Abstract
Background
Vascularized composite allotransplantation of the eye is an appealing, novel method for reconstruction of the nonfunctioning eye. The authors’ group has established the first orthotopic model for eye transplantation in the rat. With advancements in immunomodulation strategies together with new therapies in neuroregeneration, parallel development of human surgical protocols is vital for ensuring momentum toward eye transplantation in actual patients.
Methods
Cadaveric donor tissue harvest (n = 8) was performed with orbital exenteration, combined open craniotomy, and endonasal approach to ligate the ophthalmic artery with a cuff of paraclival internal carotid artery, for transection of the optic nerve at the optic chiasm and transection of cranial nerves III to VI and the superior ophthalmic vein at the cavernous sinus. Candidate recipient vessels (superficial temporal/internal maxillary/facial artery and superficial temporal/facial vein) were exposed. Vein grafts were required for all anastomoses. Donor tissue was secured in recipient orbits followed by sequential venous and arterial anastomoses and nerve coaptation. Pedicle lengths and calibers were measured. All steps were timed, photographed, video recorded, and critically analyzed after each operative session.
Results
The technical feasibility of cadaveric donor procurement and transplantation to cadaveric recipient was established. Mean measurements included optic nerve length (39 mm) and caliber (5 mm), donor artery length (33 mm) and caliber (3 mm), and superior ophthalmic vein length (15 mm) and caliber (0.5 mm). Recipient superficial temporal, internal maxillary artery, and facial artery calibers were 0.8, 2, and 2 mm, respectively; and superior temporal and facial vein calibers were 0.8 and 2.5 mm, respectively.
Conclusion
This surgical protocol serves as a benchmark for optimization of technique, large-animal model development, and ultimately potentiating the possibility of vision restoration transplantation surgery.
Restoration and even preservation of a functional eye for treatment of congenital differences, following injury, degenerative disease, or other acquired abnormality has long been a clinical challenge. Vascularized composite allotransplantation represents the latest paradigm shift in approaches to reconstruction of complex defects resulting from trauma and congenital abnormality. Face, abdominal wall, lower extremity, hand, and upper extremity transplants have now successfully been performed in a combined total of over 100 known patients in five countries.1 As this treatment continues to grow in application, so does the scope to which it might be applied.2,3
Given the highly specialized function of the eye and its unique anatomical components, vascularized composite allotransplantation of the eye is appealing for restoration, replacement, and reconstruction of the nonfunctioning eye. With the global prevalence of blindness estimated to be nearly 40 million, the potential for such treatment is vast.4 Furthermore, given that at least three patients known to have received face transplants to date have had loss of at least one functioning eye, concomitant eye transplantation is an obvious reconstructive adjunct for similar clinical scenarios in the future.5
With the advances in microsurgery and immunomodulation that have heralded successful vascularized composite allotransplantation, together with new therapies in neuroregeneration, strategies for overcoming the challenges previously thought to undermine the possibility of eye transplantation are now emerging. For vascularized composite allotransplantation of the human eye to become a clinical reality, it is necessary to develop a reliable animal model for testing of immunomodulation and neuroregeneration therapies and to perform cadaveric studies for optimization of surgical technique ahead of advancing to large-animal primate testing and ultimately eye transplantation in actual patients. Our group has established the first viable orthotopic model for vascularized eye transplantation in the rat. Maintenance of structural integrity and viability have been confirmed by slit-lamp examination, optical coherence tomography, and histology.6 Others have described the hurdles to optic nerve regeneration following injury or neurodegeneration; increasing retinal ganglion cell survival, overcoming the inhibitory environment of the optic nerve, enhancing retinal ganglion cell intrinsic axon growth potential, and optimizing the mapping of retinal ganglion cell connections back into their targets in the brain.7 Furthermore, investigation of neurotrophin mediation of retinal ganglion cell survival and optic nerve function, genetic intervention for optic nerve regeneration and protection, and prospects of optic nerve regeneration and protection by stem cells are ongoing.8 Full-length axon regeneration and partial recovery of simple visual behaviors have been demonstrated in an adult mouse optic nerve injury model.9
The present study attempts for the first time to develop surgical protocols for procurement and implantation of a transplanted eye, in a human cadaveric model, as a vital step to achieving eye transplantation in actual patients. This will improve scientific knowledge of ocular and periorbital anatomy as applied to vascularized composite allotransplantation, and introduces technical capability in approaches to ocular tissue harvest and implantation. This surgical protocol parallels our institution’s animal model investigations of eye vascularized composite allotransplantation. This will serve as a benchmark for further optimization and refinement of technique, and as a springboard for development of a large-animal primate model for study. Ultimately, the long-term objective is to potentiate the scope of face transplants to include eye tissue and revolutionize the clinical practice of management of visual impairment and blindness by introducing the possibility of vision restoration transplantation surgery.
MATERIALS AND METHODS
Preserved injected human cadaveric heads (n = 8) underwent donor and recipient procedures. Bilateral transplants were performed between two cadavers in each surgical session (a total of four transplants). A globe and periorbita model was adopted, consisting of the entire orbital contents including the globe; periorbita; optic nerve; cranial nerves III, IV, and VI; ophthalmic artery with a portion of the adjacent carotid artery; and the superior ophthalmic vein. Neurovascular pedicle lengths and calibers were measured using calipers. All measurements were verified by two investigators. All steps were timed, photographed, video recorded, and critically analyzed after each operative session. All activities were compliant with regulations of the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Decedents and the Office for Oversight of Anatomic Specimens.
