Abstract
Background/Purpose
Approximately half of patients with systemic lupus erythematosus (SLE) develop lupus nephritis (LN), a major cause of morbidity and early mortality in that disease. Prolonged renal inflammation is associated with irreversible kidney damage which confers a 30% risk of end stage renal disease (ESRD), making early, aggressive treatment mandatory. Failure to achieve therapeutic response or recurrence of renal flare often prompts repeat biopsy. However, the role of repeat biopsy in determining long-term renal prognosis remains controversial. For this reason repeat biopsies are usually not utilized unless clinical evidence of refractory or recurrent disease is already present, despite known mismatches between clinical and biopsy findings. The current study quantifies the degree to which histolopathologic worsening between first and second biopsies and duration between them predicts ESRD and death.
Methods
Medical records of 141 LN patients with more than one biopsy were obtained from a single large urban medical center. Cases were attained using billing codes for diagnosis and procedures from 1/1999–1/2015. Biopsy worsening was defined as unfavorable histopathologic classification transitions and/or increased chronicity; if neither were present, the patient was defined as non-worsening. We used Cox proportional hazard models to study the relationship between ESRD and survival adjusting for covariates which included age at first biopsy, gender, race, initial biopsy class, and initial induction therapy.
Results
Of 630 patients screened, 141 had more than one biopsy. Advancing chronicity was detected in 48 (34.0%) and a renal class switch to worse grade of pathology was found in 54 (38.3%). At least one of these adverse second biopsy features was reported in 79 (56.0%) patients. Five years following initial biopsy, 28 (35.4%) of those with worsening histopathology on second biopsy developed ESRD, compared to 6 (9.7%) of non-worsening patients and 10 (12.7%) of patients with worsening histopathology had died compared to 2 (3.2%) of non-worsening patients. Biopsy worsening was associated with a significantly greater 15-year risk of ESRD (Hazard Ratio 4.2, p=0.0001) and death (Hazard Ratio 4.3, p=0.022), adjusting for age, gender, race, biopsy class, and treatment. Time between first and second biopsies was <1 year in 32 patients, 1–5 years in 81, and >5 years in 28. Over a 15-year period, those with <1 year between first and second biopsies (presumably enriched for patients with early clinical signs of progression) had a significantly greater risk of ESRD (Hazard Ratio 13.7, p<0.0001) and death (Hazard Ratio 16.9, p=0.0022) after adjusting for age, gender, race, biopsy class, and treatment.
Conclusion
A repeat renal biopsy demonstrating worsening pathology increases the risk of ESRD and death more than four-fold compared to non-worsening patients. Given known potential mismatch between biopsy and clinical data, repeat biopsies may add important information and justify changes in treatment not considered on clinical grounds. Earlier detection of poor prognostic signs in those without early clinical deterioration might improve outcomes in enough patients to reconsider cost effectiveness of routine repeat biopsy.
Keywords: lupus nephritis, systemic lupus erythematosus, biopsy, death, end stage renal disease, kidney
1.1 INTRODUCTION
Systemic lupus erythematosus (SLE) is a complex multi-organ disease characterized by production of antibodies to cellular constituents and dysregulation of the immune system. Up to 34% of adult SLE cases [1–3] and up to 78% of those diagnosed before age 20 [4] develop LN. About 30% of LN patients progress to ESRD [5, 6], and the overall reported prevalence of ESRD caused by LN has increased 56% over the 10 year period of 2000 to 2010 [7]. If a patient has undergone one renal biopsy, a repeat biopsy might be obtained after a short duration when there is clinical evidence of poor therapeutic response, or concern that there might be a change in histopathologic classification or a significant progression of damage. Repeat biopsies after longer durations are typically performed in patients who achieved a major response or LN disease remission but later develop recurrence of proteinuria. A clinical decision to perform repeat biopsy after a short duration of treatment is indicative of refractory lupus nephritis, as is a worsening of renal histopathology regardless of the time between biopsies.
Although each of these biopsy-related measures are generally accepted as poor prognostic indicators, little quantification of the risks they confer has been performed. Because of this, most patients are not offered repeat biopsy unless there is clinical evidence of deterioration, despite the fact that biopsies may reveal either better or worse pathology than suggested by routine laboratory tests [8–11]. This raises the question of whether improved prognostic information could be obtained by routinely obtaining post-treatment biopsy in patients with LN. The aim of this study was to determine the degree of risk conferred by biopsy evidence of worsening disease by calculating the risk of ESRD and death in LN patients with or without worsening histopathology on repeat renal biopsy. We also examined the prognosis of patients with less than one year between biopsies, compared to those with a longer duration, which, under current standards of care, likely reflects refractory versus recurrent clinical evidence of disease.
2.1 MATERIAL AND METHODS
2.1.1 Patients
All cases were obtained at a single large urban medical center with expertise in SLE and LN that included both hospital-based clinics and private facilities in Dallas, Texas, USA. The study was approved through the local Institutional Review Board. Initial case finding included a retrospective detection of the combination of CPT (50200 Percutaneous Renal Biopsy) and ICD-9 (710.0 SLE) codes from 1/1999–1/2015. This was followed by a review of medical records for those identified.
