enhancer regions of oncogenes (45,46). In PDAC models, bromodomain inhibitors can also mimic the tumor suppressive roles of the SWI/SNF catalytic subunit BRG1 (47). The neddylation activating E1 enzyme NAE, which proteolytically activates NEDD8 for subsequent transfer to neddylation targets, is inhibited by MLN4924 (48). A major target is the Cullin component of RING E3 ubiquitin ligases (CRL), which are activated by neddylation to enhance ubiquitination of CRL target proteins. By inhibiting CRL, MLN4924 reduces NF-κB activity by interfering with IκK ubiquitination (49) and favors accumulation of replication licensing factor CDT1. Moreover, reversible neddylation is an important and intrinsic component of DNA damage responses including nucleotide excision repair of double-strand DNA breaks through non-homologous end joining (NHEJ) (50). At least some of the anti-neoplastic effects of MLN4924 have been attributed to DNA damage (51,52)Through these mechanisms, inhibition of NAE leads to DNA damage, DNA re-replication, and S phase or G2 arrest (53). Furthermore, MLN4924 is effective at inhibiting growth of several cancer types including osteosarcoma (54-56). Indeed, we found that this agent induces DNA damage in PDAC cell lines. ROS accumulated concurrently, and DNA damage induced by MLN4924 was reduced in the presence of TROLOX. MLN4924 similarly induces ROS-dependent DNA damage in gastric carcinoma cells and in hepatocellular carcinoma cells (27,57). Hence, MLN4924-generated ROS is a major source of DNA damage observed in the PDAC cells treated with JQ1 and MLN4924. This is consistent with our finding that JQ1 super-additively enhances the growth inhibitory capacity of piperlongumine, which also induces ROS (58).
In addition to the tumor cell-autonomous mechanisms evaluated here in tissue culture, non-tumor cell-autonomous mechanisms may increase impact of dual JQ1 and MLN4924 treatment in vivo. JQ1 can interfere with tumor stroma and cause microenvironmental remodeling that is less suitable for tumor growth (59). Recent evidence also suggests that JQ1 can also enhance the in vivo persistence of T-cells and further augment the effectiveness of chimeric antigen receptor-transduced T-cells in leukemic models (60). This suggests a pro-immunogenic function of JQ1 that can be exploited with immune therapies as well as small molecule inhibitors. MLN4924 may also work through non-tumor-autonomous anti-neoplastic functions. Consistent with our results, MLN4924 induced DNA damage in chronic lymphocytic leukemia (CLL) (53). Moreover, MLN4924 circumvented stroma-mediated resistance to several agents (49). MLN4924 can also disrupt tumor angiogenesis in xenograft and human endothelial cell culture models by disrupting the DNA damage response in endothelial cells (61).
Combination treatment regimens will be critical for improving therapeutic response in PDAC. Herein, we describe a screening strategy to maximize the combinatorial space within a small molecule library with both a broad target profile and single agent efficacy in PDAC cell lines. The interplay between ROS and DNA damage following bromodomain and neddylation inhibition suggests a novel strategy for the treatment of PDAC. Given the favorable in vivo results in this study with limited toxicity, we propose that dual bromodomain and neddylation inhibition is a tractable combination treatment regimen for PDAC patients. This combination, which includes two new drug target classes that are in human clinical trials, and many of the other 46 top-ranked combinations warrant further exploration for their potential to improve treatment for this devastating disease.
Supplementary Material
Acknowledgments
We are grateful to Sheila Umlauf and Janie Merkel, who were instrumental in implementing the single agent and combinatorial screens at the Yale Center for Molecular Discovery. We thank Huifang Zhu for contributions to the mouse xenograft experiment in Fig. 6F, and we thank Gerald Shadel for his input on the metabolic experiments in Fig. 6.