Donor Procedure
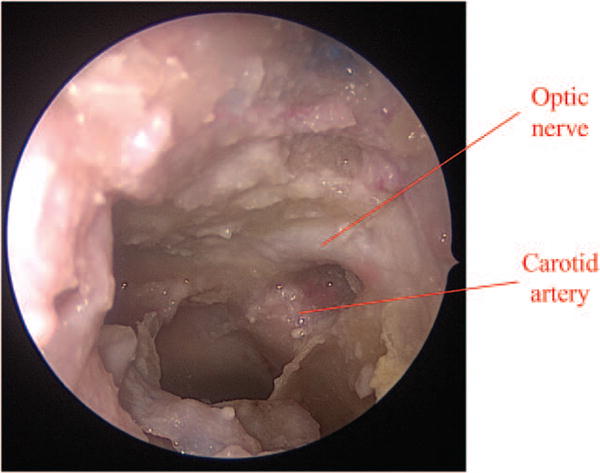
Donor procurement requires combined transorbital, endonasal, and transcranial approaches to allow for 360-degree decompression of the bony structure of the orbit and orbital apex (Fig. 1). First, endonasal resection of middle turbinate, maxillary enterostomy, total ethmoidectomy, and bilateral sphenoidotomy are performed to gain access to the medial and inferior orbit, orbital apex, and canalicular segment of the optic nerve (Fig. 2, above). Then, a small posterior septectomy is created to allow for binarial access. The lamina papyracea and medial orbital floor, orbital apex, and bony optic canal are decompressed, keeping the periorbita intact (Fig. 2, below). An endoscopic endonasal transplanum approach is performed to gain access to the optic chiasm, ophthalmic artery, and carotid artery.10 Initially, the dura is preserved to minimize the duration of the associated cerebrospinal fluid leak during the procedure. (See Video, Supplemental Digital Content 1, which shows the endonasal approach to decompress the orbital apex, http://links.lww.com/PRS/B935.) Subsequently, with a coronal incision, the temporalis is reflected and an extended pterional craniotomy is made (Fig. 3). Extradural dissection of the anterior and middle cranial fossae provides access for removal of the orbital roof and lateral orbital wall. After transection of the meningo-orbital band, the superior orbital fissure and lateral wall of the cavernous sinus are exposed. The roof of the optic canal is drilled out all the way to the medial wall where the dura had been previously exposed from the endonasal approach. The anterior clinoid process is removed to provide access to the lateral optic canal. At this point, the frontotemporal dura is opened in a curvilinear fashion. The optic nerve and internal carotid artery are identified after opening the opticocarotid cistern (Fig. 4). Transorbital exenteration is then performed to include globe, muscles, fat, suspensory ligaments, and trochlea within the periosteum of the orbit. The lateral orbital apex is decompressed, the superior orbital vein is identified and preserved, and the canthal tendons are disarticulated on each side. Next, again through an endonasal approach, the dura of the planum sphenoidale is opened to the suprasellar cistern. The optic chiasm, medial optic nerve, ophthalmic artery, and carotid artery are identified. The medial dural ring of the medial clinoid is incised to allow separation of the optic nerve from the internal carotid artery (Fig. 5). Returning transcranially, the optic nerve is followed posteriorly and transected before it reaches the optic chiasm. The oculomotor nerve is identified at its entrance into the oculomotor triangle of the cavernous sinus; the oculomotor triangle dura is opened to expose the oculomotor nerve traveling at the roof of the cavernous sinus; the transection of the nerve is completed before entering the triangle to maximize its length. Similarly, the trochlear nerve is sectioned at its cisternal segment before entering the cavernous sinus, the ophthalmic nerve is transected right after the trigeminal ganglion as it enters the cavernous sinus, and the abducens nerve is sectioned at the distal Dorello canal. The ophthalmic artery is not transected, but the internal carotid artery is ligated proximally at the paraclival-petrous segment and distally at the supraclinoidal segment just before the origin of the posterior communicating artery. Finally, en bloc mobilization of the cavernous sinus and superior orbital fissure along with the orbital apex and full globe-periorbita-orbital contents are obtained to deliver the donor specimen (Fig. 6).
Fig. 1.
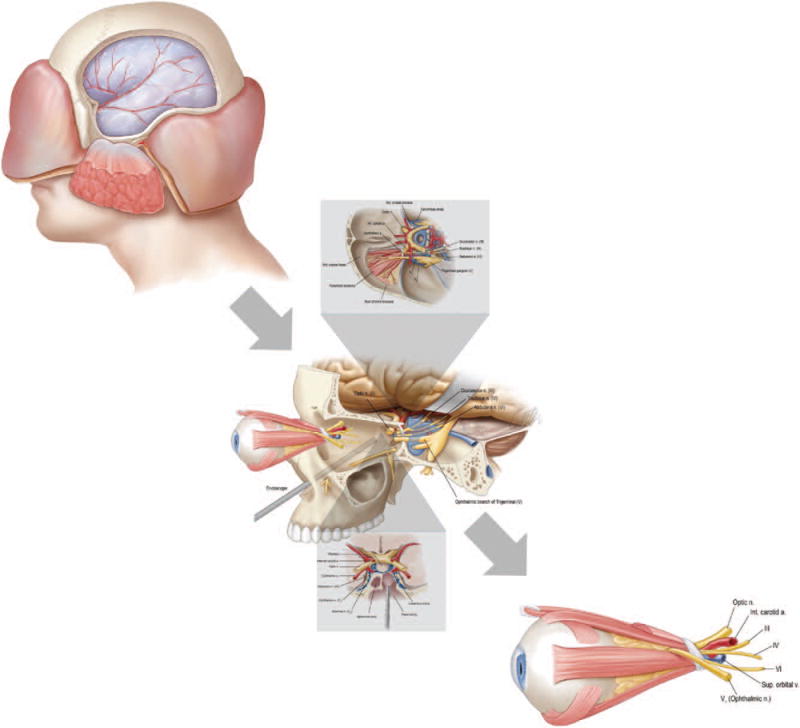
Schematic representation of donor procurement requiring combined transorbital, endonasal, and transcranial approaches to allow for 360-degree decompression of the bony structure of the orbit and orbital apex.