2.1.2 Data and Statistical Analysis
The focus of this study was on all patients who had more than 1 biopsy with findings of LN. All patients met the Systemic Lupus International Collaborating Clinics Classification Criteria for SLE [12] and the majority met the American College of Rheumatology revised criteria [13, 14]. Indications for biopsy cannot be elucidated for all patients, however all biopsies were performed for standard of care diagnosis based on proteinuria, presence of cellular casts, presence of hematuria, or worsening renal function. Biopsies were classified according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification system [15], with the addition of the activity and chronicity indices [16, 17]. Biopsies were evaluated by renal pathologists at the time of acquisition, rather than at the time of analyses, and therefore the same pathologist did not read all specimens. However, No more than five renal pathologists read the biopsies during the time frame of observation and all were part of the same faculty practice. It was hypothesized that histopathologic worsening between first and second biopsies would be an independent predictor for ESRD and death. Worsening of biopsy findings was defined as unfavorable histopathologic classification transitions (e.g. II=>III, II=>IV, II=>V, II=>VI, V=>III, V=>4, V=>VI, III=>IV, III=>VI, IV=>VI) or worsening of chronicity (e.g. Chronicity Index 2=>3, 2=>6); if neither were present, the patient was defined as non-worsening. An additional marker of refractory LN, time between biopsies, was hypothesized to be predictive of ESRD and death. Histopathologic worsening and time between biopsies are both potential surrogates for refractory disease, therefore they were analyzed separately in multivariate analyses. Additional covariates evaluated included age at first biopsy, gender, race/ethnicity, initial biopsy class, and initial induction therapy.
Kaplan-Meier survival curves were evaluated for each categorical variable, followed by breaking continuous variables into 3–5 groups (for visualization of univariate effects over time), with the time to event endpoints of ESRD or death. Cox Proportional Hazards modeling allowed adjustment of risk for covariates, and determination of partial contributions for ESRD and death. The multivariate models included the covariates listed above to adjust for factors known to influence treatment response, ESRD, and death in LN patients.
3.1 RESULTS
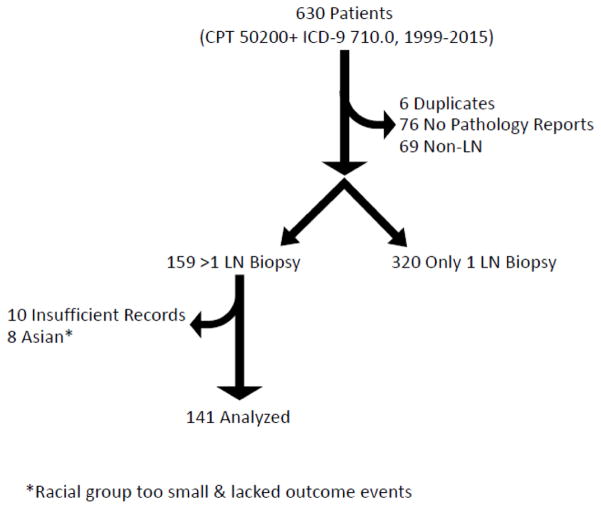
Use of billing codes identified 630 potential patients with a code for percutaneous kidney biopsy and SLE. Initial assessment noted 6 duplicates which were removed, then medical records were evaluated for presence of renal pathology reports. There were no available renal pathology reports on 76 patients, and 69 patients had non-LN kidney pathology findings including transplanted kidney biopsy, IgA nephropathy, focal segmental glomerulosclerosis, ANCA-associated disease, acute tubular necrosis, cryoglobulinemia, and diabetic nephropathy. Three hundred twenty had only one LN biopsy and 159 were left who met the criteria for greater than 1 LN biopsy. Further evaluation of this group found that 10 patients lacked sufficient records. Initial statistical evaluations noted the Asian racial group (8 patients) was too small and lacked events of ESRD and death, resulting in difficulty including race in the model. Race/ethnicity influences multiple LN outcomes, therefore the small Asian group was removed in order to include race in multivariate modeling (Figure 1). A total of 141 patients with >1 biopsy were analyzed (Table 1).
Figure 1. Flow diagram of patients.
Initial case finding utilized a combination of CPT and ICD-9 billing codes. Review of records resulted in elimination of some individuals.
Table 1. Description of Patient Cohort.
Information about domographics and variables included in the analysis are shown for the histopathologic worsening and non-worsening groups. Statistical testing included Fisher’s exact test, Chi-square, and Mann-Whitney U test, as appropriate.
