Abstract
PURPOSE
To determine the cutoffs for the interocular difference in retinal nerve fiber layer (RNFL) thickness measured with Cirrus HD-OCT (Carl Zeiss Meditec, Inc) in normal eyes.
DESIGN
Observational, clinical study.
METHODS
Scans were acquired at 7 academic glaucoma clinics from both eyes of 284 normal subjects using the Optic Disc Cube 200 × 200 protocol. The interocular differences in RNFL thickness were calculated, and normal ranges of interocular differences were determined as the 2.5th and 97.5th percentiles.
RESULTS
The average RNFL in the right eye was 0.52 μm thicker than in the left eye; the difference was marginally significant (P =.049). The temporal, nasal, and inferior quadrants had significantly thicker RNFL in the right eye, whereas the left eye showed thicker RNFL in the superior quadrant. The 2.5th and 97.5th percentile interocular difference tolerance limits for average RNFL thickness were −7.9 μm and 8.8 μm, respectively. Although the difference in average RNFL thickness correlated with differences in axial length, disc area, cup-to-disc ratio, and vertical cup-to-disc ratio, only differences in axial length (β = −0.21; P < .001) and disc area (β = 0.17; P < .001) were associated with an interocular difference of average RNFL thickness after adjustment for each other. The interocular difference remained stable despite significant decrease in RNFL thickness with aging.
CONCLUSIONS
An interocular difference in average RNFL thickness exceeding 9 μm when measured with the Cirrus HD-OCT in normal eyes may be considered statistically significant asymmetry and may be indicative of early glaucomatous damage.
Most studies of the relationship between structure and function in glaucoma have shown that structural changes to the retinal nerve fiber layer (RNFL) precede ophthalmoscopically observable changes to the optic disc and visual function loss as tested by automated perimetry.1 Thus, detecting these structural changes can be important for early diagnosis of the disease.
Because organ pairs are not always perfectly symmetric, studies have been carried out in various medical specialties to assess whether differences between organ pairs can predict or influence the localization of certain diseases. With regard to glaucoma, this approach has been used for cup-to-disc ratio (CDR) because this concept was introduced as a standardized method of assessing the optic nerve head (ONH).2 Ever since, interocular asymmetry in CDR of the same individual has been shown to be an early sign of glaucomatous damage3 or a predictor of future damage in patients with ocular hypertension.4 Similarly, other studies have shown that asymmetry in intraocular pressure (IOP) is related to the presence of glaucomatous visual field (VF) loss.5,6 Asymmetry in static perimetry also has been shown to be related to early glaucomatous damage.7 However, one of the problems with the CDR is its wide physiologic variation in normal individuals, as a result of the variability in disc and cup size, so that the CDR is larger in large discs than in small discs. Additionally, asymmetries in CDR, IOP, and visual fields do not necessarily indicate the extent to which the RNFL is damaged. Because retinal ganglion cell (RGC) death is the key pathologic event in glaucoma, it is essential that methods for early diagnosis focus on the RNFL, which is made up of RGC axons converging to form the optic nerve. The availability of objective and noninvasive imaging methods to measure the RNFL thickness in vivo offers an opportunity to diagnose glaucoma earlier than with conventional techniques. Therefore, it may be appropriate to apply the concept of interocular asymmetry in RNFL thickness for early diagnosis of glaucoma or suspected glaucoma by using these new imaging methods. A few studies have been published on this topic using time-domain optical coherence tomography (OCT)8,9 and other imaging technologies. The recent introduction of spectral-domain (SD) OCT has enabled significant improvement in image acquisition speed, rate and resolution, and accuracy in measurements compared with time-domain OCT and other imaging technologies. Therefore, interocular asymmetry in RNFL thickness measured with SD OCT may be different than previously reported. The present study was carried out to determine normal tolerance limits of the amount of asymmetry in peripapillary RNFL thickness measured with the Cirrus HD-OCT (Carl Zeiss Meditec, Inc, Dublin, California, USA) when the values from both eyes are within normal range. Determining these tolerance limits may help identify subjects having early glaucomatous axonal loss when the interocular asymmetry tolerance limit is exceeded.
METHODS
SUBJECTS
Male and female normal subjects at least 18 years of age were recruited at 7 academic ophthalmology practices and were invited to participate in the study.
Each subject was informed of the nature of the study, and the subjects’ willingness to participate required a written consent form.
Exclusion criteria included contraindication to dilation or intolerance to topical anesthetics or mydriatics; IOP of 22 mm Hg or more or any type of glaucoma, including normal-tension glaucoma, in either eye; intraocular surgery in the study eye (except cataract or refractive surgery if performed more than 1 year before testing); best-corrected visual acuity worse than 20/40; evidence of diabetic retinopathy, macular edema, or other vitreoretinal disease in either eye; or evidence of optic nerve abnormality in either eye. Subjects with CDR asymmetry of 0.2 or more were excluded to avoid including glaucoma suspects. Subjects also were excluded if their scans showed algorithm failure, or if either eye had a signal strength of less than 6. Each subject underwent a complete ophthalmologic evaluation, including measurement of visual acuity, slit-lamp examination, IOP measurement, and dilated fundus examination.