Fig. 2.
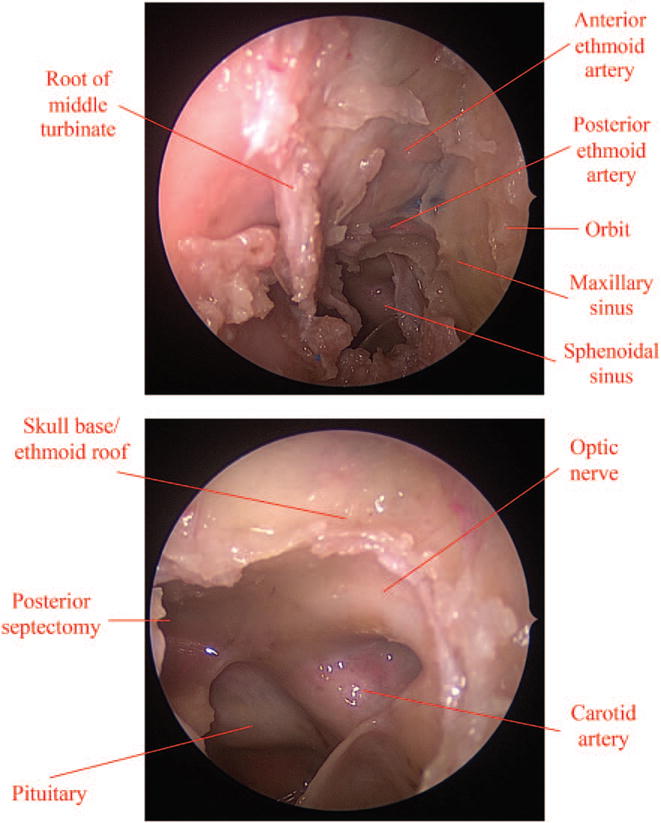
Donor procurement requires a combined endonasal and transcranial approach to decompress the orbital apex. (Above) First, endonasal resection of middle turbinate, maxillary enterostomy, total ethmoidectomy. and bilateral sphenoidotomy are performed. (Below) A small posterior septectomy, the lamina propecia, medial orbital floor, orbital apex, and bony optic canal are decompressed.
Video 1.
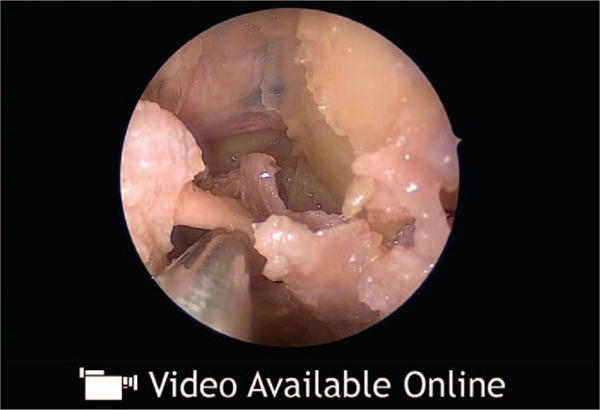
Supplemental Digital Content 1 shows the endonasal approach to decompress the orbital apex, http://links.lww.com/PRS/B935.
Fig. 3.
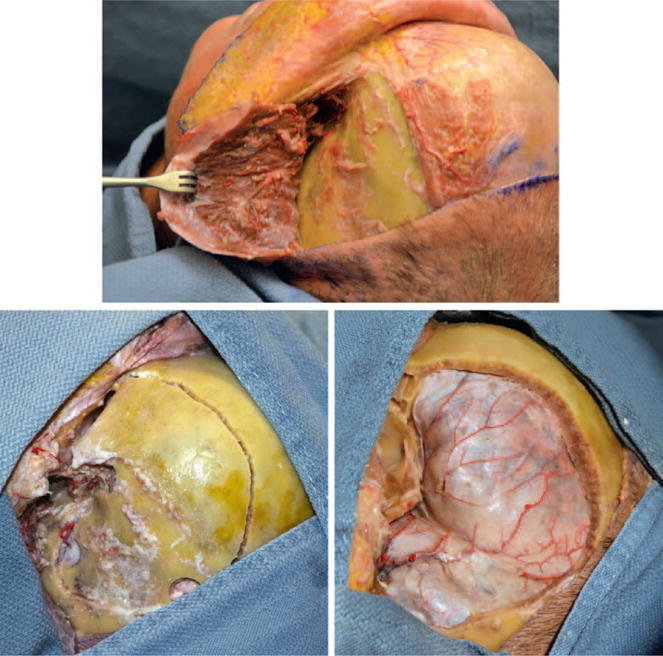
With a coronal incision, the temporalis is reflected and an extended pterional craniotomy is made.
Fig. 4.