| Worsening | Non-Worsening | p-Value | ||||
|---|---|---|---|---|---|---|
| Total (n,%) | 79 | 56.0% | 62 | 44.0% | 0.06 | |
| Demographics | ||||||
| Female (n, %) | 65 | 82.3% | 53 | 85.5% | 0.65 | |
| Race/Ethnicity (n, %) | ||||||
| Black/African American | 39 | 49.4% | 22 | 35.5% | 0.26 | |
| Hispanic/Latino (Majority Mexican) | 32 | 40.5% | 32 | 51.6% | ||
| White/Caucasian | 8 | 10.1% | 8 | 12.9% | ||
| Age in Years at First Biopsy (median, IQR) | 25.8 | [20.3–34.3] | 26.2 | [20.6–38.2] | 0.51 | |
| Initial Biopsy ISN/RPS Class (n, %) | ||||||
| Proliferative (III, IV, III+V, IV+V) | 55 | 69.6% | 47 | 75.8% | 0.31 | |
| -Proliferative only (III, IV) | 40 | 50.6% | 27 | 43.5% | ||
| -Proliferative and membranous (III+V, IV+V) | 15 | 19.0% | 20 | 32.3% | ||
| Membranous only (V) | 14 | 17.7% | 10 | 16.1% | ||
| Mesangial Proliferative (II) | 10 | 12.7% | 5 | 8.1% | ||
| Activity Index (Proliferative Only) | 11 | [7–1] | 12 | [6–15] | 0.80 | |
| Chronicity Index (Proliferative Only) | 2 | [2–4] | 2 | [1–3] | 0.63 | |
| Second Biopsy ISN/RPS Class (n, %) | ||||||
| Proliferative (III, IV, III+V, IV+V) | 63 | 79.7% | 29 | 46.8% | <0.0001 | |
| -Proliferative only (III, IV) | 34 | 43.0% | 19 | 30.6% | ||
| -Proliferative and membranous (III+V, IV+V) | 29 | 36.7% | 10 | 16.1% | ||
| Membranous only (V) | 8 | 10.1% | 23 | 37.1% | ||
| Mesangial Proliferative (II) | 3 | 3.8% | 10 | 16.1% | ||
| Advanced Sclerosing (VI) | 5 | 6.3% | 0 | 0.0% | ||
| Activity Index (Proliferative Only) | 10 | [8–14] | 6 | [4–10] | 0.0002 | |
| Chronicity Index (Proliferative Only) | 4 | [3–7] | 2 | [2–4] | 0.008 | |
| Initial Induction Immunosuppression (n, %) | ||||||
| Mycophenolate Mofetile (MMF) | 27 | 34.2% | 30 | 48.4% | 0.23 | |
| Cyclophosphamide IV (CYC) | 37 | 46.8% | 26 | 41.9% | ||
| Other [Azathioprine or Cyclosporine] | 3 | 3.8% | 2 | 3.2% | ||
| None or Unknown | 12 | 15.2% | 4 | 6.5% | ||
| Time Between First & Second Biopsies in Years (median, IQR) | 2.1 | [0.9–4.3] | 2.2 | [1.2–4.5] | 0.45 | |
| Events and Follow-Up Time | ||||||
| End Stage Renal Disease (n, %) | 38 | 48.1% | 10 | 16.1% | <0.0001 | |
| Follow-UpTime in Years (median, IQR) | 7.3 | [3.4–10.5] | 8.0 | [5.2–11.8] | ||
| Deaths (n, %) | 18 | 22.8% | 3 | 4.8% | 0.004 | |
| Follow-Up Time in Years (median, IQR) | 8.8 | [6.0–12.1] | 9.0 | [5.8–11.9] | ||
Increasing chronicity in subsequent biopsy was detected in 48 (34.0%) and a renal class switch to higher grade of pathology was found in 54 (38.3%). At least one of these biopsy findings was reported in 79 (56.0%) patients.
Initial evaluation of the age at biopsy variable noted a U-shaped curve, with greater risk of ESRD and death for the youngest and oldest patients. A similar bimodal pattern has been previously described for early and late mortality in SLE patients [18]. In order to include the age at biopsy as a covariate, the higher risk extremes of age were combined (age <18 and >50) and referenced to the lower risk group (age 18–50).
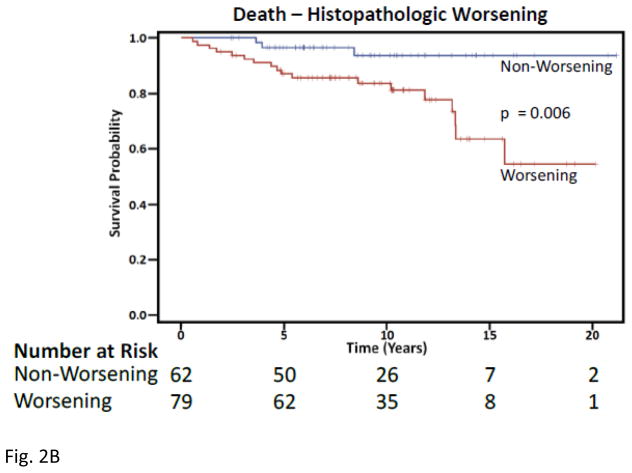
Five years following initial biopsy, 28 (35.4%) of those with worsening histopathology on second biopsy developed ESRD, compared to 6 (9.7%) of non-worsening patients (Figure 2). After only five years, 10 (12.7%) of the worsening histopathology patients had died compared to 2 (3.2%) of non-worsening patients. Over a 15-year period, those with worsening of nephritis at second biopsy had a significantly greater risk of ESRD (Hazard Ratio 4.2, p=0.0001) and death (Hazard Ratio 4.3, p=0.022) after adjusting for age, gender, race, biopsy class, and treatment.