An exclusionary diagnosis of glaucoma was made by the investigators at the study sites and was based on glaucomatous ONH abnormalities or VF defects as detected by 2 reliable Swedish interactive threshold algorithm standard 24-2 Humphrey Visual Field examinations. Subjects were excluded if their optic nerve showed abnormalities suggestive of glaucoma (cup-to-disc ratio ≥ 0.5 in either eye, cup-to disc ratio asymmetry ≥ 0.2, optic disc hemorrhage, or focal thinning of the rim). A visual field was considered glaucomatous if the glaucoma hemifield test results were outside normal limits, the pattern standard deviation had a P value of less than .05, or if there was a cluster of 3 or more points in the pattern deviation plot in a single hemifield (superior or inferior) with P values less than .05, one or more of which with a P value of less than .01.
OPTICAL COHERENCE TOMOGRAPHY SCANNING PROCEDURE
The pupils of all subjects were dilated with tropicamide 1% and phenylephrine 2.5% drops. The same instrument was used for both eyes of the same subject, and only 1 scan was acquired for each eye. At each participating center, all scans were obtained by the same operator with Cirrus HD-OCT version 3.0 using the Optic Disc 200 × 200 axial scans protocol. This protocol generates a 6 × 6-mm cube of data after a series of 200 B-scans with 200 A-scans per B-scan (40 000 points) in approximately 1.5 seconds (27 000 A-scans/second). The instrument uses the intrinsic algorithms to delineate the boundaries of the RNFL automatically and to calculate the RNFL thickness at each point on the 1.73-mm radius circle around the optic disc. The RNFL thickness measurements (overall average, quadrants, and clock hours) as well as the signal strength scaled from 0 (worst) to 10 (best) are reported on the printout. Only scans with signal strength of 6 or more, without eye movement or blinking artifacts within a 1.73-mm radius around the ONH or without algorithm segmentation failure, were used for analysis. The following ONH parameters were measured automatically by the Carl Zeiss ONH analysis algorithm developed for Cirrus HD-OCT (software version 5.0): disc area, rim area defined as the difference between disc area and cup area, vertical rim thickness (VRT; the total rim thickness, in micrometers, measured in the vertical meridian and corresponds to the summation of the rim width at the superior and inferior positions), cup-to-disc area ratio (CDR; ratio of cup area to disc area), vertical cup-to-disc ratio (VCDR; ratio of vertical line through the cup center to the same vertical line extending to the disc margin), horizontal cup-to disc ratio (HCDR; ratio of the horizontal line through the cup center to the same line extending to the disc margin), and cup volume.
STATISTICAL METHODS AND DATA ANALYSIS
All analyses were performed with SPSS software version 17.0 (SPSS, Inc, Chicago, Illinois, USA). Mean measurements of the 2 eyes were compared using the Student t test for paired samples. Interocular differences were determined by subtracting the measurements of left eyes from those of right eyes. Normal ranges for interocular differences were established as the 2.5th and 97.5th percentiles for average and sectoral (quadrants and clock hours) RNFL thickness. The relationships between interocular difference in average RNFL thickness and age, interocular differences in refraction expressed as spherical equivalent, IOP, central corneal thickness, axial length, disc area, CDR, HCDR, and VCDR were investigated using univariate and multivariate linear regressions. These variables first were fitted in a univariate model, then variables with P values less than .05 were entered in a multivariate analysis to determine the independence of effects. The interocular difference in average RNFL thickness was regressed against age to verify the hypothesis that the symmetry of RNFL thickness measurements varies as a function of age. For all analyses, a P value < .05 was considered statistically significant.
RESULTS
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS
Data for 284 subjects (135 males and 149 females) were included in the analysis. There were 122 (43%) white persons, 35 (12.3%) Hispanic persons, 51 (18%) black persons, 67 (23.7%) Asians, and 6 (2.1%) others. Their mean age was 46.13 ± 16.87 years (range, 18 to 84 years). The main clinical features for right and left eyes are shown in Table 1. Right eyes (23.96 mm) were slightly longer than left eyes (23.90 mm; P = .002), whereas HCDR was slightly greater in left eyes (0.51) compared with right eyes (0.49; P = .003). No statistically significant interocular differences were observed with regard to refraction, central corneal thickness, IOP, CDR, VCDR, disc area, integrated rim area, and VRT.
TABLE 1.
Clinical Features for Right and Left Eyes
| Variables | Right Eye | Left Eye | P Value |
|---|---|---|---|
| Refraction (diopters)a | −0.89 (2.11) | −0.88 (2.06) | .88 |
| CCT (μm) | 550.28 (36.60) | 549.81 (36.35) | .22 |
| IOP (mm Hg) | 14.01 (2.49) | 14.06 (2.42) | .52 |
| Axial length (mm) | 23.96 (1.03) | 23.90 (1.05) | .002 |
| Disc area (mm2) | 1.83 (0.35) | 1.84 (0.36) | .42 |
| CDR | 0.48 (0.17) | 0.49 (0.17) | .08 |
| Vertical CDR | 0.45 (0.16) | 0.46 (0.16) | .32 |
| Horizontal CDR | 0.49 (0.19) | 0.51 (0.19) | .003 |
| Cup volume (mm3) | 0.15 (0.15) | 0.15 (0.14) | .92 |
| IRA (mm2) | 1.32 (0.23) | 1.31 (0.24) | .30 |
| VRT (μm) | 843.42 (224.87) | 842.05 (232.61) | .8 |
CCT = central corneal thickness; CDR = cup-to-disc ratio; IOP = intraocular pressure; IRA = integrated rim area; VRT = vertical rim thickness.