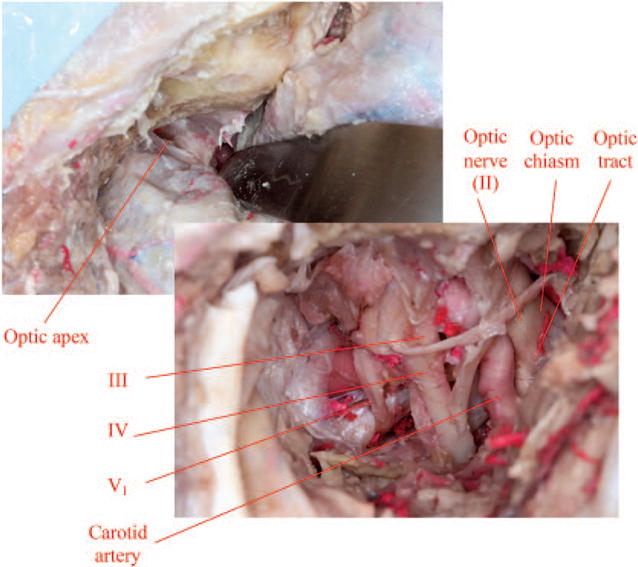
Anterior clinoidectomy with removal of the orbital roof exposes the cavernous sinus, superior orbital fissure, optic nerve, and internal carotid artery (note that cranial nerve VI lies deepest and is not visualized in this image).
Fig. 5.
The medial dural ring around the optic nerve is cut to separate it from the internal carotid artery.
Fig. 6.
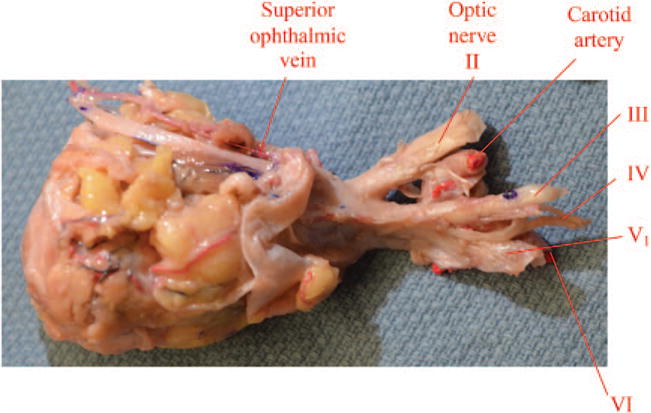
Transection of the optic nerve, occulomotor nerve, trochlear nerve, ophthalmic nerve, and abducens nerve is performed at the cavernous sinus transcranially and the carotid artery is ligated in the paraclival space to deliver the donor specimen.
Recipient Procedure
A recipient defect model was recreated by the same methods described for donor harvest, with combined transorbital, endonasal, and transcranial orbital apex decompression and exenteration, such that cranial nerves III, IV, V1, and VI are isolated at the entrance into the cavernous sinus, and the optic nerve is isolated at the cisternal space before joining the chiasm. Similarly, the ophthalmic artery is not directly ligated, but the carotid artery is isolated proximal and distal to the ophthalmic artery origin. The recipient procedure thereafter requires harvesting of vein grafts for both arterial and venous anastomoses, recipient vessel exposure, inset of donor tissue, arterial and venous anastomoses, and sequential coaptation of cranial nerves (Fig. 7). Choice of recipient vessels would in clinical reality be determined based on recipient anatomy. Candidate recipient vessels exposed include the superficial temporal artery and vein if available, although these could be damaged in any potential injury. Another option for the recipient artery is the internal maxillary artery; exposed by the coronal incision and turndown of the temporalis muscle with an extended lateral orbitotomy approach as in middle cerebral artery bypass.11 However, the accompanying venous plexus here is not amenable to venous anastomosis. Alternatively, the facial artery and vein are exposed through a Risdon incision and are a good size match, but require long vein grafts. Vein grafts from recipient facial vessels are tunneled through the temporalis. All candidate recipient vessels would require vein grafting to provide sufficient length for anastomosis to the donor pedicle. Vein graft anastomoses to recipient artery and vein are performed (Fig. 8) before inset of donor tissue, which is secured with 3-0 permanent Prolene sutures (Ethicon, Inc., Somerville, N.J.) parachuted into drill holes in four orbital quadrants. Arterial anastomosis is then performed from vein grafted recipient vessel to donor carotid artery transcranially using standard microsurgical techniques with 9-0 Ethilon sutures (Ethicon) (Fig. 9). Similarly, venous anastomosis to the donor superior ophthalmic vein is performed transcranially using standard microsurgical techniques with 9-0 Ethilon sutures (Fig. 10). Then, sequential coaptation of cranial nerves from deep to superficial (cranial nerves VI, V1, IV, III, and II) is performed (Fig. 11). (See Video, Supplemental Digital Content 2, which shows the transcranial optic nerve coaptation, http://links.lww.com/PRS/B936.) Finally, the bone graft is replaced, the temporalis resuspended, and the coronal incision closed. Endoscopic endonasal nasoseptal flap repair of the skull base can then aid in cerebrospinal fluid leak prevention.
Fig. 7.
