Abstract
BACKGROUND
Mouse sensitization and exposure are associated with uncontrolled asthma, but whether they are associated with asthma severity, an intrinsic disease characteristic and long-term outcome predictor, is unclear.
OBJECTIVE
To examine relationships between mouse sensitization and/or exposure and asthma severity in urban children.
METHODS
A total of 645 children (5-17 years) with uncontrolled asthma underwent mouse sensitization evaluation. Sensitized children had mouse allergen measured in bedroom dust. Relationships between mouse sensitization, allergen levels, and asthma severity measures (treatment step and Composite Asthma Severity Index [CASI]) were examined using regression models adjusted for age, sex, atopy, study site, race, ethnicity, and insurance.
RESULTS
The study population was predominantly minority (69.6% black, 20.8% Hispanic), low income (61.8%), and mouse sensitized (54.4%). Mean ± SD treatment step was 3.2 ± 1.6, equivalent to medium-dose inhaled corticosteroid. Mean ± SD CASI was 6.5 ± 3.4, reflecting moderate persistent asthma. Mouse sensitization was associated with higher treatment step (3.5 vs 2.9, mouse-sensitized vs nonsensitized, P < .001), independent of potential confounders (β [95% CI], 0.36 [0.07-0.64]; P = .01). Mouse sensitization was associated independently with CASI (β [95% CI], 0.82 [0.16-1.47]; P = .02). Among mouse-sensitized participants, higher bedroom floor and bed Mus m 1 were independently associated with treatment step (β [95% CI], 0.26 [0.09-0.43]; P [ .002 and β [95% CI], 0.22 [0.01-0.43]; P = .04), respectively. Higher bedroom floor Mus m 1 was independently associated with CASI (β [95% CI], 0.43 [0.05-0.81]; P = .03).
CONCLUSIONS
Mouse sensitization and exposure are associated with asthma severity, among low-income, minority children. Further studies are needed to determine whether reducing allergen exposure among mouse-sensitized patients with asthma can reduce severity, ultimately altering childhood asthma natural history.
Keywords: Inner-city asthma, Childhood asthma, Mouse sensitization, Mouse allergen, Indoor allergens
Although asthma controller medication is very effective in improving asthma control and reducing morbidity, studies have shown repeatedly that inhaled corticosteroids have little effect on the natural history of the disease.1,2 In addition, allergen immunotherapy appears to have little effect on the natural history of disease among children with asthma,3-5 and there is limited long-term data on the effect of omalizumab on the natural history of asthma. There is a need, then, to develop a better understanding of the modifiable risk factors for poor long-term outcomes to develop approaches aimed at altering the natural history of the disease. Studying risk factors for long-term outcomes is difficult because the natural history of childhood asthma plays out over several decades. However, examining current asthma severity among children can lend insight into outcomes in adulthood because asthma severity in childhood is a predictor of poor outcomes in adulthood.3,6-9
Because indoor allergen exposure causes airway inflammation and decrements in lung function among sensitized children with established asthma,10,11 it is possible that it is responsible for greater disease severity, which is a strong predictor of long-term outcomes, including adult lung function and asthma that persists in adulthood.3,6,7 Although many previous studies have examined relationships between allergic sensitization and/or exposure and asthma control, few studies have examined relationships between allergen sensitization and/or exposure and asthma severity among children with established asthma. Because childhood asthma severity is a predictor of worse long-term outcomes, the question of whether an indoor allergen, such as mouse or cockroach allergen, contributes to asthma severity has important implications for the potential role of allergen exposure reduction in modifying the natural history of the disease. We therefore tested the hypothesis that indoor allergen sensitization and/or exposure contributes to asthma severity by examining relationships between mouse allergen sensitization and/or exposure and markers of disease severity in a population of low-income, predominantly minority children and adolescents with persistent asthma. We also examined the relationship between cockroach allergen sensitization and markers of disease severity because cockroach is also known to be associated with asthma morbidity in this population.12
METHODS
Study population and recruitment procedures
As part of screening for a multicenter, randomized controlled trial of an environmental intervention, the Mouse Allergen and Asthma Intervention Trial (MAAIT), 746 children aged 5 to 17 years with persistent asthma and a recent exacerbation completed a screening clinic visit. Six hundred forty-five children had valid skin test data and were included. The analyses related to mouse and cockroach sensitization. Participants with a mouse-specific IgE level of 0.10 kU/L or more or a positive mouse skin test result (wheal size ≥ 3 mm) were eligible for the baseline home visit. Four hundred ninety-five participants completed the baseline home visit, and 461 of these participants met the more stringent criteria for mouse sensitization of a mouse-specific IgE level of 0.35 kU/L or more or a positive mouse skin test result and comprised the analysis population for the exposure analyses (Figure 1).