Figure 2. Kaplan-Meier Curves for Histopathologic Worsening.
A) Time to end stage renal disease, Upper blue line are those lacking worsening from first to second biopsy and lower red line depicts worsening histopathology. B) Time to death
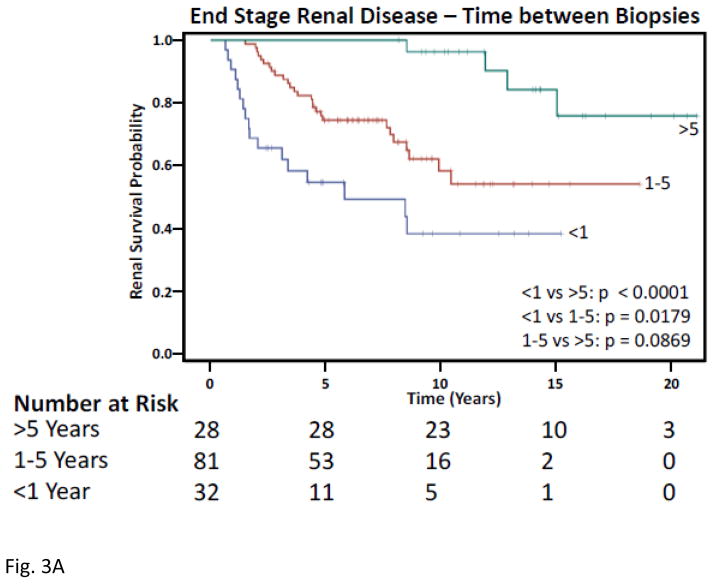
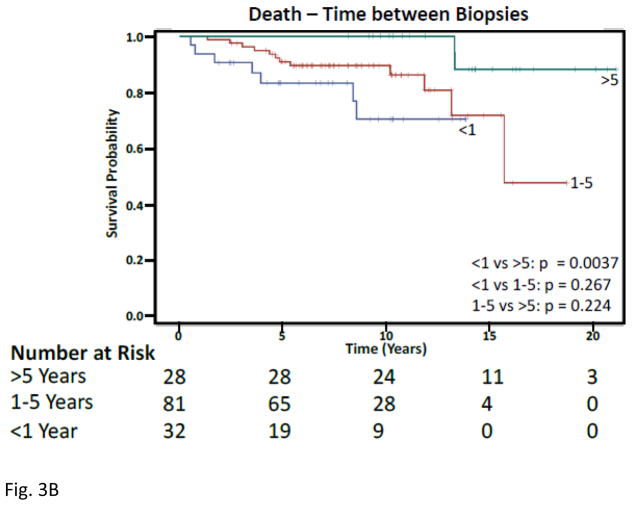
Five years following initial biopsy, 14 (43.8%) of those with < 1 year between biopsies, and 20 (24.7%) of those with 1–5 years between biopsies developed ESRD (Figure 3). Similarly, after only 5 years, 5(15.6%) of those < 1 year between biopsies and 7(8.6%) of those with 1–5 years between biopsies had died. Over a 15-year period, those with <1 year between first and second biopsies had a significantly greater risk of ESRD (Hazard Ratio 13.7, p<0.0001) and death (Hazard Ratio 16.9, p=0.0022) following adjusting for age, gender, race, biopsy class, and treatment.
Figure 3. Kaplan-Meier Curves for Time Between First and Second Biopsies.
A) Time to end stage renal disease, Upper green line are those with > 5 years between biopsies, red middle is 1–5 years, and lower blue is < 1 year. B) Time to death
4.1 DISCUSSION
SLE patients with LN are known to have significantly higher morbidity and earlier mortality than SLE patients without LN, with almost a third of them progressing to ESRD [5, 6]. The Systemic Lupus Collaborating Clinic (SLICC) international inception cohort identified LN in 700 patients (38.3% of the cohort population). Approximately 81% (n=566) presented with LN at enrollment which occurred within 18 months of SLE diagnosis, and only 134 (19.1%) developed nephritis during follow-up. Cox regression analysis of that cohort found that once a patient develops LN, they have a higher risk of ESRD (Hazard Ratio 44.7) and death (Hazard Ratio 3.2), after adjustment for gender, age at enrollment, and race/ethnicity [19]. In the United States, 12,344 incident cases of ESRD due to LN from 1995–2006 were identified; and each 3 year interval had 27% mortality with no improvement in risk over the 12 year time period [20]. LN patients with ESRD on hemodialysis have 10-year mortality rates of 45% [21, 22] and compared to gender- and age- matched local general population, LN patients with ESRD mortality risk is 26-fold in LN-related ESRD [23].