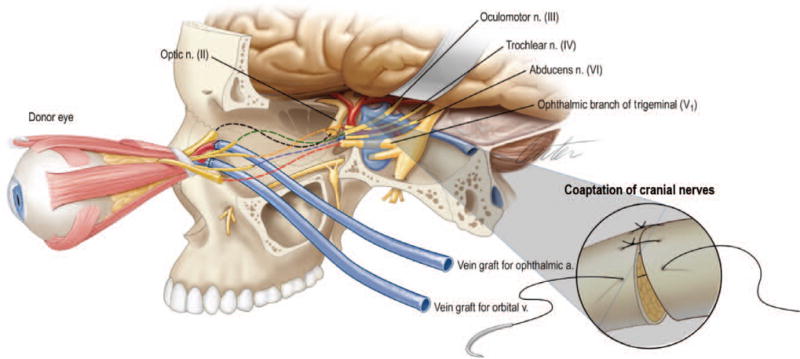
Schematic representation of the recipient procedure, which requires harvesting of vein grafts for both arterial and venous anastomoses, recipient vessel exposure, inset of donor tissue, arterial and venous anastomoses, and sequential coaptation of cranial nerves.
Fig. 8.
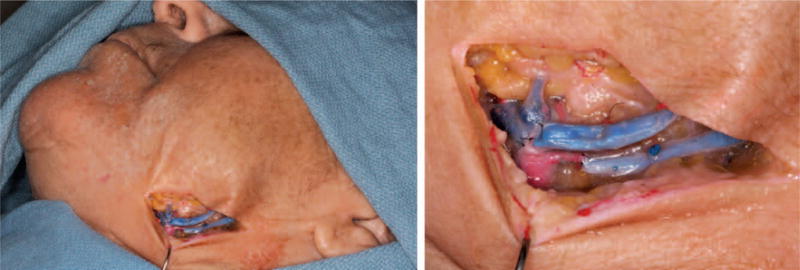
Recipient procedure, which requires harvesting of vein grafts for both arterial and venous anastomoses, recipient vessel exposure, inset of donor tissue, arterial and venous anastomoses, and sequential coaptation of cranial nerves. Vein graft anastomoses to the recipient facial artery and vein.
Fig. 9.
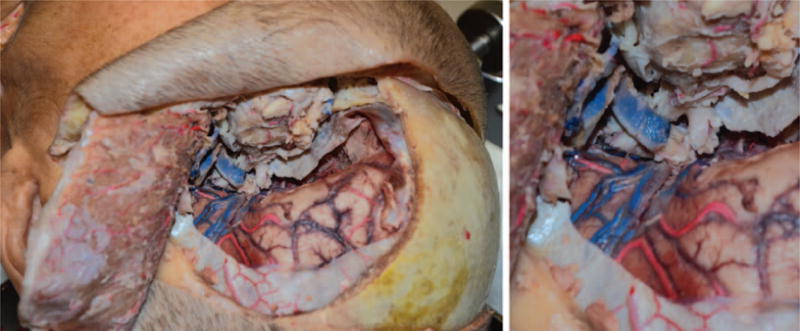
Arterial anastomosis from vein grafted recipient vessel to donor carotid artery.
Fig. 10.
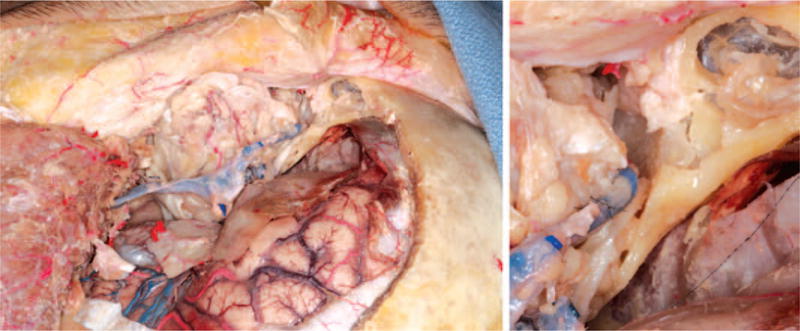
Venous anastomosis to donor superior ophthalmic vein.
Fig. 11.
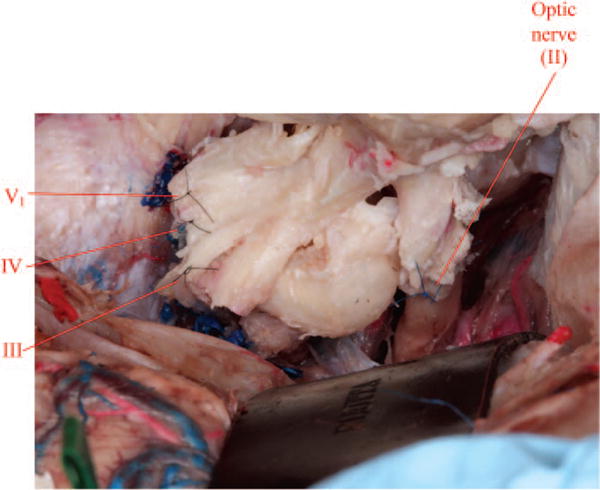
Coaptation of cranial nerves from deep to superficial (VI, V1, IV, III, and II) is performed (note that cranial nerve VI lies deepest and is not visualized in this image).
Video 2.
Supplemental Digital Content 2 shows the transcranial optic nerve coaptation, http://links.lww.com/PRS/B936.
RESULTS
The technical feasibility of cadaveric donor procurement and transplantation to cadaveric recipient was established with donor and recipient procedures as described. Mean measurements of donor neurovascular pedicle length and caliber are listed in Table 1. Similarly, recipient vessel calibers and lengths of vein graft required are listed in Table 2. Donor and recipient procurement timings are listed in Table 3 and Table 4 respectively.
Table 1.