FIGURE 1.
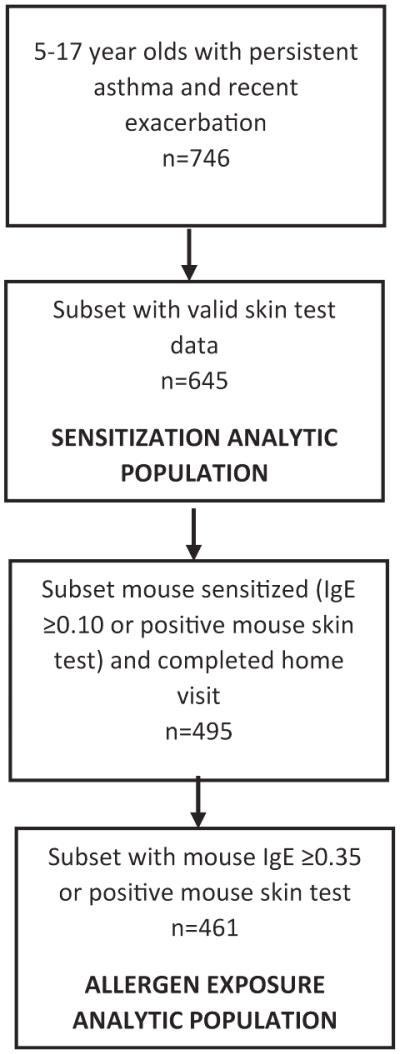
Flow diagram depicting derivation of study population used for the sensitization and exposure analyses.
Recruitment occurred between December 2010 and August 2014. Participants were recruited from pediatric emergency rooms, primary care clinics, and specialty clinics and had physician-diagnosed asthma at least 1 year before the screening visit. Persistent asthma was defined as use of a long-term controller medication or fulfillment of National Asthma Education and Prevention Program guidelines for persistent disease.13 An exacerbation was defined as an asthma-related emergency room or urgent care visit, overnight hospitalization, or oral steroid burst in the last 12 months. Children were screened for eligibility over the phone and those who met initial inclusion criteria were invited for a screening clinic visit. The study was approved by the Johns Hopkins Medicine and Boston Children’s Hospital institutional review boards. Written, informed consent was obtained from parents or guardians of participants.
Clinic visit procedures
A questionnaire was administered to collect socioeconomic, clinical, and environmental data. Trained research assistants administered the asthma symptoms and health care use questionnaire to primary caregivers of children aged 5 to 11 years and to adolescents aged 12 to 17 years.12,14 Questionnaires that captured sociodemographic and home environmental data were administered to the primary caregiver. Medication use was captured by a questionnaire and information obtained from medications that were brought to the clinic visit by study participants.
Participants underwent skin testing for 14 aeroallergens using the MultiTest II device (Lincoln Diagnostics, Decatur, Ill): dog, cat, Dermatophagoides pteronyssinus, Dermatophagoides farinae, rat epithelia, German cockroach, American cockroach, mouse epithelia, tree mix, grass mix, Alternaria, Aspergillus, common ragweed, and Cladosporium. A skin test result was considered positive if the net wheal diameter was 3 mm or greater. Atopy was defined as the number of positive skin test results, excluding mouse or cockroach, depending on which allergen was being analyzed. Blood was collected via venipuncture for measurement of mouse urine specific IgE levels using the ImmunoCAP system (ThermoFisher, Uppsala, Sweden).
Prebronchodilator and postbronchodilator spirometry data were obtained using a Koko spirometer (Longmont, Colo). Postbronchodilator spirometry data were obtained approximately 15 minutes after the administration of albuterol by nebulizer (2.5 mg/3 mL). Hankinson equations were used to generate percent of predicted values for participants 8 years and older.15 Spirometry data were reviewed for acceptability by the investigators and in accordance with American Thoracic Society guidelines.16 Bronchodilator reversibility was defined as a 12% or more increase in FEV1.13 Postbronchodilator lung function tests were examined for evidence of fixed airway obstruction, using cutoffs for abnormal lung function in children defined by the National Asthma Education and Prevention Program guidelines: FEV1/FVC less than 85% and FEV1 less than 80% predicted. These measures were restricted to participants 8 years and older because the Hankinson equations are valid down to the age of 8 years. Less than 10% of our analysis population met the criteria for FEV1 less than 80% predicted, so this outcome was not examined in final analyses.