Financial information: C.G.Langdon, J. T. Platt, M.A. Held, and D.F. Stern were supported by a gift from the Hixon Foundation; C.G.Langdon, J. T. Platt, R.E. Means, Mamillapalli, P.Iyidogan, M.A. Held, and D.F. Stern by a Biomedical Research Grant from the Connecticut Department of Public Health (2013-0200), and H. Hochster by the GI Cancer Research Fund. C.G. Langdon was sponsored by NIH training grant USPHS T32GM007324. Work at the Yale Center for Molecular Discovery was supported by US NCI grant P30 CA16359 awarded to Yale Cancer Center.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kosmidis C, Sapalidis K, Kotidis E, Mixalopoulos N, Zarogoulidis P, Tsavlis D, et al. Pancreatic cancer from bench to bedside: molecular pathways and treatment options. Ann Transl Med. 2016;4(9):165. doi: 10.21037/atm.2016.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werner J, Combs SE, Springfeld C, Hartwig W, Hackert T, Buchler MW. Advanced-stage pancreatic cancer: therapy options. Nature reviews Clinical oncology. 2013;10(6):323–33. doi: 10.1038/nrclinonc.2013.66. [DOI] [PubMed] [Google Scholar]
- 4.Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016;30(4):355–85. doi: 10.1101/gad.275776.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 6.Garcia PL, Miller AL, Kreitzburg KM, Council LN, Gamblin TL, Christein JD, et al. The BET bromodomain inhibitor JQ1 suppresses growth of pancreatic ductal adenocarcinoma in patient-derived xenograft models. Oncogene. 2016;35(7):833–45. doi: 10.1038/onc.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguirre AJ, Brennan C, Bailey G, Sinha R, Feng B, Leo C, et al. High-resolution characterization of the pancreatic adenocarcinoma genome. Proc Natl Acad Sci U S A. 2004;101(24):9067–72. doi: 10.1073/pnas.0402932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 10.Pettazzoni P, Viale A, Shah P, Carugo A, Ying H, Wang H, et al. Genetic events that limit the efficacy of MEK and RTK inhibitor therapies in a mouse model of KRAS-driven pancreatic cancer. Cancer Res. 2015;75(6):1091–101. doi: 10.1158/0008-5472.CAN-14-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manchado E, Weissmueller S, Morris JPt, Chen CC, Wullenkord R, Lujambio A, et al. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature. 2016;534(7609):647–51. doi: 10.1038/nature18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gysin S, Salt M, Young A, McCormick F. Therapeutic strategies for targeting ras proteins. Genes Cancer. 2011;2(3):359–72. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 14.Paul J, Soujon M, Wengner AM, Zitzmann-Kolbe S, Sturz A, Haike K, et al. Simultaneous Inhibition of PI3Kdelta and PI3Kalpha Induces ABC-DLBCL Regression by Blocking BCR-Dependent and -Independent Activation of NF-kappaB and AKT. Cancer Cell. 2017;31(1):64–78. doi: 10.1016/j.ccell.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Atefi M, von Euw E, Attar N, Ng C, Chu C, Guo D, et al. Reversing Melanoma Cross-Resistance to BRAF and MEK Inhibitors by Co-Targeting the AKT/mTOR Pathway. PLoS ONE. 2011;6(12):e28973. doi: 10.1371/journal.pone.0028973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwong LN, Costello JC, Liu H, Jiang S, Helms TL, Langsdorf AE, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat Med. 2012;18(10):1503–10. doi: 10.1038/nm.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai CJ, Huang MT, Wu CH, Wang CK, Tai CJ, Chang CC, et al. Combination of Two Targeted Medications (Bevacizumab Plus Cetuximab) Improve the Therapeutic Response of Pancreatic Carcinoma. Medicine (Baltimore) 2016;95(15):e3259. doi: 10.1097/MD.0000000000003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Held MA, Langdon CG, Platt JT, Graham-Steed T, Liu Z, Chakraborty A, et al. Genotype-selective combination therapies for melanoma identified by high-throughput drug screening. Cancer discovery. 2013;3(1):52–67. doi: 10.1158/2159-8290.CD-12-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roller D, Axelrod M, Capaldo B, Jensen K, Mackey A, Weber MJ, et al. Synthetic lethal screening with small molecule inhibitors provides a pathway to rational combination therapies for melanoma. Mol Cancer Ther. 2012 doi: 10.1158/1535-7163.MCT-12-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize Implements and enhances circular visualization in R. Bioinformatics. 2014;30(19):2811–2. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 21.