Donor Measurements
| Mean Pedicle Length (mm) | Mean Caliber (mm) | |
|---|---|---|
| Ophthalmic artery | 13.5 ± 0.5 | 1 ± 0.04 |
| Ophthalmic artery with cuff of internal carotid | 33 ± 1.6 | 3 ± 0.2 |
| Superior ophthalmic vein | 15 ± 2.6 | 0.5 ± 0.2 |
| Optic nerve (II) | 14 ± 1.5 | 5 ± 0.4 |
| Occulomotor nerve (III) | 10 ± 1.8 | 3 ± 0.7 |
| Trochlear nerve (IV) | 12 ± 1.0 | 0.8 ± 0.2 |
| Ophthalmic nerve (V1) | 14 ± 1.0 | 2 ± 0.03 |
| Abducens nerve (VI) | 10 ± 0.7 | 4 ± 0.5 |
Table 2.
Recipient Measurements
| Mean Caliber (mm) | Mean Vein Graft Length Required (mm) | |
|---|---|---|
| Superficial temporal artery | 0.8 ± 0.03 | 50 ± 10 |
| Superficial temporal vein | 0.8 ± 0.05 | 70 ± 10 |
| Internal maxillary artery | 2 ± 0.0 | 30 ± 0 |
| Facial artery | 2 ± 0.1 | 120 ± 5 |
| Facial vein | 2.5 ± 0.5 | 150 ± 5 |
Table 3.
Donor Procedure Timings
| Mean Time (min) | |
|---|---|
| Endonasal approach and decompression | 57 ± 11 |
| Transcranial approach and decompression | 63 ± 16 |
| Exenteration | 27 ± 3 |
| Dissection and transection of nerves and vessels | 30 ± 7 |
| Back-table preparation | 13 ± 3 |
Table 4.
Recipient Procedure Timings
| Mean Time (min) | |
|---|---|
| Harvest of vein grafts | 12 ± 2 |
| Superficial temporal artery and vein exposure | 7 ± 2 |
| Internal maxillary artery exposure | 29 ± 7 |
| Facial artery and vein exposure | 16 ± 4 |
| Inset of donor tissue | 22 ± 5 |
| Arterial anastomosis | 11 ± 3 |
| Venous anastomosis | 13 ± 3 |
| Cranial nerve coaptation (VI, V1, IV, III, and II) | 61 ± 12 |
Notably, mean donor ophthalmic artery pedicle length and caliber were 13.5 ± 0.5 and 1 ± 0.04 mm, respectively; however, with a stem of paraclival internal carotid artery, these values can be increased to 33 ± 1.6 and 3 ± 0.2 mm. Mean optic nerve was 25 ± 1.5 mm from the orbital apex to the annulus of Zinn and 14 ± 0.4 mm from the annulus of Zinn to the optic chiasm, essentially 14 mm of mobile pedicle. Similarly, cranial nerves III to VI had mobile pedicle lengths of 10 to 14 mm. Recipient internal maxillary and facial artery were the closest size match to donor ophthalmic artery with supraclinoidal carotid artery origin. The internal maxillary artery required the shortest vein graft but had no accompanying usable vein for venous anastomosis. Facial vessels required the longest vein grafts. Superficial temporal vessels were the smallest in caliber.
DISCUSSION
Surgical solutions to blindness are lacking; treatments for globe avulsion, anophthalmia, composite traumatic defects of the eye, and neurodegenerative disease are limited and compromise the fundamental reconstructive principle of “replacing like with like,” which vascularized composite allotransplantation could potentially afford.12–16 Previously, the concept of eye transplantation had been thought of as unfeasible because of the inability of retinal ganglion cells to recover from injury, inadequate restoration of circulation, and immune rejection.17 Now, with success in other modalities of vascularized composite allotransplantation, together with animal models of eye transplantation,6,18 and emerging nerve regeneration strategies,7–9 the need to elucidate the technical details of the surgical procedures for eye transplantation is also an important forerunner to a clinical eye transplant program.
The cadaveric studies presented in this article are a further springboard toward a clinical reality for vascularized composite allotransplantation of the eye. Use of cadavers to model actual clinical scenarios carries the limitation that cadaveric tissues are not the equivalent of living tissue but rather have the artifact of processing that to some extent undermines any measurements obtained, and the assumption that surgical procedures in cadaveric tissue can be replicated in living humans is always an oversimplification. Nonetheless, our anatomical measurements are comparable to those described in the literature.19–23 As a cadaver study, this investigation is also limited by the surgical considerations of eye transplantation not addressed, including patient selection and the inability to test or measure any outcomes of viability or function; nor does it address the neuroregeneration and immunomodulation challenges essential to development of clinical eye transplantation. These are undergoing investigation in other studies at our institution. A globe and periorbita–only transplant model was selected as opposed to concomitant transplant of the eyelids or bony orbit or as part of a larger facial subunit. This model also assumes one “injury pattern” that the transplant procedure aims to reconstruct. The reality is that the multiple causes for visual impairment and blindness result in a multitude of differing anatomical and physiologic reconstructive needs. Nonetheless, this study aims to develop principles that hold relevance to all approaches to eye transplantation. Alterations to this protocol would be required for harvest as part of a larger composite face transplant. Depending on the extent and requirements of the donor tissue, a larger transplant unit may change the access requirements, potentially eliminating the need to decompress the orbital apex through an endonasal approach, for example. If a box osteotomy was used to procure the entire bony orbit, the need for transorbital exenteration would also be eliminated. The perfusion strategy would also likely need to be altered, as a single vessel would unlikely be able to supply both the face and ocular structures. The inclusion of the eyelids introduces the ideal of restoring the blink reflex; cranial nerve V1 is preserved in our current protocol, but cranial nerve VII would also need to be part of the procured tissue. These considerations are part of ongoing investigations at our institution.