Asthma severity was measured using an adaptation of the Composite Asthma Severity Index (CASI),17 a validated asthma severity tool. CASI was developed by the Inner City Asthma Consortium and therefore was validated in a similar study population. The total CASI score ranges from 0 to 20, with 0 being the lowest and indicating less severe disease and 20 being the highest and indicating more severe disease. The score is generated from the following data: daytime asthma symptoms, nighttime asthma symptoms, lung function, controller treatment step, and exacerbations. We followed the scoring as outlined in the validated CASI tool, with the following exceptions: In the original CASI scoring system, both nights of symptoms in the past 2 weeks and nights of albuterol use in the past 2 weeks were used to create the nighttime component score. The questionnaire used in MAAIT did not capture the number of nights in the last 2 weeks that the participants used albuterol, but instead captured the number of nights in the last 2 weeks that participants woke with symptoms. Therefore, for the nighttime component of CASI, we used nights the patient woke with asthma symptoms alone to generate the nighttime component score. In addition, the MAAIT questionnaire captured information on oral steroid bursts and hospitalizations over the last 3 months, as compared with 2 months as outlined in the CASI questionnaire. All other sections were scored as outlined in the CASI questionnaire.
Home assessment visit
Four hundred sixty-one participants were included in the analyses of exposure as they met the more stringent criteria for mouse sensitization (mouse-specific IgE level of ≥0.35 kU/L or a positive mouse skin test result) and completed a home assessment visit. Household dust samples were collected from the bed and bedroom floor using a handheld vacuum cleaner using standard methods.18-20 The samples were analyzed for Mus m 1 by ELISA.
Statistical analysis
All analyses were performed using Stata SE 13.1 (College Station, Texas). Six hundred forty-five participants had valid skin test data and were included in the sensitization analyses. Relationships between mouse sensitization variables and CASI score and treatment step were modeled using linear regression, and relationships with dichotomous lung function outcomes were modeled using logistic regression. Multivariate models included age, sex, insurance, race, Hispanic ethnicity, study site, and atopy. Three hundred eighty-four mouse-sensitized participants had bed dust samples and 349 had bedroom floor dust samples for analysis of Mus m 1 content. Mus m 1 concentrations were log10-transformed. Relationships between home Mus m 1 levels and CASI and treatment step were analyzed using linear regression and relationships with dichotomous lung function outcomes were analyzed using logistic regression. Multivariate models included age, sex, insurance, race, Hispanic ethnicity, study site, atopy, and mouse specific IgE. A 2-tailed P level of less than .05 was considered statistically significant.
RESULTS
Study population
A total of 645 children were included in the analyses examining relationships between mouse sensitization and measures of asthma severity and their characteristics are depicted in Table I. Participants ranged in age from 5 to 17 years, 60.2% were males, 69.6% were black or African American, and 20.8% were of Hispanic ethnicity. Approximately 65% of the participants were from Baltimore and 35% were from Boston. They were predominantly on public health insurance (82.6%), and most participants had an annual family income of less than $30,000 (61.8%). Approximately 84% of the study population was atopic, defined as having at least 1 positive skin test result, and 54.4% were mouse skin prick test positive and 50.6% were mouse-specific IgE positive. The population generally had moderately severe asthma, as the mean CASI score was 6.5 ± 3.4, and a wide spectrum of asthma severity was represented because 38.5% of participants were on high-dose inhaled corticosteroids ± long-acting beta2 agonist. Nineteen percent of the population reported being prescribed only albuterol as needed, but qualified for the study because of the frequency of their symptoms. Almost 80% had had an emergency department visit and 23% had been hospitalized for asthma in the previous year. Approximately 59% had an obstructive pattern, defined as a prebronchodilator FEV1/FVC% of less than 85.
TABLE I.