Values are expressed as mean (standard deviation).
Presented as spherical equivalent.
ASYMMETRY IN RETINAL NERVE FIBER LAYER THICKNESS
Table 2 shows the mean differences in RNFL thickness between right and left eyes. The average RNFL in the right eye was 0.52 ± 4.4 μm thicker than in the left eye; the difference was marginally significant (P < .049). Similarly, all quadrants showed statistically significant interocular differences in RNFL thickness (P < .05). The RNFL in the right eye was thicker in the temporal, nasal, and inferior quadrants, whereas the left eye showed thicker RNFL in the superior quadrant. All clock hours revealed statistically significant interocular differences in RNFL thickness, with exception for clock hours 4, 5, and 6. The left eye had thicker RNFL only at clock hours 12 and 1; the RNFL was thicker in the right eye in the remaining 7 clock hours. There was no significant difference in mean signal strength between right eyes (9.201 ± 0.63) and left eyes (9.203 ± 0.68; P ± .94). The mean RNFL symmetry score, as provided by the manufacturer’s software, was 0.89 ± 0.06. The percentile distribution of the interocular differences in peripapillary RNFL thickness parameters is displayed in Table 3, and the mean distribution of interocular differences in average RNFL thickness is shown in Figure 1. The 2.5th and 97.5th percentiles of interocular difference tolerance limits for average RNFL thickness were −7.9 μm and 8.8 μm, respectively, depending on whether the RNFL was thicker in the left or right eye. Cutoff points for quadrants (10.1 μm and 22.6 μm) and clock-hour sectors (10.8 μm and 39.8 μm) were higher than those of average RNFL thickness.
TABLE 2.
Retinal Nerve Fiber Layer Thickness (μm) of Right and Left Eye and Interocular Differences (Right Eye Minus Left Eye)
| Parameters | Right Eye (SD) | Left Eye (SD) | Difference (SD) | P Value |
|---|---|---|---|---|
| Average RNFL | 93.09 (9.33) | 92.57 (9.86) | 0.52 (4.44) | 0.049 |
| TP quadrant | 64.52 (11.67) | 61.92 (10.42) | 2.60 (6.03) | <.001 |
| SP quadrant | 115.73 (14.69) | 119.41 (15.90) | −3.68 (9.07) | <.001 |
| NS quadrant | 70.51 (11.61) | 68.66 (11.47) | 1.85 (7.30) | <.001 |
| IF quadrant | 121.61 (16.05) | 120.29 (16.01) | 1.31 (9.95) | 0.027 |
| Clock-hr 1/11 | 102.20 (20.32) | 113.22 (21.59) | −11.02 (15.40) | <.001 |
| Clock-hr 2/10 | 88.13 (18.76) | 85.58 (18.58) | 2.55 (14.39) | 0.003 |
| Clock hour 3/9 | 57.41 (9.54) | 55.27 (9.13) | 2.14 (8.67) | <.001 |
| Clock hour 4/8 | 66.00 (13.17) | 65.13 (14.00) | 0.87 (9.94) | 0.141 |
| Clock hour 5/7 | 98.54 (20.69) | 99.24 (20.94) | −0.70 (13.48) | 0.386 |
| Clock hour 6 | 134.26 (23.77) | 132.47 (25.05) | 1.79 (18.70) | 0.108 |
| Clock hour 7/5 | 132.01 (23.01) | 129.16 (24.09) | 2.85 (17.67) | 0.007 |
| Clock hour 8/4 | 64.95 (14.77) | 62.03 (14.34) | 2.91 (10.15) | <.001 |
| Clock hour 9/3 | 51.19 (9.22) | 49.74 (8.29) | 1.44 (6.10) | <.001 |
| Clock hour 10/2 | 77.41 (16.07) | 73.99 (13.38) | 3.42 (10.99) | <.001 |
| Clock hour 11/1 | 127.60 (21.59) | 122.88 (22.85) | 4.72 (16.64) | <.001 |
| Clock hour 12 | 117.40 (24.75) | 122.14 (24.68) | −4.74 (17.30) | <.001 |
IF = inferior; NS = nasal; RNFL = retinal nerve fiber layer; SD = standard deviation; SP = superior; TP = temporal.
TABLE 3.