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langdon CG, Held MA, Platt JT, Meeth K, Iyidogan P, Mamillapalli R, et al. The broad-spectrum receptor tyrosine kinase inhibitor dovitinib suppresses growth of BRAF-mutant melanoma cells in combination with other signaling pathway inhibitors. Pigment Cell Melanoma Res. 2015;28(4):417–30. doi: 10.1111/pcmr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483(7391):570–5. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iorio F, Knijnenburg TA, Vis DJ, Bignell GR, Menden MP, Schubert M, et al. A Landscape of Pharmacogenomic Interactions in Cancer. Cell. 2016;166(3):740–54. doi: 10.1016/j.cell.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haibe-Kains B, El-Hachem N, Birkbak NJ, Jin AC, Beck AH, Aerts HJ, et al. Inconsistency in large pharmacogenomic studies. Nature. 2013;504(7480):389–93. doi: 10.1038/nature12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatzis C, Bedard PL, Birkbak NJ, Beck AH, Aerts HJ, Stem DF, et al. Enhancing reproducibility in cancer drug screening: how do we move forward? Cancer Res. 2014;74(15):4016–23. doi: 10.1158/0008-5472.CAN-14-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, Hou D, Luo Z, Chen P, Lv B, Wu L, et al. The novel protective role of P27 in MLN4924-treated gastric cancer cells. Cell death & disease. 2015;6:e1867. doi: 10.1038/cddis.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haverty PM, Lin E, Tan J, Yu Y, Lam B, Lianoglou S, et al. Reproducible pharmacogenomic profiling of cancer cell line panels. Nature. 2016;533(7603):333–7. doi: 10.1038/nature17987. [DOI] [PubMed] [Google Scholar]
- 29.Awasthi N, Schwarz MA, Schwarz RE. Combination effects of bortezomib with gemcitabine and EMAP II in experimental pancreatic cancer. Cancer Biol Ther. 2010;10(1):99–107. doi: 10.4161/cbt.10.1.12169. [DOI] [PubMed] [Google Scholar]
- 30.Furuyama T, Tanaka S, Shimada S, Akiyama Y, Matsumura S, Mitsunori Y, et al. Proteasome activity is required for the initiation of precancerous pancreatic lesions. Scientific reports. 2016;6:27044. doi: 10.1038/srep27044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei D, Li H, Yu J, Sebolt JT, Zhao L, Lawrence TS, et al. Radiosensitization of human pancreatic cancer cells by MLN4924, an investigational NEDD8-activating enzyme inhibitor. Cancer Res. 2012;72(1):282–93. doi: 10.1158/0008-5472.CAN-11-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan N, Wong M, Nannini MA, Hong R, Lee LB, Price S, et al. Bcl-2/Bcl-xL inhibition increases the efficacy of MEK inhibition alone and in combination with PI3 kinase inhibition in lung and pancreatic tumor models. Mol Cancer Ther. 2013;12(6):853–64. doi: 10.1158/1535-7163.MCT-12-0949. [DOI] [PubMed] [Google Scholar]
- 33.Dineen SP, Roland CL, Greer R, Carbon JG, Toombs JE, Gupta P, et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010;70(7):2852–61. doi: 10.1158/0008-5472.CAN-09-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sideras K, Braat H, Kwekkeboom J, van Eijck CH, Peppelenbosch MP, Sleijfer S, et al. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev. 2014;40(4):513–22. doi: 10.1016/j.ctrv.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(1):105–20. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhu L, Mundade R, Korc M, Loehrer PJ, Lu T. Critical role of NF-kappaB in pancreatic cancer. Oncotarget. 2014;5(22):10969–75. doi: 10.18632/oncotarget.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langdon CG, Wiedemann N, Held MA, Mamillapalli R, Iyidogan P, Theodosakis N, et al. SMAC mimetic Debio 1143 synergizes with taxanes, topoisomerase inhibitors and bromodomain inhibitors to impede growth of lung adenocarcinoma cells. Oncotarget. 2015;6(35):37410–25. doi: 10.18632/oncotarget.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71(14):5020–9. doi: 10.1158/0008-5472.CAN-11-0908. doi:0008-5472.CAN-11-0908. [pii] 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong J, Xie J, Bedolla R, Rivas P, Chakravarthy D, Freeman JW, et al. Combined targeting of STAT3/NF-kappaB/COX-2/EP4 for effective management of pancreatic cancer. Clin Cancer Res. 2014;20(5):1259–73. doi: 10.1158/1078-0432.CCR-13-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puyol M, Martin A, Dubus P, Mulero F, Pizcueta P, Khan G, et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18(1):63–73. doi: 10.1016/j.ccr.2010.05.025. doi:S1535-6108(10)00237-0. [DOI] [PubMed] [Google Scholar]