This is the first description of combined transorbital, endonasal, and transcranial approaches to fully decompress the orbital apex. Significantly, the exposure of this approach facilitates en bloc mobilization of the cavernous sinus and superior orbital fissure and allows for the addition of a stem of carotid artery to the ophthalmic artery pedicle, increasing length and caliber to ease subsequent arterial anastomosis. This technique of en bloc harvesting of cavernous sinus, superior orbital fissure, and orbital apex has been previously performed in cases of aggressive surgical resection of benign (meningiomas) or malignant (sarcomas, carcinomas) tumors with curative intent. A further potential application of eye vascularized composite allotransplantation is in these cases of aggressive tumor resection affecting the only working eye following the surgical technique described in this article. The superior ophthalmic vein is used as the donor vein, rather than the inferior ophthalmic vein, as this is the dominant of the two.
The crux of the recipient procedure is the identification of optimal recipient artery and vein. The advantage of the superficial temporal vessels is that their exposure requires no further incision beyond the coronal incision already made and thus are the least invasive of the candidate vessels. However, their small caliber creates a size mismatch. The internal maxillary artery is an appealing option given its size match and proximity and therefore shortest vein graft requirement of the vessels considered for arterial inflow. However, disadvantages of this are that it necessitates an extended lateral orbitotomy for exposure; even then, the artery is positioned deep, rendering anastomosis technically challenging. Moreover, the accompanying venous plexus is not amenable to anastomosis and thus would require exposure of a vein at another site. The facial vessels, although requiring lengthy vein grafts, present a good size match and are easily exposed through a Risdon incision. Ultimately, the propensity for these different candidate vessels to support patent anastomoses requires testing in actual physiologic circulation such as in nonhuman primate studies. The extended pterional craniotomy allows for considerable access to coapt the cranial nerves; given its deeper anatomical location, the abducens nerve must first be coapted, followed sequentially by cranial nerves V1, IV, III, and II working deep to superficial. Importantly, this recipient protocol we believe to be safe, as each step replicates tested techniques, albeit in a novel context.
Ultimately, the success of any transplantation protocol depends on expeditious harvest and transfer to reduce ischemia time. This remains true in this transplant modality as with others. Retinal function of an enucleated eye is greatly decreased or absent within 5 minutes. Maintenance and restoration of retinal function as measured by electroretinography has been demonstrated ex vivo in isolated perfused eyes for up to 10 hours. In these studies, eyes were enucleated, and perfusion was initiated shortly thereafter. The eyes were perfused under positive pressure with various buffered perfusates.24–28 The sequencing of our proposed protocol is such that the recipient can be prepared before any ischemia time such that, on availability of the donor tissue, inset, vessel anastomosis, and nerve coaptation can begin immediately. The timings outlined in Tables 3 and 4 would obviously be longer in actual clinical scenarios (e.g., with physiologic bleeding) but nonetheless demonstrate the spectra of this surgery to be within the realm of the achievable.
CONCLUSION
This surgical protocol serves as a benchmark for optimization of technique, potentiating the scope of face transplants to include eye tissue and revolutionizing the clinical management of visual impairment and blindness by introducing the possibility of vision restoration transplantation surgery.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the VA Pittsburgh Healthcare System.
Footnotes
CLINICAL QUESTION/LEVEL OF EVIDENCE: Therapeutic, V.
Supplemental digital content is available for this article. Direct URL citations appear in the text; simply type the URL address into any Web browser to access this content. Clickable links to the material are provided in the HTML text of this article on the Journal’s website (www.PRSJournal.com).
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
A “Hot Topic Video” by Editor-in-Chief Rod J. Rohrich, M.D., accompanies this article. Go to PRSJournal.com and click on “Plastic Surgery Hot Topics” in the “Videos” tab to watch. On the iPad, tap on the Hot Topics icon.