Percentile Distribution of Interocular Differences (Right Eye Minus Left Eye) in Retinal Nerve Fiber Layer Thickness (μm)
| Parameters | Percentiles | |||
|---|---|---|---|---|
|
| ||||
| 2.5 | 5 | 95 | 97.5 | |
| Average RNFL | −7.9 | −6.7 | 7.2 | 8.8 |
| TP quadrant | −10.1 | −6.9 | 12.2 | 14.9 |
| SP quadrant | −22.6 | −18.4 | 10.7 | 15.4 |
| NS quadrant | −11.9 | −10.9 | 12.4 | 16.4 |
| IF quadrant | −16.2 | −13.2 | 15.7 | 19.1 |
| Clock hour 1/11 | −39.4 | −35.6 | 15.2 | 20.9 |
| Clock hour 2/10 | −26.9 | −21.5 | 25 | 30.4 |
| Clock hour 3/9 | −12.5 | −10.6 | 16 | 19.4 |
| Clock hour 4/8 | −22.5 | −16.7 | 16.1 | 19.1 |
| Clock hour 5/7 | −27.6 | −23.4 | 24.2 | 31.3 |
| Clock hour 6 | −32.4 | −26.0 | 34.6 | 39.2 |
| Clock hour 7/5 | −39.8 | −27.6 | 27.7 | 34.8 |
| Clock hour 8/4 | −21.6 | −14.9 | 18.4 | 21.5 |
| Clock hour 9/3 | −10.8 | −7.7 | 11.3 | 14.4 |
| Clock hour 10/2 | −17.3 | −14.0 | 22.1 | 27.1 |
| Clock hour 11/1 | −26.1 | −23.3 | 33.3 | 38.5 |
| Clock hour 12 | −39.2 | −31.8 | 23.7 | 26.9 |
IF = inferior; NS = nasal; RNFL = retinal nerve fiber layer; SP = superior; TP = temporal.
FIGURE 1.
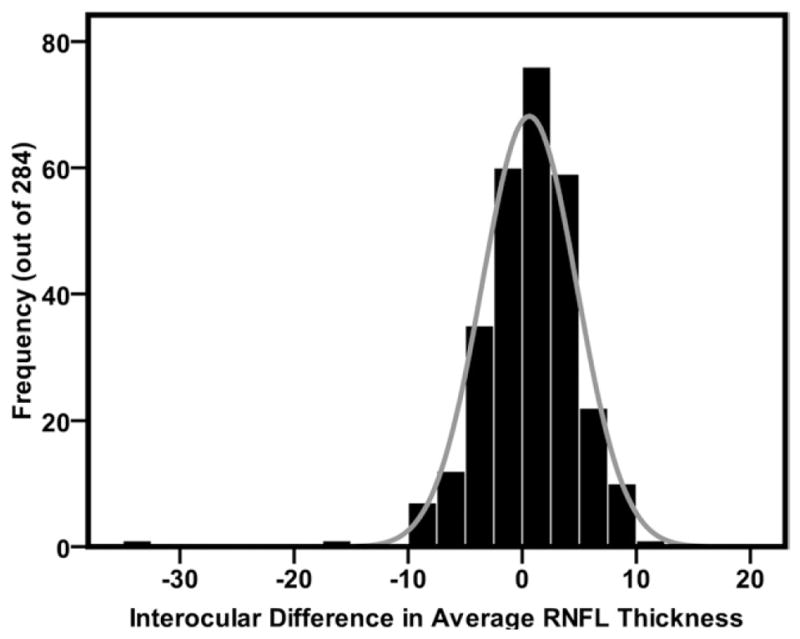
Graph showing the frequency distribution of mean interocular difference (right eye minus left eye) in average retinal nerve fiber layer (RNFL) thickness (n = 284 pairs of eyes). The difference was mostly positive because more right eyes had thicker average RNFL, which is indicated by a slight skewness to the left.
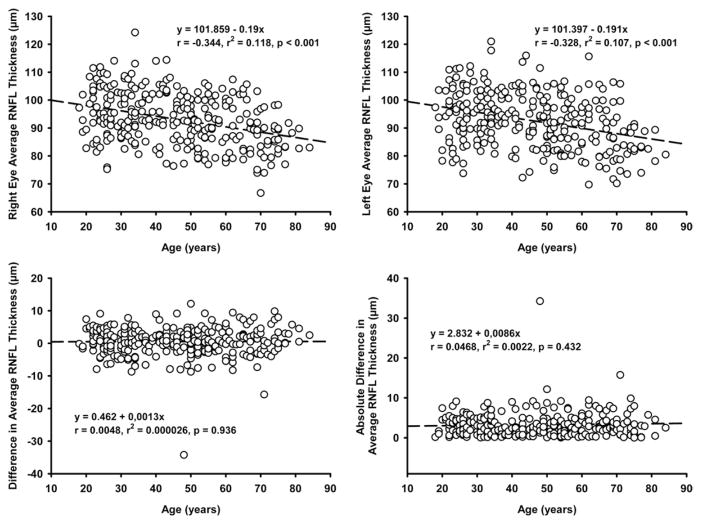
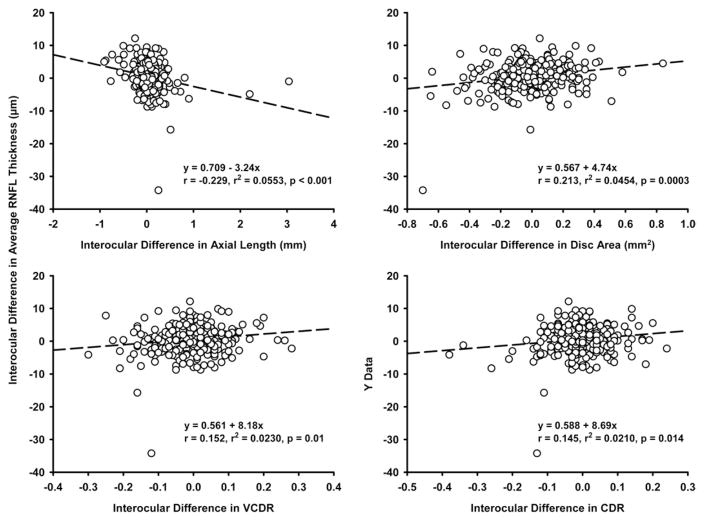
Average RNFL thickness highly correlated in both eyes (r = 0.89; P = .001). RNFL thickness of homonymous quadrants also correlated well (r = 0.81 to 0.86; all P = .001). For clock hours, the correlation coefficients ranged between 0.70 and 0.79, except for clock hour 3 (r = 0.57). Weak but significant correlations were found between signal strength and average RNFL thickness in both eyes (right eyes: r = 0.172, P = .004; left eyes: r = 0.199, P = .001). Increasing age was associated with decrease in average RNFL thickness in both eyes (right eyes: r = −0.34, P < .001; left eyes: r = −0.33, P < .001), but the effect of age on RNFL thickness was significant only in subjects 50 years old and older (n = 126; right eyes: r = −0.231, P = .009; left eyes: r = −0.246, P = .005). Both signed and absolute interocular differences in RNFL thickness remained stable with increasing age (Figure 2), and this trend did not change when data from younger (age, 18 to 49 years; n = 158) and older (n = 126) subjects were analyzed separately. In univariate regression, interocular difference in average RNFL thickness significantly correlated with interocular differences in axial length (r = −0.229; P < .001), CDR (r = 0.145; P = .014), disc area (r = 0.213; P < .001), and VCDR (r = 0.152; P < .001), but not interocular difference in signal strength (r = 0.064; P = .28) or the RNFL symmetry score (r = 0.01; P = .87). However, these correlations could explain only 2.1% to 5.3% of the interocular differences in average RNFL thickness (Figure 3). A multiple regression analysis including these 4 variables revealed that altogether they only accounted for 9.4% of the interocular variability in average RNFL thickness and that differences in axial length (β = −0.21; P = .001) and disc area (β = 0.17; P < .001) were associated independently with the difference in RNFL thickness.