The intensity and duration of renal inflammation correlates with chronic renal lesions and predicts ESRD [5]. Poor response to therapy at 6 months also correlates with worse renal outcome [24], suggesting that any underestimation of disease at that critical time point could have a significant impact on prognosis. Kidney biopsies remain the foundation for diagnosis and categorization in developing treatment strategies for LN. Although the histopathologic findings of classification, level of activity, and level of chronicity correlate poorly with urine protein and serum creatinine, there is reluctance to pursue biopsy unless there is already strong clinical evidence of refractory disease. In one study, LN patients underwent a repeat biopsy after immunosuppressive induction therapy regardless of clinical response. Clinically quiescent patients were frequently found to have histopathologic activity persisting and many of those with poor clinical response had no active inflammatory activity. This confirms that histopathology and clinical activity correlated very poorly [11], challenging the avoidance of routine repeat biopsy. Furthermore, frequent changes of histopathologic class in LN is well documented. In fact a study of Chinese LN patients found that 75% of repeat biopsies had histopathologic class transformation [25].
The current report confirms that results of repeat kidney biopsy provide independent predictors of ESRD and death after correcting for factors of age at biopsy, gender, race/ethnicity, the initial histopathologic classification, and initial induction immunosuppression therapy received. Previously reported predictors of ESRD included duration of nephritis, elevated serum creatinine, proliferative histopathology, and elevated chronicity index (as well as some of its components including glomerular sclerosis, interstitial fibrosis, and tubular atrophy) [5]. Further, race and ethnicity emerged as predictors of therapeutic response, ESRD, and death [1, 26–29]. Additional associations with poor renal outcome included hypertension, anemia, increased urine protein, reduced complement 3, tubulointerstitial inflammation, and vascular lesions on renal biopsy [6, 17, 30–33]. The majority of patients received current standard of care immunosuppression induction regimens of mycophenolate mofetil 57 (40%) or cyclophosphamide 63 (45%). Forty-eight (34.0%) patients progressed to ESRD during the period of study, which also confirms previous reports of high morbidity and damage even under optimal medical management at a major academic hospital. This is the first multivariate survival analysis evaluating histopathologic worsening and/or time between biopsies with the quantification of risk that could form the basis for evaluation of the cost/risk/effectiveness of routine repeat biopsies and/or models for optimization of treatment.
Limitations of this study include the retrospective data analysis and the single region evaluated. Although the significant risk conferred by deterioration on biopsy is clearly demonstrated, this population received repeat biopsies only through clinical decisions based upon standard of care practices, so the potential impact of early biopsy for patients without clinical deterioration cannot be assessed.. Although our methodology of case finding provided an unbiased selection of patients, many identified biopsies lacked findings consistent with LN or had no biopsy reports available for review. Laboratory data at the time of initial biopsy were not readily available for the majority of patients due to use of non-consolidated records systems for this information. In the future such data may be more consistently available in electronic records. It would have been desirable to have urine protein, serum creatinine, urine casts, and urine red blood cell data available at baseline, as these clinical data points are utilized by clinicians to determine the need for kidney biopsy or repeat kidney biopsy, and are therefore, to some extent collinear both with each other and with other variables that were included in our analysis. We also lacked complete information related to comorbidities, such as hypertension and were therefore unable to incorporate these data into our models
The patient population reported here reflects the overall population of LN except for the exclusion of the Asian racial group, which was underrepresented in our cohort. An advantage of the large urban hospital complex at which this project was conducted was its ability to provide a wide range of subjects from different socioeconomic groups and types of insurance. Additional benefit was the inclusion of Mexican nationals and other patients who might not be found in Medicaid and Medicare data sets. The same group of nephrologists and rheumatologists consult at both the public and private facilities attached to this center, and the urban hospital had its own county-supported insurance program allowing access to the same therapies, ability to obtain biopsies, and ability to regularly follow patients in outpatient clinics. Therefore, indications for renal biopsy and immunosuppression regimens at each center were presumably comparable.
5.1 CONCLUSIONS
In conclusion, worsening of LN at second biopsy has a quantifiable risk of ESRD (Hazard Ratio 4.2, p=0.0001) and death (Hazard Ratio 4.3, p=0.022) after adjusting for age, gender, race, biopsy class, and treatment. Less than 1 year between first and second renal biopsies, which likely represents patients with early clinical deterioration, also confers a significantly greater risk of ESRD (Hazard Ratio 13.7, p<0.0001) and death (Hazard Ratio 16.9, p=0.0022) after adjusting for age, gender, race, biopsy class, and treatment. Although these data cannot determine the extent to which routine repeat biopsies might detect earlier and more reversible lesions in patients not sent for biopsy under current guidelines the dismal outcomes of current standards of care suggest the importance of studies to improve upon it.
Table 2. Histopathologic Worsening Multivariate Cox Proportional Hazard Ratio Analysis.