References
- 1.Petyruizzo P, Lanzetta M, Dubernard JM, et al. The International Registry on Hand and Composite Tissue Transplantation. Transplantation. 2010;90:1590–1594. doi: 10.1097/TP.0b013e3181ff1472. [DOI] [PubMed] [Google Scholar]
- 2.Couzin-Frankel J. Second sight: Eye transplants are science fiction. A team of researchers wants to change that. Science. 2015;347:1194–1197. doi: 10.1126/science.347.6227.1194. [DOI] [PubMed] [Google Scholar]
- 3.Davidson EH, Wang EW, Komatsu C, et al. Clinical considerations in vascularized composite allotransplantation of the eye. J Craniofac Surg. 2016;27:1622–1628. doi: 10.1097/SCS.0000000000002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 5.Carty MJ, Bueno EM, Lehmann LS, Pomahac B. A position paper in support of face transplantation in the blind. Plast Reconstr Surg. 2012;130:319–324. doi: 10.1097/PRS.0b013e3182589b27. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Komatsu C, Wang B, et al. Evaluation of viability, structural integrity, and functional outcome after whole eye transplantation. Plast Reconstr Surg. 2015;135(Suppl):82. [Google Scholar]
- 7.Moore DL, Goldberg JL. Four steps to optic nerve regeneration. J Neuroophthalmol. 2010;30:347–360. doi: 10.1097/WNO.0b013e3181e755af. [DOI] [PubMed] [Google Scholar]
- 8.Limb GA, Martin KR, Sixth ARVO/Pfizer Ophthalmics Research Institute Conference Working Group Current prospects in optic nerve protection and regeneration: Sixth ARVO/Pfizer Ophthalmics Research Institute conference. Invest Ophthalmol Vis Sci. 2011;52:5941–5954. doi: 10.1167/iovs.10-6894. [DOI] [PubMed] [Google Scholar]
- 9.de Lima S, Koriyama Y, Kurimoto T, et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci USA. 2012;109:9149–9154. doi: 10.1073/pnas.1119449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koutourousiou M, Fernandez-Miranda JC, Stefko ST, Wang EW, Snyderman CH, Gardner PA. Endoscopic endonasal surgery for suprasellar meningiomas: Experience with 75 patients. J Neurosurg. 2014;120:1326–1339. doi: 10.3171/2014.2.JNS13767. [DOI] [PubMed] [Google Scholar]
- 11.Nossek E, Costantino PD, Eisenberg M, et al. Internal maxillary artery-middle cerebral artery bypass: Infratemporal approach for subcranial-intracranial (SC-IC) bypass. Neurosurgery. 2014;75:87–95. doi: 10.1227/NEU.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiratli H, Tümer B, Bilgiç S. Management of traumatic luxation of the globe: A case report. Acta Ophthalmol Scand. 1999;77:340–342. doi: 10.1034/j.1600-0420.1999.770319.x. [DOI] [PubMed] [Google Scholar]
- 13.Schneck M, Oshry T, Marcus M, Lifshitz T. Attempted bilateral manual enucleation (gouging) during a physical assault. Ophthalmology. 2003;110:575–577. doi: 10.1016/S0161-6420(02)01768-2. [DOI] [PubMed] [Google Scholar]
- 14.Gundlach KK, Guthoff RF, Hingst VH, Schittkowski MP, Bier UC. Expansion of the socket and orbit for congenital clinical anophthalmia. Plast Reconstr Surg. 2005;116:1214–1222. doi: 10.1097/01.prs.0000181653.38200.eb. [DOI] [PubMed] [Google Scholar]
- 15.Schusterman MA, Reece GP, Miller MJ. Osseous free flaps for orbit and midface reconstruction. Am J Surg. 1993;166:341–345. doi: 10.1016/s0002-9610(05)80328-9. [DOI] [PubMed] [Google Scholar]
- 16.Jackson IT, Tolman DE, Desjardins RP, Brånemark PI. A new method for fixation of external prostheses. Plast Reconstr Surg. 1986;77:668–672. doi: 10.1097/00006534-198604000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Ellenberg D, Shi J, Jain S, et al. Impediments to eye transplantation: Ocular viability following optic-nerve transection or enucleation. Br J Ophthalmol. 2009;93:1134–1140. doi: 10.1136/bjo.2008.155267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polat RM, Zor F, Isik S, et al. Evaluation of the optic nerve regeneration in anew orbital composite tissue allotransplantation model. Plast Reconstr Surg. 2012;130(Suppl):72–73. [Google Scholar]
- 19.Lang J, Kageyama I. The ophthalmic artery and its branches, measurements and clinical importance. Surg Radiol Anat. 1990;12:83–90. doi: 10.1007/BF01623328. [DOI] [PubMed] [Google Scholar]
- 20.Vilensky J, Robertson W, Suarez-Quian C. The Clinical Anatomy of the Cranial Nerves: The Nerves of “On Olympus Towering Top”. Ames, Iowa: Wiley-Blackwell; 2015. [Google Scholar]
- 21.Pinar YA, Govsa F. Anatomy of the superficial temporal artery and its branches: Its importance for surgery. Surg Radiol Anat. 2006;28:248–253. doi: 10.1007/s00276-006-0094-z. [DOI] [PubMed] [Google Scholar]
- 22.Nagase T, Kobayashi S, Sekiya S, Ohmori K. Anatomic evaluation of the facial artery and vein using color Doppler ultrasonography. Ann Plast Surg. 1997;39:64–67. doi: 10.1097/00000637-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Choi J, Park HS. The clinical anatomy of the maxillary artery in the pterygopalatine fossa. J Oral Maxillofac Surg. 2003;61:72–78. doi: 10.1053/joms.2003.50012. [DOI] [PubMed] [Google Scholar]
- 24.Shi J, Ellenberg D, Kim JY, et al. Restoration of electroretinogram activity in exenterated swine eyes following ophthalmic artery anastomosis. Restor Neurol Neurosci. 2009;27:351–357. doi: 10.3233/RNN-2009-0485. [DOI] [PubMed] [Google Scholar]
- 25.Gouras P, Hoff M. Retinal function in an isolated, perfused mammalian eye. Invest Ophthalmol. 1970;9:388–399. [PubMed] [Google Scholar]
- 26.Peachey NS, Green DJ, Ripps H. Ocular ischemia and the effects of allopurinol on functional recovery in the retina of the arterially perfused cat eye. Invest Ophthalmol Vis Sci. 1993;34:58–65. [PubMed] [Google Scholar]
- 27.Niemeyer G. The function of the retina in the perfused eye. Doc Ophthalmol. 1975;39:53–116. doi: 10.1007/BF00578759. [DOI] [PubMed] [Google Scholar]
- 28.Niemeyer G. Retinal research using the perfused mammalian eye. Prog Retin Eye Res. 2001;20:289–318. doi: 10.1016/s1350-9462(00)00029-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