FIGURE 2.
Scattergrams showing linear regression analysis between age and (Top left) right eye average retinal nerve fiber layer (RNFL) thickness, (Top right) left eye average RNFL thickness, (Bottom left) signed interocular difference in average RNFL thickness, and (Bottom right) absolute interocular difference in average RNFL thickness. The top plots show a decline in RNFL thickness with increasing age, and the bottom plots show that interocular difference in RNFL thickness is not influenced by increasing age.
FIGURE 3.
Scattergrams showing linear regression analysis between interocular difference in average retinal nerve fiber layer (RNFL) thickness and (Top left) interocular differences in axial length, (Top right) disc area, (Bottom left) vertical cup-to-disc ratio (VCDR), and (Bottom right) cup-to-disc ratio (CDR). All interocular difference values derived from right minus left eye values.
In both eyes, weak but positive correlations were found between superior (right eye: r = 0.278, P < .001; left eye: r = 0.297, P < .001), nasal (right eye: r = 0.303, P < .001; left eye: r = 0.247, P < .001), inferior (right eye: r = 0.234, P = .001; left eye: r = 0.231, P = .001) quadrants RNFL thickness and disc area. The temporal quadrant RNFL thickness did not correlate with disc area in either eye. With regard to interocular differences, RNFL thickness of the superior (r = 0.171; P = .004) and inferior (r = 0.16; P = .007) quadrants also weakly correlated with disc area.
DISCUSSION
THE RNFL IS KNOWN TO BE THE SITE OF EARLY GLAUCOMAtous damage, which may occur first before structural change can be observed ophthalmoscopically and before loss of visual function can be detected on standard automated perimetry. Thus, early detection of structural damage to the RNFL is useful for the management of the disease. To achieve this, several techniques, such as scanning laser polarimetry, scanning laser ophthalmoscopy, and OCT, have been developed recently. Grant and Burke stated that eyes having a visual field deficit when treatment is started are more likely to progress to blindness than are eyes in which treatment is started without visual field deficit.10 This implies that it is beneficial to detect glaucoma and start treatment before visual field deficits have occurred. In keeping with early detection of glaucoma, Caprioli and Miller estimated that examination of the RNFL is more important than the appearance of the optic nerve.11 Compared with other imaging techniques, SD OCT has proven to provide higher resolution and more detailed images, which should result in more precise quantitative analysis of the RNFL. The present study was designed around 2 concepts. First, in normal eyes, the interocular difference in RNFL thickness, similar to the CDR and IOP, is small. Second, glaucoma is usually bilateral, but often asymmetric at presentation and in its progression. Therefore, if a cutoff value can be determined for normal interocular difference in RNFL thickness, then it may help in early detection of glaucoma. Values of interocular difference beyond the cutoff point may be indicative of early glaucomatous thinning of the RNFL or a predictive factor for later development of glaucomatous optic neuropathy.