- 41.Alagesan B, Contino G, Guimaraes AR, Corcoran RB, Deshpande V, Wojtkiewicz GR, et al. Combined MEK and PI3K inhibition in a mouse model of pancreatic cancer. Clin Cancer Res. 2015;21(2):396–404. doi: 10.1158/1078-0432.CCR-14-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian F, Engst S, Yamaguchi K, Yu P, Won KA, Mock L, et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res. 2009;69(20):8009–16. doi: 10.1158/0008-5472.CAN-08-4889. [DOI] [PubMed] [Google Scholar]
- 43.Moritz A, Li Y, Guo A, Villen J, Wang Y, MacNeill J, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3(136) doi: 10.1126/scisignal.2000998. ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153(2):320–34. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy N, Malik S, Villanueva KE, Urano A, Lu X, Von Figura G, et al. Brg1 promotes both tumor-suppressive and oncogenic activities at distinct stages of pancreatic cancer formation. Genes Dev. 2015;29(6):658–71. doi: 10.1101/gad.256628.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458(7239):732–6. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 49.Godbersen JC, Humphries LA, Danilova OV, Kebbekus PE, Brown JR, Eastman A, et al. The Nedd8-activating enzyme inhibitor MLN4924 thwarts microenvironment-driven NF-kappaB activation and induces apoptosis in chronic lymphocytic leukemia B cells. Clin Cancer Res. 2014;20(6):1576–89. doi: 10.1158/1078-0432.CCR-13-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown JS, Lukashchuk N, Sczaniecka-Clift M, Britton S, le Sage C, Calsou P, et al. Neddylation promotes ubiquitylation and release of Ku from DNA-damage sites. Cell reports. 2015;11(5):704–14. doi: 10.1016/j.celrep.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blank JL, Liu XJ, Cosmopoulos K, Bouck DC, Garcia K, Bernard H, et al. Novel DNA damage checkpoints mediating cell death induced by the NEDD8-activating enzyme inhibitor MLN4924. Cancer Res. 2013;73(1):225–34. doi: 10.1158/0008-5472.CAN-12-1729. [DOI] [PubMed] [Google Scholar]
- 52.Zhou L, Chen S, Zhang Y, Kmieciak M, Leng Y, Li L, et al. The NAE inhibitor pevonedistat interacts with the HDAC inhibitor belinostat to target AML cells by disrupting the DDR. Blood. 2016;127(18):2219–30. doi: 10.1182/blood-2015-06-653717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paiva C, Godbersen JC, Berger A, Brown JR, Danilov AV. Targeting neddylation induces DNA damage and checkpoint activation and sensitizes chronic lymphocytic leukemia B cells to alkylating agents. Cell death & disease. 2015;6:e1807. doi: 10.1038/cddis.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Shi CC, Zhang HP, Li GQ, Li SS. MLN4924 suppresses neddylation and induces cell cycle arrest, senescence, and apoptosis in human osteosarcoma. Oncotarget. 2016 doi: 10.18632/oncotarget.9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan H, Tang Z, Jin H, Sun Y. Neddylation inhibitor MLN4924 suppresses growth and migration of human gastric cancer cells. Scientific reports. 2016;6:24218. doi: 10.1038/srep24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knorr KL, Schneider PA, Meng XW, Dai H, Smith BD, Hess AD, et al. MLN4924 induces Noxa upregulation in acute myelogenous leukemia and synergizes with Bcl-2 inhibitors. Cell Death Differ. 2015;22(12):2133–42. doi: 10.1038/cdd.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen P, Hu T, Liang Y, Jiang Y, Pan Y, Li C, et al. Synergistic inhibition of autophagy and neddylation pathways as a novel therapeutic approach for targeting liver cancer. Oncotarget. 2015;6(11):9002–17. doi: 10.18632/oncotarget.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475(7355):231–4. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Yamamoto K, Tateishi K, Kudo Y, Hoshikawa M, Tanaka M, Nakatsuka T, et al. Stromal remodeling by the BET bromodomain inhibitor JQ1 suppresses the progression of human pancreatic cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kagoya Y, Nakatsugawa M, Yamashita Y, Ochi T, Guo T, Anczurowski M, et al. BET bromodomain inhibition enhances T cell persistence and function in adoptive immunotherapy models. J Clin Invest. 2016;126(9):3479–94. doi: 10.1172/JCI86437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao WT, Wu JF, Yu GY, Wang R, Wang K, Li LH, et al. Suppression of tumor angiogenesis by targeting the protein neddylation pathway. Cell death & disease. 2014;5:e1059. doi: 10.1038/cddis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.