Upper section evaluates time to ESRD, adjusting for multiple factors and lower evaluates time to death.
| ESRD
| |||
|---|---|---|---|
| Parameter Description | Regression Coefficient | p-Value | Hazard Ratio |
| Age at First Renal Biopsy | |||
| Age 18–50 | ref. | ref. | |
| Age<18 & Age>50 at First Renal Biopsy | −1.036 | 0.037 | 0.355 |
| Gender | |||
| Female | ref. | ref. | |
| Male | 0.091 | 0.830 | 1.095 |
| Race/Ethnicity | |||
| White | ref. | ref. | |
| Hispanic Ethnicity | 0.620 | 0.325 | 1.859 |
| Black Race | 0.458 | 0.459 | 1.580 |
| First Biopsy Histopathologic Classification | |||
| Mesangial (II) | ref. | ref. | |
| Membranous (V) | 0.873 | 0.291 | 2.395 |
| Proliferative (III, IV, III+V, IV+V) | 1.484 | 0.048 | 4.411 |
| Induction Therapy | |||
| Other (AZA, CYA) | ref. | ref. | |
| CYC | 0.208 | 0.845 | 1.231 |
| MMF | 0.438 | 0.681 | 1.549 |
| None or Unknown Induction Therapy | −0.464 | 0.689 | 0.629 |
| Second Biopsy Compared to First | |||
| Same or Improved | ref. | ref. | |
| Worse | 1.426 | 0.0001 | 4.160 |
| DEATH
| |||
|---|---|---|---|
| Parameter Description | Regression Coefficient | p-Value | Hazard Ratio |
| Age at First Renal Biopsy | |||
| Age 18–50 | ref. | ref. | |
| Age<18 & Age>50 at First Renal Biopsy | −0.562 | 0.281 | 1.754 |
| Gender | |||
| Female | ref. | ref. | |
| Male | 0.495 | 0.397 | 1.641 |
| Race/Ethnicity | |||
| White | ref. | ref. | |
| Hispanic Ethnicity | 0.049 | 0.954 | 1.050 |
| Black Race | 0.206 | 0.796 | 1.229 |
| First Biopsy Histopathologic Classification | |||
| Mesangial (II) | ref. | ref. | |
| Membranous (V) | 0.185 | 0.835 | 1.203 |
| Proliferative (III, IV, III+V, IV+V) | 0.108 | 0.895 | 1.114 |
| Induction Therapy | |||
| Other (AZA, CYA) | ref. | ref. | |
| CYC | 0.377 | 0.746 | 1.457 |
| MMF | 0.312 | 0.799 | 1.366 |
| None or Unknown Induction Therapy | 1.165 | 0.344 | 3.206 |
| Second Biopsy Compared to First | |||
| Same or Improved | ref. | ref. | |
| Worse | 1.458 | 0.022 | 4.297 |
Table 3.
Time In Years Between First and Second Biopsies Multivarite Cox Proportional Hazard Ratio Analysis.Upper section evaluates time to ESRD, adjusting for multiple factors and lower evaluates time to death.
| ESRD
| |||
|---|---|---|---|
| Parameter Description | Regression Coefficient | p-Value | Hazard Ratio |
| Age at First Renal Biopsy | |||
| Age 18–50 | ref. | ref. | |
| Age<18 & Age>50 at First Renal Biopsy | −0.162 | 0.773 | 0.850 |
| Gender | |||
| Female | ref. | ref. | |
| Male | 0.267 | 0.517 | 1.307 |
| Race/Ethnicity | |||
| White | ref. | ref. | |
| Hispanic Ethnicity | 0.997 | 0.121 | 2.711 |
| Black Race | 0.832 | 0.190 | 2.298 |
| First Biopsy Histopathologic Classification | |||
| Mesangial (II) | ref. | ref. | |
| Membranous (V) | 0.869 | 0.294 | 2.385 |
| Proliferative (III, IV, III+V, IV+V) | 1.339 | 0.070 | 3.815 |
| Induction Therapy | |||
| Other (AZA, CYA) | ref. | ref. | |
| CYC | 0.777 | 0.466 | 2.174 |
| MMF | 0.634 | 0.555 | 1.886 |
| None or Unknown Induction Therapy | −0.196 | 0.867 | 0.822 |
| Time Between First and Second Biopsies | |||
| >5 Years | ref. | ref. | |
| <1 Year | 2.614 | 0.0001 | 13.65 |
| >1–5 Years | 1.530 | 0.0028 | 4.619 |
| DEATH
| |||
|---|---|---|---|
| Parameter Description | Regression Coefficient | p-Value | Hazard Ratio |
| Age at First Renal Biopsy | |||
| Age 18–50 | ref. | ref. | |
| Age<18 & Age>50 at First Renal Biopsy | 1.221 | 0.027 | 3.391 |
| Gender | |||
| Female | ref. | ref. | |
| Male | 0.743 | 0.218 | 2.102 |
| Race/Ethnicity | |||
| White | ref. | ref. | |
| Hispanic Ethnicity | 0.092 | 0.913 | 1.097 |
| Black Race | 0.340 | 0.671 | 1.405 |
| First Biopsy Histopathologic Classification | |||
| Mesangial (II) | ref. | ref. | |
| Membranous (V) | 0.343 | 0.699 | 1.409 |
| Proliferative (III, IV, III+V, IV+V) | 0.042 | 0.958 | 1.043 |
| Induction Therapy | |||
| Other (AZA, CYA) | ref. | ref. | |
| CYC | 1.053 | 0.383 | 2.866 |
| MMF | 0.711 | 0.576 | 2.095 |
| None or Unknown Induction Therapy | 1.847 | 0.143 | 6.340 |
| Time Between First and Second Biopsies | |||
| >5 Years | ref. | ref. | |
| <1 Year | 2.825 | 0.002 | 16.860 |
| >1–5 Years | 2.093 | 0.011 | 8.106 |
Highlights.