We have found that interocular differences in RNFL thickness for average, three quadrants, and most clock hours were statistically significant, with the right eye showing significantly thicker RNFL than the left eye. Park and associates investigated the interocular and intraocular hemiretinal RNFL asymmetry in normal subjects using Stratus OCT.9 With regard to interocular comparison, they also found that the right eye had significantly thicker average and sectoral RNFL, with a mean difference in average RNFL thickness 6 times higher than that in our study. Budenz assessed the interocular symmetry in RNFL thickness after scanning 108 pairs of normal eyes using the standard and fast RNFL thickness acquisition protocols of the Stratus OCT.8 The average RNFL of the right eye was significantly thicker than that of the left eye by 1.3 μm on the standard scan (P = .004) and by 1.2 μm on the fast scan (P = .026). In sectoral analysis, the left eye had thicker RNFL in only 1 clock hour, whereas the right eye’s RNFL was thicker in 6 clock hours. In the Sydney Childhood Eye Study,12 a population-based study including 1172 6-year-old children, measurements of RNFL thickness obtained with Stratus OCT showed a nonsignificant difference of 0.4 μm between the 2 eyes.
The cutoff limit of interocular difference normalcy of 8.8 μm for average RNFL thickness found in the present study is lower than 11.7 μm earlier found by Budenz and 16 to 17 μm found in 95% of children who participated in the Sydney Childhood Eye Study.8,12 Although our value is lower as a result of higher-resolution images, automatic circle placement, and measurement accuracy provided by Cirrus HD-OCT compared with Stratus OCT, the greater interocular difference found in the Sydney Childhood Eye Study is not well understood.12 Budenz postulated that their greater difference in interocular symmetry may be the result of a decrease in symmetry with age.8 Quadrant and clock-hour RNFL thicknesses in the Sydney Childhood Eye Study showed greater interocular differences than average, as reported by Budenz and in the current study.8,12 Although interocular asymmetry in RNFL thickness is not a new finding, the use of SD OCT in the current study revealed that the amount of interocular difference is lower than that previously reported using other imaging technologies. Therefore, our results are clinically relevant and are worth communicating.
The interocular symmetry in RNFL thickness also has been studied using other imaging devices. Essock and associates and Kurimoto and associates found no difference between the right and left eye average RNFL thickness measured by scanning laser polarimetry.13,14 Although the former study found similar RNFL thickness measurements in the inferior retina of both eyes, but significantly thicker RNFL in the superior retina of the left eye, the latter reported that the right eye RNFL was significantly thicker in 4 temporal sectors, whereas the left eye RNFL was thicker in only 2 nasal sectors. It is worth noting that in these 2 studies, the peripapillary RNFL circle was divided into 16 sectors of 22.5 degrees each compared with 12 sectors of 30 degrees each in Stratus- or Cirrus-OCT-based studies, making it difficult to compare their sectoral RNFL thickness measurements directly with ours. Gherghel and associates measured the RNFL thickness with scanning laser ophthalmoscopy in 314 normal eyes of 117 subjects and found statistically significant interocular differences in the average and nasal RNFL thickness, with the left eye displaying higher values.15 Scanning laser ophthalmoscopy also was used in another study that included 882 eyes of 1764 normal individuals.16 Significantly higher average RNFL values were observed in right eyes than left eyes. Overall, there is inconsistency as to which eye has thicker RNFL than the other, suggesting the lateral predominance varies from one individual to another. The mechanism behind the interocular differences in RNFL thickness in healthy eyes is not well understood. However, significant interocular asymmetries have been demonstrated histologically by comparing the density of RGC axons of the 2 eyes,17 suggesting the anatomic origin of interocular asymmetry in RNFL thickness. Because the RNFL is made of RGC axons assembled into fiber bundles separated by tunnels of tissue made of glial and Müller cells,18 the asymmetry may be the result of the difference in the density of RGC, glial and Müller cells, or both. Indeed, several previous studies have provided evidence that there is a wide range of RNFL thicknesses in the normal population, probably resulting from the known variation in total number of RGC axons among normal individuals.19 –21 Thus, it seems logical to speculate that a larger number of RGC axons is associated with more glial cells. If this is true, then normal eyes probably differ not only in the number of RGC axons, but also in the number of glial cells within the RNFL. The contribution of other structures such as blood vessels to thickness of the RNFL also might be accounted for, as recently shown by Hood and associates.22 Thus, the difference across studies may be the result of RNFL morphologic differences in study populations.