Study population characteristics (n = 645)
| Characteristic | n (%) |
|---|---|
| Age (y), mean ± SD | 9.9 ± 3.2 |
|
| |
| Sex: Male | 388 (60.2) |
|
| |
| Race | |
|
| |
| Black/African American | 449 (69.6) |
|
| |
| White | 114 (17.7) |
|
| |
| Other | 82 (12.7) |
|
| |
| Hispanic ethnicity | 134 (20.8) |
|
| |
| Socioeconomic measures | |
|
| |
| Public insurance | 533 (82.6) |
|
| |
| Annual income <$30K | 397 (61.8) |
|
| |
| Study site | |
|
| |
| Baltimore | 416 (64.5) |
|
| |
| Boston | 229 (35.5) |
|
| |
| Skin prick test (SPT) sensitization | |
|
| |
| ≥1 +SPT | 543 (84.2) |
|
| |
| No. +SPTs, mean ± SD | 4.5 ± 3.5 |
|
| |
| Mouse | 351 (54.4) |
|
| |
| Cockroach | 252 (39.1) |
|
| |
| Cat | 252 (39.1) |
|
| |
| Dust mite | 240 (37.2) |
|
| |
| Dog | 104 (16.1) |
|
| |
| Asthma severity measures | |
|
| |
| Composite asthma severity score,* mean ± SD | 6.5 ± 3.4 |
|
| |
| Treatment step | |
|
| |
| 0: No treatment | 0 (0) |
|
| |
| 1: Albuterol as needed | 120 (18.6) |
|
| |
| 2: Low-dose ICS or montelukast | 136 (21.1) |
|
| |
| 3: Low-dose ICS þ LABA or medium-dose ICS | 110 (17.0) |
|
| |
| 4: Medium-dose ICS þ LABA | 31 (4.8) |
|
| |
| 5: High-dose ICS ± LABA | 248 (38.5) |
|
| |
| Asthma-related ED visits and hospitalizations, last 12 mo | |
|
| |
| ED visit | 507 (78.6) |
|
| |
| Hospitalization | 147 (22.8) |
|
| |
| Lung physiology | |
|
| |
| Prebronchodilator FEV1 <80% predicted† | 82 (20.9) |
|
| |
| Prebronchodilator FEV1/FVC% <85%‡ | 351 (58.9) |
ED, Emergency department; ICS, inhaled corticosteroid; LABA, long-acting beta2 agonist.
n = 538.
n = 591.
n = 392, restricted to ≥8 y.
n = 596.
Associations between mouse sensitization and asthma severity score and treatment step
Mouse sensitization, defined as having either a positive skin prick test result (net wheal ≥3 mm) or mouse-specific IgE level of 0.35kU/L or more, was independently associated with a higher CASI score (β [95% CI], 0.82 [0.16-1.47]; P = .02) and a higher prescribed treatment step (β [95% CI], 0.36 [0.07-0.64]; P = .01) (Table II). These analyses were adjusted for age, sex, site, health insurance, atopy (defined by the number of positive skin test results excluding mouse), race, and Hispanic ethnicity. When additionally adjusted for cockroach skin test sensitivity, the relationships remained statistically significant without a significant change in the point estimates. The mouse-specific IgE level, expressed as log2(IgE), was also independently associated with a higher CASI score (β [95% CI], 0.08 [0.01-1.15 ]; P = .04) and a higher prescribed treatment step (β [95% CI], 0.03 [0.001-0.06]; P = .04). There was no relationship between mouse wheal size and CASI score or treatment step. In these models, there was a positive association between cockroach skin test sensitivity and treatment step, but not CASI score (see Table E1 in this article’s Online Repository at www.jaci-inpractice.org). The magnitude of the relationship between cockroach skin test sensitivity and treatment step was similar to that observed between mouse skin test sensitivity and treatment step. There was no association between dog, cat, or dust mite sensitivity and CASI or treatment step.
TABLE II.