Over one-third of lupus nephritis patients with more than one renal biopsy progressed to end stage renal disease during the time frame of study
Over half of the studied patients were found to have worsening histopathology on repeat kidney biopsy
Repeat renal biopsy with features of worsening histopathology confers a 4-fold increased risk of end stage renal disease or death, after adjusting for age, gender, race/ethnicity, initial biopsy class, and initial induction therapy
Refractory disease resulting in the clinical decision to obtain a repeat renal biopsy within one year confers more than a 10-fold risk of end stage renal disease and death, after adjusting for age, gender, race/ethnicity, initial biopsy class, and initial induction therapy
Acknowledgments
Funding:
CA received support or services from the following while extracting data, analyzing, drafting, and completing this manuscript: Mallinckrodt/Questcor Pharmaceuticals Research Fellowship Award Program, U.S. Department of Health and Human Services >National Institutes of Health >National Institute of Diabetes and Digestive and Kidney Diseases T32 DK007257-32, U.S. Department of Health and Human Services >National Institutes of Health >National Center for Advancing Translational Sciences UL1TR001105 (UTSW CTSA), U.S. Department of Health and Human Services >National Institutes of Health >National Institute of General Medical Sciences U54 GM104938-04 (OU OSCTR), U.S. Department of Health and Human Services >National Institutes of Health >National Institute of Arthritis and Musculoskeletal and Skin Diseases>P30 AR053483-10<=OMRF, U.S. Department of Health and Human Services >National Institutes of Health >National Institute of Allergy and Infectious Diseases U01 AI101934-05<=OMRF, and U.S. Department of Health and Human Services > National Institutes of Health >National Institute of Allergy and Infectious Diseases U19 AI082714-08<=OMRF.
Abbreviations
- CPT
current procedural terminology
- ESRD
end stage renal disease
- ICD-9
international classification of disease-ninth revision
- LN
lupus nephritis
- SLE
systemic lupus erythematosus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, Winkelmayer WC, Costenbader KH. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis and rheumatism. 2013;65:753–763. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: the georgia lupus registry. Arthritis & rheumatology. 2014;66:357–368. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somers EC, Marder W, Cagnoli P, Lewis EE, Deguire P, Gordon C, Helmick CG, Wang L, Wing JJ, Dhar JP, Leisen J, Shaltis D, McCune WJ. Population-based incidence and prevalence of systemic lupus erythematosus: the michigan lupus epidemiology and surveillance program. Arthritis & rheumatology. 2014;66:369–378. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fish AJ, Blau EB, Westberg NG, Burke BA, Vernier RL, Michael AF. Systemic lupus erythematosus within the first two decades of life. The American journal of medicine. 1977;62:99–117. doi: 10.1016/0002-9343(77)90355-2. [DOI] [PubMed] [Google Scholar]
- 5.Faurschou M, Starklint H, Halberg P, Jacobsen S. Prognostic factors in lupus nephritis: diagnostic and therapeutic delay increases the risk of terminal renal failure. The Journal of rheumatology. 2006;33:1563–1569. [PubMed] [Google Scholar]
- 6.Singh S, Zhou XJ, Ahn C, Saxena R. A retrospective analysis of clinical presentation of lupus nephritis. The American journal of the medical sciences. 2011;342:467–473. doi: 10.1097/MAJ.0b013e3182199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.N.I.o.H. National Institute of Diabetes and Digestive and Kidney Diseases, United States Renal Data System. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2010 www.usrds.org.
- 8.Christopher-Stine L, Siedner M, Lin J, Haas M, Parekh H, Petri M, Fine DM. Renal biopsy in lupus patients with low levels of proteinuria. The Journal of rheumatology. 2007;34:332–335. [PubMed] [Google Scholar]
- 9.De Rosa MA, Toblli JE, De Rosa GE, Marini A. Could Proteinuria Less Than 500 mg/day Be an Actual Indicator of Lupus Nephritis Biopsy? Journal of the American Society of Nephrology: JASN, Annual Meeting Abstracts. 2013:PO720. [Google Scholar]
- 10.Baranowska-Daca E, Choi YJ, Barrios R, Nassar G, Suki WN, Truong LD. Nonlupus nephritides in patients with systemic lupus erythematosus: a comprehensive clinicopathologic study and review of the literature. Hum Pathol. 2001;32:1125–1135. doi: 10.1053/hupa.2001.28227. [DOI] [PubMed] [Google Scholar]