Although interocular difference in RNFL thickness significantly correlated with differences in axial length, CDR, disc area, and VCDR, the relationships accounted for less than 6% of the variability. Interocular difference in VCDR was the only variable showing a significant relationship with difference in RNFL thickness in the study by Budenz.8 Further analysis in our study revealed that only differences in axial length and disc area were significant predictors of interocular asymmetry in RNFL thickness, as also reported by Budenz and associates.23 In contrast, Park and associates and Kurimoto and associates could not demonstrate the relationship between interocular difference in average RNFL thickness and difference in axial length.2,14 Clinically, our finding signifies that interocular differences in axial length and disc area should be accounted for when considering interocular asymmetry in RNFL thickness for diagnosing early glaucoma. There is abundant evidence from histologic and imaging studies that RNFL thickness declines with increasing age, as also shown by our results. Our finding that interocular difference in average RNFL thickness remained stable with increasing age suggests that aging evenly affects the RNFL in both eyes of normal subjects. One earlier study reported a nonsignificant slight tendency toward increased differences,8 whereas another found that aging significantly increased the interocular difference in average RNFL thickness.24 Although it is not exactly known why these discrepancies across studies exist, we believe our observation is likely to be plausible because there is no basis to believe that in normal eyes, RGC axons in one eye die at a faster rate than in the other, unless a pathologic event comes into play.
The present study may have some limitations, one of which is the relatively small number of participants in different ethnic groups, which may make one question the generalizability of our conclusions. However, we strongly believe that the results of the current study are suggestive and need to be considered, particularly in view of the lack of effect of ethnicity on interocular difference in average RNFL thickness. Nevertheless, other studies with larger sample sizes may be warranted to confirm our findings.
To summarize, interocular difference in average RNFL thickness of normal individuals should not exceed 9 μm if measured with Cirrus HD-OCT. Differences greater than this value should be considered suggestive of early glaucomatous optic neuropathy if used in association with other clinical parameters or risk factors for glaucoma.
Acknowledgments
PUBLICATION OF THIS ARTICLE WAS SUPPORTED BY AN UNRESTRICTED GRANT FROM THE RESEARCH TO PREVENT BLINDNESS, Inc, New York, New York, and unrestricted research support from Carl Zeiss Meditec, Inc, Dublin, California. The following investigators have disclosed a financial interest in Carl Zeiss Meditec: Jeffrey M. Liebmann, grant support; Mary K. Durbin, employee of Carl Zeiss Meditec. Involved in: Design of study (D.L.B.); Collection and management of data (D.L.B., Robert T. Chang, O’Rese J. Knight, Jeffery M. Liebmann, Christopher K. Leung, Dennis S. Lam, James H. Peace, Christopher A. Girkin, John S. Werner, John L. Keltner, Esther Kim, Gadi Wollstein, Joel S. Schuman, Hiroshi Ishikawa, Robert J. Noecker); Analysis and interpretation of data (J.-C.M., D.L.B., M.K.D.); Preparation of the manuscript and statistical analysis and interpretation (J.-C.M., M.K.D., D.L.B.); Review and approval of the manuscript (D.L.B.; R.T.C., O.J.K., J.M.L., C.K.L., D.S.L., J.H.P., C.A.G., J.S.W., J.L.K., E.K., G.W., J.S.S., H.I., R.J.N.). The manufacturing company did not play any role other than providing equipment for normative data collection and making the data set available. The study design and the decision to publish were those of the corresponding author. The study was approved by the institutional review board at each participating center and adhered to the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
Biography

Jean-Claude Mwanza, MD, MPH, PhD, is an Assistant Scientist in the Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida. His research interests include imaging in glaucoma, early glaucoma detection and glaucoma progression.
APPENDIX
THE CIRRUS OCT NORMATIVE DATA STUDY GROUP
Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida, USA: Donald L. Budenz (Principal Investigator), Robert T. Chang, and O’Rese J. Knight.
New York Eye and Ear Infirmary, New York, New York, USA: Jeffery M. Liebmann (Principal Investigator).
Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong, China: Christopher K. Leung (Principal Investigator), Dennis S. Lam.
United Medical Research Institute, Inglewood, California, USA: James H. Peace (Principal Investigator).
Department of Ophthalmology, University of Alabama, Birmingham, Alabama, USA: Christopher A. Girkin (Principal Investigator).
Department of Ophthalmology & Visual Science, University of California Davis, Sacramento, California, USA: John S. Werner (Principal Investigator), John L. Keltner, Esther Kim.
UPMC Eye Center, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA: Gadi Wollstein (Principal Investigator), Joel S. Schuman, Hiroshi Ishikawa, Robert J. Noecker.