Associations between mouse sensitization and severity score and treatment step
| CASI score*
β (95% CI) P value |
Treatment stepf β (95% CI) P value |
Prebronchodilator FEV1/FVC 85%‡
OR (95% CI) P value |
Post-FEV1/FVC <85%§
OR (95% CI) P value |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Mouse sensitization variable | Crude | Adjusted∥ | Crude | Adjusted∥ | Crude | Adjusted∥ | Crude | Adjusted∥ |
| Wheal size (mm) |
0.10 (0.01 to 0.18)
P = .02 |
0.07 (−0.03 to 0.17) P = .17 |
0.06 (0.02 to 0.10)
P = .002 |
0.03 (−0.02 to 0.07) P = .21 |
1.05 (1.00 to 1.11)
P = .045 |
1.01 (0.95 to 1.08) P = .68 |
1.05 (0.99 to 1.12) P = .10 |
1.02 (0.94 to 1.10) P = .68 |
|
| ||||||||
| log2(IgE) (kU/L) |
0.10 (0.03 to 0.16)
P = .003 |
0.08 (0.01 to 0.15)
P = .04 |
0.06 (0.03 to 0.09)
P < .001 |
0.03 (0.001 to 0.06)
P = .04 |
1.01 (0.97 to 1.05) P = .64 |
1.00 (0.95 to 1.04) P = .89 |
1.00 (0.96 to 1.06) P = .87 |
0.98 (0.92 to 1.04) P = .50 |
|
| ||||||||
| +SPT or +IgE |
0.92 (0.36 to 1.49)
P = .001 |
0.82 (0.16 to 1.47)
P = .02 |
0.56 (0.31 to 0.81)
P < .001 |
0.36 (0.07 to 0.64)
P = .01 |
1.43 (1.02 to 1.99)
P = .04 |
1.16 (0.78 to 1.74) P = .46 |
1.40 (0.92 to 2.13) P = .12 |
1.11 (0.67 to 1.86) P = .68 |
SPT, Skin prick test.
n = 591 for wheal size and +SPT/IgE, n = 581 for log2(IgE).
n = 645 for wheal size and +SPT/IgE, n = 635 for log2(IgE).
n = 596 for wheal size and +SPT/IgE, n = 586 for log2(IgE), n values for crude models.
Restricted to participants 8 y and older because Hankinson equations are valid down to 8 y. n = 381-382 for wheal size and +SFl7IgE, n = 375-376 for log2(IgE).
Adjusted for age, sex, insurance, site, atopy (number of positive skin test results excluding mouse), race, and Hispanic ethnicity.
TABLE E1.
Associations between cockroach sensitization and severity score and treatment step
| CASI score*
β (95% CI) P value |
Treatment step†
β (95% CI) P value |
Prebronchodilator FEV1/FVC 85%‡
OR (95% CI) P value |
||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Cockroach sensitization variable | Crude | Adjusted§ | Crude | Adjusted§ | Crude | Adjusted§ |
| Positive skin prick test result |
0.66 (0.10 to 1.23)
P = .02 |
0.22 (−0.43 to 0.86) P = .51 |
0.42 (0.18 to 0.67)
P = .001 |
0.32 (0.05-0.61)
P = .02 |
1.47 (1.05-2.06)
P = .03 |
1.10 (0.74-1.64) P = .65 |
n = 591.
n = 645.
n = 596.
Adjusted for age, sex, insurance, site, atopy (number of positive skin test results excluding cockroach), race, and Hispanic ethnicity.
Associations between mouse sensitization and lung function
We examined relationships between mouse wheal size, mouse log2(IgE), and mouse sensitization and indicators of obstruction on either prebronchodilator or postbronchodilator lung function. None of the measures of mouse sensitization was associated with any of the measures of obstruction, which included prebronchodilator FEV1/FEV of less than 85% and postbronchodilator FEV1/FEV of less than 85% (Table II). We also examined whether mouse sensitization modified the relationship between age and prebronchodilator FEV1/FVC. There was a statistically significant lower prebronchodilator FEV1/FVC with increasing age, for mouse sensitized, but not nonsensitized participants (P < .001 and .26, respectively), and this interaction was statistically significant (P = .05; Figure 2).
FIGURE 2.

Relationship between age and FEV1/FVC, by mouse sensitization. Mouse sensitization defined as positive SPTresult or mouse-specific IgE. SPT, Skin prick test. Gray shading represents 95% CIs. P value derived from interaction term (Mouse sensitization × Age) included in a linear regression model.
Association between mouse allergen exposure and CASI score, treatment step, and lung function, among mouse-sensitized children
Higher mouse allergen exposure among mouse-sensitized participants was associated with a higher CASI score as well as a higher treatment step. Log10(bedroom floor mouse allergen) was positively associated with CASI score and treatment step, and log10(bed mouse allergen) was positively associated with treatment step (Table III). These relationships were independent of age, sex, site, insurance, race, Hispanic ethnicity, mouse-specific IgE, and atopy (number of positive skin test results excluding mouse). We also examined the relationship between mouse allergen exposure and measures of obstruction and no associations were found (Table III).
TABLE III.