- 11.Zickert A, Sundelin B, Svenungsson E, Gunnarsson I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med. 2014;1:e000018. doi: 10.1136/lupus-2014-000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sanchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG, Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G, Jr, Magder LS. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis and rheumatism. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 15.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M N. International Society of Nephrology Working Group on the Classification of Lupus, N. Renal Pathology Society Working Group on the Classification of Lupus. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney international. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 16.Austin HA, 3rd, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, Decker JL, Balow JE. Prognostic factors in lupus nephritis. Contribution of renal histologic data. The American journal of medicine. 1983;75:382–391. doi: 10.1016/0002-9343(83)90338-8. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis care & research. 2011;63:865–874. doi: 10.1002/acr.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. The American journal of medicine. 1976;60:221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 19.Hanly JG, O’Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, Bae SC, Bernatsky S, Clarke AE, Wallace DJ, Merrill JT, Isenberg DA, Rahman A, Ginzler EM, Fortin P, Gladman DD, Sanchez-Guerrero J, Petri M, Bruce IN, Dooley MA, Ramsey-Goldman R, Aranow C, Alarcon GS, Fessler BJ, Steinsson K, Nived O, Sturfelt GK, Manzi S, Khamashta MA, van Vollenhoven RF, Zoma AA, Ramos-Casals M, Ruiz-Irastorza G, Lim SS, Stoll T, Inanc M, Kalunian KC, Kamen DL, Maddison P, Peschken CA, Jacobsen S, Askanase A, Theriault C, Thompson K, Farewell V. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology. 2016;55:252–262. doi: 10.1093/rheumatology/kev311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, Massarotti E, Lu B, Solomon DH, Winkelmayer WC. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis and rheumatism. 2011;63:1681–1688. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang SH, Chung BH, Choi SR, Lee JY, Park HS, Sun IO, Choi BS, Park CW, Kim YS, Yang CW. Comparison of clinical outcomes by different renal replacement therapy in patients with end-stage renal disease secondary to lupus nephritis. The Korean journal of internal medicine. 2011;26:60–67. doi: 10.3904/kjim.2011.26.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro FM, Fabris CL, Bendet I, Lugon JR. Survival of lupus patients on dialysis: a Brazilian cohort. Rheumatology. 2013;52:494–500. doi: 10.1093/rheumatology/kes298. [DOI] [PubMed] [Google Scholar]
- 23.Yap DY, Tang CS, Ma MK, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrology, dialysis transplantation: official publication of the European Dialysis and Transplant Association-European Renal Association. 2012;27:3248–3254. doi: 10.1093/ndt/gfs073. [DOI] [PubMed] [Google Scholar]
- 24.Nived O, Hallengren CS, Alm P, Jonsen A, Sturfelt G, Bengtsson AA. An observational study of outcome in SLE patients with biopsy-verified glomerulonephritis between 1986 and 2004 in a defined area of southern Sweden: the clinical utility of the ACR renal response criteria and predictors for renal outcome. Scandinavian journal of rheumatology. 2013;42:383–389. doi: 10.3109/03009742.2013.799224. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Tam LS, Lai FM, Kwan BC, Choi PC, Li EK, Chow KM, Li PK, Szeto CC. Repeat renal biopsy in lupus nephritis: a change in histological pattern is common. American journal of nephrology. 2011;34:220–225. doi: 10.1159/000330356. [DOI] [PubMed] [Google Scholar]
- 26.Litwic AE, Sriranganathan MK, Edwards CJ. Race and the response to therapies for lupus: How strong is the evidence? International Journal of Clinical Rheumatology. 2013;8:471–481. [Google Scholar]
- 27.Richman IB, Taylor KE, Chung SA, Trupin L, Petri M, Yelin E, Graham RR, Lee A, Behrens TW, Gregersen PK, Seldin MF, Criswell LA. European genetic ancestry is associated with a decreased risk of lupus nephritis. Arthritis and rheumatism. 2012;64:3374–3382. doi: 10.1002/art.34567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barr RG, Seliger S, Appel GB, Zuniga R, D’Agati V, Salmon J, Radhakrishnan J. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrology, dialysis transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18:2039–2046. doi: 10.1093/ndt/gfg345. [DOI] [PubMed] [Google Scholar]
- 29.Alarcon GS, McGwin G, Jr, Bastian HM, Roseman J, Lisse J, Fessler BJ, Friedman AW, Reveille JD. Systemic lupus erythematosus in three ethnic groups. VII. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis and rheumatism. 2001;45:191–202. doi: 10.1002/1529-0131(200104)45:2<191::AID-ANR173>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C. The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:2531–2539. doi: 10.1093/ndt/gfm245. [DOI] [PubMed] [Google Scholar]
- 31.Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J, Rohde RD. Factors predictive of outcome in severe lupus nephritis. Lupus Nephritis Collaborative Study Group. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2000;35:904–914. doi: 10.1016/s0272-6386(00)70262-9. [DOI] [PubMed] [Google Scholar]
- 32.Fatemi A, Kazemi M, Sayedbonakdar Z, Farajzadegan Z, Karimzadeh H, Moosavi M. Long-term outcome of biopsy-proven lupus nephritis in Iran. International journal of rheumatic diseases. 2013;16:739–746. doi: 10.1111/1756-185X.12228. [DOI] [PubMed] [Google Scholar]
- 33.Wu LH, Yu F, Tan Y, Qu Z, Chen MH, Wang SX, Liu G, Zhao MH. Inclusion of renal vascular lesions in the 2003 ISN/RPS system for classifying lupus nephritis improves renal outcome predictions. Kidney international. 2013;83:715–723. doi: 10.1038/ki.2012.409. [DOI] [PubMed] [Google Scholar]