References
- 1.Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41(3):741–748. [PubMed] [Google Scholar]
- 2.Armaly MF. Cup-disc ratio in early open-angle glaucoma. Doc Ophthalmol. 1969;26(1):526–533. doi: 10.1007/BF00944008. [DOI] [PubMed] [Google Scholar]
- 3.Fishman RS. Optic disc asymmetry. A sign of ocular hypertension. Arch Ophthalmol. 1970;84(5):590–594. doi: 10.1001/archopht.1970.00990040592006. [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA, Enger C, Katz J, Sommer A, Scott R, Gilbert D. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112(5):644– 649. doi: 10.1001/archopht.1994.01090170088028. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright MJ, Anderson DR. Correlation of asymmetric damage with asymmetric intraocular pressure in normal-tension glaucoma (low-tension glaucoma) Arch Ophthal-mol. 1988;106(7):898–900. doi: 10.1001/archopht.1988.01060140044020. [DOI] [PubMed] [Google Scholar]
- 6.Lewis RA, Johnson CA, Adams AJ. Automated perimetry and short wavelength sensitivity in patients with asymmetric intraocular pressures. Graefes Arch Clin Exp Ophthalmol. 1993;231(5):274–278. doi: 10.1007/BF00919105. [DOI] [PubMed] [Google Scholar]
- 7.Feuer WJ, Anderson DR. Static threshold asymmetry in early glaucomatous visual field loss. Ophthalmology. 1989;96(9):1285–1297. doi: 10.1016/s0161-6420(89)32724-2. [DOI] [PubMed] [Google Scholar]
- 8.Budenz DL. Symmetry between the right and left eyes of the normal retinal nerve fiber layer measured with optical coherence tomography (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:252–275. [PMC free article] [PubMed] [Google Scholar]
- 9.Park JJ, Oh DR, Hong SP, Lee KW. Asymmetry analysis of the retinal nerve fiber layer thickness in normal eyes using optical coherence tomography. Korean J Ophthalmol. 2005;19(4):281–287. doi: 10.3341/kjo.2005.19.4.281. [DOI] [PubMed] [Google Scholar]
- 10.Grant WM, Burke JF., Jr Why do some people go blind from glaucoma? Ophthalmology. 1982;89(9):991–998. doi: 10.1016/s0161-6420(82)34675-8. [DOI] [PubMed] [Google Scholar]
- 11.Caprioli J, Miller JM. Measurement of relative nerve fiber layer surface height in glaucoma. Ophthalmology. 1989;96(5):633–639. doi: 10.1016/s0161-6420(89)32837-5. [DOI] [PubMed] [Google Scholar]
- 12.Huynh SC, Wang XY, Burlutsky G, Mitchell P. Symmetry of optical coherence tomography retinal measurements in young children. Am J Ophthalmol. 2007;143(3):518–520. doi: 10.1016/j.ajo.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 13.Essock EA, Sinai MJ, Fechtner RD. Interocular symmetry in nerve fiber layer thickness of normal eyes as determined by polarimetry. J Glaucoma. 1999;8(2):90–98. [PubMed] [Google Scholar]
- 14.Kurimoto Y, Matsuno K, Kaneko Y, Umihira J, Yoshimura N. Asymmetries of the retinal nerve fibre layer thickness in normal eyes. Br J Ophthalmol. 2000;84(5):469– 472. doi: 10.1136/bjo.84.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gherghel D, Orgul S, Prunte C, et al. Interocular differences in optic disc topographic parameters in normal subjects. Curr Eye Res. 2000;20(4):276–282. [PubMed] [Google Scholar]
- 16.Hermann MM, Theofylaktopoulos I, Bangard N, Jonescu-Cuypers C, Coburger S, Diestelhorst M. Optic nerve head morphometry in healthy adults using confocal laser scanning tomography. Br J Ophthalmol. 2004;88(6):761–765. doi: 10.1136/bjo.2003.028068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dichtl A, Jonas JB, Naumann GO. Retinal nerve fiber layer thickness in human eyes. Graefes Arch Clin Exp Ophthalmol. 1999;237(6):474– 479. doi: 10.1007/s004170050264. [DOI] [PubMed] [Google Scholar]
- 18.Radius RL, Anderson DR. The histology of retinal nerve fiber layer bundles and bundle defects. Arch Ophthalmol. 1979;97(5):948–950. doi: 10.1001/archopht.1979.01020010506027. [DOI] [PubMed] [Google Scholar]
- 19.Sony P, Sihota R, Tewari HK, Venkatesh P, Singh R. Quantification of the retinal nerve fibre layer thickness in normal Indian eyes with optical coherence tomography. Indian J Ophthalmol. 2004;52(4):303–309. [PubMed] [Google Scholar]
- 20.Mikelberg FS, Drance SM, Schulzer M, Yidegiligne HM, Weis MM. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. 1989;96(9):1325–1328. doi: 10.1016/s0161-6420(89)32718-7. [DOI] [PubMed] [Google Scholar]
- 21.Jonas JB, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Histomorphometry of the human optic nerve. Invest Ophthalmol Vis Sci. 1990;31(4):736–744. [PubMed] [Google Scholar]
- 22.Hood DC, Anderson S, Rouleau J, et al. Retinal nerve fiber structure versus visual field function in patients with ischemic optic neuropathy. A test of a linear model. Ophthalmology. 2008;115(5):904–910. doi: 10.1016/j.ophtha.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114(6):1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funaki S, Shirakashi M, Funaki H, et al. Relationship between age and the thickness of the retinal nerve fiber layer in normal subjects. Jpn J Ophthalmol. 1999;43(3):180–185. doi: 10.1016/s0021-5155(99)00015-5. [DOI] [PubMed] [Google Scholar]