Associations between mouse allergen exposure and severity score and treatment step, among mouse sensitized
| CASI score β (95% CI) P value |
Treatment step β (95% CI) P value |
Prebronchodilator FEV1/FVC 85% OR (95% CI) P value |
Post-FEV1/FVC <85%*
OR (95% CI) P value |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Mus m 1 | Crude | Adjusted† | Crude | Adjusted† | Crude | Adjusted† | Crude | Adjusted† |
| log10(bed) |
0.60 (0.13 to 1.06)
P = .01 |
0.29 (−0.19 to 0.77)
P = 2A |
0.28 (0.08 to 0.49)
P = .006 |
0.22 (0.01 to 0.43)
P = .04 |
1.01 (0.76 to 1.35) P = .92 |
1.11 (0.81 to 1.53) P = .50 |
1.09 (0.77 to 1.56) P = .62 |
0.99 (0.67 to 1.48) P = .98 |
|
| ||||||||
| log10(bedroom floor) |
0.48 (0.10 to 0.86)
P = .01 |
0.43 (0.05 to 0.81)
P = .03 |
0.29 (0.12 to 0.45)
P = .001 |
0.26 (0.09 to 0.43)
P = .002 |
0.86 (0.69 to 1.09) P = .21 |
0.98 (0.76 to 1.26) P = .87 |
0.84 (0.63 to 1.14) P = .26 |
0.86 (0.62 to 1.21) P = .39 |
Restricted to participants 8 y and older because Hankinson equations are valid down to 8 y. n = 223-229 for log10(bed) and n = 197-202 for log10(bedroom floor).
Adjusted for age, sex, insurance, site, atopy (number of positive skin test results excluding mouse), race, Hispanic ethnicity, and mouse-specific IgE. n = 358-384 for log10(bed) and n = 325-349 for log10(be droom floor).
DISCUSSION
Our study’s objective was to determine whether there is an association between indoor allergen sensitization and/or exposure and asthma severity, focusing on mouse sensitization and exposure, a relevant allergen among low-income, minority children with asthma.19 In this study population, we found associations between higher mouse allergen exposure and more severe asthma, as captured by asthma severity score and prescribed treatment step. We also found that mouse sensitization was associated with not only asthma severity but also a greater decrease in FEV1/FVC with increasing age compared with nonsensitized peers. Together these findings support a link between mouse sensitization and exposure and asthma severity, which suggests that intervening on these factors may improve certain features of asthma severity, such as controller medication requirements. Any improvement in asthma severity has the potential to reduce the risks of long-term manifestations of childhood asthma such as lung function decline. Although each indoor allergen has its own characteristics, these findings with respect to mouse allergen suggest that other indoor allergens may also play a role in determining asthma severity among other populations of children with asthma.
Our findings suggest that when compared with nonsensitized peers with asthma, mouse-sensitized children with asthma have more severe asthma, as indicated by higher CASI scores and higher prescribed controller medication treatment step. These results show that, on average, mouse-sensitized children had a CASI score approximately 0.9 points higher than did non-sensitized children. A difference of 1 point in CASI score corresponds to an increase in treatment step by 1 step or an FEV1 % predicted below 85%.16 Our findings also suggest that mouse-sensitized patients with asthma are prescribed a higher treatment step, equal to approximately 100 to 150 μg of fluticasone per day. When we examined relationships between age and FEV1/FVC, we found a greater decrease in FEV1/FVC with older age among mouse-sensitized versus non—mouse-sensitized participants, suggesting that mouse sensitization modifies the relationship between FEV1/FVC and age, with greater declines in FEV1/FVC among mouse-sensitized children and adolescents. This finding suggests that sensitization may be associated with worsening lung function over time in children with established asthma. Although some studies have suggested that indoor allergen exposure early in life is associated with lower lung function,21 mouse allergen was not evaluated in these studies. Future prospective studies directed at following lung function over time in mouse-sensitized versus nonsensitized children with asthma would provide further insight into the potential role of mouse sensitization in the natural history of asthma and persistence into adulthood.
We also examined the effects of mouse allergen exposure, a more readily modifiable risk factor than sensitization, on asthma severity. We found higher exposure to be associated with more severe asthma among mouse-sensitized participants, as indicated by a higher CASI score and a higher prescribed treatment step. For every 10-fold increase in mouse allergen bedroom floor levels, there was an approximately 0.5-point increase in CASI score. For everyone 10-fold increase in mouse allergen bed and bedroom levels, there was an approximately 0.25 increase in treatment step. For example, a mouse-sensitized child with a Mus m 1 concentration of 10 μg/g would be expected to be prescribed 60 to 110 μg more inhaled fluticasone per day than a mouse-sensitized child exposed to 1 μg/g of Mus m 1.
Previous studies have described a link between mouse sensitization and exposure and markers of asthma control,22-25 such as symptoms and exacerbations, but to our knowledge, no studies have expressly linked mouse allergen sensitization and/or exposure to features of severity such as CASI score, a tool first validated in 2012, and controller medication requirements. There have, however, been a handful of studies that have linked mouse sensitization and exposure to poorer lung function.19 Although we found no associations between either mouse allergen sensitization or exposure and measures of fixed obstruction (postbronchodilator lung function indices), the association between mouse sensitization and lower prebronchodilator FEV1/FVC with increasing age is consistent with these previous studies and this relationship was similar, although not statistically significant, for postbronchodilator FEV1/FVC. Studies of other common indoor allergens, such as dust mite, dog, cat, or cockroach, have also described an association with sensitization and exposure and lower lung function,3,21 as well as persistent asthma and asthma severity, based on FEV1 and asthma medication use.26
Because childhood asthma severity and lung function are associated with worse outcomes in adulthood,3,6-9 reducing indoor allergen exposure has potential to improve long-term outcomes of asthma by altering the natural history of the disease. It is important to note, though, that this link between higher mouse allergen exposure and asthma severity has been observed in children with established asthma, and not in children at risk for developing asthma. In fact, a recent study found that early allergen exposure, including to mouse, in the first year of life is associated with decreased risk of recurrent wheeze at age 3 years,27 so that mouse allergen exposure reduction would not be expected to be an effective primary prevention approach. However, although the protective associations observed between early life mouse allergen exposure and incident asthma are intriguing, it is not yet clear that they are causal. It is possible, for example, that mouse allergen exposure is a surrogate marker for microbial exposures that influence immune development. In contrast, among children with established asthma and mouse sensitization, there is growing evidence that mouse allergen exposure causes asthma morbidity, and does so through an IgE-dependent mechanism.
Our study has several notable strengths and limitations. Although our findings are not generalizable to all children with asthma, the findings are likely applicable to similar populations of low-income, predominantly minority children and adolescents with persistent asthma. Although the study focuses on an allergen that is highly relevant to this high-risk population, the findings, along with the body of work focused on other major indoor allergens, suggest that other indoor allergens could also be modifiable risk factors for asthma severity in children and so these other allergens should be examined in future studies. We found no association between positive skin prick test results to dog, cat, or dust mite with either CASI or treatment step in this population, which likely reflects the fact that these allergens have generally not been associated with asthma outcomes in this population for whom pest allergens are most relevant.12,19,28
In conclusion, among low-income, urban minority children and adolescents with persistent asthma, mouse sensitization is associated with greater disease severity and prescribed treatment step. Increasing age may be associated with decreasing FEV1/FVC among mouse-sensitized, but not non—mouse-sensitized children. In addition, mouse-sensitized children with higher home mouse allergen levels had greater controller medication requirements and disease severity. Together, these findings support the hypothesis that intervening upon mouse sensitization or exposure among those with persistent asthma may alter the natural history of the disease, and thereby improve long-term outcomes. More broadly, these findings suggest that indoor allergen sensitization and exposure may be important predictors of asthma severity, and that intervening upon these risk factors may alter the natural history of childhood asthma.
What is already known about this topic? Mouse sensitization and allergen exposure have been linked to poor asthma control.
What does this article add to our knowledge? In this study, mouse sensitization and allergen exposure are also associated with asthma severity, which is a predictor of poor outcomes in adulthood.
How does this study impact current management guidelines? Intervening upon mouse sensitization or exposure in this population has the potential to reduce asthma severity.
Acknowledgments
We are grateful to the Inner-city Asthma Consortium and Jeremy Wildfire for providing information about the Composite Asthma Severity Index so that we could adapt its use for this study.
This study is registered under NCT01251224 at clinicaltrials.gov. This study was supported by the National Institutes of Health (grant nos. U01 Al 083238, K24 AI 106822, K24 AI114769 R01 ES023447, P50ES015903, and P01 ES018176).
M. Perzanowski and R. Miller have received research support from the National Institutes of Health (NIH). M. E. Bollinger has received research support from the NIH/National Institute of Allergy and Infectious Diseases (grant no. RO1 NCT01251224). E. Matsui has received research support from the NIH; has received consultancy fees from the Environmental Defense Fund, Dwight, and Church LLC; and has received lecture fees from Thermo-Fisher.
Abbreviations used
- CASI
Composite Asthma Severity Index
- FVC
Forced vital capacity
- MAAIT
Mouse Allergen and Asthma Intervention Trial
Footnotes
Conflicts of interest:
The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Anselmo M. Pediatric asthma controller therapy. Paediatr Drugs. 2011;13:11–7. doi: 10.2165/11533730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Strunk RC, Sternberg AL, Szefler SJ, Zeiger RS, Bender B, Tonascia J. Long-term budesonide or nedocromil treatment, once discontinued, does not alter the course of mild to moderate asthma in children and adolescents. J Pediatr. 2009;154:682–7. doi: 10.1016/j.jpeds.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim SL, Brown KC, Wood RA, Wise RA, Eggleston PA, Tonascia J, et al. Adult asthma severity in individuals with a history of childhood asthma. J Allergy Clin Immunol. 2005;115:61–6. doi: 10.1016/j.jaci.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Maestrelli P, Zanolla L, Pozzan M, Fabbri LM. Effect of specific immunotherapy added to pharmacologic treatment and allergen avoidance in asthmatic patients allergic to house dust mite. J Allergy Clin Immunol. 2004;113:643–9. doi: 10.1016/j.jaci.2003.12.586. [DOI] [PubMed] [Google Scholar]
- 5.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186. doi: 10.1002/14651858.CD001186.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Savage JH, Matsui EC, McCormack M, Litonjua AA, Wood RA, Keet CA. The association between asthma and allergic disease and mortality: a 30-year follow-up study. J Allergy Clin Immunol. 2014;133:1484–7. doi: 10.1016/j.jaci.2014.01.028. 1487.e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sears MR. Lung function decline in asthma. Eur Respir J. 2007;30:411–3. doi: 10.1183/09031936.00080007. [DOI] [PubMed] [Google Scholar]
- 8.Limb SL, Brown KC, Wood RA, Wise RA, Eggleston PA, Tonascia J, et al. Irreversible lung function deficits in young adults with a history of childhood asthma. J Allergy Clin Immunol. 2005;116:1213–9. doi: 10.1016/j.jaci.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Tai A, Tran H, Roberts M, Clarke N, Gibson AM, Vidmar S, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. 2014;133:1572–1578. doi: 10.1016/j.jaci.2013.12.1033. e3. [DOI] [PubMed] [Google Scholar]
- 10.Sordillo JE, Webb T, Kwan D, Kamel J, Hoffman E, Milton DK, et al. Allergen exposure modifies the relation of sensitization to fraction of exhaled nitric oxide levels in children at risk for allergy and asthma. J Allergy Clin Immunol. 2011;127:1165–72. doi: 10.1016/j.jaci.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A. Exposure and sensitization to indoor allergens: association with lung functions, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol. 2003;112:362–8. doi: 10.1067/mai.2003.1654. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 13.Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, et al. Inner-city Asthma Consortium. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010;65:1414–22. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the US general population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 16.Standardization of Spirometry, 1994 Update American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 17.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index—an outcome measure for use in children and adolescents. J Allergy Clin Immunol. 2012;129:694–701. doi: 10.1016/j.jaci.2011.12.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood RA, Eggleston PA, Lind P, Ingemann L, Schwartz B, Graveson S, et al. Antigenic analysis of household dust samples. Am Rev Respir Dis. 1988;137:358–63. doi: 10.1164/ajrccm/137.2.358. [DOI] [PubMed] [Google Scholar]
- 19.Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, et al. Mouse allergen is the major allergen of public health relevance in Baltimore city. J Allergy Clin Immunol. 2013;132:830–5. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98:167–76. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U. Perennial allergen sensitization early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 22.Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97:514–20. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 23.Pongracic JA, Visness CM, Gruchalla RS, Evans R, III, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. 2008;101:35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- 24.Phipatanakaul W, Eggleston PA, Wright EC, Wood RA. National Cooperative Inner-City Asthma Study Mouse allergen II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106:1075–80. [Google Scholar]
- 25.Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, et al. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010;65:1414–22. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarpong SB, Karrison T. Skin test reactivity to indoor allergens as a marker of asthma severity in children with asthma. Ann Allergy Asthma Immunol. 1998;80:303–8. doi: 10.1016/S1081-1206(10)62973-0. [DOI] [PubMed] [Google Scholar]
- 27.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134:593–601. doi: 10.1016/j.jaci.2014.04.018. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–70. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]


